Abstract
Background
Genetic variants of the warfarin sensitivity gene CYP2C9 have been associated with increased bleeding risk during warfarin initiation. Studies also suggest that such patients remain at risk throughout treatment.
Objective
Would testing patients with non-valvular atrial fibrillation (AF) for CYP2C9 before initiating warfarin improve outcomes?
Design
Markov state transition decision model.
Setting
Ambulatory or inpatient settings necessitating new initiation of anticoagulation.
Patients
The base case was a 69-year-old man with newly diagnosed non-valvular AF. Interventions included: (1) warfarin, (2) aspirin, or (3) no antithrombotic therapy without genetic testing; and genetic testing followed by (4) aspirin or (5) no antithrombotic therapy in those with culprit CYP2C9 alleles.
Measures
Quality-adjusted life years (QALYs).
Results
In the base case, testing and treating patients with CYP2C9*2 and/or CYP2C9*3 with aspirin rather than warfarin was best (8.97 QALYs). However, warfarin without genetic testing was a close second (8.96 QALYs), a difference of roughly 5 days. Sensitivity analyses demonstrated that genetic testing followed by aspirin was best for patients at lower risk of embolic events. Warfarin without testing was preferred if the rate of embolic events was greater than 5% per year, or the risk of major bleeding while receiving warfarin was lower.
Conclusion
For patients at average risk for ischemic stroke due to AF and at average risk for major hemorrhage, treatment based on genetic testing offers no benefit compared to warfarin initiation without testing. The gain from testing may be larger in patients at lower risk of embolic events or at greater risk of bleeding.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-009-0927-7) contains supplementary material, which is available to authorized users.
Key words: anticoagulant therapy, warfarin, genetic testing, pharmacogenetics, decision analysis, atrial fibrillation, stroke
INTRODUCTION
While the effectiveness of anticoagulant therapy has been established clearly for patients with atrial fibrillation at average risk for thromboembolism, the decision to treat patients at low or moderate risk for ischemic stroke remains complex.1–3 The balance between the risk of bleeding and the benefit appreciated by preventing stroke becomes particularly important in these lower risk patients. The identification of factors, be they clinical or genetic, that predict an increased risk of bleeding can inform this decision, and may affect the choice of antithrombotic therapy in patients at lower risk for ischemic stroke.
Genetic variation in warfarin metabolism and sensitivity genes recently has been associated with both a decreased warfarin dosing requirement and increased risk of bleeding.4–7 Several studies have demonstrated that patients possessing certain variant alleles of vitamin K epoxide reductase, VKORC1 and cytochrome P450 CYP2C9 have greater instability in anticoagulation intensity8, as measured by the international normalized ratio (INR), and increased bleeding risk during the initiation phase of anticoagulant therapy.9–14 While the increased risk of bleeding attributable to VKORC1 appears to be limited to the initiation phase of anticoagulant therapy, several studies have presented evidence suggesting that variant alleles of CYP2C9 also are associated with a continued risk of bleeding during the maintenance phase of anticoagulant therapy.4,11,15,16
Therefore, we developed a decision analytic model to address the clinical question of whether testing for CYP2C9 should be performed routinely before initiating anticoagulant therapy in patients with non-valvular atrial fibrillation. The Federal Drug Administration already has endorsed new labeling for Coumadin™/warfarin, suggesting that clinicians consider performing genetic testing before initiating warfarin to aid in initial dose selection, and indeed warfarin dose titration algorithms have been developed to incorporate the results of such testing.17–20 Our intent is not to focus on this issue, but rather to examine whether testing for CYP2C9 is useful in informing the decision of whether or not to treat with anticoagulant therapy.
METHODS
Review of Data
CYP2C9 Genotype and Bleeding Events An association between the CYP2C9 genotype and bleeding risk and anticoagulation status was first suggested by Ogg21 and confirmed by Higashi et al.11 Variation in the activity of this hepatic microsomal enzyme, which comprises the primary metabolic pathway for S-warfarin, leads to significant differences in patients’ responses to warfarin. The *2 and *3 polymorphisms lead to decreased enzymatic activity of 30% and 80%, respectively; thus, patients with these genetic variants metabolize warfarin more slowly. In Higashi’s study of 185 patients studied between 1990 and 2001, those with at least one culprit allele had a 1.4-fold increased risk of supratherapeutic INRs [95% confidence interval (CI), 1.03–1.90], and required a longer time to stable dosing. In addition, such patients had a significantly increased risk of serious or life-threatening bleeding, both during the initiation phase (1st 3 months) (HR, 3.94; 95% CI, 1.29–12.06) and during the entire follow-up period (HR, 2.39; 95% CI, 1.18–4.86).Subsequently, a systematic review and meta-analysis has noted that patients with either CYP2C9*2 or CYP2C9*3 variant have lower mean daily warfarin dose requirements and a greater risk of bleeding [relative risk (RR), 2.26; 95% CI, 1.36–3.75].4 Several studies have related the risk of bleeding in patients with culprit alleles to intensity of anticoagulation.11 However, in separate studies by Margaglione12 and Taube13, such an association between higher risk of bleeding and elevated INRs was not seen. In a large Scandinavian study following 7,983 patients receiving either acenocoumarol or phenprocoumon, coumarins not commonly used in the United States, variant alleles of CYP2C9 were not associated with an increased risk of bleeding events during the initiation phase of anticoagulation.14 Furthermore, patients receiving phenprocoumon had no significantly increased risk of major bleeding during the entire study period. However, patients with the variant alleles who received acenocoumarol had an increased relative hazard of major bleeding events (HR, 1.83; 95% CI, 1.01–3.32), consistent with studies of warfarin.Most recently, Limdi et al. reported on the association of genotype and bleeding risk in 446 patients taking warfarin with over 555 person-years of follow-up.15 In Cox proportional hazards models adjusting for age, gender, race, BMI, VKORC1 genotype, vitamin K and alcohol intake, warfarin dose, interacting drugs, significant comorbid illnesses, and INR at the time of hemorrhage, they calculated an overall hazard ratio of 3.0 (95% CI, 1.22–7.54). The availability of longitudinal data with 2-year follow-up allowed them to calculate relative hazards for major bleeding both before stabilization of therapy (HR, 5.3; 95% CI, 0.47–58.7) and the after first stabilization of therapy (HR, 2.2; 95% CI, 0.72–6.57), although these results were not statistically significant, and there was no significant increase in risk of minor hemorrhage (HR, 1.3; 95% CI, 0.8–2.1). These results are similar to those described in the earlier study by Higashi, although neither study recorded any intracranial hemorrhages among their major hemorrhages.11
Relative Hazard of Major Hemorrhage in Patients Receiving Anticoagulant Therapy In a review of 16 trials examining the use of anticoagulant and antiplatelet agents for the prevention of stroke, the rate of major extracranial hemorrhage averaged 0.6% per year in patients not receiving anticoagulant therapy. The relative risk for major extracranial hemorrhage in patients receiving anticoagulant therapy was 2.4, resulting in an average rate of 1.4% per year for trial participants.22,23 Higher rates of hemorrhage have been described in other populations.24,25In a seminal multicenter study examining risk factors for complications of chronic anticoagulant therapy, Fihn et al. described a 1.9-fold (95% CI, 1.3–3.0) increased risk of serious bleeding during the first 3 months of treatment compared with the rest of the 1st year.26 Fang et al. recently described the rates of intracranial hemorrhage in 13,559 AF patients in Kaiser Permanente’s ATRIA cohort.27 They noted a strong correlation between increasing age and risk of intracranial hemorrhage. Patients between 60 and 69 years of age had 0.07% (95% CI, 0.02%–0.27%) per year rate of intracranial hemorrhage off warfarin and a 0.40% (95% CI,.023%–0.68%) per year rate of intracranial hemorrhage on warfarin (relative hazard — 5.7). These results are consistent with those described in van Walraven’s individual patient meta-analysis of six published randomized AF trials.28 Other studies have found similar increases in risk of intracranial hemorrhage.29–35 The risk of intracranial hemorrhage while receiving aspirin is roughly 0.6 that of patients receiving warfarin.28,36–38
Description of Decision Model We developed a Markov state transition model 39 using a standard computer program (DECISION MAKER)40 to analyze decision trees and to perform sensitivity analyses. We considered five strategies for a hypothetical 69-year-old man with newly diagnosed non-valvular atrial fibrillation at average risk for ischemic stroke (Fig. 1) (see online appendix for decision model and description). Regardless of the strategy, patients may or may not possess a culprit allele of CYP2C9. Patients with the variants face approximately a four-fold increased risk of major bleeding versus those with the “wild-type” allele during warfarin initiation (the first 3 months) and a more than two-fold increased risk during the maintenance phase of treatment. We assumed that patients have a 1.9-fold increased risk of major bleeding during the initiation phase as described by Fihn and others. 24,26 Since the observed increased risk is a weighted average of higher risk in CYP2C9 variants and lower risk in CYP2C9 wild type, this resulted in a relative hazard of 4.7 for those with the culprit alleles and a lower relative hazard of 1.2 in those without the culprit alleles during the initiation phase versus the maintenance phase of warfarin therapy. In a similar manner, we calculated a lower relative hazard of major bleeds during the maintenance phase of therapy in those without the culprit alleles (0.65). We stratified major bleeding events into intracerebral hemorrhage, subdural hematoma, and extracranial bleeding. Intracerebral hemorrhage was further categorized into lobar and deep hemispheric locations, as previously described.30,41The Markov model contains 28 states of health (Supplementary Figure 1. During each monthly cycle, patients face the chance of thromboembolic events, and the chance of hemorrhagic events (intracerebral hemorrhage, subdural hematoma, and non-CNS bleeds). All of these events may lead to death, severe or mild permanent morbidity, or resolution. These sequences of chance events filter patients in each cycle of the simulation from one state to the state they occupy in the next cycle. Baseline values for parameters used in the decision analytic model are summarized in Table 1.
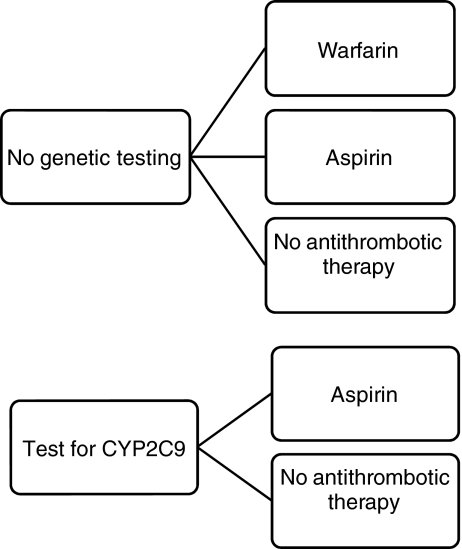
Figure 1.
Strategies examined for prevention of thromboembolism in a 69-year-old man with newly diagnosed non-valvular atrial fibrillation. In the top panel treatment is initiated without knowledge of CYP2C9 genotype. In the lower panel treatment is based on the results of CYP2C9 genotyping. Those who are negative for the variant alleles receive warfarin, whereas in those who test positive, we examine treatment with either aspirin or no antithrombotic therapy.
Table 1.
Data Required in the Analysis—Probabilities, Rates, Quality of Life
| Parameter | Value | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene frequency of CYP2C9*2 and/or CYP2C9*3 | 0.20 | 4,11,54–56 | ||||||
| Relative hazard of major bleed in variants versus “wild-type” CYP2C9 variants | ||||||||
| Initiation phase | 3.94 | 11,15 | ||||||
| Maintenance phase | 2.39 | 11,15 | ||||||
| Relative hazard of major bleed during initiation phase versus maintenance phase - | ||||||||
| General population | 1.9 | 26 | ||||||
| CYP2C9 variants | 4.7 | Calculated | ||||||
| CYP2C9 “wild-type” | 1.2 | Calculated | ||||||
| Rate of thromboembolism - untreated (%/year) | 0.045 | 35,57 | ||||||
| Efficacy of treatment | ||||||||
| With warfarin | 0.68 | 35 | ||||||
| With aspirin | 0.22 | 23,57 | ||||||
| Rate of thromboembolism - treated with warfarin (%/year) | 0.014 | Calculated | ||||||
| Rate of thromboembolism - treated with aspirin (%/year) | 0.04 | Calculated | ||||||
| Probable outcome from thromboembolic event | ||||||||
| Death | 0.27 | 35 | ||||||
| Permanent sequelae | 0.44 | 35,58 | ||||||
| With severe disability | 0.29 | 35,59,60 | ||||||
| With mild disability | 0.71 | 35,59,60 | ||||||
| Good recovery | 0.29 | 35,58 | ||||||
| Location of hemorrhage (value) (reference) | ||||||||
| Lobar ICH | Deep ICH | Extra-cranial | Subdural hematoma | |||||
| Rate of bleeding —untreated (%/year) | 0.00035 | 46 | 0.00035 | 46 | 0.006 | 22 | 0.00025 | 29,35 |
| Probable outcome from bleeding event - without warfarin* | ||||||||
| Death | 0.190 | 41 | 0.207 | 41 | 0.13 | 0.20 | 61 | |
| Severe long-term disability — GOS=3† | 0.428 | 41 | 0.436 | 41 | 0.07‡ | |||
| Mild long-term disability — GOS=4† | 0.196 | 41 | 0.187 | 41 | 0.40‡ | |||
| Good recovery - GOS=5† | 0.185 | 41 | 0.170 | 41 | 0.17‡ | |||
| Relative hazard of bleeding on anticoagulants | 5.7 | 62 | 5.7 | 62 | 2.4 | 63 | 4.0 | 29,32 |
| Rate of bleeding on anticoagulants (%/year) | 0.002 | 46 | 0.002 | 46 | 0.014 | 0.001 | 35 | |
| Probable outcome from bleeding event - on warfarin | ||||||||
| Death | 0.379 | 0.405 | 0.15 | 63 | 0.20 | 61 | ||
| Severe long-term disability - GOS=3† | 0.429 | 0.420 | 0.09 | 61 | ||||
| Mild long-term disability — GOS=4† | 0.111 | 0.103 | 0.50 | 61 | ||||
| Good recovery - GOS=5† | 0.080 | 0.073 | 0.20 | 61 | ||||
| Variable | Quality of life | |||||||
| Value | Reference | |||||||
| Long-term morbidities | ||||||||
| Well | 1.0 | |||||||
| Well while receiving anticoagulants | 0.99 | 64 | ||||||
| Severe long-term disability | 0.11 | 64 | ||||||
| Mild long-term disability | 0.76 | 64 | ||||||
| Dead | 0.0 | |||||||
| Short-term morbidities in patients with resolution | ||||||||
| Extracranial bleeding event§ | 0.84 | |||||||
| Intracerebral hemorrhage║ | 0.79 | |||||||
| Thromboembolic event║ | 0.79 | |||||||
*Assume outcomes of bleeding events for aspirin-treated patients are the same as for untreated patients
†GOS — Glascow Outcomes Score at 3 months
‡ Assume same distribution of neurological outcomes in survivors as in anticoagulated patients with subdural hematoma
§Assume Q = 0 for duration of hospitalization; length of stay (LOS) for gastrointestinal hemorrhage (DRG 174) =4.9 days
‖LOS for specific cerebrovascular disorders except transient ischemic attack (DRG 14) =6.4 days
RESULTS
In the base case (see Table 2, for a 69-year-old man at average risk for ischemic stroke due to AF and at average risk for major hemorrhage, testing and treating patients with CYP2C9*2 and/or CYP2C9*3 with aspirin rather than warfarin is best, yielding an expected utility of 8.97 quality-adjusted life years (QALYs). Anticoagulant therapy without prior testing for culprit alleles of CYP2C9 is a close second, yielding 8.96 QALYs, a difference of roughly 5 days.
Table 2.
Results of Base Case Analysis
| Strategy | Effectiveness |
|---|---|
| (QALYs) | |
| Testing for CYP2C9 followed by aspirin if (+) | 8.97 |
| Anticoagulation without prior testing | 8.96 |
| Testing for CYP2C9 followed by no antithrombotic therapy if (+) | 8.95 |
| Aspirin without prior testing | 8.60 |
| No antithrombotic therapy | 8.47 |
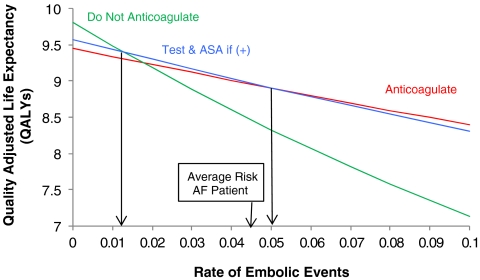
We performed sensitivity analyses to examine the impact of both uncertainty in parameter estimates and patient-to-patient variability in risk profiles for stroke and bleeding. While the average risk of stroke in patients with atrial fibrillation is 4.5% per year35, there is a wide spectrum of risk depending upon other co-morbidities, such as diabetes mellitus, hypertension, congestive heart failure, etc.42–44 Figure 2 examines the impact of changes in the risk of embolic events. The effectiveness of all strategies declines with increasing risk of embolic events. However, there are two thresholds of interest. Above an embolic rate of 5% per year (CHADS2 score of approximately 3 or greater)45, warfarin without prior testing is preferred; below a threshold of 1.3% per year no antithrombotic therapy is preferred. However, in the range between these two thresholds, testing followed by aspirin therapy in patients with culprit alleles is best. Thus, for the practicing physician who judges his or her patient to have an annual risk of thromboembolism between 1.3% per year and 5% per year, learning the results of CYP2C9 genotyping could alter the decision to anticoagulate.
Figure 2.
Quality-adjusted life expectancy (y-axis) predicted for three of the five strategies as a function of the estimated annual risk of thromboembolism (x-axis). At the base case value (4.5% annual rate of thromboembolism) for “average risk” patients with atrial fibrillation, testing and treating those found to possess the culprit alleles of CYP29C with aspirin is best. For patients having less than a 1.3% per year risk of embolic events, no antithrombotic therapy is best, while for those having greater than a 5% per year risk anticoagulation without prior testing is best. Thus, for individuals judged to have an annual risk of thromboembolism between 1.3% and 5% per year, testing and treating those found to possess culprit alleles of CYP29C with aspirin yields best outcomes, although by a very small margin.
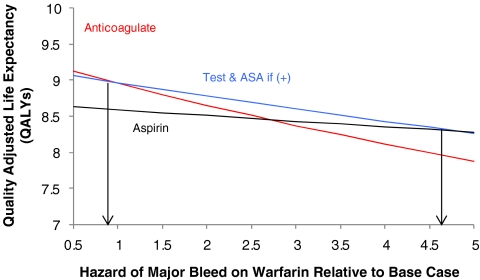
Patients may also vary in their underlying risk of major bleeding. In sensitivity analyses examining the risk of bleeding on warfarin, relative to the base hazards of intracerebral, subdural, and extracranial bleeding, testing is preferred unless the relative hazard of such events is less than 0.9, in which case anticoagulant therapy is best (Fig. 3. We also examined how an increased risk of intracranial hemorrhage associated with advancing age would impact the screening decision. In the base case we used an annual intracranial hemorrhage incidence of 0.07%.27 However, older patients (75–84 years of age) have an increased annual incidence of 0.11%, while those 85 years or older have an annual incidence approaching 0.2%.46 Older patients may also face an increased risk of thromboembolism (8.1%/year) if they have one or more risk factors beyond advanced age.35 In elderly patients without additional risk factors for thromboembolism who are between the ages of 70 and 79 years of age, or 80 years of age or older, testing followed by aspirin in those with genetic variants yields increasing benefit compared with warfarin. For the more typical elderly patients (75–84 years of age) with risk factors for thromboembolism, warfarin is preferred unless the relative hazard of major bleeding exceeds 2.8 during the maintenance phase of anticoagulant therapy, in which case genetic testing would be best.
Figure 3.
Quality-adjusted life expectancy (y-axis) predicted as a function of varying the hazard of major bleeding on warfarin (x-axis). Hazard of major bleeding on warfarin is relative to the baseline hazard estimate drawn from prior published reports. Thus, a hazard of 1.0 on the x-axis indicates relative hazards of 5.7, 4.0, and 2.4, respectively, for intracerebral, subdural, and extracranial bleeding while receiving warfarin. For patients in whom this hazard is judged to be less than 0.9, anticoagulation without prior testing yields superior outcomes. Aspirin without prior testing is best if the relative hazard exceeds 4.7, while genetic testing followed by aspirin for those possessing culprit alleles is favored in the range between these two values.
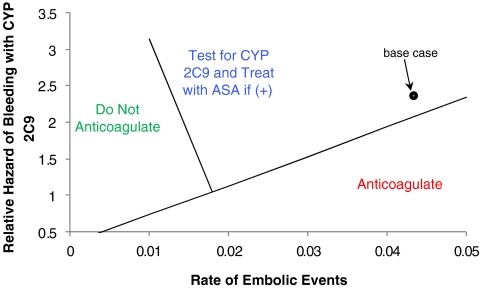
The evidence supporting an increased hazard of bleeding during the maintenance phase of warfarin therapy in patients with culprit alleles remains mixed. While testing was preferred at the base case relative hazard of 2.39, warfarin without prior testing would be preferred at lower relative hazards, less than 2.13. Figure 4 shows a two-way sensitivity analysis examining the effect of varying both the rate of embolic events and the relative hazard of major bleeding during the maintenance phase of anticoagulant therapy. Testing is favored in the region into which individuals fall if they are at lower risk of embolic events and, because of their possession of culprit variants of CYP2C9, at higher relative hazard of hemorrhage in the maintenance phase. While the base case falls within the region in which genetic testing is preferred, it is close to the threshold line. For patients at lower risk of thromboembolic events and at higher risk of bleeding, no antithrombotic therapy is favored.
Figure 4.
Two-way sensitivity analysis examining the effect of varying the annual risk of embolic events (x-axis) and the relative hazard of major bleeding associated with culprit alleles of CYP2C9 during the maintenance phase of anticoagulant therapy (y-axis). Hypothetical patients can therefore be assigned risk estimates for each of these two parameters. The area on the graph where they fall then allows assignment of a strategy either of (1) “Do not anticoagulate,” (2) genetic testing followed by aspirin for those individuals found to possess CYP2C9*2 and/or CYP2C9*3 culprit alleles, or (3) anticoagulation without genetic testing. Patients whose risk for thromboembolism is judged to be high are placed in the region to the right. For these patients, if the relative hazard for bleeding conferred by CYP2C9 is low, anticoagulation without prior testing is favored. For patients in whom the rate of embolic events is judged to be low (but not less than 1% per year) and the relative hazard conferred by CYP2C9 is high, genetic testing followed by aspirin in those found to possess the culprit genetic variants is best. For our base case 69-year-old man with nonvalvular AF, estimates for these two parameters place him just within the genetic testing region.
The efficacy of screening is dependent upon the frequency of the variant alleles; hence, the benefit accrued by treating such patients with a potentially more appropriate therapy (such as aspirin) is diluted by the outcomes in the majority of patients who don’t possess the culprit alleles. Therefore, we also examined outcomes of different treatments among patients known to have either the CYP2C9 *2 or *3 alleles. In such patients, aspirin yielded the greatest expected utility, 8.60 QALYs, while warfarin and no antithrombotic therapy fared somewhat worse, yielding 8.53 and 8.47 QALYs, respectively. Thus, were a physician to already know that a patient possessed one or more of these culprit alleles, treatment with aspirin would be best.
Finally, we examined an alternative scenario in which a safer, novel antithrombotic therapy becomes available that is not affected by CYP2C9-related pathways, such as the direct thrombin inhibitors. We assumed the novel agent: (1) had an efficacy and overall bleeding risk similar to that of warfarin in patients who do not possess culprit CYP2C9 alleles and (2) was not associated with an increased relative hazard of major bleeding during the initiation phase of treatment. This strategy yielded an expected utility of 9.04 QALYs, a gain of 0.09 QALYs vs. warfarin therapy.
DISCUSSION
Our analysis suggests that the benefit of testing for culprit alleles of CYP2C9 as an adjunct to decision making for antithrombotic therapy in patients with non-valvular atrial fibrillation depends upon several factors, particularly the risk of thromboembolic events for the individual patient. For the “average” patient with nonvalvular AF who would be assigned a CHADS2 score of 247, not otherwise at increased risk for major hemorrhage, the strategy of choosing warfarin or aspirin based on the results of genetic testing for CYP2C9 yields essentially equivalent outcomes compared to the strategy of initiating warfarin without genetic screening. For individuals at higher risk of thromboembolic stroke (i.e., CHADS2 score 3–6), treatment selection in response to genetic testing would yield inferior outcomes. Testing followed by treatment with aspirin in those found to have the culprit alleles only becomes preferred for patients at low risk of thromboembolism (between 1.3% and 5% per year). Bleeding risk also influences the benefit of genetic testing, with the gain from testing increasing as the relative hazard of major hemorrhage increases.
Among the testing strategies, treatment with aspirin in those found to possess the genetic variant is always better than not treating unless the risk of embolic events is less than roughly 1% per year, in which case no antithrombotic therapy is best. Although aspirin has a lower associated risk of major hemorrhage and is not impacted by the metabolic pathways governed by CYP2C9, it has only moderate efficacy (22%)23 in preventing embolic events. However, should safer novel antithrombotic therapies that are not impacted by CYP2C9-related pathways, such as the direct thrombin inhibitors, become available, testing followed by treatment with these drugs might be a beneficial strategy, even for patients at average risk for embolic events. Alternatively, if increasing the frequency of INR measurements during initiation as well as maintenance of warfarin therapy were demonstrated to reduce the risk of hemorrhage conferred by high-risk CYP2C9 alleles, then altering the INR monitoring strategy, rather than withholding warfarin altogether, might prove of benefit to affected individuals. Clearly, the adequacy of INR monitoring is an important determinant of the efficacy of warfarin for prevention of thromboembolism.48 Future research is needed to determine what role more vigilant monitoring may play in pharmacogenetic dosing strategies.
This analysis did not examine a strategy of pharmacogenetic-based dosing, in which the dose of warfarin is adjusted in response to genetic information. Several prior decision analyses have examined this question with varying results.49–51 In a separate study, we examined the cost-effectiveness of such a dosing strategy and found that for patients at average risk for ischemic stroke due to AF and at average risk for major hemorrhage, genotype-guided dosing resulted in better outcomes, but at a high cost.52 The marginal cost-effectiveness of testing versus standard induction was more than $170,000 per QALY. Further, we found there was only a 10% chance that genotype-guided dosing would likely be “cost-effective” (i.e., <$50,000 per QALY). We also identified several key parameters beyond the efficacy of pharmacogenetic-based dosing on bleeding risk that would influence the cost-effectiveness of this strategy, including the cost of genotyping and the delay in starting warfarin necessitated by waiting for testing results. Indeed, if future studies demonstrate efficacies above 32% in preventing major hemorrhages during warfarin initiation, then genotyping strategies that cost less than $200 and provide results quickly enough to avoid delays in initiating therapy could make genotype-guided dosing “cost-effective.”
Like all decision analyses, ours is limited by the data that are available. We have incorporated published data on risk of hemorrhage in anticoagulated patients who have been genotyped. There is even less information on whether CYP2C9 variants may influence risk of thromboembolism. Ongoing randomized trials are examining the efficacy of genotype-based dosing strategies for anticoagulation. Until these are completed, however, models such as ours can serve to guide bedside decision-making in an environment where genetic tests are widely available.
The discovery of genetic variants that influence the risk of bleeding during long-term anticoagulation holds the promise of improving the risk-benefit ratio of anticoagulation for the millions of individuals worldwide with atrial fibrillation and other conditions that increase risk for thromboembolism. It is important to recall, however, that the overwhelming majority of long-term morbidity and mortality related to bleeding on anticoagulants is due to intracranial hemorrhage53 and that fully 2/3 of these hemorrhages occur in the absence of supratherapeutic anticoagulation.41 Thus, if any genes are likely to play a major role in the decision to anticoagulate, they may well be unrelated to warfarin sensitivity or drug metabolism in general, but instead be discovered through their role in the underlying conditions that predispose to hemorrhage independent of the presence of anticoagulation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 25.5 KB)
(GIF 62.6 KB)
Acknowledgements
Mark H. Eckman: National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK075599), National Heart, Lung, and Blood Institute (K30 HL078581-01), and Foundation for Informed Medical Decision Making.
Steven M. Greenberg: National Institutes of Neurological Disorders and Stroke (R01 NS059727).
Jonathan Rosand: National Institutes of Neurological Disorders and Stroke (R01 NS059727), and the Deane Institute for Integrative Study of Atrial Fibrillation and Stroke.
We are grateful to Dr. Brian F. Gage in the Division of General Medical Sciences, Washington University in St. Louis for his help and generous contributions.
Conflict of Interest None disclosed.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-009-0927-7) contains supplementary material, which is available to authorized users.
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 14(7):e257–354. [DOI] [PubMed]
- 2.Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):429S–456S. [DOI] [PubMed]
- 3.Rockson SG, Albers GW. Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J Am Coll Cardiol. 2004;43(6):929–35. [DOI] [PubMed]
- 4.Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7(2):97–104. [DOI] [PubMed]
- 5.Yin T, Miyata T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1 - rationale and perspectives. Thromb Res. 2007;120(1):1–10. [DOI] [PubMed]
- 6.Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121(1):23–34. [DOI] [PMC free article] [PubMed]
- 7.Linder MW. Genetic mechanisms for hypersensitivity and resistance to the anticoagulant Warfarin. Clin Chim Acta. 2001;308(1–2):9–15. [DOI] [PubMed]
- 8.Muszkat M, Blotnik S, Elami A, Krasilnikov I, Caraco Y. Warfarin metabolism and anticoagulant effect: a prospective, observational study of the impact of CYP2C9 genetic polymorphism in the presence of drug-disease and drug-drug interactions. Clin Ther. 2007;29(3):427–37. [DOI] [PubMed]
- 9.Adcock DM, Koftan C, Crisan D, Kiechle FL. Effect of polymorphisms in the cytochrome P450 CYP2C9 gene on warfarin anticoagulation. Arch Pathol Lab Med. 2004;128(12):1360–3. [DOI] [PubMed]
- 10.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353(9154):717–9. [DOI] [PubMed]
- 11.Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. Jama. 2002;287(13):1690–8. [DOI] [PubMed]
- 12.Margaglione M, Colaizzo D, D’Andrea G, et al. Genetic modulation of oral anticoagulation with warfarin. Thromb Haemost. 2000;84(5):775–8. [PubMed]
- 13.Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood. 2000;96(5):1816–9. [PubMed]
- 14.Visser LE, van Schaik RH, van Vliet M, et al. The risk of bleeding complications in patients with cytochrome P450 CYP2C9*2 or CYP2C9*3 alleles on acenocoumarol or phenprocoumon. Thromb Haemost. 2004;92(1):61–6. [DOI] [PubMed]
- 15.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T Genotype on the Risk of Hemorrhagic Complications in African-American and European-American Patients on Warfarin. Clin Pharmacol Ther. 2007. [DOI] [PMC free article] [PubMed]
- 16.Visser LE, van Vliet M, van Schaik RH, et al. The risk of overanticoagulation in patients with cytochrome P450 CYP2C9*2 or CYP2C9*3 alleles on acenocoumarol or phenprocoumon. Pharmacogenetics. 2004;14(1):27–33. [DOI] [PubMed]
- 17.Gage BF. Pharmacogenetics-based coumarin therapy. Hematology Am Soc Hematol Educ Program. 2006:467–73. [DOI] [PubMed]
- 18.Kamali F, Pirmohamed M. The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol. 2006;61(6):746–51. [DOI] [PMC free article] [PubMed]
- 19.Krynetskiy E, McDonnell P. Building individualized medicine: prevention of adverse reactions to warfarin therapy. J Pharmacol Exp Ther. 2007;322(2):427–34. [DOI] [PubMed]
- 20.Millican EA, Lenzini PA, Milligan PE, et al. Genetic-based dosing in orthopedic patients beginning warfarin therapy. Blood. 2007;110(5):1511–5. [DOI] [PMC free article] [PubMed]
- 21.Ogg MS, Brennan P, Meade T, Humphries SE. CYP2C9*3 allelic variant and bleeding complications. Lancet. 1999;354(9184):1124. [DOI] [PubMed]
- 22.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501. [DOI] [PubMed]
- 23.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67. [DOI] [PubMed]
- 24.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95(3):315–28. [DOI] [PubMed]
- 25.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713–9. [DOI] [PubMed]
- 26.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118(7):511–20. [DOI] [PubMed]
- 27.Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54(8):1231–6. [DOI] [PMC free article] [PubMed]
- 28.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. Jama. 2002;288(19):2441–8. [DOI] [PubMed]
- 29.Wintzen AR, de Jonge H, Loeliger EA, Bots GT. The risk of intracerebral hemorrhage during oral anticoagulant treatment: a population study. Ann Neurol. 1984;16(5):553–8. [DOI] [PubMed]
- 30.Woo D, Broderick JP. Spontaneous intracerebral hemorrhage: epidemiology and clinical presentation. Neurosurg Clin N Am. 2002;13(3):265–79, v. [DOI] [PubMed]
- 31.Furlan AJ, Whisnant JP, Elveback LR. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 1979;5(4):367–73. [DOI] [PubMed]
- 32.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26(8):1471–7. [DOI] [PubMed]
- 33.Woo D, Kaushal R, Chakraborty R, et al. Association of apolipoprotein E4 and haplotypes of the apolipoprotein E gene with lobar intracerebral hemorrhage. Stroke. 2005;36(9):1874–9. [DOI] [PubMed]
- 34.Albers GW, Sherman DG, Gress DR, Paulseth JE, Petersen P. Stroke prevention in nonvalvular atrial fibrillation: a review of prospective randomized trials. Ann Neurol. 1991;30(4):511–8. [DOI] [PubMed]
- 35.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–57. [PubMed]
- 36.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. Jama. 1998;280(22):1930–5. [DOI] [PubMed]
- 37.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med. 1996;156(4):409–16. [PubMed]
- 38.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):234S–264S. [DOI] [PubMed]
- 39.Detsky A, Naglie G, Krahn M, Naimark D, Redelmeier D. Primer on medical decision analysis: Part 1—Getting started. Med Decis Making. 1997; 17: 123–125. [DOI] [PubMed]
- 40.Lau J, Kassirer JP, Pauker SG. Decision Maker 3.0. Improved decision analysis by personal computer. Med Decis Making. 1983;3(1):39–43. [DOI] [PubMed]
- 41.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164(8):880–4. [DOI] [PubMed]
- 42.Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116(1):6–12. [DOI] [PubMed]
- 43.Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators [see comments]. Ann Intern Med. 1992;116(1):1–5. [DOI] [PubMed]
- 44.Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158(12):1316–20. [DOI] [PubMed]
- 45.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285(22):2864–70. [DOI] [PubMed]
- 46.Broderick JP. Natural history of primary intracerebral hemorhage. In: Whisnant JP, ed. Population-based clinical epidemiology of stroke. Oxford: Butterworth-Heinmann; 1993: 154–173.
- 47.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285(22):2864–70. [DOI] [PubMed]
- 48.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–37. [DOI] [PubMed]
- 49.Health care savings from personalized medicine using genetic testing: the case of warfarin. http://www.aei-brookings.org/publications/abstract.php?pid= 1127. 1/15/2008.
- 50.You JH, Chan FW, Wong RS, Cheng G. The potential clinical and economic outcomes of pharmacogenetics-oriented management of warfarin therapy - a decision analysis. Thromb Haemost. 2004;92(3):590–7. [DOI] [PubMed]
- 51.Veenstra DL. The cost-effectiveness of warfarin pharmacogenomics. J Thromb Haemost. 2007;5(9):1974–5. [DOI] [PubMed]
- 52.Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-Effectiveness of Pharmacogenetic-Based Dosing of Warfarin in Patients with Atrial Fibrillation. Ann Intern Med. 2009; 150: 73–83. [DOI] [PubMed]
- 53.Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120(8):700–5. [DOI] [PMC free article] [PubMed]
- 54.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12(3):251–63. [DOI] [PubMed]
- 55.Stubbins MJ, Harries LW, Smith G, Tarbit MH, Wolf CR. Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics. 1996;6(5):429–39. [DOI] [PubMed]
- 56.Xie HG, Prasad HC, Kim RB, Stein CM. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev. 2002;54(10):1257–70. [DOI] [PubMed]
- 57.The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. The Atrial Fibrillation Investigators. Arch Intern Med. 1997;157(11):1237–40. [PubMed]
- 58.Penado S, Cano M, Acha O, Hernandez JL, Riancho JA. Stroke severity in patients with atrial fibrillation. Am J Med. 2002;112(7):572–4. [DOI] [PubMed]
- 59.Man-Son-Hing M, Laupacis A. Balancing the risks of stroke and upper gastrointestinal tract bleeding in older patients with atrial fibrillation. Arch Intern Med. 2002;162(5):541–50. [DOI] [PubMed]
- 60.Tengs TO, Yu M, Luistro E. Health-related quality of life after stroke a comprehensive review. Stroke. 2001;32(4):964–72. [DOI] [PubMed]
- 61.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. [DOI] [PubMed]
- 62.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33(5):1190–5. [DOI] [PubMed]
- 63.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception- cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348(9025):423–8. [DOI] [PubMed]
- 64.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–36. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 25.5 KB)
(GIF 62.6 KB)