Summary
Preclinical and clinical studies of CYP gene-directed enzyme-prodrug therapy have focused on anticancer prodrugs activated by CYP2B enzymes, which have low endogenous expression in human liver; however, the gene therapeutic potential of CYP3A enzymes, which are highly expressed in human liver, remains unknown. This study investigated methoxymorpholinyl-doxorubicin (MMDX), a novel CYP3A-activated anticancer prodrug. Retroviral transfer of CYP3A4 increased 9L gliosarcoma cell chemosensitivity to MMDX 120-fold (IC50=0.2nM). In CHO cells, overexpression of P450 reductase in combination with CYP3A4 enhanced chemosensitivity to MMDX, and to ifosfamide, another CYP3A4 prodrug, 11–23-fold compared to CYP3A4 expression alone. CYP3A4 expression and MMDX chemosensitivity were increased in human lung (A549) and brain (U251) tumor cells infected with replication-defective adenovirus encoding CYP3A4. Co-infection with Onyx-017, a replication-conditional adenovirus that co-amplifies and co-replicates the Adeno-3A4 virus, led to large increases in CYP3A4 RNA but only modest increases in CYP3A4 protein and activity. MMDX induced remarkable growth delay of 9L/3A4 tumors, but not 9L tumors, in immunodeficient mice administered low-dose MMDX either i.v. or by direct intratumoral injection (60µg/kg, every 7-days ×3), with the intratumoral route being substantially less toxic to the mouse host. No antitumor activity was observed with i.p. MMDX treatment, suggesting a substantial hepatic first pass effect, and with activated MMDX metabolites formed in the liver having poor access to the tumor site. These studies demonstrate that human CYP3A4 has strong potential for MMDX prodrug activation therapy, and suggest that endogenous tumor cell expression of CYP3A4, and not hepatic CYP3A4 activity, is a key determinant of responsiveness to MMDX therapy in cancer patients in vivo.
Keywords: Methoxymorpholinyl doxorubicin, nemorubicin, CYP3A4, gene-directed enzyme-prodrug therapy, prodrug-activation gene therapy
Introduction
Genes encoding prodrug activation enzymes, when expressed in tumor cells, confer the ability to metabolize an inactive anti-cancer prodrug into a potent cytotoxin. The cytotoxic metabolite not only kills the enzyme-expressing cells in situ, but may also diffuse into neighboring cells, thereby inducing a bystander cytotoxic response 1, 2. This strategy serves as the basis for prodrug-activation gene therapy using liver cytochrome P450 (CYP)-activated anticancer prodrugs, such as cyclophosphamide, which has the potential to achieve selective delivery of activated prodrugs to tumor tissue while minimizing systemic metabolism, thereby reducing host toxicity 3. Enzyme-prodrug combinations that have been evaluated in preclinical studies include CYP2B enzymes together with P450 reductase 4 in combination with cyclophosphamide 5–8, herpes simplex virus thymidine kinase with ganciclovir as the prodrug, cytosine deaminase with 5-fluorocytosine, carboxylesterase with irinotecan, thymidine phosphorylase with 5-deoxy-fluorouridine, purine nucleoside phosphorylase with 6-methylpurine derivatives, and nitroreductase with CB1954 9–11. Thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine have been tested in clinical trials 12–14 as has CYP2B6 in combination with oral cyclophosphamide in patients with advanced breast cancer or malignant melanoma, where clinical indications of efficacy were reported 15. Other P450 prodrugs investigated in preclinical gene therapy studies include ifosfamide (IFA) 16, 17, an isomer of cyclophosphamide, and the bioreductive drugs tirapazamine 18 and AQ4N 19.
Methoxymorpholinyl doxorubicin (MMDX) is a derivative of doxorubicin that displays enhanced cytotoxicity toward human tumor cells and hematopoietic progenitors when incubated with liver microsomes, which contain high P450 metabolic activity 20, 21. The potentiation of MMDX activity is due to metabolic activation by liver-expressed CYP3A enzymes 22–24. The P450-generated active metabolite of MMDX was recently identified and shown to have much higher potency toward cultured cells, and remarkable effectiveness toward tumor xenografts in nude mice, compared to MMDX 25. Activated MMDX retains activity against tumor cells with different mechanisms of resistance to classical anticancer agents, including MMDX itself 26, 27. Troleandomycin, a CYP3A enzyme-selective inhibitor, blocks hepatic MMDX activation, thereby decreasing anti-tumor activity and bone marrow toxicity, demonstrating that activated MMDX contributes to both antitumor activity and host toxicity in vivo 28. Consequently, strategies to reduce systemic MMDX exposure need to be developed to optimize the therapeutic index of this novel anthracycline prodrug.
In a previous study, CYP3A4 was shown to be the most active catalyst of MMDX activation in a panel of rat and human CYP3A enzymes 27. Presently, we evaluate the therapeutic impact of introducing CYP3A4 into tumor cells in combination with MMDX treatment in vitro and in vivo. In addition, we employ a replication-defective adenovirus to facilitate CYP3A4 gene transfer to human tumor cells. The potential utility of a replication-conditional adenovirus to enhance CYP3A4 gene delivery was also investigated. Our findings demonstrate the striking therapeutic potential of CYP3A4 in combination with MMDX treatment, and furthermore, suggest that endogenous tumor expression of CYP3A4 in individual patients may serve as an important determinant of responsiveness to MMDX in vivo.
Materials and Methods
Supplementary Materials and Methods
Materials, and methods used for Western blotting, P450 reductase assay, qPCR analysis, CYP3A4 adenovirus preparation and adenovirus-mediated RNA transcription, protein expression and enzyme activity are available on-line as Supplementary Materials.
Cell lines
CHO/HR, CHO/3A4 and CHO/3A4/HR cells 29 were obtained from Dr. Thomas Friedberg (Biomedical Research Centre, University of Dundee, UK) and were grown in α-MEM culture medium containing dialyzed 10% FBS (CHO/3A4 and CHO/3A4/HR cells) or in DMEM containing 10% FBS, 10mM hypoxanthine and 1.6mM thymidine (Invitrogen). 9L and 9L/3A4 cells (see below) were cultured in DMEM + 10% FBS. Human tumor cell lines U251 (brain tumor) and A549 (lung cancer) were obtained from Dr. Dominic Scudiero (NCI, Bethesda, MD).
Generation of 9L/3A4 cells by retroviral infection
CYP3A4 cDNA, obtained by reverse transcriptase PCR of human liver RNA and cloned in the XhoI/XbaI sites of pDHFR to yield pDHFR/3A4, was kindly provided by Dr. Thomas Friedberg. The nucleotide sequence flanking the AUG initiator codon of CYP3A4 was modified to conform to the conserved Kozak sequence XX(A/G)XXAUGG, required for efficient initiation of eukaryotic protein synthesis 30. A retroviral plasmid of the pBabe series, which encodes a puromycin resistance gene transcribed from the SV40 early promoter, was obtained from Dr. B. Spiegelman (Dana-Farber Cancer Institute, Boston, MA). CYP3A4 excised from pDHFR/3A4 with XhoI and XbaI was blunt-ended and subcloned into the blunt-ended SnaBI site of pBabe-puro to yield pBabe-puro-3A4, where CYP3A4 transcription is regulated by the retroviral long terminal repeat promoter. To generate infectious retroviral particles, the ecotropic packaging cell line Bosc 23 was cultured at 2.5 × 106 cells/60-mm dish in 4 ml of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS), 100U/ml penicillin and 100µg/ml streptomycin (Invitrogen). pBabe-puro-3A4 DNA (5µg) and pKAT DNA (2µg) 31 were co-transfected into Bosc 23 cells using Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Cells were co-transfected with pBabe-puro + pKAT as a P450-deficient control. 24hr later, the culture medium was replaced with 4ml of fresh DMEM containing 10% FBS. After a second 24-h period, the culture medium, containing infectious retroviral particles, was gently removed with a sterile pipette and added to 9L gliosarcoma cells (5 × 105 cells in a 100-mm dish) in the presence of 4 µg/ml of polybrene (Sigma-Aldrich, St. Louis, MO). Three hr later the medium was supplemented with 7 ml of fresh DMEM + 10% FBS. The infected 9L cells were trypsinized 48 hr later, split into four 100-mm dishes and treated with 2 µg/ml puromycin for 3 d. Approximately 80% of the infected 9L cells acquired resistance to puromycin, indicating a high efficiency of retroviral gene delivery. Pools of puromycin-resistant cells were grown in puromycin-free medium and assayed for CYP3A4 protein (Western blot analysis) and MMDX sensitivity. To select clonal 9L/3A4 cells, a puromycin-resistant 9L/3A4 cell pool was trypsinized and diluted to a nominal density of 1, 2 and 4 cells per 200 µl of DMEM containing 10% FBS and plated in 96-well plates. Wells containing single colonies were identified ~ 2 wk later using a light microscope, trypsinized and divided into duplicate wells of a 24-well plate. One well derived from each colony was kept untreated, and the second well was treated with 5 nM MMDX for 4d. Six of the 30 colonies examined were killed by MMDX with high efficiency (100% cell killing after 4d). Wells containing untreated cells derived from the corresponding colonies were trypsinized and replated into 6-well plates for propagation and further characterization. All experiments using 9L/3A4 cells were carried out using 9L/3A4 clone 5 (see Fig. 1A, below). The term “9L cells” refers to a pool of puromycin-resistant 9L cells infected with pBabe-puro, which was used as a P450-deficient control.
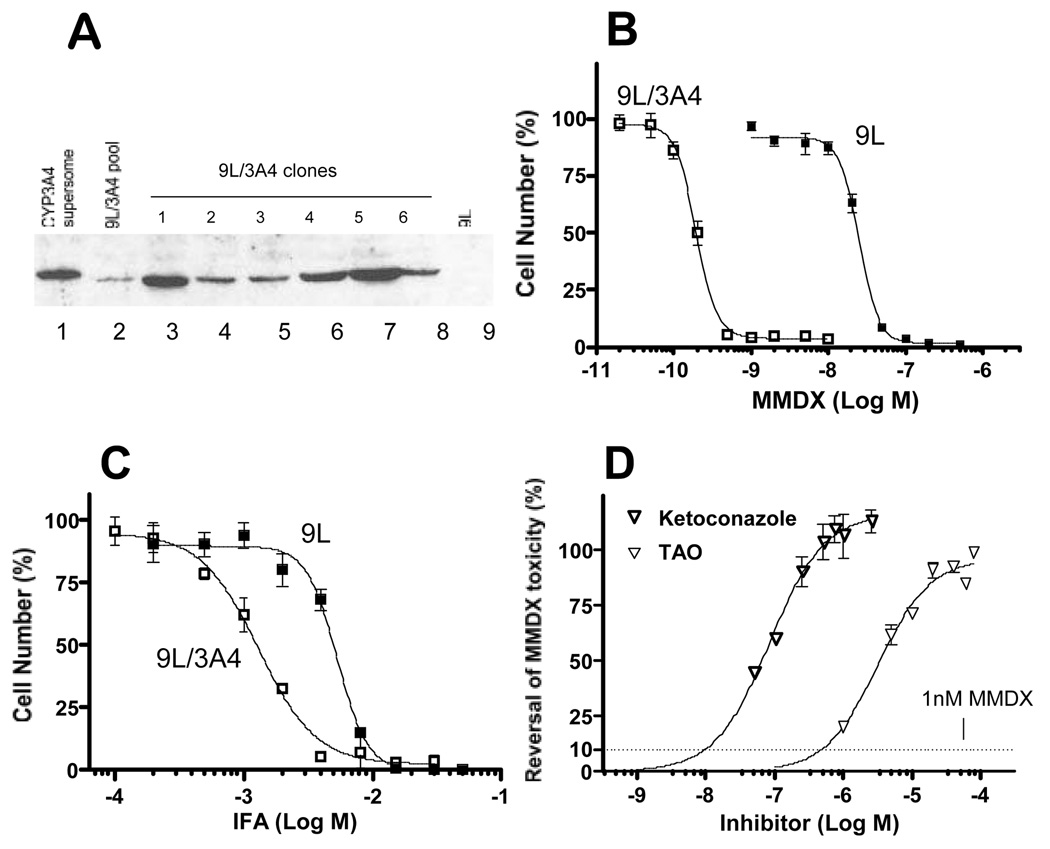
Fig. 1. Chemosensitivity of 9L/3A4 cells to MMDX and IFA.
A) CYP3A4 Western blot of total cellular protein (60 µg/well; 10% SDS-polyacrylamide gel). Lane 1, Baculovirus-expressed CYPA3A4 (Supersomes™) protein standard (0.5 pmol P450); lane 2, original puromycin-selected 9L/3A4 retroviral pool; lanes 3–8, individual 9L/3A4 clones, numbered 1 to 6 and selected based on enhanced MMDX sensitivity; lane 9, wild-type 9L cells. Quantitation of CYP3A4 protein levels using ImageQuant™ software (Molecular Dynamics, Sunnyvale, CA) based on lymphoblast-expressed CYP3A4 standard yield a CYP3A4 protein content of 15.3 pmol P450/mg cellular protein in clone 5. B) and C) – Chemosensitivity of 9L/3A4 and 9L cells to MMDX (B) and IFA (C) determined in a 4 d growth inhibition assay. Relative cell number, expressed as a percentage of drug-free controls, was determined by crystal violet staining. IC50 values determined in this and similar experiments are listed in Table 1. D) Cytotoxicity of 1 nM MMDX toward 9L/3A4 cells in a 4 day growth inhibition assay was blocked in a concentration-dependent manner by the CYP3A inhibitors troleandomycin (TAO) and ketoconazole with EC50 values of 5 µM and 50 nM, respectively. Horizontal dashed line represents 90% cell killing of 9L/3A4 cells at 1 nM MMDX. Data shown are mean values ± SD (n=3).
Cell growth inhibition assay
9L and CHO cells were plated in triplicate wells of a 96-well plate at 3000 cells per well 24 hr prior to drug treatment. Cells were treated with various concentrations of MMDX or IFA for 4d. Cells were then stained with crystal violet (A595) and relative cell survival was calculated 27. IC50 values were determined from a semi-logarithmic graph of the data points using Prism 4 (Graphpad Software Inc., San Diego, CA).
Quantitation of 4-OH-IFA production and active MMDX formation by tumor cells expressing CYP3A4
9L/3A4 cells were plated in 12-well culture plates at 1.5 × 105 cells/well in 1.5ml culture medium. Twenty-four hr later, IFA at various concentrations was added to the cells together with 5mM semicarbazide to trap and stabilize the 4-OH-IFA metabolite. After 4 hr treatment, an aliquot of culture medium (0.5ml) was removed from each well, and stored at −80°C until ready for 4-OH-IFA analysis. Cells remaining on the plate were washed with PBS and stained with crystal violet. A C18 HPLC assay was used to quantify 4-OH-IFA by fluorescence after derivatization of its by-product acrolein to 7-hydroxyquinoline 32. Standard curves for 4-OH CPA were generated using 4-OOH-CPA dissolved in cell culture medium (0–40 µM) 32. Cellular IFA 4-hydroxylase activity was calculated from integrated peak areas determined by Millennium32 software.
CYP3A4-activated MMDX metabolite released into the culture medium was assayed as follows. 9L and CHO cells expressing CYP3A4, and CYP3A4-deficient control cells, were plated in 12-well plates at 1.5 × 105 cells/well. Twenty-four hr later, MMDX at various concentrations was added to the cells in 1.5ml culture medium for 2hr, at which time 0.5ml of the culture supernatant was removed from the 9L and 9L/3A4 cell cultures and mixed with 0.5 ml of fresh α-MEM + 10% dialysed FBS. In parallel, 0.5 ml of culture supernatant was removed from the MMDX-treated CHO cell lines and mixed with 0.5 ml of fresh DMEM + 10% FBS. A 0.2ml aliquot of each sample was added to triplicate wells of 9L cells, seeded 24 hr earlier at 3000 cells/well in 96-well plates (‘9L indicator cells’). The 9L indicator cells were cultured for 4 d in the 0.2 ml medium containing MMDX metabolites, and then stained with crystal violet to determine relative cell numbers as an index of the level of active MMDX metabolite formed by each cell line during the initial 2hr MMDX incubation period.
Adenoviral infection of human tumor cell lines and MMDX cytotoxicity assays
A549 and U251 cells were plated in 24-well plates at 14,000 cells/well and infected 24 hr later with Adeno-3A4 (MOI 0 to 400) either alone or in combination with Onyx-017 (MOI 0, 0.7, 2). The cells were incubated with the viruses for 4 hr in 0.2 ml culture medium/well, after which 0.8 ml of fresh medium was added to each well. The virus was removed after 24 hr and 1 ml of fresh medium containing MMDX (0 to 8 nM) was added to the cells. After 2 d of MMDX treatment, the medium was replaced with 1 ml of fresh MMDX-containing medium for an additional 4 d. Surviving cells were stained with crystal violet.
Tumor growth delay assay
9L and 9L/3A4 cells were grown as solid tumors in male ICR/Fox Chase SCID mice (Taconic, Inc., Germantown, NY) using procedures approved by the Boston University Institutional Animal Care and Use Committee. Cells cultured in DMEM medium to 75% confluence were trypsinized and washed in PBS and then adjusted to 2 × 107 cells/ml of FBS-free DMEM. Four-week-old SCID mice (18–20 g) were implanted with either 9L or 9L/3A4 tumor cells by injection of 4 × 106 cells/0.2 ml of cell suspension, s.c. on each hind flank. Tumor sizes (length and width) were measured twice a week using Vernier calipers beginning 7d after tumor implantation. When the average tumor size reached 300 to 400 mm3, MMDX dissolved in PBS was administered by tail vein injection (i.v.) or by direct intratumoral (i.t.) injection (three injections spaced 7 d apart, each at 60 µg MMDX per kg body weight, except as noted). Intratumoral injections were performed using a syringe pump (model 70–2212, Dual Syringe Pump with Serial Communication, Harvard Apparatus, Inc. Holliston, MA) set a 1 µl s−1 with a 30-gauge needle. Each i.t. treatment dose was divided into three injections per tumor, with the injected volume set at 50 µl per tumor per 25 g mouse. Thus, for a 30 g mouse, a total of 120 µl of 15 µg/ml of MMDX solution was administered: 20 µl per site × 3 sites per tumor × 2 tumors/mouse. For a 25 g mouse, a total of 100 µl of 15 µg/ml MMDX solution was administered: 50 µl per tumor, 16.7 µl per site × 3 sites per tumor × 2 tumors/mouse. Drug-free controls were injected i.t. with the same vol of PBS. In some experiments, MMDX was administered by i.p. injection at 40 or 60 µg/kg body weight. Tumor sizes and body weights were measured twice/wk for the duration of the study. Tumor volumes were calculated using the formula: V = π/6 (L × W)3/2. Percent tumor regression was calculated as 100 × (V1–V2)/V1, where V1 is the tumor vol on the day of drug treatment and V2 is the vol on the day when the largest the decrease in tumor size is seen following drug treatment. Tumor doubling time was calculated as the time required for tumors to double in vol after drug treatment.
Results
Retroviral expression of human CYP3A4 chemosensitizes 9L gliosarcoma cells to MMDX and IFA
Retrovirus encoding CYP3A4 cDNA was used to infect 9L tumor cells, which were selected based on their acquired resistance to puromycin. CYP3A4 protein was detected in the resultant pool of puromycin-resistant cells, as shown by Western blotting (Fig. 1A). Selection of cells showing increased sensitivity to MMDX yielded clon es with elevated levels of CYP3A4 protein. 9L/3A4 clone 5 had the highest CYP3A4 protein content, 15.3 pmol CYP3A4/mg total cellular protein and was used in all subsequent experiments. The IC50 value of MMDX toward 9L/3A4 cells, 0.2 nM, was 120-fold lower than that of P450-deficient 9L cells (IC50 = 23.9 nM) (Fig. 1B and Table 1). This potent cytotoxicity of activated MMDX contrasts with that of the CYP3A4 prodrug IFA, which required millimolar concentrations to kill 9L/3A4 cells (Fig. 1C and Table 1). To verify the role of CYP3A4 metabolism in the activation of MMDX to cytotoxic metabolites, 9L/3A4 cells were treated with MMDX (1 nM) together with increasing concentrations of the CYP3A4 inhibitors ketoconazole and troleandomycin. MMDX cytotoxicity was fully blocked by ketoconazole (0.5 µM) and troleandomycin (20 µM) (Fig. 1D).
Table 1. Cytotoxicity of MMDX and IFA and their activated metabolites toward 9L and CHO tumor cell lines.
Shown are IC50 values determined with 9L and CHO cell lines that expressed CYP3A4 and/or human P450 reductase (HR). CHO/HR cells and 9L cells infected with the P450-deficient retroviral vector pBabe-puro were used as CYP3A4-negative controls. Assays were carried out: with MMDX; with MMDX + dexamethasone-induced rat liver microsomes (2 µg microsomal protein/well) plus 0.3 mM NADPH, generating activated MMDX in situ 24; with IFA; and with 4-OOH-IFA, which spontaneously decomposes to the active IFA metabolite, 4-OH-IFA. Each IC50 value was determined in a 4 day growth inhibition assay from dose-response curves that include triplicate samples at each of 9 drug concentrations. Data shown are representative of two or more independent experiments similar to those shown in Fig. 1B and 1C. Values shown for CHO/3A4 cells are for two independent clones with similar CYP3A4 protein content. Values shown in parenthesis are fold differences in IC50 values for 9L/3A4 cells vs. 9L cells, and for CHO/3A4/HR cells vs. CHO/3A4 cells.
| IC50 (nM) | IC50 (µM) | |||
|---|---|---|---|---|
| MMDX | MMDX+Dex Microsomes | IFA | 4-OOH-IFA | |
| 9L | 23.9 | 0.6 | 4900 | 8.9 |
| 9L/3A4 | 0.2 (120x) | N.D. | 1230 (4x) | 4.6 |
| CHO/HR | 58.8 | 2.2 | 4460 | 9.1 |
| CHO/3A4 | 2.2, 4.4 | N.D. | 3460, 3550 | 4.7, 4.7 |
| CHO/3A4/HR | 0.29 (11.3x) | N.D. | 151 (23x) | 6.6 |
N.D., not determined
P450 reductase overexpression enhances cytotoxicity of MMDX and IFA
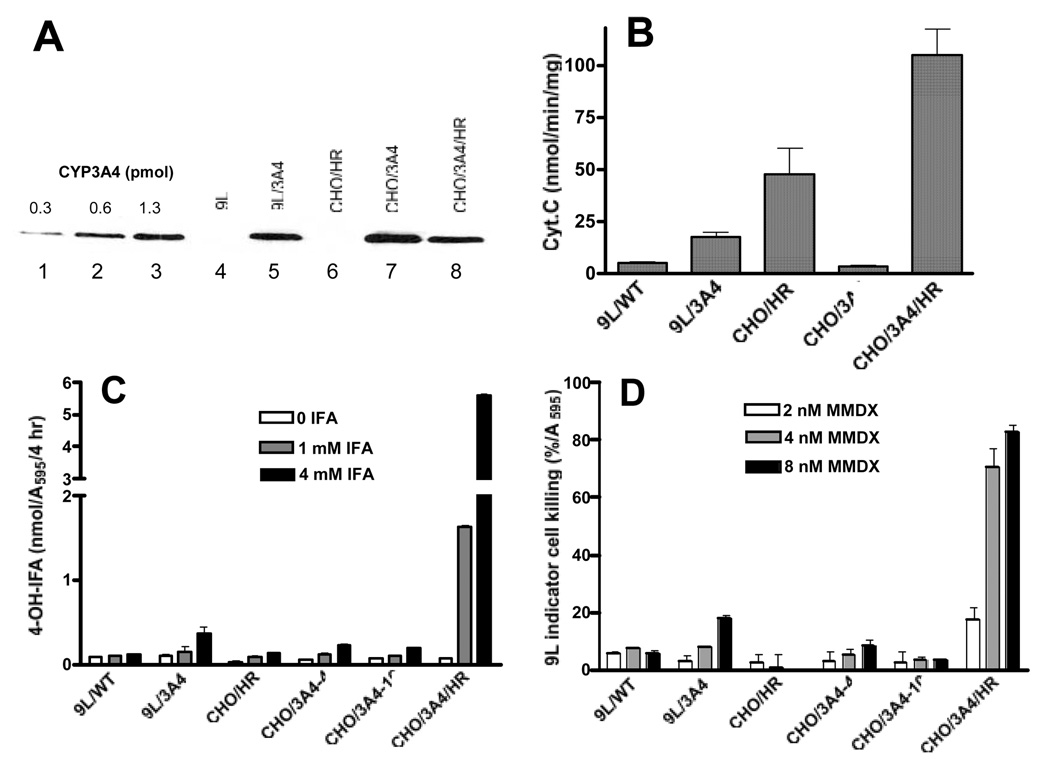
P450 reductase is a rate-limiting component for microsomal P450 reactions 33, including those catalyzed by CYP3A enzymes. We therefore investigated whether the cytotoxicity of MMDX could be further increased by overexpression of P450 reductase. Efforts to augment the endogenous P450 reductase of 9L/3A4 cells by infection with retrovirus encoding rat P450 reductase were unsuccessful and resulted in a small decrease in CYP3A4 protein (data not shown). We therefore examined CHO cell lines engineered to express CYP3A4 alone (CHO/3A4 cells) or in combination with P450 reductase (CHO/3A4/HR cells) 29. These cells have CYP3A4 protein levels similar to each other and to 9L/3A4 cells (Fig. 2A). P450 reductase activity was 31-fold higher in CHO/3A4/HR cells than in CHO/3A4 cells; it was also 6-fold higher than in 9L/3A4 cells (Fig. 2B). Correspondingly, CHO/3A4/HR cells showed a large increase in IFA metabolic activity, 28-fold compared to CHO/3A4 cells and 15-fold compared to 9L/3A4 cells (Fig. 2C). CHO/3A4/HR cells also showed increased MMDX activation, as determined by the formation of metabolites cytotoxic toward 9L indicator cells. Thus, at 4 nM MMDX, CHO/3A4/HR cells produced sufficient active metabolite to kill >70% of the 9L indicator cells, whereas <10% indicator cell killing was observed with CHO/3A4 and 9L/3A4 cell lines under the same conditions (Fig. 2D). Moreover, CHO/3A4/HR cells were ~11.3-fold more sensitive to MMDX than CHO/3A4 cells (Table 1), where the low endogenous P450 reductase limits P450 metabolic activity. CHO/3A4/HR cells also exhibited 8 to 23-fold greater sensitivity to IFA, as compared to HO/3A4 and 9L/3A4 cells (Table 1). All three cells lines exhibited similar intrinsic sensitivities to activated IFA (<2-fold difference in IC50 values; Table 1, last column) as determined using 4-OOH-IFA, a chemically activated form of IFA. Thus, the endogenous CHO tumor cell level of P450 reductase is rate-limiting for CYP3A4-catalyzed activation of both MMDX and IFA.
Fig. 2. Metabolic activation of IFA and MMDX by CHO and 9L cells expressing CYP3A4 and/or P450 reductase (HR).
A) Western blot of CYP3A4 protein levels in CYP3A4 standards (lanes 1–3) and in each of the indicated cell lines (lanes 4–8), and B) P450 reductase activity (cytochrome c reduction) were both determined in total cell lysates prepared from cells plated overnight in 6-well tissue culture plates at 300,000 cells/well, and collected in 0.2 ml of 50 µM KPi buffer pH 7.4 containing 0.1 mM EDTA and 20% glycerol, then lysed by sonication. C) 4-OH-IFA production by cultured cell lines. Cells were plated overnight in 12-well tissue culture plates at 150,000 cells/well and then incubated for 4 hr in 1.5 ml fresh medium containing 0, 1 or 4 mM IFA, plus 5 mM semicarbazide to stabilize the 4-hydroxy metabolite. An aliquot (0.5 ml) of culture medium was removed, derivatized, and assayed for 4-OH-IFA formation and for relative cell number (crystal violet staining, A595). Data shown are nmol 4-OH-IFA formed/A595 cells during the 4 hr incubation (mean ± range, n=2). D) Active MMDX metabolite formation, determined by the killing of 9L indicator cells in a 4 d growth inhibition assay. 9L indicator cells plated overnight at 3,000 cells/well in 96-well plate were incubated for 4 d with cultured supernatants from 9L, 9L/3A4 or CHO/HR, CHO/3A4, CHO/3A4/HR cells incubated for 2 hr with 2, 4 or 8 nM MMDX. 9L indicator cell killing expressed as a percentage of MMDX-free controls was determined by crystal violet staining (mean ± range, n=2). CHO/3A4-4 and CHO/3A4–10 correspond to two independent clones with similar CYP3A4 protein levels (not shown) and similar sensitivity to MMDX (see Table 1).
Adeno-3A4 infection of human tumor cell lines
Adeno-3A4, an E1 and E3 region-deleted, replication-defective adenovirus encoding full length CYP3A4 cDNA, was used to induce CYP3A4 expression in two human tumor cell lines, A549 lung and U251 brain cancer cells. U251 cells are more susceptible to adenovirus infection than A549 cells, as determined using adenovirus encoding bacterial β-galactosidase, visualized by staining the infected cells with the chromophoric substrate X-gal (data not shown). At an Adeno-3A4 MOI of 150, CYP3A4 RNA increased up to ~6000-fold in both cell lines, as determined by qPCR (Fig. S1A). CYP3A4 RNA levels in the Adeno-3A4-infected cells (MOI 75) were similar to those found in 9L/3A4 cells. Adeno-3A4 also induced a dose-dependent increase in CYP3A4 protein (Fig. S1B and Fig. S2) and metabolic activity, assayed by the formation and release of 4-OH-IFA into the culture medium of cells incubated with IFA (Fig. S1C). Overall, 4-OH-IFA production per pmol CYP3A4 protein was 2 to 4-fold higher in 9L/3A4 cells than in the Adeno-3A4-infected A549 and U251 cells.
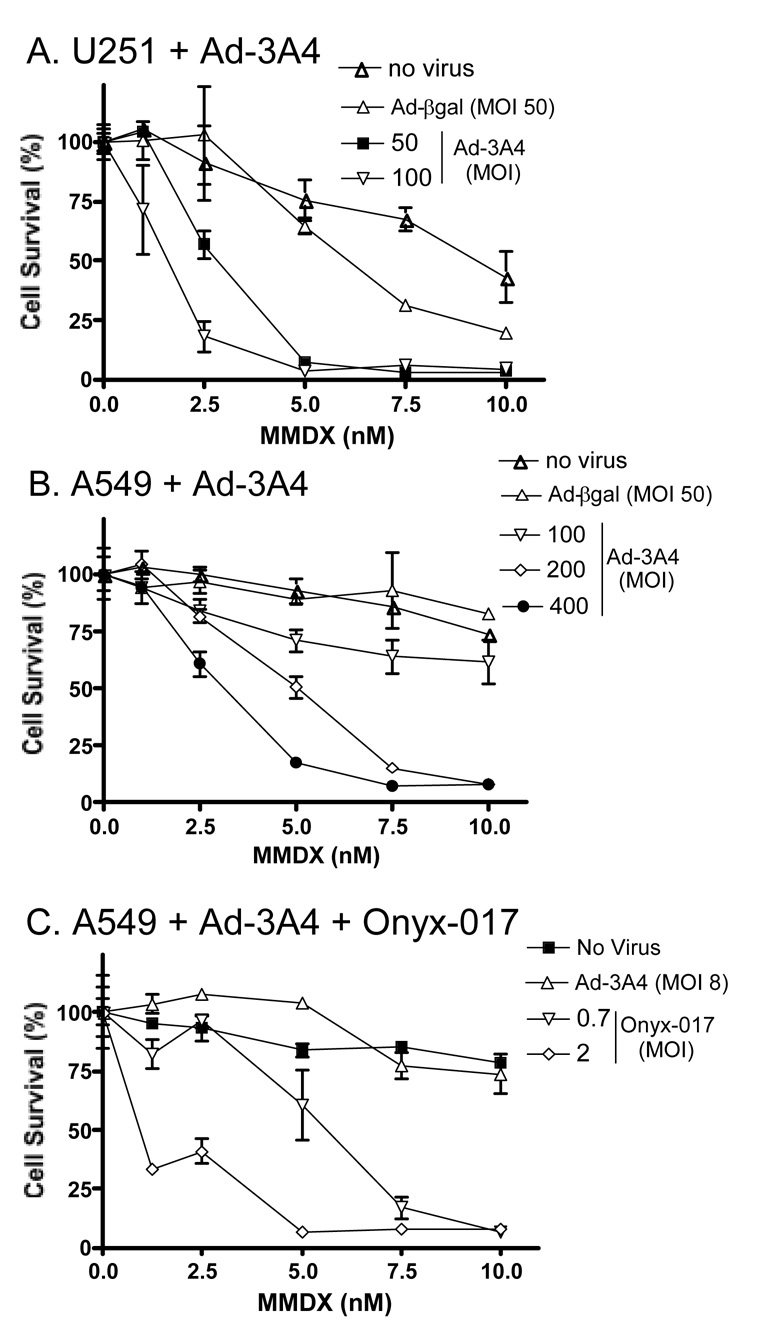
Adeno-3A4 infection conferred dose-dependent increases in MMDX toxicity towards U251 cells (IC50 = 1.4 nM at 100 MOI Adeno-3A4; Fig. 3A). MMDX was cytotoxic to the Adeno-3A4-infected A549 cells, but only at high viral doses (IC50 = 4.7 nM at 200 MOI; Fig. 3B) (c.f., IC50 (MMDX) = 24 nM in A549 controls 27). In an effort to chemosensitize the cells to MMDX at lower Adeno-3A4 MOIs, cells were co-infected with Onyx-017, an E1B-55kd-deleted oncolytic adenovirus that selectively replicates in p53-deficient tumor cells and can be used as a helper virus to co-amplify and increase the expression and cellular transmission of replication-defective virus encoding CYPs 2B6 and 2B11 6, 34. Co-infection of A549 or U251 tumor cells with Adeno-3A4 + Onyx-017 resulted in up to a 50–60-fold increase in CYP3A4 RNA compared to infection with Adeno-3A4 alone (Fig. S3A) accompanied by large increases in adenoviral E3 RNA, derived from Onyx-017 (Fig. S3B). However, only modest increases in CYP3A4 protein were obtained, although increased expression of a higher molecular weight CYP3A4 immunoreactive protein was also observed in cells infected with both viruses (Fig. S2). Onyx-017 increased Adeno-3A4-dependent CYP3A4 metabolic activity (Fig. S3C) and CYP3A4-dependent MMDX cytotoxicity (Fig. 3C), albeit to a much lesser extent than the increase in CYP3A4 RNA (Fig. S3A).
Fig. 3. Cytotoxicity of MMDX toward Adeno-3A4-infected U251 (A) and A549 cells (B, C), without (A, B) or with (C) Onyx-017 co-infection.
Cells seeded overnight in 24-well plates (14,000 cells/well) were infected with Adeno-3A4 or Adeno-βgal at the indicated MOI for 24 hr, either alone (A, B) or in combination with Onyx-017 (C; at MOIs 0.7 and 2). Cells were treated with MMDX in fresh culture medium at the indicated concentrations beginning 24 hr after infection. The culture medium was replaced with medium containing fresh MMDX 2 d later to minimize the intrinsic toxicity of the virus, and the incubation was continued for an additional 5 d (total of 7 d MMDX treatment). Data are expressed as percent cell survival compared to the corresponding drug-free controls, determined by crystal violet staining, mean ± SD (n=3). In the absence of MMDX, Adeno-3A4 was moderately toxic to U251 cells (≤30% cell killing at MOI 50 and 100) but not to A549 cells (<16% killing at MOI 200 and 400) (data not shown). The IC50 (MMDX) in A549 cells was 1 nM at 8 MOI Adeno-3A4 + 2 MOI Onyx-017 vs. no effect in A459 cells infected with 8 MOI Adeno-3A4 alone.
Impact of CYP3A4 on MMDX anti-tumor activity in vivo
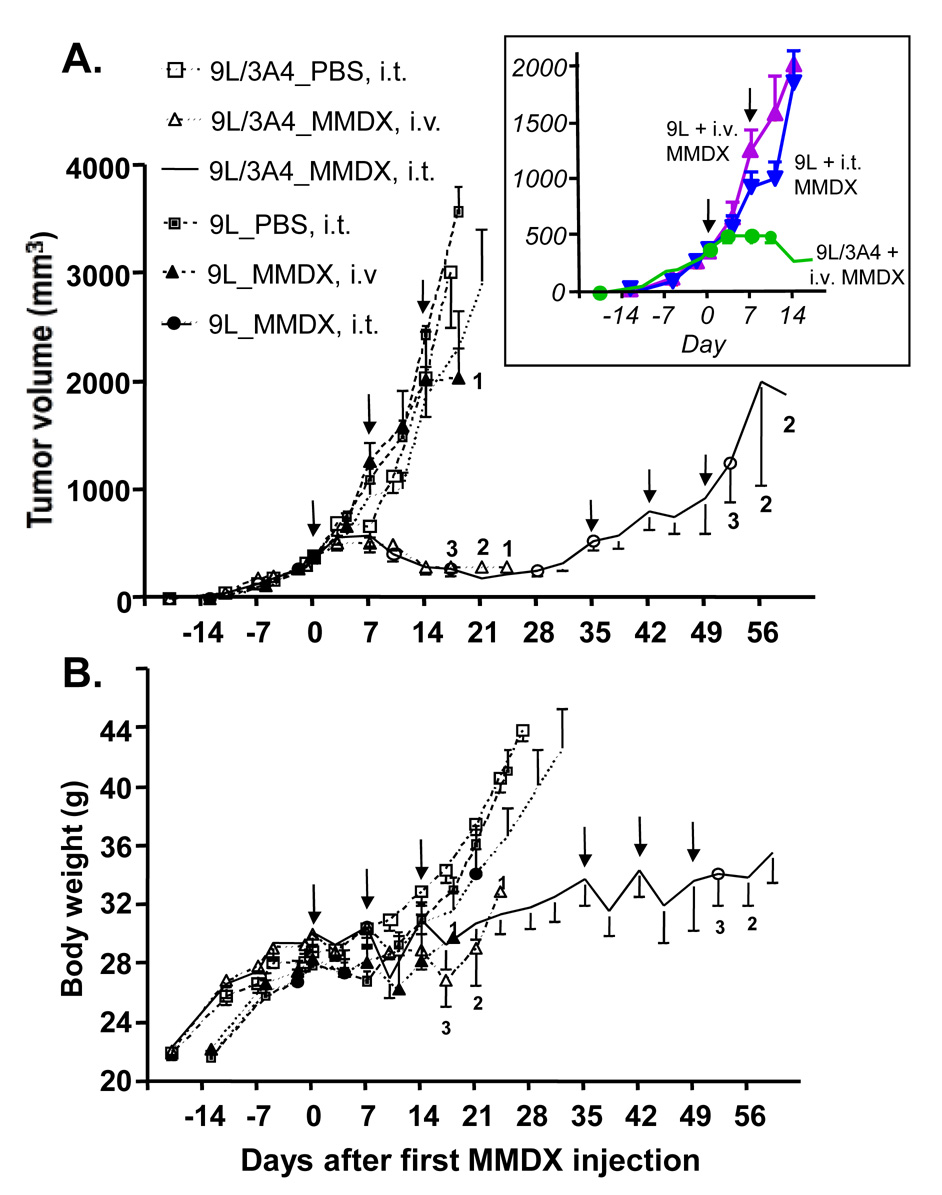
9L/3A4 cells were used as an ex vivo gene transfer model to evaluate the impact of CYP3A4 gene delivery on the chemosensitivity of 9L tumors to MMDX in vivo in the context of endogenous liver MMDX metabolism. Fig. 4 shows the results of a tumor growth delay study comparing the antitumor activity of MMDX toward 9L tumors vs. 9L/3A4 tumors. Mice were given a series of 3 weekly injections of MMDX (60 µg/kg body weight) by i.v. injection or by direct intratumoral (i.t.) injection. 9L tumors did not show any sustained growth delay in response to MMDX treatment via either injection route, although a modest but transient growth inhibitory effect was apparent in the case of the i.t. treatment group 3 d after the second MMDX injection (Fig. 4A, inset). In contrast, 9L/3A4 tumors exhibited a substantial and prolonged regression following the first MMDX treatment cycle, either by the i.v or i.t. route (Fig. 4A and Table 2). Regrowth of the MMDX-treated 9L/3A4 tumors was not apparent until day 35, at which time a second cycle of 3 weekly MMDX treatments was initiated. Four of the 8 tumors in this group did not double in size by the time the experiment was terminated on day 59; the doubling time for the other 4 tumors was 35 days for the first cycle of MMDX treatment and 17 d for the second MMDX treatment cycle, i.e., 6-fold and 3-fold longer than the 5.8 d doubling time of untreated 9L/3A4 tumors.
Fig. 4. Tumor growth delay induced by MMDX in scid mice bearing 9L and 9L/3A4 tumors.
9L and 9L/3A4 tumors were implanted s.c. and grown in male scid mice. Tumor vol (A) and body weight (B) were measured twice a week. In the absence of drug treatment, 9L/3A4 tumors grew somewhat slower than 9L tumors (doubling time of 5.8 d vs. 4.6 d; Table 2), as was observed for the corresponding cell lines in culture (data not shown). Arrows indicate days on which each of three weekly doses of MMDX (60 µg/kg) was administered, either by i.v. or i.t. injection, as indicated, beginning when the tumors reached 300–400 mm3 in size. Numbers shown alongside individual data points indicate the number of mice remaining alive at each time point; data points not numbered indicate no deaths occurred, i.e., 4 mice alive/drug-treated group. Tumor vol and body weight data are mean ± SE values based on n=6 tumors (n=3 mice) (drug-free controls) or n=8 tumors (n=4 mice) (MMDX treatment groups). MMDX was highly toxic by the i.v. administration route, with 4 out of 4 i.v. injected 9L tumor-bearing mice dying by day 21 (i.e., 7 d after completing the cycle of three weekly MMDX injections), and 3 out of 4 i.v. MMDX-injected 9L/3A4 tumor-bearing mice dying by day 24. Drug toxicity was not observed for the i.t. MMDX 9L/3A4 tumor group until after completion of a second cycle of three weekly MMDX injections, with 1 mouse dying on day 52 and a second mouse on day 56. The inset in (A) highlights the transient growth delay seen in 9L tumors given MMDX by i. t. injection.
Table 2. Effect of MMDX treatment on tumor doubling time, tumor regression and host toxicity.
9L and 9L/3A4 tumors grown in scid mice, as in Fig. 4, were treated with MMDX using the indicated dosing regimen, route and dose. Tumor-doubling time corresponds to the time in days required for the tumor vol to double relative to the vol on day 0, i.e., the first day of MMDX treatment. Tumor regression was calculated as described in Materials and Methods. Toxicity indicates the number of mice that died over the course of a cycle of MMDX treatment (i.e., a series 3, 4 or 5 MMDX injections spaced 6 or 7 days apart, as indicated under Dosing Regimen) compared to the total number of treated animals, which ranged from 2 to 10, as shown in the last column.
| Tumor | MMDX Dosing Regimen | Dosing route | MMDX Dose (µg/kg) | Doubling Time (day) | Tumor Regression (%) | Toxicity |
|---|---|---|---|---|---|---|
| 9L | q7d × 3 | i.t. | 0 | 4.6 | 0 | 0/3 |
| 9L | q6d × 5 | i.p | 60 | 5.9 | 0 | 0/10 |
| 9L | q7d × 3 | i.t. | 60 | 6.5 | 0 | 0/4 |
| 9L | q7d × 3 | i.v. | 60 | 4.9 | 0 | 4/4 |
| 9L/3A4 | q6d × 4 | i.p. | 0 | 4.7 | 0 | 0/2 |
| 9L/3A4 | q7d × 3 | i.t. | 0 | 5.8 | 0 | 0/3 |
| 9L/3A4 | q6d × 5 | i.p. | 40 | 5.7 | 0 | 0/10 |
| 9L/3A4 | q6d × 4 | i.p. | 60 | 6.3 | 0 | 0/2 |
| 9L/3A4 | q7d × 3 | i.t. | 60 | ~ 39 | 57 | 0/4 |
| 9L/3A4 | q7d × 3 | i.v. | 60 | ~ 38 | > 46 | 3/4 |
In separate experiments, mice bearing 9L/3A4 tumors were treated with MMDX by i.p. injection every 6 d, either as a series of 5 injections at 40 µg/kg body weight, or a series of 4 injections at 60 µg/kg body weight. No antitumor activity, and no host toxicity, was seen with the i.p. route of MMDX administration (Table 2). In contrast, i.v. MMDX treatment induced strong systemic toxicity, with 4 out of 4 mice dying within 7 d (9L tumors) and 3 out of 4 mice dying within 10 d (9L/3A4 tumors) after completing the first MMDX treatment cycle (Fig. 4A and Table 2). This toxicity was also apparent from the body weight loss of 3–7 g seen for the 9L and 9L/3A4 tumor-bearing mice following i.v. MMDX injection (Fig. 4B). Although a 2–4 g body weight loss was observed in the 9L/3A4 tumor-bearing mice on days 10 and 17 after the first i.t. MMDX injection, body weights recovered and no drug-induced deaths occurred (Fig. 4B). Some toxicity was observed after the second cycle of i.t. MMDX treatment, with two mice dying after the third MMDX injection. Thus, MMDX given by direct intratumoral delivery is effective in regressing 9L tumors that express CYP3A4, with dramatically decreased host toxicity compared to i.v. drug treatment.
Discussion
CYP3A4 is the major cytochrome P450 in human liver, where it plays a dominant role in the metabolism of several anti-cancer drugs, including epipodophyllotoxins, tamoxifen, taxol, vinca alkaloids and IFA 35. Drugs such as vinca alkaloids and irinotecan are metabolized by CYP3A4 to less active or inactive metabolites 36, 37, whereas other anti-cancer drugs, including IFA 32, the bioreductive agent AQ4N 19 and the anthracycline MMDX, are metabolized by CYP3A4 to more toxic metabolites. These and other anti-cancer P450 prodrugs are potential candidates for use in P450-based gene therapy for cancer 38. Preclinical P450 prodrug-activation studies to date have focused on CYP2B enzymes in combination with cyclophosphamide and IFA, and initial clinical trials have been promising 15, 39. However, the therapeutic potential of other P450-prodrug combinations, in particular those that involve CYP3A enzymes, which are distinguished from CYP2B enzymes by their high level expression in liver, is largely unknown. The present study investigated whether CYP3A4 may be used together with the CYP3A prodrug MMDX for gene-directed enzyme prodrug therapy (GDEPT) applications in cancer. Our primary goal was to determine whether CYP3A4 can be delivered to tumor cells at a level that is sufficient to sensitize the cells to MMDX, not only in cell culture, but in vivo, where MMDX is extensively metabolized in the liver by endogenous hepatic CYP3A enzymes.
MMDX has several features that make it an attractive prodrug candidate for GDEPT applications. In particular, the cell membrane permeability 24 and long half-life of activated MMDX 15 both indicate that the activated metabolite has the potential to confer a strong bystander effect, which is essential for any GDEPT strategy to be effective in vivo. The potential utility of MMDX for GDEPT for cancer treatment was investigated using CYP3A4, which displays the highest rate of MMDX activation in a panel of CYP3A enzymes 27. A 120-fold increase in MMDX sensitivity was obtained in 9L gliosarcoma cells infected with retrovirus expressing CYP3A4 as compared to CYP3A4-deficient 9L cell controls. Intratumoral expression of CYP3A4 dramatically increased chemosensitivity to MMDX in vivo, as revealed by a tumor growth delay study carried out in a scid mouse xenograft model. Notably, intratumoral expression of CYP3A4 increased MMDX antitumor activity dramatically, even though MMDX itself has substantial intrinsic anticancer activity, and despite the fact that MMDX-activating CYP3A enzymes are already expressed endogenously at a high level in mouse liver 28, as they are in human liver 27. CYP3A4 in combination with MMDX is thus a promising enzyme-prodrug combination that warrants further development and preclinical evaluation. The strong chemosensitization of 9L/3A4 tumors to MMDX reported here further suggests that MMDX may be particularly active against tumors that express CYP3A4 endogenously, i.e., without the introduction of a gene therapy vector. CYP3A4 expression has been reported in a broad range of primary human tumor tissues including those from patients with colon cancer 40, breast cancer 41, lung cancer 42, renal cell cancer 43, and bladder cancer 44. MMDX could be an agent of choice for patients with tumors characterized by high CYP3A4 levels, insofar as those tumors are likely to be resistant to drugs such as irinotecan/CPT-11, paclitaxel and docetaxel, which are inactivated by CYP3A4 metabolism 36, 40, 45.
CYP3A4-expressing 9L cells grew ~2-fold slower in culture than the corresponding P450-deficient cells. Slower growth rates were also reported in response to the introduction of CYP3A4 in human Caco-2 intestinal cells and HepG2 hepatoma cells, both derived from tissues that normally express CYP3A4 endogenously 46, 47. In the case of Caco-2/3A4 cells, cell growth improved with the loss of CYP3A4 during cell passage 46. Cells with high CYP3A4 activity may thus be at a growth disadvantage and ultimately be replaced by more rapidly proliferating cells with a lower level of CYP3A4. This hypothesis may help explain the difficulty we encountered in co-expressing CYP3A4 and P450 reductase at high levels in 9L cells, insofar as cells with lower CYP3A4 metabolic activity (i.e., cells that do not overexpress P450 reductase and/or have a low level of CYP3A4) may have a growth advantage over cells in the population with higher levels of CYP3A4 activity. Conceivably, high levels of CYP3A4 activity could slow cell growth by metabolizing an essential endogenous cell growth regulator. However, an increase in growth rate was not seen when 9L/3A4 cells were cultured in the presence of the CYP3A-selective inhibitor TAO (10 to 100 µM) (data not shown). Further studies are needed to fully understand the effects of CYP3A4 expression on tumor cell growth and viability and their impact on efforts to induce CYP3A4 expression in human tumors in vivo using viral or other gene therapy vectors.
CHO/3A4/HR cells are characterized by a 31-fold increase in P450 reductase activity compared to CHO/3A4 cells, which translated into a 28-fold higher CYP3A4 metabolic activity (IFA 4-hydroxylation) and an 11-fold (MMDX) or 23-fold (IFA) increase in prodrug cytotoxicity (Table 1). These findings support our earlier observation that coexpression of P450 reductase with CYP2B1 or CYP2B6 substantially improves GDEPT activity when combined with CPA or IFA treatment 4, 7. In some tumor cell lines, however, there may be minimal or even no improvement in CYP metabolic activity upon co-expression of P450 reductase 48, perhaps due to an already high molar ratio of endogenous P450 reductase to exogenous CYP, which is expected to saturate CYP metabolic activity 49. Efforts to use retrovirus to increase the level of P450 reductase in 9L/3A4 cells were unsuccessful, and resulted in a decrease in CYP3A4 protein levels without an increase in MMDX toxicity (data not shown). A decrease in CYP3A4 protein content was also seen when P450 reductase was introduced into CHO/3A4 cells, although in that case a large increase in overall CYP3A metabolic activity was obtained (Fig. 2 and Ref. 29). Thus, the impact of P450 reductase gene transfer on tumor cell capacity for P450 prodrug activation may be dependent on the endogenous tumor cell level of P450 reductase and its relation to the overall tumor cell P450 protein content following introduction of the gene therapy vector. Gene therapy vectors that give high levels of P450 expression (e.g., adenoviral vectors) 34 may be particularly demanding in terms of the requirement for P450 reductase, particularly in the case of CYPs that have a low apparent affinity for P450 reductase 50. The expression in tumor cells of other enzymes that utilize endogenous P450 reductase, which include cytochrome b5, heme oxygenase and the fatty acid hydroxylation system, may also influence the requirement of exogenous P450 reductase for efficient GDEPT activity.
The toxicity of activated MMDX is manifest at nanomolar prodrug concentrations, which enabled us to carry out the present studies at concentrations of MMDX that are >10,000-fold lower than the Km for MMDX, 16 µM 25. Nevertheless, despite the very low concentration of MMDX, there is sufficient formation of the active MMDX metabolite to effect tumor cell killing. This high potency of MMDX was retained in vivo, where low drug doses (60 µg/kg body weight) effected a substantial anti-tumor response, despite the fact that circulating MMDX concentrations are typically < 10 nM following bolus MMDX administration 51, i.e., >1000-fold lower than the Km (MMDX) for CYP3A4 metabolism. In contrast, IFA cytotoxicity required millimolar concentrations of prodrug, consistent with the Km (IFA) of ~ 1 mM exhibited by CYP3A4 32.
Human tumor cells infected with Adeno-3A4 exhibited a modest increase in MMDX sensitivity compared to that seen in the case of the retrovirus-infected 9L cells. Efforts to further chemosensitize the tumor cells using the tumor cell replicating adenovirus Onyx-017 to promote the replication and increase the expression from the replication-defective adenoviral P450 vector 6, 34 were only partially successful. Although co-infection of tumor cells with Onyx-017 + Adeno-3A4 resulted in a 50 to 60-fold increase in CYP3A4 RNA compared to Adeno-3A4 infection alone, only modest increases in CYP3A4 protein and activity were achieved. Further work will be needed to develop strategies for increasing intratumoral CYP3A4 protein and activity in vivo, e.g., by taking advantage of the recently described stabilizing effect of NFκB on CYP3A4 protein 52, before this CYP3A4 gene therapy can be implemented in the clinic. Nevertheless, the present study provides proof-of-concept for the potential of CYP3A4 for prodrug activation-based gene therapy in the context of a high background of liver CYP3A activity.
P450-based GDEPT using cyclophosphamide-activating CYP2B enzymes improves intratumoral 4-OH-cyclophosphamide pharmacokinetics and enhances antitumor activity in vivo, with the greatest improvements obtained when cyclophosphamide is administered by directly intratumoral injection 53, 54. In the present study, host toxicity associated with MMDX treatment was dramatically decreased when MMDX was directly delivered into tumors using a syringe pump. 9L/3A4 tumors did not respond to MMDX administered by i.p. injection, suggesting there is a significant first pass hepatic effect, which not only leads to MMDX activation but also inactivates MMDX and/or its active metabolite. Low host toxicity was observed when MMDX was administered by direct i.t. injection, in contrast to i.v. MMDX treatment, which resulted in 7/8 deaths after the first cycle of MMDX treatment, perhaps due to intrinsic toxicities associated with exposure to the unmetabolized parent drug. Direct i.t. delivery of MMDX might also help overcome the low efficiency of drug uptake that characterizes many solid tumors, although in the present study there was no difference in 9L/3A4 antitumor activity between i.v. and i.t. MMDX administration. While intratumoral chemotherapy is not suitable for all solid tumors and may impose certain practical limitations, it has been used in the clinic for treatment of a number of diseases, including head and neck cancer, lung cancer, and breast cancer 55–57, and could prove to be particularly effective in the case of CYP3A4-expressing tumors and MMDX.
In summary, the present proof-of-concept studies establish the therapeutic efficacy of MMDX treatment in combination with CYP3A4 gene transfer and demonstrate the strong anti-cancer potential of this novel gene-prodrug combination. However, the efficiency of CYP3A4 protein expression in tumor cells needs to be improved in order for this strategy to be implemented in the clinic. The present studies also highlight the importance of intratumoral, rather than hepatic, CYP3A4 expression for effective chemotherapeutic responses to MMDX in the absence of a gene therapeutic, and suggest that patient tumor biopsies should be screened for CYP3A4 protein levels to identify individuals who are most likely to benefit from treatment with MMDX and perhaps other CYP3A4 prodrugs.
Supplementary Material
Acknowledgements
The authors thank Jie Ma for assistance with intravenous injections and Dr. Thomas Friedberg (Univ. of Dundee) for providing CYP3A4 cDNA and CHO cells expressing CYP3A4.
Abbreviations
- MMDX
methoxymorpholinyl doxorubin, also known as nemorubicin
- CYP or P450
cytochrome P450
- IFA
ifosfamide
- FBS
fetal bovine serum
- DMEM
Dulbecco’s modified Eagle’s medium
- qPCR
quantitative, real-time PCR
- MOI
multiplicity of infection
- GDEPT
gene-directed enzyme prodrug therapy
Footnotes
Supported in part by NIH grant CA49248 (to DJW).
References
- 1.Michael M, Doherty MM. Tumoral drug metabolism: overview and its implications for cancer therapy. J Clin Oncol. 2005;23(1):205–229. doi: 10.1200/JCO.2005.02.120. [DOI] [PubMed] [Google Scholar]
- 2.Moolten FL. Drug sensitivity ("suicide") genes for selective cancer chemotherapy. Cancer Gene Ther. 1994;1(4):279–287. [PubMed] [Google Scholar]
- 3.Roy P, Waxman DJ. Activation of oxazaphosphorines by cytochrome P450: application to gene-directed enzyme prodrug therapy for cancer. Toxicol In Vitro. 2006 Mar;20(2):176–186. doi: 10.1016/j.tiv.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Yu LJ, Waxman DJ. Potentiation of cytochrome P450/cyclophosphamide-based cancer gene therapy by coexpression of the P450 reductase gene. Cancer Res. 1997;57(21):4830–4837. [PubMed] [Google Scholar]
- 5.Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998 May;16(5):444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 6.Jounaidi Y, Chen CS, Veal GJ, Waxman DJ. Enhanced antitumor activity of P450 prodrug-based gene therapy using the low Km cyclophosphamide 4-hydroxylase P450 2B11. Mol Cancer Ther. 2006 Mar;5(3):541–555. doi: 10.1158/1535-7163.MCT-05-0321. [DOI] [PubMed] [Google Scholar]
- 7.Jounaidi Y, Hecht JE, Waxman DJ. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58(19):4391–4401. [PubMed] [Google Scholar]
- 8.Tyminski E, Leroy S, Terada K, et al. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005 Aug 1;65(15):6850–6857. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 9.Niculescu-Duvaz I, Springer CJ. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol. 2005 May;30(1):71–88. doi: 10.1385/MB:30:1:071. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Parker WB, Sorscher EJ, Ealick SE. PNP anticancer gene therapy. Curr Top Med Chem. 2005;5(13):1259–1274. doi: 10.2174/156802605774463105. [DOI] [PubMed] [Google Scholar]
- 11.Searle PF, Chen MJ, Hu L, et al. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin Exp Pharmacol Physiol. 2004 Nov;31(11):811–816. doi: 10.1111/j.1440-1681.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 12.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther. 2001;12(12):1594–1596. [PubMed] [Google Scholar]
- 14.Freytag SO, Stricker H, Peabody J, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007 Mar;15(3):636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 15.Braybrooke JP, Slade A, Deplanque G, et al. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin Cancer Res. 2005;11(4):1512–1520. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Waxman DJ. Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res. 1995;55(3):581–589. [PubMed] [Google Scholar]
- 17.Samel S, Keese M, Lux A, et al. Peritoneal cancer treatment with CYP2B1 transfected, microencapsulated cells and ifosfamide. Cancer Gene Ther. 2006 Jan 1;13(1):65–73. doi: 10.1038/sj.cgt.7700849. [DOI] [PubMed] [Google Scholar]
- 18.Jounaidi Y, Waxman DJ. Combination of the bioreductive drug tirapazamine with the chemotherapeutic prodrug cyclophosphamide for P450/P450-reductase-based cancer gene therapy. Cancer Res. 2000;60(14):3761–3769. [PubMed] [Google Scholar]
- 19.McCarthy HO, Yakkundi A, McErlane V, et al. Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther. 2003;10(1):40–48. doi: 10.1038/sj.cgt.7700522. [DOI] [PubMed] [Google Scholar]
- 20.Ghielmini M, Colli E, Bosshard G, et al. Hematotoxicity on human bone marrow-and umbilical cord blood-derived progenitor cells and in vitro therapeutic index of methoxymorpholinyldoxorubicin and its metabolites. Cancer Chemother Pharmacol. 1998;42(3):235–240. doi: 10.1007/s002800050810. [DOI] [PubMed] [Google Scholar]
- 21.Kuhl JS, Duran GE, Chao NJ, Sikic BI. Effects of the methoxymorpholino derivative of doxorubicin and its bioactivated form versus doxorubicin on human leukemia and lymphoma cell lines and normal bone marrow. Cancer Chemother Pharmacol. 1993;33(1):10–16. doi: 10.1007/BF00686016. [DOI] [PubMed] [Google Scholar]
- 22.Lau DH, Duran GE, Lewis AD, Sikic BI. Metabolic conversion of methoxymorpholinyl doxorubicin: from a DNA strand breaker to a DNA cross-linker. Br J Cancer. 1994;70(1):79–84. doi: 10.1038/bjc.1994.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis AD, Lau DH, Duran GE, Wolf CR, Sikic BI. Role of cytochrome P-450 from the human CYP3A gene family in the potentiation of morpholino doxorubicin by human liver microsomes. Cancer Res. 1992;52(16):4379–4384. [PubMed] [Google Scholar]
- 24.Baldwin A, Huang Z, Jounaidi Y, Waxman DJ. Identification of novel enzyme-prodrug combinations for use in cytochrome P450-based gene therapy for cancer. Arch Biochem Biophys. 2003;409(1):197–206. doi: 10.1016/s0003-9861(02)00453-8. [DOI] [PubMed] [Google Scholar]
- 25.Quintieri L, Geroni C, Fantin M, et al. Formation and antitumor activity of PNU-159682, a major metabolite of nemorubicin in human liver microsomes. Clin Cancer Res. 2005;11(4):1608–1617. doi: 10.1158/1078-0432.CCR-04-1845. [DOI] [PubMed] [Google Scholar]
- 26.Alvino E, Gilberti S, Cantagallo D, et al. In vitro antitumor activity of 3'-desamino-3'(2-methoxy-4-morpholinyl) doxorubicin on human melanoma cells sensitive or resistant to triazene compounds. Cancer Chemother Pharmacol. 1997;40(2):180–184. doi: 10.1007/s002800050644. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Waxman DJ. Antitumor activity of methoxymorpholinyl doxorubicin: potentiation by cytochrome P450 3A metabolism. Mol Pharmacol. 2005;67(1):212–219. doi: 10.1124/mol.104.005371. [DOI] [PubMed] [Google Scholar]
- 28.Quintieri L, Rosato A, Napoli E, et al. In vivo antitumor activity and host toxicity of methoxymorpholinyl doxorubicin: role of cytochrome P450 3A. Cancer Res. 2000;60(12):3232–3238. [PubMed] [Google Scholar]
- 29.Ding S, Yao D, Burchell B, Wolf CR, Friedberg T. High levels of recombinant CYP3A4 expression in Chinese hamster ovary cells are modulated by coexpressed human P450 reductase and hemin supplementation. Arch Biochem Biophys. 1997;348(2):403–410. doi: 10.1006/abbi.1997.0405. [DOI] [PubMed] [Google Scholar]
- 30.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 31.Finer MH, Dull TJ, Qin L, Farson D, Roberts MR. kat: a high-efficiency retroviral transduction system for primary human T lymphocytes. Blood. 1994;83(1):43–50. [PubMed] [Google Scholar]
- 32.Chen CS, Jounaidi Y, Waxman DJ. Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos. 2005 Sep;33(9):1261–1267. doi: 10.1124/dmd.105.004788. [DOI] [PubMed] [Google Scholar]
- 33.Miwa GT, West SB, Lu AY. Studies on the rate-limiting enzyme component in the microsomal monooxygenase system. Incorporation of purified NADPH-cytochrome c reductase and cytochrome P-450 into rat liver microsomes. J Biol Chem. 1978;253(6):1921–1929. [PubMed] [Google Scholar]
- 34.Jounaidi Y, Waxman DJ. Use of replication-conditional adenovirus as a helper system to enhance delivery of P450 prodrug-activation genes for cancer therapy. Cancer Res. 2004;64(1):292–303. doi: 10.1158/0008-5472.can-03-1798. [DOI] [PubMed] [Google Scholar]
- 35.Kivisto KT, Kroemer HK, Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br J Clin Pharmacol. 1995;40(6):523–530. doi: 10.1111/j.1365-2125.1995.tb05796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haaz MC, Rivory L, Riche C, Vernillet L, Robert J. Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res. 1998 Feb 1;58(3):468–472. [PubMed] [Google Scholar]
- 37.Yao D, Ding S, Burchell B, Wolf CR, Friedberg T. Detoxication of vinca alkaloids by human P450 CYP3A4-mediated metabolism: implications for the development of drug resistance. J Pharmacol Exp Ther. 2000;294(1):387–395. [PubMed] [Google Scholar]
- 38.Chen L, Waxman DJ. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Curr Pharm Des. 2002;8(15):1405–1416. doi: 10.2174/1381612023394566. [DOI] [PubMed] [Google Scholar]
- 39.Salmons B, Lohr M, Gunzburg WH. Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: results of a phase I/II clinical trial. J Gastroenterol. 2003 Mar;38 Suppl 15:78–84. [PubMed] [Google Scholar]
- 40.Martinez C, Garcia-Martin E, Pizarro RM, Garcia-Gamito FJ, Agundez JA. Expression of paclitaxel-inactivating CYP3A activity in human colorectal cancer: implications for drug therapy. Br J Cancer. 2002;87(6):681–686. doi: 10.1038/sj.bjc.6600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt R, Baumann F, Knupfer H, et al. CYP3A4, CYP2C9 and CYP2B6 expression and ifosfamide turnover in breast cancer tissue microsomes. Br J Cancer. 2004;90(4):911–916. doi: 10.1038/sj.bjc.6601492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kivisto KT, Fritz P, Linder A, Friedel G, Beaune P, Kroemer HK. Immunohistochemical localization of cytochrome P450 3A in human pulmonary carcinomas and normal bronchial tissue. Histochem Cell Biol. 1995 Jan;103(1):25–29. doi: 10.1007/BF01464472. [DOI] [PubMed] [Google Scholar]
- 43.Murray GI, McFadyen MC, Mitchell RT, Cheung YL, Kerr AC, Melvin WT. Cytochrome P450 CYP3A in human renal cell cancer. Br J Cancer. 1999 Apr;79(11–12):1836–1842. doi: 10.1038/sj.bjc.6690292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray GI, Taylor VE, McKay JA, et al. Expression of xenobiotic metabolizing enzymes in tumours of the urinary bladder. Int J Exp Pathol. 1995 Aug;76(4):271–276. [PMC free article] [PubMed] [Google Scholar]
- 45.Engels FK, Ten Tije AJ, Baker SD, et al. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004 May;75(5):448–454. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Crespi CL, Penman BW, Hu M. Development of Caco-2 cells expressing high levels of cDNA-derived cytochrome P4503A4. Pharm Res. 1996;13(11):1635–1641. doi: 10.1023/a:1016428304366. [DOI] [PubMed] [Google Scholar]
- 47.Yoshitomi S, Ikemoto K, Takahashi J, Miki H, Namba M, Asahi S. Establishment of the transformants expressing human cytochrome P450 subtypes in HepG2, and their applications on drug metabolism and toxicology. Toxicol In Vitro. 2001;15(3):245–256. doi: 10.1016/s0887-2333(01)00011-x. [DOI] [PubMed] [Google Scholar]
- 48.Lengler J, Omann M, Duvier D, et al. Cytochrome P450 reductase dependent inhibition of cytochrome P450 2B1 activity: Implications for gene directed enzyme prodrug therapy. Biochem Pharmacol. 2006 Sep 28;72(7):893–901. doi: 10.1016/j.bcp.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Kaminsky LS, Guengerich FP. Cytochrome P-450 isozyme/isozyme functional interactions and NADPH-cytochrome P-450 reductase concentrations as factors in microsomal metabolism of warfarin. Eur J Biochem. 1985 Jun 18;149(3):479–489. doi: 10.1111/j.1432-1033.1985.tb08950.x. [DOI] [PubMed] [Google Scholar]
- 50.Shimada T, Mernaugh RL, Guengerich FP. Interactions of mammalian cytochrome P450, NADPH-cytochrome P450 reductase, and cytochrome b(5) enzymes. Arch Biochem Biophys. 2005;435(1):207–216. doi: 10.1016/j.abb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Vasey PA, Bissett D, Strolin-Benedetti M, et al. Phase I clinical and pharmacokinetic study of 3'-deamino-3'-(2-methoxy-4-morpholinyl)doxorubicin (FCE 23762) Cancer Res. 1995;55(10):2090–2096. [PubMed] [Google Scholar]
- 52.Zangar RC, Bollinger N, Verma S, Karin NJ, Lu Y. The nuclear factor-kappa B pathway regulates cytochrome P450 3A4 protein stability. Mol Pharmacol. 2008 Jun;73(6):1652–1658. doi: 10.1124/mol.107.043976. [DOI] [PubMed] [Google Scholar]
- 53.Chen CS, Jounaidi Y, Su T, Waxman DJ. Enhancement of intratumoral cyclophosphamide pharmacokinetics and antitumor activity in a P450 2B11-based cancer gene therapy model. Cancer Gene Ther. 2007 Dec;14(12):935–944. doi: 10.1038/sj.cgt.7701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichikawa T, Petros WP, Ludeman SM, et al. Intraneoplastic polymer-based delivery of cyclophosphamide for intratumoral bioconversion by a replicating oncolytic viral vector. Cancer Res. 2001 Feb 1;61(3):864–868. [PubMed] [Google Scholar]
- 55.Duvillard C, Polycarpe E, Romanet P, Chauffert B. Intratumoral chemotherapy: experimental data and applications to head and neck tumors. Ann Otolaryngol Chir Cervicofac. 2007 Jun;124(2):53–60. doi: 10.1016/j.aorl.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008 Jul;61(1):1–12. doi: 10.1016/j.lungcan.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Almond BA, Hadba AR, Freeman ST, et al. Efficacy of mitoxantrone-loaded albumin microspheres for intratumoral chemotherapy of breast cancer. J Control Release. 2003 Aug 28;91(1–2):147–155. doi: 10.1016/s0168-3659(03)00214-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.