Abstract
Astrocytes participate in signaling via Ca2+ transients that spread from cell to cell across a multicellular syncytium. The effect, if any, of these Ca2+ waves on the transcription of CREB-dependent genes is not known. We report here that, unlike neurons, increasing intracellular Ca2+ in cultured mouse cortical astrocytes failed to activate CREB-dependent transcription, even though CREB was phosphorylated at serine 133. In contrast, both CREB phosphorylation and CREB-dependent transcription were robustly stimulated by increasing cAMP. The failure of Ca2+-activated transcription in astrocytes was correlated with the absence of CaMKIV, a Ca2+-dependent protein kinase required for Ca2+-stimulated gene transcription in neurons. The inability of Ca2+ to signal via CaMKIV may insulate CREB-dependent gene transcription in astrocytes from activation by Ca2+ waves.
Keywords: Ca2+/calmodulin-dependent protein kinase, Ca2+ imaging, Ca2+ waves, cyclic AMP, okadaic acid, protein phosphatase
INTRODUCTION
Although Ca2+ ions comprise a universal signaling mechanism in the nervous system, they play different roles in neurons and astrocytes. In neurons, action potentials and excitatory synaptic activity lead to brief Ca2+ transients, due to entry of Ca2+ through voltage-gated Ca2+ channels and Ca2+-permeant neurotransmitter receptors, as well as Ca2+ release from intracellular endoplasmic reticulum stores. As mediators of neurotransmitter release and dendritic integration, these neuronal Ca2+ signals are generally transient and localized due to rapid intracellular buffering, extrusion across the plasma membrane, and uptake into intracellular stores. Nevertheless, increased duration and/or intensity of excitatory stimulation can raise intracellular [Ca2+] to levels high enough to induce transcription of activity-dependent genes, a process required for learning and memory.
In contrast to neurons, astrocytes also utilize Ca2+ for intercellular signaling. Stimulation of cultured astrocytes leads to the generation of Ca2+ transients that are substantially longer than those of neurons and that can propagate from cell to cell across a multicellular syncytium (Cornell-Bell et al., 1990; Charles et al., 1991; Scemes and Giaume, 2006). These propagating waves are mediated by gap junctions (Finkbeiner, 1992; Scemes and Giaume, 2006) and by release of ATP, which raises intracellular [Ca2+] in adjacent astrocytes by activating purinergic P2 receptors (James and Butt, 2001). Thus, these Ca2+ waves provide a mechanism for intercellular signaling across astrocyte networks that may operate in parallel with faster communication among neurons mediated by action potentials and synaptic transmission.
These astrocytic Ca2+ waves have the potential to induce sustained activation of Ca2+-dependent genes, which, in neurons, would occur only transiently in response to excitatory input. In this report we investigated the extent to which Ca2+ can activate gene expression in astrocytes. Surprisingly, we found that in astrocytes, transcriptional activation regulated by the transcription factor, Ca2+/cAMP-regulatory element binding protein (CREB), failed to respond to increased intracellular [Ca2+], even though it was strongly activated by cAMP. Our results indicate that astrocytes lack the intracellular Ca2+ signaling cascades required to induce CREB-dependent gene transcription in neurons.
MATERIALS AND METHODS
Cell Culture and Transfection
Astrocytes were isolated from embryonic day 16 or postnatal day 1 mouse cerebral cortex (C57Bl/6J, Jackson Laboratories, Bar Harbor, ME). Cells were mechanically dissociated by trituration, plated onto poly-L-lysine-coated (Sigma-Aldrich Co., St. Louis, MO) T75 flasks (2 brains/flask) and were maintained in DMEM/F12 medium (1/1) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and penicillin G (100 U/ml) and streptomycin (100 μg/ml) (both Invitrogen, Carlsbad, CA) in 5% CO2 at 37°C. Starting on day 3 after plating, medium was replaced every two days. After 10–13 days, cells were trypsinized and replated in same medium without penicillin/streptomycin at 5 x 105/well in 24-well plates for luciferase assays and 2.0 x 106/well in 35-mm dishes for RNA and protein analyses.
Luciferase Assays
Astrocytes were transiently transfected 1 day after replating using a Lipofectamine 2000 diluted 1:33 in Opti-MEM (Invitrogen). 4xCRE- (Stratagene, La Jolla, CA) and TRKB P2-(Kingsbury et al., 2003) luciferase reporters were introduced at 250 ng/well; cells were cotransfected with 35 ng/well TK Renilla plasmid (Promega, Madison, WI). One day after transfection, cultures were stimulated for 5 h, washed with PBS and harvested in 110 μl Renilla luciferase lysis buffer (Promega). Five μl of extract were assayed for reporter (luciferase assay reagent, Promega) and Renilla (Renilla luciferase assay system; Promega) activity. Promoter-driven luciferase activity was normalized to Renilla activity to correct for variability among wells.
Western Blot
Cells were harvested directly into 2X Laemmli sample buffer (Sigma-Aldrich), heated to 100°C for 5 min, fractionated by SDS-PAGE on 4–12% Bis-Tris gels run in MOPS buffer; gels were transferred to a PVDF membrane (Invitrogen). Western blotting was conducted using antibodies to CREB (Cell Signaling; 1:1000), phospho-CREB (specific for phospho-Ser133; Cell Signaling; 1:1000), CaMKIV (Cell Signaling; 1:1000), and actin (Sigma-Aldrich; 1:2000). Secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was used at 1:5000. Blots were incubated with antibodies overnight at 4°C in blocking buffer (TBS-t, 5% bovine serum albumin and 5% dehydrated milk), developed with ECL Plus Western Blotting Detection System (Amersham Biosciences, Piscataway, New Jersey) and analyzed on an Image Station 2000R (Eastman Kodak, Rochester, NY).
Quantitative, Real-time PCR (Q-PCR)
Following stimulation, astrocytes were harvested using Qia-shredder (Qiagen, Germantown, MD), and RNA was isolated using RNeasy RNA extraction kit (Qiagen). 0.5–1 μg was used for reverse transcription with Superscript II (Invitrogen) for 50 min at 42°C. RNA processed in the absence of Superscript II served as control. Q-PCR was conducted using a LightCycler 480 Q-PCR thermal cycler (Roche, Basel, Switzerland) SYBR Green I Mastermix (Roche), and a final primer concentration of 500 nM. The Q-PCR protocol included an initial hold step for 5 min at 95°C, and cycles of 10 sec at 95°C, 15 sec at 58°C, and 1 min at 72°C, repeated for 45 cycles. Final samples were analyzed by gel electrophoresis to confirm that the correct products were amplified. Forward and reverse primer sequences were: for CaMKIV, GATGCAGGTGTAAAAGAGG and CATCCTGCTGTGGAACCC and for β-actin, ATCGTGGGCCGCCCTAGGCA and TGGCCTTAGGGTTCGAGAGG (obtained from Invitrogen or IDT, Coralville, IA).
Ca2+ Imaging
Intracellular Ca2+ levels were determined using fura-2 imaging as described by Bambrick et al. (1997).
Reagents
ATP, KCl, ionomycin and okadaic acid were purchased from Sigma-Aldrich; forskolin, cyclopiazonic acid and BAPTA-AM from Calbiochem (San Diego, CA); 4-Br A23187 from Alexis Biochemicals (San Diego, CA); and [Phe(2),Orn(8)]-oxytocin from Bachem Bioscience, Inc. (King of Prussia, PA).
RESULTS
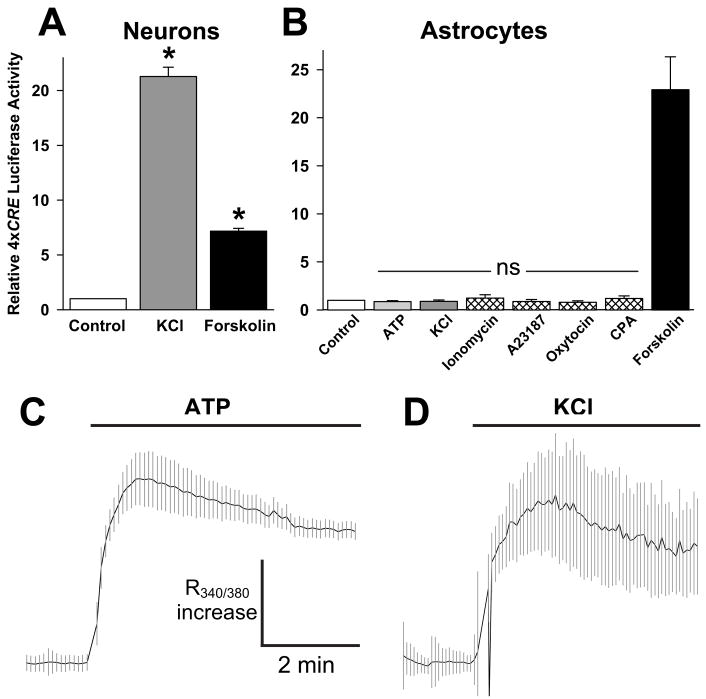
To compare the regulation of CREB-dependent genes in astrocytes and neurons, cultures were transfected with a luciferase reporter construct driven by four tandem CRE sequences (4xCRE). In neurons, both depolarization by addition of 50 mM KCl and increasing cAMP by addition of forskolin stimulated 4xCRE reporter activity (Fig. 1A). Depolarization-induced gene transcription in neurons was blocked by Ca2+ chelators and L-type Ca2+-channels antagonists, confirming that the effect is mediated by Ca2+ (Kingsbury et al., 2003). In contrast, ATP (10 μM) and depolarization with elevated KCl, which raised [Ca2+] in astrocytes by 3–6-fold (Fig. 1C,D), failed to stimulate 4xCRE in astrocytes (Fig. 1B). Higher concentrations of ATP did not significantly stimulate 4xCRE expression (+10 μM ATP: 1.5 ± 0.4, p>0.05; +30 μM ATP: 1.1 ± 0.1, p>0.05; +100 μM ATP: 1.6 ± 0.3, p>0.05; +10 μM forskolin: 17.8 ± 3.1, p<0.001; n=3). Moreover, additional stimuli reported to raise intracellular [Ca2+], including the ionophores, ionomycin and 8-Br-A23187, the endocrine hormone, oxytocin, and cyclopiazonic acid, an inhibitor of uptake of Ca2+ into internal stores, all failed to stimulate 4xCRE expression (Fig. 1B). It should be noted that 10 μM 8-Br-A23187 is used in the calibration of Ca2+ imaging experiments in astrocytes (Parpura and Haydon, 2000) in order to set the intracellular Ca2+ concentration at a very high level. Although Ca2+ failed to induce 4xCRE expression in astrocytes, forskolin stimulated 4xCRE expression by 40-fold (Fig. 1B). We also examined the effects of forskolin and ATP on 4xCRE expression in astrocyte cultures prepared from postnatal (P1) mouse cortex. As with embryonic cortex (Fig. 1B), the results demonstrated that ATP did not induce CREB-dependent gene transcription in P1 astrocytes, while forskolin induced a robust stimulation (+10 μM ATP: 0.91 ± 0.1, p>0.05; +50 μM forskolin: 20.3 ± 8.0, p<0.05; n=3). Thus, CREB-dependent gene transcription is not stimulated by increased intracellular [Ca2+] in astrocytes, even though cAMP can induce robust stimulation.
Fig. 1. Ca2+ activated 4xCRE-luciferase reporter in neurons but not astrocytes.
(A) 4xCRE was activated in neurons by both depolarization (50 mM KCl) and forskolin (50 μM). Error bars show mean ± sem. *, significantly different from control (p<0.05). (B) Multiple stimuli that raise [Ca2+]i failed to activate 4xCRE-luciferase in astrocytes. None of the treatments, which included ATP (10 μM; n = 7 experiments), KCl (50 mM; n = 7), ionomycin (2.5 μM; n = 3), A23187 (10 μM; n = 3), [Phe(2), Orn(8)]-oxytocin (10 nM; n = 6), and cyclopiazonic acid (10 μM; n = 3), significantly stimulated 4xCRE activity. In contrast, forskolin stimulated 4xCRE activity by 20-fold. Error bars show mean ± sem. ns, not significantly different from control (p>0.05) (C,D) ATP (10 μM, panel C) and KCl (50 mM, panel D) each caused a rapid rise in [Ca2+]i that was maintained for the duration of the treatment. Vertical lines show ± sem for 30 cells (each experiment was repeated 3 times with similar results). The vertical calibration bar indicates a 1.5- or 3-fold increase in R340/380 in C and D, respectively.
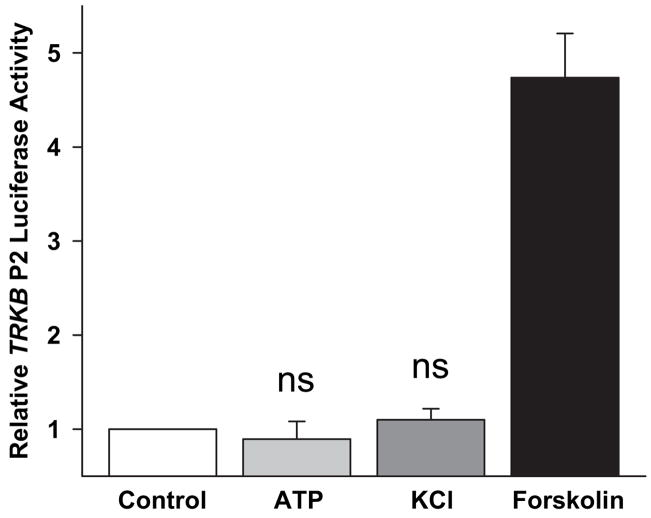
The presence of CREs in gene promoters confers cAMP- and Ca2+-responsiveness to many genes in multiple cell types (Mayr and Montminy, 2001; Zhang et al., 2005). The observation that 4xCRE is not activated by Ca2+ in astrocytes suggests that the promoters of endogenous genes requiring CREB activation for Ca2+-responsiveness will not be activated by Ca2+ in astrocytes. To test this, we measured luciferase reporter activity driven by the downstream promoter (P2) of the gene encoding the brain-derived neurotrophic factor (BDNF) receptor, TRKB (Barettino et al., 1999). P2 contains two CREs and, as previously reported, is activated by both cAMP and Ca2+ in neurons (Kingsbury et al., 2003). Neither ATP (10 μM) nor KCl (50mM) activated the TRKB P2-luciferase reporter in astrocytes, while forskolin treatment induced a 4–5-fold stimulation (Figure 2). The ability of cAMP, but not Ca2+, to activate TRKB P2-luciferase matches the stimulus pattern of 4xCRE-luciferase, consistent with the notion that the inability of Ca2+ to activate CRE-luciferase reflects a difference in Ca2+ signaling mechanism in astrocytes rather than a unique property of 4xCRE.
Fig. 2. TRKB P2 was not activated by ATP in astrocytes.
ATP and KCl did not significantly activate TRKB P2. Error bars show mean ± sem (n = 4 experiments). ns, not significantly different from control (p>0.05).
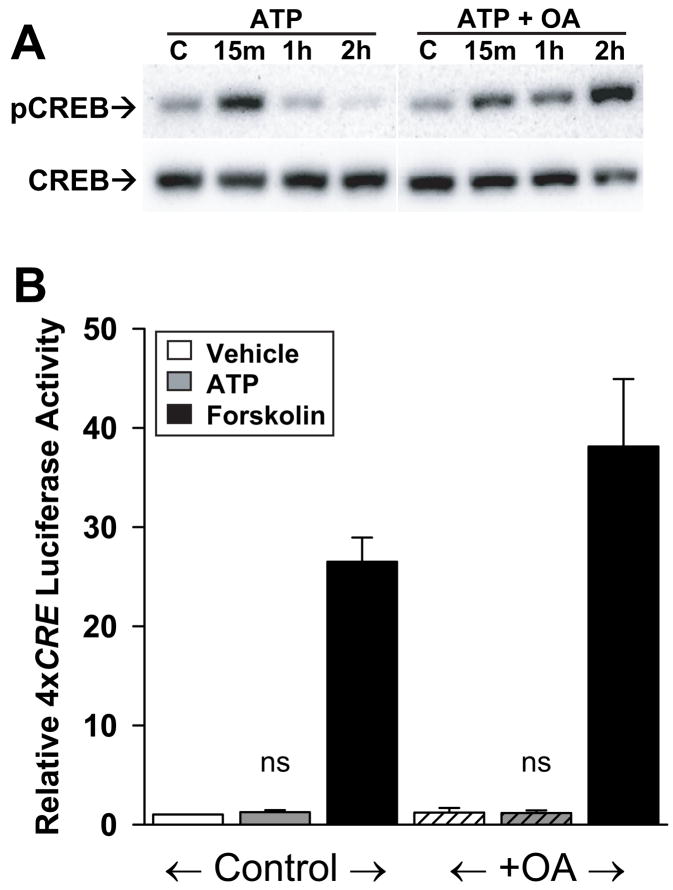
Since phosphorylation of serine 133 is required for CREB-dependent gene transcription (Mayr and Montminy, 2001), we determined if ATP was capable of inducing CREB phosphorylation in astrocytes. CREB phosphorylation was monitored by Western blot analysis using an antibody that binds CREB only when it is phosphorylated at serine 133. Both ATP and forskolin stimulated CREB phosphorylation, however, whereas forskolin treatment resulted in phospho-CREB levels that remained significantly above control at least 2 hr, ATP-induced phospho-CREB levels had returned to baseline within 1 hr (Figure 3A,B). Preincubation with the membrane permeant Ca2+ chelator BAPTA-AM (150 μM) for 30 min inhibited the ATP-induced increase in phospho-CREB (Figure 3C). Taken together, these results demonstrate that, although ATP can induce Ca2+-dependent CREB phosphorylation, the time-course is transient compared to that induced by forskolin.
Fig. 3. ATP-induced CREB phosphorylation was transient compared to that induced by forskolin.
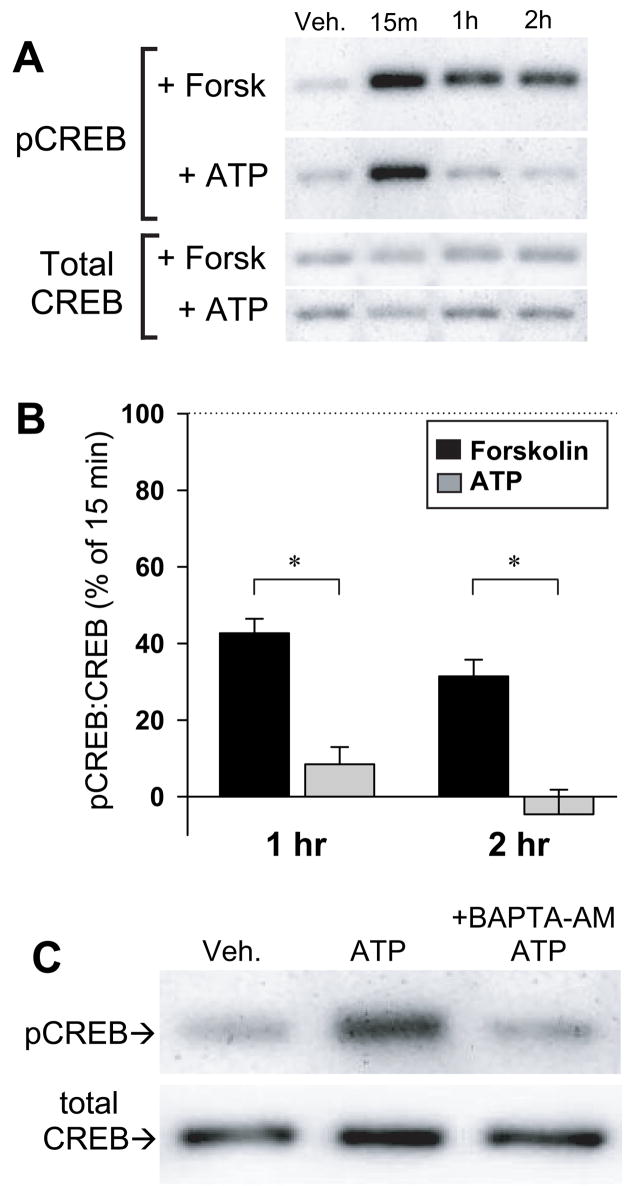
(A) Western blots showing time-course of CREB phosphorylation in the presence of forskolin (50 μM) and ATP (10 μM). Blots were probed with an antibody against CREB phosphorylated at serine 133 (top), stripped, and reprobed with an antibody against total CREB (bottom). (B) Analysis of four experiments. For each experiment, the magnitude of the Phospho-CREB:total CREB ratio in the presence of ATP or forskolin minus the unstimulated value was set to 100%. CREB phosphorylation at 1 hr and 2 hr is expressed as a percentage of this 15 min value. Although both forskolin and ATP rapidly raise phospho-CREB within 15 min (A), forskolin induced CREB phosphorylation remained significantly elevated up to 2 hr, whereas ATP-induced phospho-CREB levels returned to baseline levels by 1 hr. *, p < 0.0001 for forskolin vs. ATP at 1 hr (n = 5 experiments) and at 2 hrs (n = 4 experiments). (C) Western blot showing Ca2+-dependence of ATP-induced CREB phosphorylation in astrocytes. Cells were pretreated for 30 min with BAPTA-AM (150 μM) and then treated with vehicle (DMSO), forskolin (50 μM) or ATP (10 μM) for 15 min as indicated.
Transient CREB phosphorylation may not be sufficient to induce CREB-dependent gene transcription (Bito et al., 1996). To test the hypothesis that the failure of ATP to activate 4xCRE is due to acid (OA), an inhibitor of protein phosphatases PP1 and PP2A, which account for the majority of cellular protein phosphatase activity (Cohen et al., 1990; Shenolikar, 1994; Bito et al., 1996). OA prolonged ATP-induced CREB serine 133 phosphorylation, with phospho-CREB levels still elevated at 2 hrs as they were following forskolin treatment (Figure 4A). Nevertheless, ATP still failed to stimulate 4x CRE activity in the presence of OA (Figure 4B). Forskolin increased 4xCRE activity over 30-fold (Figure 4B), confirming that the failure of the combined OA and ATP treatment to activate 4xCRE expression was not due to nonspecific inhibitory effects of OA. Thus insufficient duration of CREB phosphorylation, alone, cannot account for the failure of ATP to activate 4xCRE.
Fig. 4. Extending duration of CREB phosphorylation did not enable ATP-induced transcription.
(A) Western blot analysis revealed that OA pretreatment prolonged ATP-induced CREB phosphorylation. Blots were analyzed as described in Fig. 3. This experiment was conducted four times with similar results. (B) Even after pretreatment with OA, ATP did not significantly affect 4xCRE activity. Error bars show mean ± sem (n = 6 experiments). ns, not significantly different from control (p>0.05).
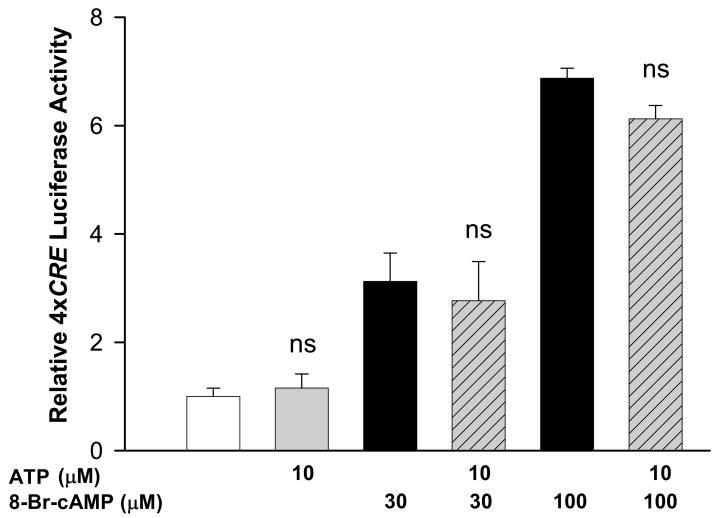
Another possible explanation for the failure of ATP to stimulate 4xCRE expression is that Ca2+ may activate a parallel pathway that inhibits CREB-dependent gene transcription. For example, it has been reported that Ca2+/calmodulin-dependent kinase II (CaMKII) phosphorylates CREB at serine 142 in addition to serine 133 in response to Ca2+ resulting in inhibition of CREB-dependent gene transcription (Sun et al., 1994). In cultured hippocampal neurons, Ca2+ entering via extrasynaptic NMDA receptors has been reported to antagonize activation of CREB-dependent genes induced by Ca2+ entering through synaptic NMDA receptors (Hardingham et al., 2002). To test the possible role of Ca2+ in initiating a CREB inhibitory-pathway, astrocytes were treated with ATP and the membrane-permeant cAMP analog, 8-Br-cAMP, alone or in combination (Fig. 5). If Ca2+ activates a CREB-inhibitory pathway, ATP should antagonize stimulation of CREB-dependent gene transcription by cAMP. Like forskolin, 30 and 100 μM 8-Br-cAMP treatment resulted in 4xCRE activation. Importantly, activation of 4xCRE by ATP + 8-Br-cAMP is not significantly different from that by 8-Br-cAMP alone. Thus, the failure of ATP to induce CREB-dependent transcription in astrocytes is likely due to the absence of a necessary transcription-activating signal and not to the stimulation of a Ca2+-dependent CREB-inhibitory pathway.
Fig. 5. ATP did not inhibit activation of 4xCRE by cAMP.
Error bars show mean ± sem for 4 replicate determinations. ns, not significantly different from the 4xCRE activity in the absence of ATP (p>0.05).
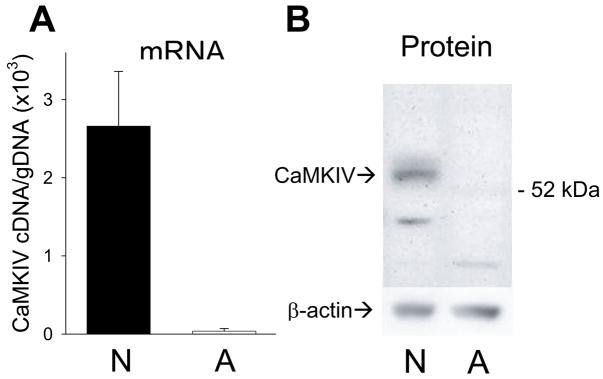
Phosphorylation of CREB at serine 133 is generally necessary but not sufficient for activation of CRE-containing genes. CREB phosphorylation leads to the recruitment of a co-activator such as CREB binding protein (CBP) to the transcription initiation complex. In neurons, Ca2+-induced activation of CREB-dependent gene transcription requires concurrent activation of CBP by CaMKIV (Chaw-la et al., 1998; Impey et al., 2002). We postulated that failure to activate CBP by CaMKIV-mediated phosphorylation could explain the inability of Ca2+ to stimulate CREB-dependent gene transcription in astrocytes. As shown in Figure 6, CaMKIV expression was virtually undetectable in astrocytes. Using Q-PCR, CaMKIV mRNA was easily detected in neurons, but was expressed only marginally above genomic DNA expression in astrocytes (Fig. 6A), determined by omitting reverse transcriptase from the reaction generating cDNA. Consistent with the absence of CaMKIV mRNA in astrocytes, we were unable to detect significant CaMKIV protein expression by Western blot analysis (Fig. 6B). As a positive control, analysis of cortical neurons revealed a strong a double band at the expected molecular weights of 61 and 63 kDa, corresponding to the CaMKIVα and CaMKIVβ isoforms (Ohmstede et al., 1989). Given its vital role in the Ca2+-dependent activation of CREB in neurons, the lack of CaMKIV expression in astrocytes provides a plausible explanation for the failure of Ca2+ to activate CREB-dependent gene transcription in astrocytes.
Fig. 6. Astrocytes did not express CaMKIV.
CaMKIV mRNA and protein expression was analyzed in neurons (N) and astrocytes (A). (A) CaMKIV RNA expression determined by Q-PCR, relative to the signal obtained when reverse transcriptase was omitted (gDNA). Error bars show mean ± sem, n = 4 experiments. (B) Western blot analysis of CaMKIV expression, which was detected in neurons (left lane) but not in astrocytes (right lane). Neuronal bands at 61 and 63 kDa corresponded to the α- and β-isoforms of CaMKIV. Blots were stripped and reprobed for β-actin. CaMKIV protein expression in astrocytes, normalized to β-actin expression, was 3.2 ± 2.4% of that in neurons (n = 3 experiments).
DISCUSSION
Ca2+ fails to activate CREB-dependent gene transcription in astrocytes
Our results reveal that, in cultured cortical astrocytes, elevated [Ca2+] does not activate either a simple, synthetic CREB-dependent promoter (4xCRE) or a complex promoter of an endogenous gene (TRKB P2), despite the fact that both are robustly stimulated by cAMP. The use of multiple stimuli to raise intracellular [Ca2+], validated by Ca2+ imaging (Fig. 1), and the demonstration that ATP stimulated CREB phosphorylation in a Ca2+-dependent manner (Fig. 3C), confirmed that [Ca2+] was both elevated and biologically active under conditions where the CREB-dependent reporters were not activated. Thus, Ca2+ fails to stimulate both synthetic (Fig. 1) and endogenous (Fig. 2) CREB-dependent promoters, leading to the conclusion that the results obtained are due to a Ca2+ signaling deficit in astrocytes. Notably, in neurons from the same embryonic mouse cortices, Ca2+ stimulated both 4xCRE (Fig. 1A) and TRKB P2 (Kingsbury et al., 2003) expression. Even under conditions where cAMP and Ca2+ induced comparable increases in CREB phosphorylation in astrocytes (Fig. 4A), only cAMP activated CREB-dependent gene transcription (Fig. 4B). Consequently, the failure of Ca2+ to stimulate CREB-dependent gene transcription is most likely due to the lack of Ca2+-dependent coactivator activation, a signaling step accomplished by CaMKIV in neurons (Impey et al., 2002).
Absence of Ca2+ signaling pathways in astrocytes
Although this is the first report of the failure of Ca2+ to regulate CREB-dependent genes in astrocytes, there is evidence in the literature suggesting that astrocytic Ca2+ signaling pathways may be defective. Watterson et al. (2001) reported that CaMKIV, was undetectable on Western blots from rat hippocampal astrocytes. Recently, Cahoy et al. (2008) published a transcriptome database tabulating the genes expressed by neurons, astrocytes and other brain cells. Consistent with the results reported here, Ca2+ signaling pathways were highly enriched in neurons compared to astrocytes, and CaMKIV transcripts in particular were strongly expressed in neurons but nearly undetectable in astrocytes.
Ca2+-dependent genes can be regulated in astrocytes by other signaling pathways
Even though the CREB-dependent promoters utilized in this study were insensitive to Ca2+ in astrocytes, those promoters were still activated by forskolin (Fig. 1) and 8-Br-cAMP (Fig. 5). Thus, the cAMP-dependent signaling mechanisms (e.g., PKA) that activate CREB and necessary coactivators (c.f. Brindle et al., 1995) are intact in astrocytes. Recently, Canellada et al. (2008) reported that Ca2+ stimulates transcription of the genes encoding cyclooxygenase-2 and Rcan1–4 in astrocytes. Interestingly, this effect was mediated by the Ca2+-dependent, calcineurin/NFAT signaling pathway. In addition, the immediate early gene, c-fos, can be activated in astrocytes by Ca2+-dependent signaling mechanisms due to the presence of a serum response element and the repressor element, DRE, in its promoter (Edling et al., 2006). Thus, genes such as c-fos, which have complex promoters, may be activated by Ca2+-dependent mechanisms that are independent of CRE. Moreover, CREB-dependent genes may also be activated by the mitogen-activated protein kinase and phosphoinositide-3 kinase signaling pathways, independently of Ca2+ (Schinelli et al., 2001).
CREB-dependent gene transcription and Ca2+ waves in astrocytes
The failure of Ca2+ to regulate CREB-dependent genes in astrocytes may reflect the unique mechanism of Ca2+ signaling that arises as intracellular transients, which can propagate from cell to cell across a multicellular astrocytic syncytium. If intracellular Ca2+ signaling were intact, as it is in neurons, these Ca2+ waves would be expected to provide constitutive drive on CREB-dependent genes, overexpression of which could be detrimental to normal cell function. There are hundreds, and possibly thousands, of CREB-dependent genes (Zhang et al., 2005) that potentially would be stimulated as a result of these Ca2+ transients, representing such diverse cellular functions as intracellular signaling, transcriptional regulation, energy metabolism and cellular differentiation (Su et al., 2000; Mayr and Montminy, 2001; Lonze and Ginty, 2002, Impey et al., 2004; Takanaga et al., 2004). Based on the analysis of 4xCRE and TRKB P2 promoter activation reported here, we suggest that many of these genes may effectively be insulated from the astrocytic Ca2+ waves, because the absence of CaMKIV expression prevents Ca2+ from activating CREB-dependent gene transcription. Nevertheless, these genes need not always be silenced in astrocytes; they can still be activated by cAMP.
Acknowledgments
The authors thank L. L. Bambrick for technical assistance with Ca2+ imaging and L. L. Bambrick, R. J. Bloch, G. M. Fiskum, F. L. Margolis, M. M. McCarthy, and S. M. Thompson for helpful suggestions. Supported by grants from NIH (R01NS048095) and DOD (W81XWH-04-1-0176) to B.K.K. PDM was supported by NIH predoctoral training grant T32GM08181. T.J.K. was a BIRCWH Scholar supported by NICHD/ ORWH/NIDDK584 (NICHD K12 HD43489).
References
- Bambrick LL, Golovina VA, Blaustein MP, Yarowsky PJ, Krueger BK. Abnormal calcium homeostasis in astrocytes from the trisomy 16 mouse. Glia. 1997;19:352–358. doi: 10.1002/(sici)1098-1136(199704)19:4<352::aid-glia8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Barettino D, Pombo PMG, Espliguero G, Rodriguez-Pena A. The mouse neurotrophin receptor trkB gene is transcribed from two different promoters. Biochim Biophys Acta. 1999;1446:24–34. doi: 10.1016/s0167-4781(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Brindle P, Nakajima T, Montminy M. Multiple protein kinase A-regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci USA. 1995;92:10521–10525. doi: 10.1073/pnas.92.23.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellada A, Ramirez BG, Minami T, Redondo JM, Cano E. Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1–4 and cyclooxygenase-2 as NFAT target genes. Glia. 2008 doi: 10.1002/glia.20647. Published online Feb. 21, 2008. [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–340. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Calcium waves in astrocytes – filling in the gaps. Neuron. 1992;8:1101–1108. doi: 10.1016/0896-6273(92)90131-v. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Impey S, Fong AL, Wang Y, Cardinaux J-R, Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR, Goodman RH. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34:235–244. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2X and P2Y purinoreceptors mediate ATP-evoked calcium signaling in optic nerve glia in situ. Cell Calcium. 2001;30:251–259. doi: 10.1054/ceca.2001.0232. [DOI] [PubMed] [Google Scholar]
- Kingsbury TJ, Murray PD, Bambrick LL, Krueger BK. Ca2+-dependent regulation of trkB expression in neurons. J Biol Chem. 2003;278:40744–4074. doi: 10.1074/jbc.M303082200. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Ohmstede CA, Jensen KF, Sahyoun NE. Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. J Biol Chem. 1989;264:5866–5875. [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what are they and what do they do? Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinelli S, Zanassi P, Paolillo M, Wang H, Feliciello A, Gallo V. Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways. J Neurosci. 2001;21:8842–8853. doi: 10.1523/JNEUROSCI.21-22-08842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S. Protein serine/threonine phosphatases – new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- Su G, Haworth RA, Dempsey RJ, Sun D. Regulation of Na+-K+-Cl− cotransporter in primary astrocytes by dibutyryl cAMP and high [K+]o. Am J Physiol Cell Physiol. 2000;279:C1710–1721. doi: 10.1152/ajpcell.2000.279.6.C1710. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Yoshitake T, Hara S, Yamasaki C, Kunimoto M. cAMP-induced astrocytic differentiation of C6 glioma cells is mediated by autocrine interleukin-6. J Biol Chem. 2004;279:15441–15447. doi: 10.1074/jbc.M311844200. [DOI] [PubMed] [Google Scholar]
- Watterson DM, Mirzoeva S, Guo L, Whyte A, Bourguignon J-J, Hibert M, Haiech J, Van Eldik LJ. Ligand modulation of glial activation: cell permeable, small molecule inhibitors of serine-threonine protein kinases can block induction of interleukin 1b and nitric oxide synthase II. Neuro-chem Int. 2001;39:459–468. doi: 10.1016/s0197-0186(01)00053-5. [DOI] [PubMed] [Google Scholar]
- Zhang X, Odom DT, Koo S-H, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]