Abstract
Using the T-REx™ (Invitrogen, CA) gene switch technology, we previously generated a dominant-negative HSV-1 recombinant, CJ83193, capable of inhibiting its own replication as well as that of wild-type HSV-1 and HSV-2. It has been further demonstrated that CJ83193 is an effective vaccine against HSV-1 infection in a mouse ocular model. To ensure its safety and augment its efficacy, we generated an improved CJ83193-like HSV-1 recombinant, CJ9-gD, which contains a deletion in an HSV-1 essential gene and encodes an extra copy of gene-encoding glycoprotein D (gD) driven by the tetO-bearing hCMV major immediate-early promoter. Unlike CJ83193, which exhibits limited plaque-forming capability in Vero cells and expresses little gD in infected cells, CJ9-gD is completely replication defective, yields high-level expression of gD following infection, and cannot establish detectable infection in mouse trigeminal ganglia following intranasal and ocular inoculation. Mice immunized with CJ9-gD produced 3.5-fold higher HSV-1 neutralizing antibody titer than CJ83193-immunized mice, and were completely protected from herpetic ocular disease following corneal challenge with wild-type HSV-1. Moreover, immunization of mice with CJ9-gD elicited a strong HSV-1-specific T-cell response and led to an 80% reduction in latent infection by challenge wild-type HSV-1 compared with the mock-immunized control.
INTRODUCTION
The major clinical significance of herpes simplex virus type 1 and type 2 (HSV-1 & 2) is their ability to cause acute primary infection and to reactivate periodically from latency and cause recurrent infection. Although HSV infections are often asymptomatic, their clinical manifestations include orofacial infections, genital herpes, neonatal herpes, keratoconjunctivitis and herpes encephalitis (Koelle and Ghiasi, 2005; Stanberry et al., 2000; Whitley et al., 1998). HSV-2 is the primary cause of genital ulcer disease. HSV-1 infection often associates with orofacial blisters and herpetic ocular disease, which is the leading cause of virus-induced blindness in developed as well as less developed countries (Group, 1998; Kovac-Kovacic and Skaleric, 2000; Xu et al., 2002). Notably, there has been a significant increase in HSV-1-related genital herpes in recent years and in some developed countries or populations, HSV-1 infection has become a common cause of genital herpes (Lafferty et al., 2000; Lowhagen et al., 2000; Nilsen and Myrmel, 2000; Ribes et al., 2001; Roberts, 2005; Scoular et al., 2002; Tran et al., 2004). Although the severity of most symptomatic HSV infections can be reduced by antiviral treatment, there is no effective medication that can prevent primary HSV infections nor decrease the incidence of recurrences except for daily suppressive therapy. Thus, there is a strong need for a safe and effective vaccine against HSV infections (Koelle and Corey, 2008; Stanberry, 2004).
HSV gene expression was classified into three major phases during productive infection, named immediate-early (α), early (β), and late (γ), with late genes being further divided into two groups, γ1 and γ2 (Roizman, 2001). The expression of α genes requires no de novo protein synthesis and is activated by the virion-associated protein VP16 together with cellular transcription factors (Roizman, 2001). Whereas the expression of viral α and β genes does not depend on viral DNA replication, expression of γ genes is highly influenced by de novo viral DNA synthesis. Specifically, de novo viral DNA replication leads to increased expression of γ1 genes and inhibition of viral DNA replication blocks expression of γ2 genes.
Safety and efficiency in eliciting an effective host immune response are two major criteria in developing recombinant viral vaccines against wild-type viral infections. In the last decade, various forms of HSV replication-defective viruses and neuroattenuated, replication-competent mutants have been tested as potential live vaccines against HSV infection in several different animal models (Boursnell et al., 1997; Brehm et al., 1997; Da Costa et al., 1999; Farrell et al., 1994; Keadle et al., 2002; McLean et al., 1994; Meignier et al., 1988; Meignier et al., 1990; Morrison and Knipe, 1994; Nguyen et al., 1992; Osorio and Ghiasi, 2003, 2005; Parker et al., 2006; Prichard et al., 2005; Spector et al., 1998; Walker and Leib, 1998). Given that both replication-defective viruses and neuroattenuated mutants are replication competent in the context of wild-type virus, their use as a vaccine in humans does pose a safety concern, particularly in individuals who harbor latent HSV infection (Koelle and Ghiasi, 2005). Using the T-REx™ (Invitrogen, CA) gene switch technology developed in this laboratory and the dominant-negative mutant polypeptide UL9-C535C of HSV-1 origin binding protein UL9, we have established a new strategy for development of a safe and effective recombinant HSV vaccine against HSV-1 infection. Specifically, we constructed a replication-defective HSV recombinant, CJ83193, capable of inhibiting the replication of wild-type HSV-1 and HSV-2 in cell cultures (Yao and Eriksson, 1999, 2002) and in the central nervous system of mice co-inoculated with HSV-1 and CJ83193 (Augustinova and Yao, unpublished data). We demonstrate further that CJ83193 is an effective vaccine against HSV-1 infection in mice and is capable of eliciting long-term humoral and cell-mediated immunity comparable with that induced by wild-type HSV-1 (Augustinova et al., 2004).
HSV-1 encodes at least 12 glycoproteins, among which gB, gC, gD, gH, and gL are most abundantly expressed in infected cells and constitute the major HSV-1 glycoproteins on the viral envelope (Handler et al., 1996). gD is the major target for neutralizing antibodies against HSV infection (Cohen et al., 1984; Minson et al., 1986; Para et al., 1985; Sim and Watson, 1973). The role of gD in eliciting host T-cell response has also been illustrated (Koelle et al., 1994; Koelle et al., 1998b; Mikloska and Cunningham, 1998; Zarling et al., 1986a; Zarling et al., 1986b). We have shown that the expression of gD, a γ gene product, is significantly lower in CJ83193-infected cells than cells infected with wild-type HSV-1. We thus hypothesize that the vaccine efficacy of CJ83193 can be elevated if the expression of gD can be substantially increased.
Here we describe construction of a CJ83193-derived dominant-negative and replication-defective HSV-1 recombinant, CJ9-gD, which has a deletion in the essential UL9 gene and encodes an extra copy of the gD gene controlled by the tetO-bearing hCMV major immediate-early promoter. Thus, unlike CJ83193, which exhibits very limited plaque-forming ability in Vero cells and expresses little gD, CJ9-gD is completely replication defective and capable of expressing high levels of gD in infected cells. We show that CJ9-gD is a safer and more effective vaccine than CJ83193 in a mouse model of HSV-1 infection.
RESULTS
Construction and in vitro characterization of CJ9-gD
To introduce the HSV-1 gD gene under control of the tetO-hCMV IE promoter into the CJ9-lacZ genome at the UL9 locus (Fig. 1A), we transfected U2CEP4R-11 cells with 6 μg of Mfe I-linearized p9DNATO-gD and 0.1 μg of pcDNA-UL9 followed by CJ9-lacZ super-infection at an MOI of 5 PFU/cell. Progeny viruses were harvested at 24 h post infection and plaque assays were performed on RUL9-8 cell monolayers in the presence of neutral red and X-Gal. White plaques, indicating replacement of the Lac Z gene by the gD gene-containing fragment of p9DNATO-gD, were isolated and plaque purified three more times on RUL9-8 cell monolayers. CJ9-gD is a viral recombinant obtained after four rounds of plaque purification that exhibits no blue plaques in plaque assay in the presence of X-Gal, and expresses no detectable gD in infected U2CEP4R-11 cells in the absence of tetracycline and a high level of gD in the presence of tetracycline (unpublished data, Yao et. al), which demonstrates that the detected gD expression in CJ9-gD-infected U2CEP4R-11 cells is driven by the tetO-bearing hCMV major immediate-early promoter (Yao et al., 1998). Western blot analysis (Fig. 2A) shows that CJ9-gD expresses significantly higher levels of gD than CJ83193 and CJ9-lacZ in infected Vero cells. The replacement of the Lac Z gene by the gD gene at the UL9 locus in CJ9-gD was confirmed by PCR analysis of CJ83193, CJ9-lacZ, and CJ9-gD viral DNA with the UL9 sequence-specific primers that flank the lac Z gene- and the gD gene-containing DNA insert (data not shown). No viral plaques were detected on Vero cell monolayers when a total of 1.1 × 108 PFU of CJ9-gD virus was assayed, whereas an average of 1 plaque per 1 × 106 PFU of CJ83193 virus was detected on Vero cell monolayers.
Figure 1. Schematic diagram of genomes of HSV-1 recombinant CJ9-gD.
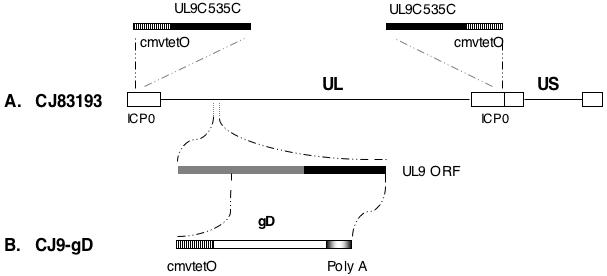
UL and US represent the unique long and unique short regions of the HSV-1 genome, respectively, which are flanked by their corresponding inverted repeat regions (open boxes). A. The replacement of the ICP0 coding sequences with DNA sequences encoding the DNA binding domain of UL9, UL9-C535C (black box), under control of the tetO-bearing hCMV major immediate-early promoter (vertical line box) in CJ83193 is shown above the diagram of the HSV-1 genome. An expanded UL9 ORF from UL9 locus is shown below the diagram. B. CJ9-gD was generated by replacing the Xcm I - Mlu I fragment encoding UL9 amino acids 217 to 803 within the UL9 ORF in CJ83193 with the HSV-1 gD gene under control of the tetO-bearing hCMV immediate-early promoter. The gradient box shows the poly A signal sequence of bovine growth hormone gene.
Figure 2. High-level expression of gD following CJ9-gD infection of Vero cells.
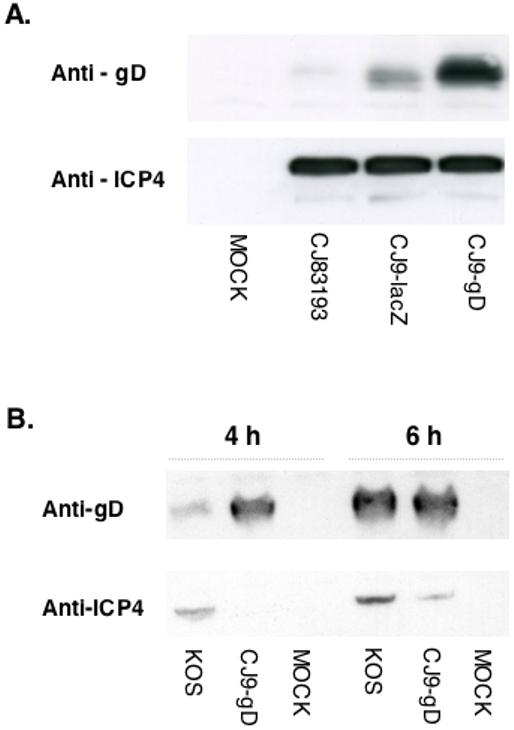
A.Vero cells were seeded at 1 × 106 cells per 60-mm dish. At 23 h after seeding, cells in duplicate were either mock-infected or infected with CJ83193, CJ9-lacZ, or CJ9-gD at an MOI of 5 PFU/cell. Infected cell extracts were prepared at 24 h post-infection. B. Vero cells were infected with the wild-type HSV-1 strain KOS, or CJ9-gD at an MOI of 20 PFU/cell. Infected cell extracts were prepared at 4 h and 6 h post-infection. Proteins in infected cell extracts were resolved on SDS-PAGE, followed by immunoblotting with a monoclonal antibody specific for ICP4 or a polyclonal antibody specific for gD.
The results shown in Fig. 2B demonstrate that a significantly higher level of gD was detected in CJ9-gD-infected cells than in wild-type virus KOS-infected cells at 4 h post-infection although levels of ICP4, the major immediate-early regulatory protein of HSV-1, were lower in CJ9-gD-infected Vero cells than in cells infected with KOS. Similar levels of gD were expressed in KOS- and CJ9-gD-infected Vero cells at 6 h post-infection.
Inhibition of wild-type HSV-1 replication by CJ9-gD
To assess the dominant-negative effect of UL9-C535C encoded by CJ9-gD on the replication of wild-type HSV-1, co-infection experiments were performed (Fig, 3A). As shown, co-infection of cells with CJ9-gD at an MOI of 3 and 5 PFU/cell led to a 70- and 145-fold decrease in wild-type virus production, respectively, compared with cells singly infected by KOS. Little reduction in wild-type virus yield was detected when a similar co-infection experiment was performed with a traditional HSV-1 replication-defective recombinant (Yao and Eriksson, 1999).
Figure 3. Trans-dominant-negative effect of CJ9-gD on replication of wild-type herpes simplex virus type 1 (HSV-1).
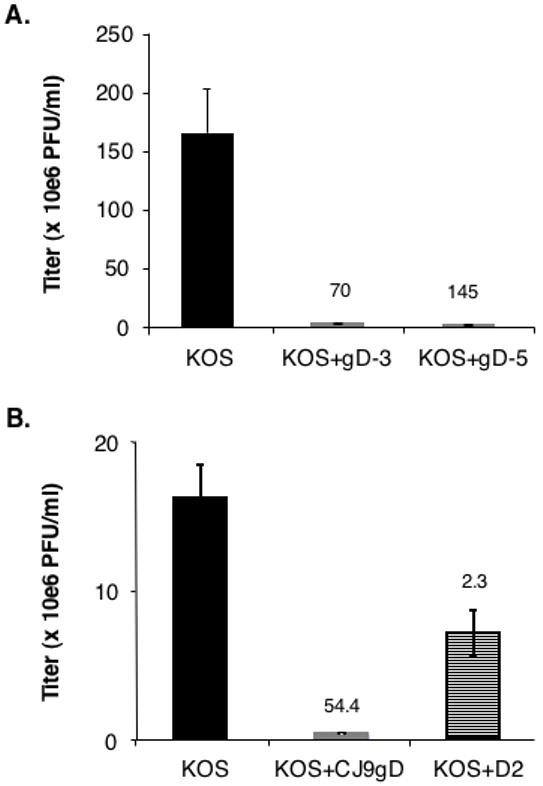
Vero cells were seeded at 7.5 × 105 per 60-mm dish. A. At 24 h post-seeding, cells in triplicate were infected with either wild-type HSV-1 strain KOS at an MOI of 1 PFU/cell, KOS at an MOI of 1 PFU/cell and CJ9-gD at an MOI of 3 PFU/cell, or KOS at an MOI of 1 PFU/cell and CJ9-gD at an MOI of 5 PFU/cell. B. Vero cells were infected with wild-type HSV-1 strain KOS at an MOI of 0.5 PFU/cell. At 1 h post-infection, cells were either mock-superinfected or superinfected with CJ9-gD, or an ICP4 deletion mutant, D2, at an MOI of 3 PFU/cell. Infected cells were harvested at 18 h post-wild-type HSV-1 infection and viral titers were determined on Vero cell monolayers. Viral titers are expressed as the mean +/- SD. Numbers on the top of the graph indicate the fold of reduction in wild-type virus yield between single infection and co-infection.
Fig. 3B represents an experiment in which monolayers of Vero cells were first infected with KOS at an MOI of 0.5 PFU/cell followed by superinfection with CJ9-gD or an HSV-1 ICP4 deletion mutant, D2, at an MOI of 3 PFU/cell at 1 h after KOS infection. It shows that CJ9-gD can also effectively prevent the wild-type HSV-1 infection in pre-infected cells; 54-fold less in wild-type virus synthesis was detected in cells superinfected with CJ9-gD than in those not super-infected, and a 2.3-fold reduction in KOS yield seen in cells superinfected with D2 is likely due to the co-synthesis of D2 virus in infected cells. D2 can not form plaque when assayed on Vero cells because of the lack of functional ICP4 protein (DeLuca and Schaffer, 1988).
CJ9-gD is more effective than CJ83193 in induction of anti-HSV-1 specific neutralization antibody response
We previously showed that mice immunized with CJ83193 at a dose of 2 × 107 PFU/mouse had ∼ 4-fold higher HSV-1 neutralizing antibody titer than mice immunized at a dose of 2 × 106 PFU/mouse (Augustinova et al., 2004) (Augustinova and Yao, unpublished data). To examine if high levels of gD expression by CJ9-gD could lead to a more effective induction of HSV-1 neutralizing antibody response, mice were either mock-immunized or immunized with CJ83193 or CJ9-gD at a dose of 2 × 106 PFU/mouse. The results (Fig. 4A) demonstrate that immunization with CJ9-gD elicited an average of 3.5-fold and 2-fold higher anti-HSV-1 specific neutralizing antibody titer than that of mice immunized with CJ83193 (p = 0.0065, unpaired t-test) and CJ9-lacZ (p = 0.02, unpaired t-test), respectively. CJ9-gD induced the same level of anti-HSV-1 specific neutralizing antibodies as wild-type HSV-1 KOS in mice (data not shown, Brans and Yao, unpublished work). The HSV-1 neutralizing antibody titer detected in the mock-immunized control was less than 2 (data not shown).
Figure 4. Induction of herpes simplex virus type 1 (HSV-1) -specific neutralizing and gD-specific antibodies.
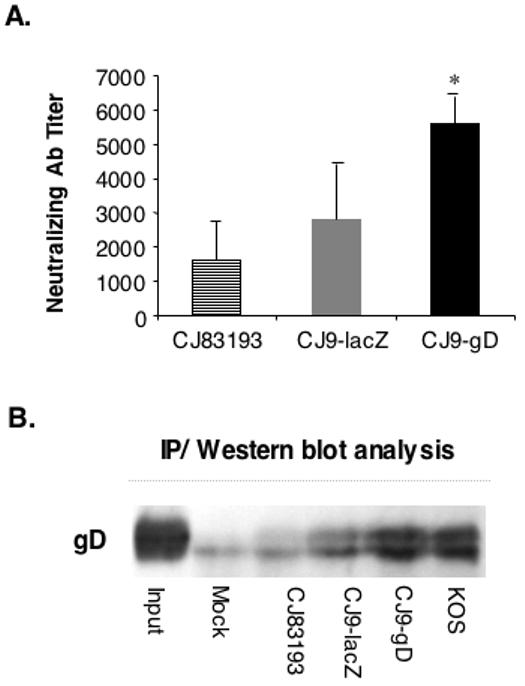
A. Blood was obtained from the tail veins of mice 4 weeks after primary immunization. Serum from mice immunized with CJ8319 (n = 4-7) and CJ9-gD (n = 4-16) was pooled and heat-inactivated. A series of two-fold dilutions of serum was made in normal growth medium in the presence of Low Tox M rabbit C. Two hundred fifty PFU of wild-type HSV-1 was added to each tube containing diluted serum and to control tubes containing titration growth medium and Low Tox M rabbit C in a final volume of 600 μl. The neutralizing titers were calculated as the final serum dilution leading to a 50% reduction in the number of HSV-1 PFU compared with that obtained in media plus complement alone. The graph represents results (the mean value +/- SD) from 4 independent experiments, which contained at least 4 mice per group. The titer of HSV-1 neutralizing antibody in mock-immunized mice was less than 2. B. Individual groups of female BALB/c mice (n = 8) were immunized with mock-infected cell lysate, CJ83193, CJ9-lacZ, CJ9-gD, or KOS at 2 × 106 PFU per mouse as described. Four weeks after primary immunization, blood was obtained from tail veins of mice and pooled. Sera from individual groups of mice were incubated with cell extract prepared from p9DNATO-gD transfected U2OS cells. gD/mouse IgG-specific complexes were precipitated with Protein A (Immunoprecipitation Starter Pack, Amersham Biosciences), resolved on SDS-PAGE, and probed with a gD-specific polyclonal antibody.
Fig. 4B shows that levels of anti-gD-specific antibodies in mice immunized with CJ9-gD were much higher than those detected in mouse serum from CJ83193 and CJ9-lacZ-immunized mice and is at levels similar to those induced in mice immunized with the wild-type HSV-1 KOS, suggesting that the elevated neutralization antibody titer in CJ9-gD immunized mice is the direct result of more efficient induction of anti-gD-specific antibodies. The enhanced effectiveness of CJ9-gD in induction of HSV-1 neutralizing antibodies has been further confirmed by the finding that CJ9-gD at a dose of 1 × 105 PFU/mouse elicits 2-fold higher HSV-1 neutralizing antibody titer than CJ83193 at a dose of 5 × 105 PFU/mouse (Brans and Yao, unpublished work).
We next examined the effectiveness of CJ9-gD in induction of HSV-1-specific T-cell immune response with cytokine assays (Fig. 5A) and CD4+ T cell (Fig. 5B) and CD8+ T cell (Fig. 5C) IFN-γ ELISPOT. Although less effective than wild-type HSV-1 (p = 0.138, statistically insignificant), immunization with CJ9-gD led to a significant induction of HSV-1-specific IFN-γ response compared with a mock-immunized control (5,768 pg/ml vs. 301 pg/ml, p = 0.014, unpaired t-test) (Fig. 5A). Similar levels of IL-2 response were detected in CJ9-gD and wild-type HSV-1 immunized mice, and the difference in levels of IL-2 expression between CJ9-gD- and CJ83193-immunized mice was statistically insignificant._ No statistical difference was seen in induction of IL-4 response among mock-immunized mice and mice immunized with KOS, CJ9-gD, and CJ83193 (Fig. 5A). In a separate experiment (data not shown) in which CJ9-gD and CJ9-lacZ were compared directly, IFN-γ response was similar, while higher IL-2 production was seen in CJ9-gD immunized mice (p = 0.56, statistically insignificant). IFN-γ ELISPOT assays (Fig. 5B) demonstrate that immunization with CJ9-gD elicited an HSV-1-specific CD4+ T-cell response similar to that seen in wild-type HSV-1, yielding 20-fold more IFN-γ spot-forming cells than the mock-immunized control (p=0.002). HSV-1-specific CD8+ T-cell response (Fig. 5C) was comparable between mice immunized with KOS and CJ9-gD (p = 0.07, statistically insignificant). Taken together, the results demonstrate that, like wild-type HSV-1, immunization with CJ9-gD can effectively elicit HSV-1-specfic Th1 T-cell response.
Figure 5. Induction of HSV-1-specific T cell response in CJ9-gD-immunized mice.
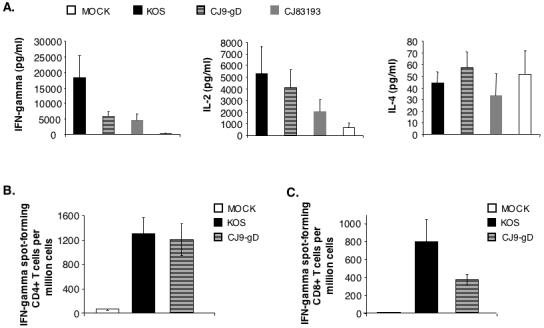
Cytokine assays (A). Female BALB/c mice were immunized with either mock-infected cell lysate, KOS, CJ9-gD or CJ83193 at 2 × 106 PFU per mouse. Splenocytes were isolated individually from mock-immunized (n = 4) and immunized mice (n = 4), and seeded in 24-well plates at 1.5 × 106 cells/well. Cells in duplicate were mock-stimulated or stimulated with UV-inactivated HSV-1 strain McKrae. Extracellular medium was collected at 72 h post-stimulation and levels of IFN-γ, IL-2, and IL-4 were determined. Cytokine production is presented as the mean concentration ± SEM in splenocytes isolated from 4 mice per group. IFN-γ ELISPOT assays (B and C). Splenocytes were prepared individually from mice (n = 4) either mock-immunized or immunized with KOS or CJ9-gD. For CD4+ T cell ELISPOT (B), CD4+ T cells were isolated from splenocytes using Dynal mouse CD-negative kit, and seeded in 96-well MultiScreen HTS™, IP sterile white filtration plates pre-coated with anti-mouse IFN-γ specific monoclonal antibody (AN18) at 5 × 104 and 1.5 × 105 cells/well. Cells in triplicate were stimulated with mock-infected or UV-inactivated HSV-1 strain McKrae infected- and mitomycin C-treated syngeneic CD11c+ BM-DCs. For detection of HSV-1-specific CD8+ T cells (C), splenocytes seeded in triplicate wells of 96-well filtration plates pre-coated with monoclonal antibody AN18 were stimulated with either mock-infected or HSV-1 strain McKrae-infected and mitomycin C-treated syngeneic CL7 cells. The IFN-γ spot-forming cells were detected as described in Materials and Methods. The HSV-1-specific IFN-γ spot-forming cells (SFC) are expressed as the mean ± SEM per million splenocytes from 4 mice per group.
Effect of immunization with CJ9-gD on acute viral replication and reactivation of latent infection by wild-type HSV-1
Four weeks after the initial immunization, individual groups of mice (n = 12) were challenged with HSV-1 strain mP following corneal scarification and eye swabs were taken on days 5 and 7 post-challenge. The degree of replication of challenge virus in trigeminal ganglia (TG) of mock-immunized and immunized mice was examined on day 6 post-challenge. Immunization with CJ83193, CJ9-lacZ, and CJ9-gD significantly reduced the replication of challenge virus in the eyes of immunized mice compared with the mock-immunized control on day 5 post-challenge (Fig. 6A). No challenge virus was detectable in eye swabs collected from immunized mice on day 7 post-challenge. On day 5 after challenge, the yield of challenge virus in eye swabs of mice immunized with mock-infected cell lysate was 13,410 PFU/ml, and no challenge virus was detected in eye swabs of mice immunized with CJ9-gD. An average of 21.25 PFU/ml and 6.87 PFU/ml of challenge virus was detected in mice immunized with CJ83193 and CJ9-lacZ, respectively.
Figure 6. Reduction of challenge wild-type HSV-1 replication in the eye and trigeminal ganglion of immunized mice.
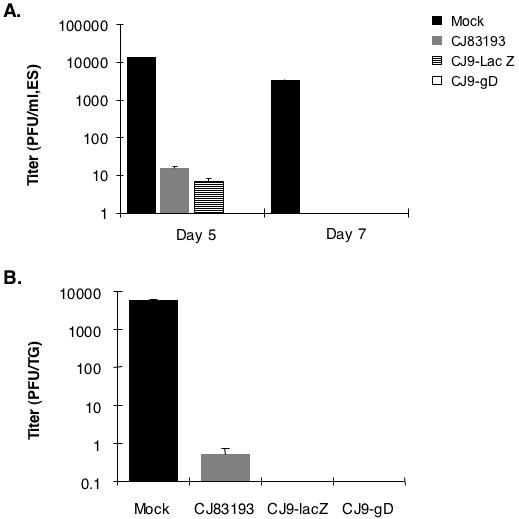
Female BALB/c mice were randomly divided into 4 groups of 12 mice each and immunized with either mock-infected cell lysate or CJ83193, CJ9-lacZ, or CJ9-gD as described. Four weeks after primary immunization, mice in all groups were challenged following corneal scarification with 2 × 105 PFU/eye of HSV-1 strain mP. Eye swabs were taken on days 5 and 7 post-challenge, while mouse trigeminal ganglia (n = 8) were prepared on day 6 post-challenge. Infectious viruses in individual eye-swab materials (A) and trigeminal ganglia (B) were assessed by standard plaque assay on Vero cell monolayers. Viral titers are expressed as the mean ± SEM in individual eye swabs and trigeminal ganglia of mice per group.
The yield of challenge virus in TG of mock-immunized mice on day 6 was 5,728 PFU/ml, and no challenge virus was detected in TG of CJ9-lacZ- and CJ9-gD-immunized mice (Fig. 6B). Among 8 TG harvested from CJ83193-immunized mice, one ganglion showed detectable challenge virus at 2 PFU/ml and no virus was present in the remaining 7 TG. In a similar experiment, we detected no challenge virus in TG (n = 8) among all immunized mice harvested on day 6 post-challenge (data not shown). Collectively, these results demonstrate that CJ9-gD is more effective than CJ83193 in prevention of acute replication of challenge wild-type HSV-1 in the corneal surface and TG of immunized mice.
Fig. 7A shows a representative of two independent experiments that demonstrate that immunization with CJ9-gD can completely prevent the development of the HSV-induced keratitis by challenge virus (n = 16), while a minor challenge virus-induced keratitis was observed in CJ83193- and CJ9-lacZ-immunized mice (n = 16) in the described experiments. It should be noted that all mock-immunized mice (n = 22) developed signs of encephalitis, and 18 mice died by day 12 post-challenge. No signs of virus-induced encephalitis were seen in immunized mice (n = 20-22).
Figure 7. Prevention of herpetic keratitis and establishment of latent infection by challenge virus in immunized mice.
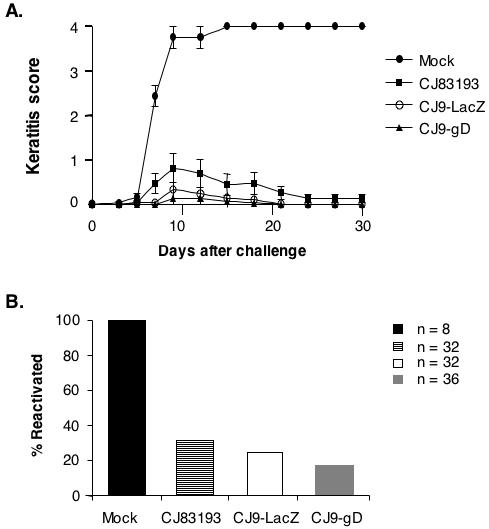
A. Female BALB/c mice were randomly divided into 4 groups of 8 mice each and immunized with mock-infected cell lysate, CJ83193, CJ9-lacZ, or CJ9-gD as described. Four weeks after initial immunization, both eyes of the mice were challenged with HSV-1 strain mP following corneal scarification. Individual eyes were scored for severity of keratitis during a 30-day follow-up period. The indicated values represent the mean score ± SEM of all eyes from each group of mice. B. Mice described in panel A and from a similar experiment in which mice were immunized with either mock-infected cell lysate (n = 10), CJ83193 (n = 8), CJ9-lacZ (n = 8), or CJ9-gD (n = 10) were euthanized 30 days after challenge. Trigeminal ganglia of mice from different groups were processed and co-cultivated individually onto Vero cell monolayers. After 5 days of co-cultivation, explants were harvested, processed, and assayed on Vero cell monolayers for the presence of infectious viruses.
Co-cultivation assays of TG harvested from mock-immunized and immunized mice (Fig. 7B) indicate that immunization with CJ83193, CJ9-lacZ, and CJ9-gD led to a 69%, 75%, and 80% lower reactivation of the challenge virus, respectively, than in mock-immunized control mice.
CJ9-gD cannot establish detectable infection in the trigeminal ganglia of mice following intranasal and ocular inoculation
Da Costa et al. (Da Costa et al., 1999) showed that deletion of two essential viral genes whose function is required for HSV-2 viral DNA replication could render the resulting viral recombinant dl5-29 unable to establish a stable latent infection in the TG of infected mice following intranasal inoculation of 1 × 106 PFU per mouse. Considering that CJ9-gD has a deletion in the essential UL9 gene and expresses a dominant-negative UL9-C535C polypeptide, which lead to either blocking (UL9 deletion) or significantly inhibit (UL9-C535C expression) viral replication, we examined whether CJ9-gD can establish latent infection in mice after intranasal and ocular inoculation. As shown (Fig. 8), while significant amounts of KOS DNA were present in TG DNA isolated from KOS inoculated mice, no viral DNA was detected in TG DNA of mice intranasally inoculated with CJ83193 and CJ9-gD (Fig. 8A). We did, however, detect an average of 8.3 copies/TG of CJ83193 viral DNA following ocular inoculation. Again, no CJ9-gD viral DNA was detectable in TG DNA of mice inoculated with CJ9-gD (Fig. 8B). These results indicate that CJ9-gD cannot establish a detectable infection in the TG of infected mice.
Figure 8. CJ9-gD cannot establish detectable infection in the mouse trigeminal ganglia after intranasal and ocular inoculation.
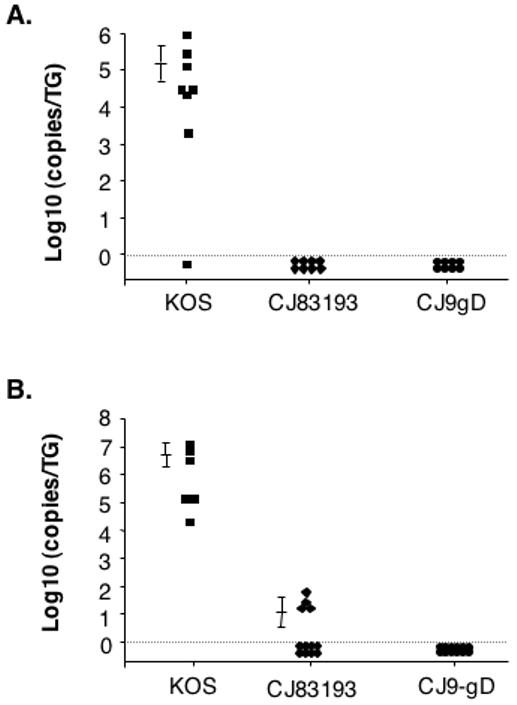
CD-1 mice were randomly assigned to 5 groups of 8 to 10 mice each and inoculated either intranasally (A) or ocularly after corneal scarification (B) at a dose of 1.5 × 106 PFU/nasal and 2 × 106 PFU/eye with KOS, 7134, d27, CJ83193, or CJ9-gD. As a negative control, CD-1 mice were also inoculated in a similar fashion with DMEM. Individual TG DNA was isolated on day 3 post-inoculation followed by real-time PCR analysis with primers specific to the HSV-1 DNA polymerase.
DISCUSSION
We constructed a new generation of dominant-negative and replication-defective HSV-1 recombinant, CJ9-gD, which has the essential UL9 gene deleted and encodes an extra copy of the gD gene under control of the tetO-bearing hCMV major immediate-early promoter. Because this promoter is fully functional in the context of the HSV-1 genome during acute viral infection and is independent of HSV-1 viral DNA replication (Johnson et al., 1992; Marconi et al., 1996; Mester et al., 1995; Stinski and Roehr, 1985; Yao and Eriksson, 1999), this design allows high and immediate-early expression of gD after CJ9-gD enters the cell. Indeed, CJ9-gD expresses much higher levels of gD than CJ83193 and CJ9-lacZ (Fig. 2A). We further showed that significantly higher amounts of gD were expressed in CJ9-gD-infected cells than that of wild-type virus-infected cells at 4 h post-infection. CJ9-gD represents the first genetically engineered HSV recombinant that expresses gD at the immediate-early phase of viral infection.
In addition to the tetO-bearing hCMV immediate-early promoter-driven gD gene inserted at the UL9 locus in the unique long region of the HSV-1 genome (Fig. 1), CJ9-gD retains the endogenous copy of gD gene in the unique short region of the viral genome. We have recently replaced the HSV-1 ICP47 gene with the Lac Z gene in CJ9-gD (Brans et al., unpublished study). Given that several Lac Z-expressing CJ9-gD isolates express similar levels of gD as CJ9-gD in infected Vero cells, and yields of CJ9-gD and CJ9-lacZ in RUL9-8 cells are similar, it seems that a single crossover homologous recombination between the two gD coding sequences within the CJ9-gD genome, which could lead to a replication-defective viral recombinant, is not a major factor that could influence the genetic stability of CJ9-gD. Alternatively, one can delete the endogenous copy of gD gene from the unique short region of CJ9-gD to eliminate this potential genetic instability.
Although CJ83193 cannot initiate acute productive infection in corneas of infected mice, nor can it reactivate from TG of mice latently infected by CJ83193 (Augustinova et al., 2004), CJ83193 does, however, possess a limited plaque-forming ability in Vero cells (Augustinova et al., 2004). The present study shows that CJ9-gD is completely replication defective in Vero cells. We detected no CJ9-gD viral DNA in the TG of mice inoculated by CJ9-gD following corneal inoculation under the described conditions, while limited copies of CJ83193 viral DNA were detected in the TG of mice inoculated with CJ83193. These results suggest that CJ9-gD is safer than CJ83193 as a vaccine candidate in clinical applications. It is worth noting that because CJ9-gD expresses high levels of the dominant-negative UL9-C535C polypeptide in normal cells, lacks the essential UL9 gene, and possesses a unique capability in inhibiting wild-type HSV infection in co-infected cells as well as cells first infected with wild-type HSV-1 (Fig. 3), it is reasonable to believe that CJ9-gD will be a safer vaccine candidate, at least in theory, than traditional replication-defective HSV recombinants that contain recessive mutations, particularly should recombinant viral vaccines be used as a therapeutic vaccine for recurrent HSV infection (Koelle and Ghiasi, 2005).
The effectiveness of CJ9-gD as a vaccine against wild-type HSV-1 infection was examined in a mouse model of HSV-1 infection. Mice immunized with CJ9-gD produce 3.5-fold higher anti-HSV-1-specific neutralizing antibodies than those detected in serum of CJ83193-immunized mice and at the same level as that induced in mice immunized with the wild-type HSV-1 KOS (Lu et al., unpublished data, Brans et al., manuscript submitted). It is further demonstrated that CJ9-gD is more efficient in inducing anti-gD-specific antibodies than CJ83193 and CJ9-lacZ in mice. We demonstrate that immunization with CJ9-gD elicits a similar degree of HSV-1-specific T cell response as KOS-immunized mice (Fig. 5). Consistent with these observations, mice immunized with CJ9-gD exhibited complete protection from herpetic disease following corneal challenge with wild-type HSV-1. The efficacy of CJ9-gD as an effective vaccine in prevention of HSV-1-induced genital disease has also been demonstrated in a mouse model of HSV-1 genital infection (Brans and Yao, manuscript study). We show further that CJ9-gD is significantly more effective than CJ83193 in protecting mice from HSV-1 genital infection. In the this study we detected no statistical difference in induction of IFN-γ and IL-2 responses between KOS and CJ9-gD, CJ9-gD and CJ83193 (Fig. 5A) or CJ9-gD and CJ9-lacZ (data not shown), suggesting that, compared with its dominant role in induction of HSV-1-specific neutralizing antibody response, gD does not appear to play a major role in eliciting overall HSV-1-specific Th-1 T-cell response during HSV-1 infection and/or following immunization with CJ9-gD in mice. This is probably not surprising, given that in addition to gD, CJ9-gD is capable of expressing various viral α, β, and γ1 gene products that effectively elicit T-cell responses (Koelle et al., 2001; Koelle et al., 1994; Koelle et al., 1998a; Mikloska and Cunningham, 1998; Mikloska et al., 2000), while gD is one of only four essential HSV-1 glycoproteins on the virus envelope that are required for virus to enter cells. Lastly, the present study indicates that immunization with CJ9-gD can lead to a significant reduction in reactivation efficiency of the challenge virus in immunized mice (20%) compared with mock-immunized controls (100%). Consistent with this observation, we recently showed that immunization with CJ9-gD prevents recurrent genital infection and disease in guinea pigs (Brans and Yao, unpublished work) and significantly reduces the frequency and magnitude of latent infection by the challenge wild-type HSV-1 in a guinea pig model of HSV-1 skin infection (Brans et al., 2008).
HSV-1 evolves several different strategies to evade host immune responses (Dubin et al., 1991; Eidson et al., 2002; Fruh et al., 1995; He et al., 1997; Hill et al., 1995; Sievers et al., 2002; York et al., 1994). ICP0 and ICP47 are the two HSV immediate-early proteins directly involved in host immune evasion following viral infection. ICP0 plays a key role in blocking IFN-induced inhibition of viral infection (Eidson et al., 2002), while ICP47 down-regulates the major histocompatibility complex (MHC) class I expression on the surface of infected cells (Fruh et al., 1995; Hill et al., 1995; York et al., 1994). The ability of CJ9-gD to express high levels of gD at the immediate-early phase of infection could lead to effective presentation of gD-specific peptide-MHC class I complexes at the cell surface before the onset of inhibition of ICP47 on MHC class I antigen presentation. Because ICP47 binds poorly to mouse and other murine transporter-associated protein (TAP) and is unable to block peptide transport and antigen presentation in mouse cells, the potential advantage of immediate-early expression of gD by CJ9-gD may not be fully appreciated in the tested mouse model. Additionally, considering the recently identified function of an HSV-1 delayed-early gene product, gB, in down-regulating the MHC class II antigen presentation in infected cells (Neumann et al., 2003) as well as the potential interference on antigen presentation by other HSV-encoded immune evasion molecules (Rouse and Kaistha, 2006), one can speculate that immediate-early expression of gD by CJ9-gD might elicit a more effective and potent gD-specific T-cell immune response than an HSV recombinant virus that expresses gD at the delayed-early phase of infection. This hypothesis is supported by an earlier study of Wachsman et al., which showed that immunization with vaccinia virus expressing HSV-1 gD by a viral early promoter was significantly more effective in induction of T-cell response as well as in protecting guinea pigs from wild-type HSV-2 infection than the vaccinia virus expressing gD under control of a viral late promoter, albeit two viral recombinants were capable of expressing similar levels of gD (Wachsman et al., 1989).
MATERIALS AND METHODS
Cells
African Green Monkey Kidney (Vero) cells and the osteosarcoma line U2OS cells were grown and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Aldrich) supplemented with 10% fetal bovine serum (FBS) in the presence of 100 U/ml penicillin G and 100 μg/ml streptomycin sulfate (Gibco)(Yao and Schaffer, 1995). U2OS cells are able to complement functionally for the HSV-1 ICP0 deletion (Yao and Schaffer, 1995). U2CEP4R11 cells are tetR-expressing U2OS cells that were maintained in DMEM plus 10% FBS and hygromycin B at 50 μg/ml (Yao et al., 1998). RUL9-8 cells were UL9-expressing U2CEP4R11 cells that were grown and maintained in DMEM plus 10% FBS supplemented with hygromycin B (50 μg/ml) and G418 (400 μg/ml) (Yao et al., 2006).
Plasmids
pcDNA-UL9 expresses UL9 from the HSV-1 UL9 promoter with the bovine growth hormone (BGH) polyadenylation signal sequence at its 3′ end (Yao et al., 2006). Plasmid pUL9BX-lacZ was generated by replacing the Xcm I-Bam HI fragment within the UL9 open reading frame in pcDNA-UL9 with DNA sequences encoding the Lac Z gene under the control of the hCMV major immediate-early promoter with the SV40 poly A signal at the 3′ end of the lac Z gene (Fig. 1). The Xcm I-Bam HI fragment encodes UL9 amino acids 217 to 535. pUL9-V is a derivative of pcDNA-UL9 with a deletion of a 17-bp Not I-Xba I fragment present in pcDNA3. Plasmid p9DNATO-B was constructed by replacing the Xcm I - Mlu I fragment containing UL9 amino acids 217 to 803 in plasmid pUL9-V with the Mlu I - Pvu II DNA fragment consisting of the tetO-hCMV-MCS-poly A transcription unit of pCDNA4-TO (Invitrogen, Carlbad, CA).
Plasmid p9DNATO-gD expresses the HSV-1 gD gene under control of the tetO-bearing hCMV major immediate-early promoter, which contains the Hind III - Ple I-gD gene-encoding fragment of pRE4 (the kind gift of Gary H. Cohen and Roselyn J. Eisenberg, University of Pennsylvania) in p9DNATO-B at the Hind III and Pme I sites.
Viruses
Wild-type HSV-1 strains, KOS and mP were propagated and plaque-assayed on Vero cells (Augustinova et al., 2004). CJ83193 is an HSV-1 recombinant in which both copies of the ICP0 gene are replaced by DNA sequences encoding UL9-C535C under the control of the tetO-bearing hCMV major immediate-early promoter (Fig. 1A) (Yao and Eriksson, 1999). It was propagated and assayed in U2CEP4R11 cells (Yao and Eriksson, 1999). D2, a KOS-derived ICP4-deletion mutant, was propagated and assayed in ICP4-expressing E5 cells (DeLuca and Schaffer, 1988). CJ9-lacZ was generated by replacing the essential UL9 gene in CJ83193 with the Lac Z gene under control of the hCMV major immediate-early promoter using plasmid pUL9BX-lacZ (Theopold et al., unpublished data).
SDS-PAGE and Western blot analysis
Vero cells seeded in 100 mm dishes at 2 × 106 cells/dish were mock-infected or infected with indicated viruses at MOIs of 10 or 20 PFU/cell. Cell extracts were prepared at 4 h, 6 h, or 15 h post-infection (Yao and Schaffer, 1994). Proteins in the cell extract were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9% acrylamide), transferred to polyvinylidene difluoride (PVDF) membranes, and probed with either a monoclonal antibody specific for HSV-1 immediate-early protein ICP4 (Fitzgerald Industries International, Inc., Concord, MA) or a rabbit polyclonal antibody (R45) specific for gD as described previously (Yao and Schaffer, 1994). R45 was the kind gift of Drs. Gary H. Cohen and Roselyn J. Eisenberg.
Mice
CD-1 mice and female BALB/c mice 6 to 8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in metal cages at four mice per cage and maintained on a 12-h light/dark cycle. Mice were allowed to acclimatize to the housing conditions for 1 week prior to experimentation. All animal experiments were conducted according to the protocols approved by Harvard Medical Area Standing Committee on Animals and the American Veterinary Medical association.
Acute infection and real-time PCR
CD-1 mice were anesthetized with sodium pentobarbital. Wild-type HSV-1 and HSV-1 recombinant viruses at indicated doses in a volume of 10 μl were inoculated either intranasally or to the eye after corneal scarification (Augustinova et al., 2004; Leib et al., 1989). Individual trigeminal ganglia (TG) were harvested on day 3 post-inoculation and TG DNA was isolated with the DNeasy tissue kit (Qiagen) and suspended in 60 μl of AE buffer. The presence of HSV-1 viral DNA was quantified by real-time PCR with a pair of primers specific to the HSV-1 DNA polymerase (Forward: 5′ GCT CGA GTG CGA AAA AAC GTT C, Reverse: 5′ CGG GGC GCT CGG CTA AC) as previously described (Brans et al., 2008)(Aldea et al., 2002). The minimal copies of HSV-1 viral DNA that can be reliably detected were 5 copies per reaction.
Immunizations and challenges
Six to 8-week-old female BALB/c mice were randomly divided into several groups and were immunized with either mock-infected cell lysate, KOS, CJ83193, CJ9-lacZ, or CJ9-gD at 2 × 106 PFU per mouse in a 20 μl volume s.c. with a 26-gauge needle. Mice were boosted s.c. with the same virus 2 weeks after primary immunization. Four weeks after the initial immunization, mice in all groups were challenged following corneal scarification with 2 × 105 PFU of HSV-1 strain mP per eye in a 10 μl volume (Morrison and Knipe, 1994). The ability of the challenge virus to cause acute infection in the eyes and TG at indicated time points was assayed on Vero cell monolayers as described previously (Augustinova et al., 2004).
In vitro co-cultivation assays were used for measuring the reactivation efficiency of challenge virus in mock-vaccinated and vaccinated mice 30 days after challenge with wild-type HSV-1 (Augustinova et al., 2004; Leib et al., 1989). In brief, at 30 days post-challenge, each trigeminal ganglion of mice from different groups was processed and co-cultivated individually onto Vero cell monolayers (Augustinova et al., 2004; Leib et al., 1989). After 5 days of co-cultivation, explants were harvested and assayed on Vero cell monolayers for the presence of infectious viruses.
Detection of HSV-1 neutralization antibodies
Blood was obtained from the tail veins of immunized or mock-immunized mice 1 day prior to immunization and 28 to 30 days after primary immunization. Neutralizing serum Ab titers were determined in the presence of complement and expressed as the final serum dilution leading to a 50% reduction in HSV-1 PFU relative to the HSV-1 PFU obtained in medium in the presence of complement (Augustinova et al., 2004; Bourne et al., 1996b).
Immunoprecipitation
U2OS cells seeded at 1 × 106 cells per 100-mm dish were mock-transfected or transfected with 10 ug of p9DNATO-gD by lipofectamine 2000 at 24 h post-seeding. Cell extracts were prepared at 24 h post-transfection (Yao and Schaffer, 1994). Immunoprecipitations were performed by mixing 5 μl of pooled serum collected from mock-immunized and immunized mice with 200 μl of cell extracts prepared above. The gD/mouse IgG-specific complexes were precipitated with Protein A (Immunoprecipitation Starter Pack, Amersham Biosciences), resolved on SDS-PAGE and probed with the anti-gD-specific polyclonal antibody, R45.
Cytokine assay
Two weeks after the second immunization, the spleens of immunized and mock-immunized mice were removed, and single cell suspensions were prepared by grinding spleens with two sterile glass slides. After removal of tissue debris and treatment of cell suspensions with ACK lysis buffer (0.15 M NH4CL, 1 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2-7.4), cells were re-suspended in DMEM/L-glutamine-plus complete medium (10% heat inactivated-FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μM 2-mercaptoethanol, and 1% non-essential amino acid) followed by Ficoll-Paque Plus (Amersham Biosciences) gradient isolation of lymphocytes. Cells were then seeded into 24-well plates at 1 × 106 cells per well in a volume of 1 ml. Cells in triplicate were either mock-stimulated with medium or with UV inactivated HSV-1 strain McKrae, at an MOI of 5 PFU/cell according to the titer prior to UV inactivation. As a positive control, cells in triplicate were stimulated with Con A at 2.5 μg/1 × 106 cells per ml. Cells were incubated at 37° C and the supernatant was collected at 72 h post-stimulation and stored at -80° C. Levels of IFN-γ, IL-2, and IL-4 were determined by an ELISA kit specific for mouse IFN-γ, IL-2, or IL-4 (Endogen, Pierce Biotechnology, Inc., Rockford, IL, USA).
IFN-γ ELISPOT assay
Splenocytes were isolated individually from mice either mock-immunized or immunized with KOS or CJ9-gD 10 to 12 weeks after primary immunization as described above. For CD4+ T cell ELISPOT assays, CD4+ T cells were isolated from splenocytes using Dynal mouse CD-negative kit and seeded in 96-well MultiScreen HTS™, IP sterile white filtration plates (Millipore Co., Billerica, MA) pre-coated with anti-mouse IFN-γ specific monoclonal antibody (AN18, 3321-3, Mabtech Inc., Cincinnati, OH) at 5 × 104 and 1.5 × 105 cells/well. Cells in triplicate were either stimulated with mock-infected or UV-inactivated HSV-1 strain McKrae-infected (5 PFU/cell) and mitomycin C-treated (50 μg/ml) syngeneic CD11c+ bone marrow-derived DCs (BM-DCs) at responder-to-stimulator ratios of 5:1 and 10:1, respectively. BM-DCs were prepared from bone marrow cells harvested from femurs and tibias of female BALB/c mice and cultured for 8 - 10 days with RPMI-1640 complete medium (10% heat inactivated-FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercaptoethanol, 2 mM L-glutamine) in the presence of GM-CSF at 20 ng/ml (PeproTech Inc., Rocky Hill, New Jersey) according to the protocol described by Lutz et al. (Lutz et al., 1999). Flow cytometric analysis using PE-labeled CD11c+ mouse-specific antibody shows that nearly 60% of day 7 and 65% of day 11 non-adherent cells in BM culture were CD11c+ DCs. For detection of HSV-1-specific CD8+ T cells, splenocytes isolated from mock-immunized and immunized mice were seeded in 96-well filtration plates pre-coated with anti-mouse IFN-γ specific monoclonal antibody (AN18) at 5 × 104 and 1.5 × 105 cells/well. Cells in triplicate were stimulated with either mock-infected or HSV-1 strain McKrae-infected (1 PFU/cell) and mitomycin C-treated (50 μg/ml) syngeneic CL7 cells (ATCC, Manassas, VA) at responder-to-stimulator ratios of 2:1 and 5:1, respectively. After incubation at 37 C for 24 h, wells were washed with PBS containing 0.05% Tween 20 followed by reaction with biotinylated IFN-γ specific monoclonal antibody (R4-6A2, Mabtech) at 250 ng/well at room temperature for 2 h. After incubation with Streptavidin-Alkaline Phosphatase (Mabtech), the IFN-γ spot-forming cells were detected by addition of BCIP/NBT substrate. Spots were counted in a dissecting microscope and the number of HSV-1-specific IFN-γ spot-forming cells (SFC) was expressed as the mean ± SEM per million splenocytes minus the SFC detected in the corresponding control wells stimulated with mock-infected BM-DCs or CL7 cells.
Clinical observations
Following immunization and subsequent challenge with wild-type HSV-1 strain mP, mice were observed daily during a 30-day follow-up period for signs of clinical illness (Augustinova et al., 2004). Eyes were examined with an ophthalmoscope for evidence of keratitis and scored for severity of disease (scale 0-4). The mean keratitis score for each group of mice was determined. A score of 0 represents a clear cornea; 1, 2, and 3 indicate corneal opacity from mild (less than 25% of corneal surface) to moderate (25-50% of cornea surface) and severe (50-75% of corneal surface), respectively. A score of 4 indicates total opacity of cornea with no posterior view.
Statistical analysis
For statistical analysis Student’s t-tests were performed. Results are considered to be statistically insignificant when the P value is greater than 0.05.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants 5RO1AI050880 and 5RO1GM051449 from the National Institutes of Health.
Abbreviations
- HSV
Herpes simplex virus
- gD
Glycoprotein D
Footnotes
The work was done in Boston, MA, USA.
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Aldea C, Alvarez CP, Folgueira L, Delgado R, Otero JR. Rapid detection of herpes simplex virus DNA in genital ulcers by real-time PCR using SYBR green I dye as the detection signal. J Clin Microbiol. 2002;40:1060–1062. doi: 10.1128/JCM.40.3.1060-1062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinova H, Hoeller D, Yao F. The dominant-negative herpes simplex virus type 1 (HSV-1) recombinant CJ83193 can serve as an effective vaccine against wild-type HSV-1 infection in mice. J Virol. 2004;78:5756–5765. doi: 10.1128/JVI.78.11.5756-5765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne N, Milligan GN, Schleiss MR, Bernstein DI, Stanberry LR. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine. 1996a;14:1230–1234. doi: 10.1016/s0264-410x(96)00027-8. [DOI] [PubMed] [Google Scholar]
- Bourne N, Stanberry LR, Bernstein DI, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996b;173:800–807. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- Boursnell ME, Entwisle C, Blakeley D, Roberts C, Duncan IA, Chisholm SE, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175:16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans R, Eriksson E, Yao F. Immunization with a Dominant-Negative Recombinant HSV Type 1 Protects against HSV-1 Skin Disease in Guinea Pigs. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.142. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Bonneau RH, Knipe DM, Tevethia SS. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GH, Dietzschold B, Ponce de Leon M, Long D, Golub E, Varrichio A, et al. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984;49:102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa XJ, Jones CA, Knipe DM. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci U S A. 1999;96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca NA, Schaffer PA. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988;62:732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G, Socolof E, Frank I, Friedman HM. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991;65:7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell HE, McLean CS, Harley C, Efstathiou S, Inglis S, Minson AC. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol. 1994;68:927–932. doi: 10.1128/jvi.68.2.927-932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, et al. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- Group THEDS Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- Handler CG, Eisenberg RJ, Cohen GH. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6070. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Morrison LA, Morris JL, Pepose JS, Stuart PM. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J Virol. 2002;76:3615–3625. doi: 10.1128/JVI.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Corey L. Herpes Simplex: Insights on Pathogenesis and Possible Vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Frank JM, Johnson ML, Kwok WW. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998a;72:7476–7483. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Ghiasi H. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr Eye Res. 2005;30:929–942. doi: 10.1080/02713680500313153. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998b;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac-Kovacic M, Skaleric U. The prevalence of oral mucosal lesions in a population in Ljubljana, Slovenia. J Oral Pathol Med. 2000;29:331–335. doi: 10.1034/j.1600-0714.2000.290707.x. [DOI] [PubMed] [Google Scholar]
- Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis. 2000;181:1454–1457. doi: 10.1086/315395. [DOI] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, et al. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowhagen GB, Tunback P, Andersson K, Bergstrom T, Johannisson G. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect. 2000;76:179–182. doi: 10.1136/sti.76.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Marconi P, Krisky D, Oligino T, Poliani PL, Ramakrishnan R, Goins WF, et al. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc Natl Acad Sci U S A. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CS, Erturk M, Jennings R, Challanain DN, Minson AC, Duncan I, et al. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994;170:1100–1109. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- Meignier B, Longnecker R, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Meignier B, Martin B, Whitley RJ, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162:313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- Mester JC, Pitha PM, Glorioso JC. Antiviral activity of herpes simplex virus vectors expressing murine alpha 1-interferon. Gene Ther. 1995;2:187–196. [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol. 1998;79:353–361. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- Mikloska Z, Ruckholdt M, Ghadiminejad I, Dunckley H, Denis M, Cunningham AL. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-gamma and IL-12 production. J Immunol. 2000;164:5167–5176. doi: 10.4049/jimmunol.164.10.5167. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- Minson AC, Hodgman TC, Digard P, Hancock DC, Bell SE, Buckmaster EA. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Knipe DM. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Eis-Hubinger AM, Koch N. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol. 2003;171:3075–3083. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Knipe DM, Finberg RW. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–7072. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen A, Myrmel H. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet Gynecol Scand. 2000;79:693–696. [PubMed] [Google Scholar]
- Osorio Y, Ghiasi H. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J Virol. 2003;77:5774–5783. doi: 10.1128/JVI.77.10.5774-5783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y, Ghiasi H. Recombinant herpes simplex virus type 1 (HSV-1) codelivering interleukin-12p35 as a molecular adjuvant enhances the protective immune response against ocular HSV-1 challenge. J Virol. 2005;79:3297–3308. doi: 10.1128/JVI.79.6.3297-3308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para MF, Parish ML, Noble AG, Spear PG. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985;55:483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JN, Pfister LA, Quenelle D, Gillespie GY, Markert JM, Kern ER, et al. Genetically engineered herpes simplex viruses that express IL-12 or GM-CSF as vaccine candidates. Vaccine. 2006;24:1644–1652. doi: 10.1016/j.vaccine.2005.09.051. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Kaiwar R, Jackman WT, Quenelle DC, Collins DJ, Kern ER, et al. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine. 2005;23:5424–5431. doi: 10.1016/j.vaccine.2005.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes JA, Steele AD, Seabolt JP, Baker DJ. Six-year study of the incidence of herpes in genital and nongenital cultures in a central Kentucky medical center patient population. J Clin Microbiol. 2001;39:3321–3325. doi: 10.1128/JCM.39.9.3321-3325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. Genital herpes in young adults: changing sexual behaviours, epidemiology and management. Herpes. 2005;12:10–14. [PubMed] [Google Scholar]
- Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe DM, aPMH, editor. Fields Virology. 4rd ed. Lippincott Williams & Wilkins; Philadelphia, Pa: 2001. pp. 2399–2459. [Google Scholar]
- Rouse BT, Kaistha SD. A tale of 2 alpha-herpesviruses: lessons for vaccinologists. Clin Infect Dis. 2006;42:810–817. doi: 10.1086/500141. [DOI] [PubMed] [Google Scholar]
- Scoular A, Norrie J, Gillespie G, Mir N, Carman WF. Longitudinal study of genital infection by herpes simplex virus type 1 in Western Scotland over 15 years. Bmj. 2002;324:1366–1367. doi: 10.1136/bmj.324.7350.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers E, Neumann J, Raftery M, SchOnrich G, Eis-Hubinger AM, Koch N. Glycoprotein B from strain 17 of herpes simplex virus type I contains an invariant chain homologous sequence that binds to MHC class II molecules. Immunology. 2002;107:129–135. doi: 10.1046/j.1365-2567.2002.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Watson DH. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973;19:217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Spector FC, Kern ER, Palmer J, Kaiwar R, Cha TA, Brown P, et al. Evaluation of a live attenuated recombinant virus RAV 9395 as a herpes simplex virus type 2 vaccine in guinea pigs. J Infect Dis. 1998;177:1143–1154. doi: 10.1086/515278. [DOI] [PubMed] [Google Scholar]
- Stanberry LR. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes. 2004;11(Suppl 3):161A–169A. [PubMed] [Google Scholar]
- Stanberry LR, Cunningham AL, Mindel A, Scott LL, Spruance SL, Aoki FY, et al. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30:549–566. doi: 10.1086/313687. [DOI] [PubMed] [Google Scholar]
- Stinski MF, Roehr TJ. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985;55:431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T, Druce JD, Catton MC, Kelly H, Birch CJ. Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex Transm Infect. 2004;80:277–279. doi: 10.1136/sti.2004.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman M, Aurelian L, Smith CC, Perkus ME, Paoletti E. Regulation of expression of herpes simplex virus (HSV) glycoprotein D in vaccinia recombinants affects their ability to protect from cutaneous HSV-2 disease. J Infect Dis. 1989;159:625–634. doi: 10.1093/infdis/159.4.625. [DOI] [PubMed] [Google Scholar]
- Walker J, Leib DA. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine. 1998;16:1–5. doi: 10.1016/s0264-410x(97)00164-3. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–553. doi: 10.1086/514600. quiz 554-545. [DOI] [PubMed] [Google Scholar]
- Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, et al. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J Infect Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- Yao F, Eriksson E. A novel anti-herpes simplex virus type 1-specific herpes simplex virus type 1 recombinant. Hum Gene Ther. 1999;10:1811–1818. doi: 10.1089/10430349950017491. [DOI] [PubMed] [Google Scholar]
- Yao F, Eriksson E. Inhibition of herpes simplex virus type 2 (HSV-2) viral replication by the dominant negative mutant polypeptide of HSV-1 origin binding protein. Antiviral Res. 2002;53:127–133. doi: 10.1016/s0166-3542(01)00207-8. [DOI] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J Virol. 1994;68:8158–8168. doi: 10.1128/jvi.68.12.8158-8168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Svensjo T, Winkler T, Lu M, Eriksson C, Eriksson E. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–1950. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- Yao F, Theopold C, Hoeller D, Bleiziffer O, Lu Z. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol Ther. 2006;13:1133–1141. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]
- York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Zarling JM, Moran PA, Burke RL, Pachl C, Berman PW, Lasky LA. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986a;136:4669–4673. [PubMed] [Google Scholar]
- Zarling JM, Moran PA, Lasky LA, Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986b;59:506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


