Abstract
BACKGROUND
Obese women experience higher postmenopausal breast cancer risk, morbidity, and mortality and may be less likely to undergo mammography.
OBJECTIVES
To quantify the relationship between body weight and mammography in white and black women.
DATA SOURCES AND REVIEW METHODS
We identified original articles evaluating the relationship between weight and mammography in the United States through electronic and manual searching using terms for breast cancer screening, breast cancer, and body weight. We excluded studies in special populations (e.g., HIV-positive patients) or not written in English. Citations and abstracts were reviewed independently. We abstracted data sequentially and quality information independently.
RESULTS
Of 5,047 citations, we included 17 studies in our systematic review. Sixteen studies used self-reported body mass index (BMI) and excluded women <40 years of age. Using random-effects models for the six nationally representative studies using standard BMI categories, the combined odds ratios (95% CI) for mammography in the past 2 years were 1.01 (0.95 to 1.08), 0.93 (0.83 to 1.05), 0.90 (0.78 to 1.04), and 0.79 (0.68 to 0.92) for overweight (25–29.9 kg/m2), class I (30–34.9 kg/m2), class II (35–39.9 kg/m2), and class III (≥40 kg/m2) obese women, respectively, compared to normal-weight women. Results were consistent when all available studies were included. The inverse association was found in white, but not black, women in the three studies with results stratified by race.
CONCLUSIONS
Morbidly obese women are significantly less likely to report recent mammography. This relationship appears stronger in white women. Lower screening rates may partly explain the higher breast cancer mortality in morbidly obese women.
KEY WORDS: obesity, mammography, screening, systematic review
INTRODUCTION
Breast cancer remains the second leading cause of cancer death among women in the United States1. Screening mammography reduces breast cancer mortality2–6, and current guidelines recommend mammography every 1–2 years for women over 40 years of age7,8.
Obesity has increased over the past 2 decades among women in the US9 and has disparate effects on pre- and postmenopausal breast cancer. Excess body weight may actually decrease the risk of premenopausal breast cancer10,11, but the relationship between obesity and premenopausal breast cancer mortality is ambiguous11,12. However, obesity is an important risk factor for both the development of10,11,13–15 and mortality from16–19 postmenopausal breast cancer. Obesity may also worsen breast cancer morbidity, including risk of breast cancer recurrence20, contralateral breast cancer21, wound complications after breast surgery22, and lymphedema23,24.
The mechanism by which obesity leads to poorer prognosis of breast cancer is not well understood and may be related to tumor characteristics, hormonal mechanisms, suboptimal diet and physical activity, or delay in diagnosis16. Studies of the relationship between obesity and stage at breast cancer diagnosis are conflicting25,26.
Several observational studies suggest that obese women may be less likely to report recent mammography27–39, but the relationship between obesity and screening mammography remains unclear40–43. Some studies suggest the problem may be confined to white women31–33,36.
Therefore, we conducted a systematic review and meta-analysis to determine whether overweight or obese women are less likely to have recent mammography than their normal-weight counterparts. We also studied the effect of race on the relationship between weight and recent mammography.
METHODS
Search Strategy
Our overall search strategy addressed a broader question regarding the association between obesity and screening for breast, cervical, and colon cancer. For this study, we searched the PubMed, CINAHL, and Cochrane Library electronic databases from inception to July 2008 to identify original articles evaluating the relationship between body weight and recent mammography in the US using search terms for breast cancer screening, breast cancer, and body weight (Appendix Table 5). We manually searched the references of included articles and the tables of contents of 11 key medical journals from August 2006 through November 2006 and then updated our manual search from April 2008 to July 2008. General medical, cancer, women’s health, and prevention journals were selected based on the origin of the included articles and the topic itself to avoid missing articles due to any delays in electronic indexing. Searchers were physician investigators and included a senior obesity researcher (J.M.C.), an investigator with systematic review experience (S.B.), and a post-doctoral epidemiology trainee with relevant clinical experience (N.M.M). Two reviewers conducted title and abstract reviews independently. If a title was selected by either investigator, it was advanced to abstract review. Title and abstract reviews were designed to be sensitive; if there was any question of an article exploring weight as a predictor of screening upon title or abstract review, we advanced the article to the next level of review. Of 273 abstracts, there were 62 conflicts (23%) in abstract review, which we resolved by consensus through discussion. Disagreements usually pertained to misreading on the part of one of the investigators, and disagreements in judgment were rare.
Table 5.
Electronic Database Search Terms*
| PubMed | |
|---|---|
| Keywords | MeSH terms |
| Breast cancer(s); breast neoplasm(s); breast tumor(s); neoplasm(s), breast; tumor(s), breast; cancer(s), breast; cancer(s) of breast; cancer(s) of the breast; mammary carcinoma(s) of breast; mammary carcinoma(s), human; carcinoma(s), mammary human; human mammary carcinoma(s); mammary neoplasm(s), human; human mammary neoplasm(s); neoplasm(s), human mammary; mammary neoplasm(s), human | Breast neoplasms |
| Breast cancer screening; mammogram; mammography; mammographies; screening mammography; screening for breast cancer | Mammography |
| Body weight(s); weight; obesity; adiposity; body mass index; Quetelet index; BMI; overweight; body measure(s); measure(s), body; index, body mass; index, Quetelet; Quetelet's index; Quetelets index; body weights and measures | Body weights and measures |
| Cancer screening | |
| CINAHL | |
| Keywords | CINAHL headings |
| Breast cancer, breast neoplasms | Breast neoplasms |
| Breast cancer screening, mammography, mammogram | Mammography |
| BMI, body mass index, obesity, Quetelet index | Body weights and measures |
| Cancer screening | Cancer screening |
| Cochrane | |
| Search all text | MeSH terms |
| Breast cancer, breast neoplasms | Breast neoplasms |
| Breast cancer screening, mammography, mammogram | Mammography |
| BMI, body mass index, Quetelet index | Body weights and measures |
| Cancer screening | |
*Our overall search strategy addressed a broader question regarding the association between obesity and screening for breast, cervical, and colon cancer. This study focuses on the relationship between weight and mammography
Study Selection
We included published original articles if they reported the prevalence of mammography by body weight in adults ≥18 years of age and were written in English. We defined original articles as articles in which the authors analyzed raw data and thus excluded reviews, commentaries, editorials, and consensus statements. We excluded studies conducted outside of the US since other countries may have different screening guidelines and resources, and the relationship between weight and mammography might differ based on cultural norms. We also excluded studies of screening in special populations since there may be different screening expectations for some populations (e.g., participants presenting to a cancer screening clinic, HIV-positive patients, those with a history of breast cancer, and those involved in a study of interventions to improve screening). Two investigators reviewed articles independently. Of 101 articles, there were 3 disagreements (3%), which were resolved through discussion.
Data Abstraction and Quality Assessment
Two reviewers sequentially abstracted the data on population characteristics, the exposure, and the outcome using standardized data abstraction forms. Two studies included body mass index (BMI) in models when exploring determinants of screening, but did not explicitly report mammography prevalence by BMI; the authors kindly provided these results34,39.
Two reviewers evaluated study quality independently using a quality form (Appendix A) based on the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement, Checklist of Essential Items version 3 (September 2005)44, which was published recently45. We assumed that the importance of any confounding variable varied according to study design. Therefore, we did not expect each study to handle confounding in the same fashion and assessed quality as being adequate, fair, or inadequate on an individual basis. We resolved disagreements in data abstraction and quality evaluation through discussion.
Data Synthesis and Analysis
First, we created tables to describe all studies qualitatively. We reported results of adjusted analyses when available. In order to obtain generalizable combined estimates for the association between weight and mammography, we conducted unstratified meta-analyses and meta-analyses stratified by white and black race for studies that: (1) had nationally representative data and (2) reported BMI in five standard categories according to the World Health Organization46 and the National Institutes of Health47: (normal: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, class I obesity: 30–34.9 kg/m2, class II obesity: 35–39.9 kg/m2, and class III obesity: ≥ 40 kg/m2). We contacted the authors of articles that did not report results for mammography by BMI in five standard categories; two authors provided the quantitative results requested28,40. Two authors were unable to provide quantitative results stratified by race30,33.
Using the DerSimonian and Laird method48, we used random-effects models to calculate combined odds ratios and 95% confidence intervals for mammography by BMI category using normal BMI as the reference category. For the study that reported adjusted proportions33, we calculated odds ratios. We converted the relative risk to an odds ratio49 for another study32. One study provided results stratified by race only31, and we included the results from the white and black cohorts separately in our main and race-specific analyses.
We tested for heterogeneity using the I2 statistic50 with an I2 value of >50% signifying “substantial heterogeneity”51. We chose a random-effects model as a more conservative approach to account for potential between-study variability.
We tested for publication bias using the tests of Begg and Mazumdar52 and Egger and colleagues53. All analyses were completed using STATA (StataCorp. 2005. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP).
We conducted several sensitivity analyses. We examined the effect of the removal of any one study on the combined estimate for the unstratified analyses. Also, two35,37 of the seven studies30–33,35,37,38 that were based on nationally representative data and reported BMI in five categories used the same 2000 National Health Interview Survey (NHIS) data but performed slightly different analyses. We included the study with more conservative results in the main meta-analysis35. We included the other, less conservative estimate from the other study37 in a separate analysis. In another analysis, we included all studies that provided BMI in five standard categories regardless of whether they were nationally representative.
RESULTS
Literature Search Results
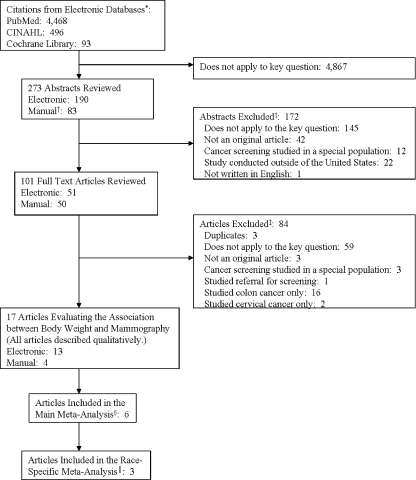
Of 5,047 titles identified in the overall search, 17 articles met our inclusion criteria and addressed mammography (Fig. 1). Seven30–33,35,37,38 of the 17 studies were sufficiently homogeneous (i.e., used nationally representative survey data and provided information for mammography by five standard categories of BMI) to include in the unstratified meta-analyses. Two of these studies were based on the same 2000 NHIS data35,37; thus, six studies were included in our main meta-analyses. Five nationally-representative studies30–33,35 reported race-stratified analyses, and two of these30,33 did not report the necessary quantitative results to allow their inclusion in the meta-analyses; thus, we included three studies in our race-stratified meta-analysis. Six studies were not nationally representative and were conducted in primarily non-white populations34,39,40,42,43 or reported race-stratified results36.
Figure 1.
Study flow diagram. *Search terms for breast cancer, cervical cancer, colon cancer, body weight, breast cancer screening, cervical cancer screening, and colon cancer screening were used to conduct the search of electronic databases. Specific terms are provided in Appendix Table 5. †Manual searching involved searching of references of included and key articles and searching of tables of contents of the following journals: Cancer, Journal of General Internal Medicine, Annals of Internal Medicine, Obesity, Ethnicity and Disease, Cancer Detection and Prevention, Journal of Health Care for the Poor and Underserved, Preventing Chronic Disease, Journal of Women’s Health, American Journal of Public Health, Preventive Medicine, and American Journal of Epidemiology. ‡Reasons for exclusion add up to more than abstracts or articles excluded since reviewers could have more than one reason for exclusion. §Studies included in the main meta-analysis reported nationally-representative results in five standard body mass index categories (normal 18–24.9 kg/m2, overweight 25–29.9 kg/m2, class I obesity 30–34.9 kg/m2, class II obesity 35–39.9 kg/m2, class III obesity ≥ 40 kg/m2). A seventh study37 met these criteria, but was based on the same data as another study35 and therefore was only included in a sensitivity analysis. ║Studies included in the race-specific meta-analysis reported nationally representative results in five standard body mass index categories (normal 18–24.9 kg/m2, overweight 25–29.9 kg/m2, class I obesity 30–34.9 kg/m2, class II obesity 35–39.9 kg/m2, class III obesity ≥40 kg/m2).
Study Characteristics
The 17 included studies, which comprised approximately 276,034 participants, are described in Tables 1 and 2. Sixteen studies were cross-sectional27–38,40–43, and one was longitudinal39. All studies used BMI as the measure of excess body weight. Thirteen studies defined the outcome as mammography in the last 2 years28–33,35–38,40,42,43, two as mammography in the last year27,34, one as mammography in the last 3 years41, and one as mammography every 2 years over a 6-year period39. Ten27,29–33,35,37,38,41 of the 17 studies (59%) were based on nationally representative surveys, the NHIS, Behavioral Risk Factor Surveillance System (BRFSS), or Health and Retirement Survey. Most subjects were white. Reported absolute screened proportions ranged from 53.2% to 85.6%29,30,32–34,36–41,43.
Table 1.
Description of Studies Included in Qualitative and Quantitative Analyses*
| Author, year | Study population | Mean age, y (range) | Race/ethnicity (%) | Exclusion criteria |
|---|---|---|---|---|
| Amonkar et al. 200227 | 9,908 respondents to the 1997 BRFSS | NR (40–80+) | White 83.8%; black 15%; Asian/Pacific Islander 0.4%; American Indian 0.4%; other 0.4% | <40 years of age |
| Amy et al. 200628 | 338 respondents to survey available in clothing stores, a convention, magazine, and research database | 45(21–80)† | White 68%‡ | <40 years of age, BMI <25 kg/m2 |
| Berz et al. 2008§38 | 105,899 respondents to the 2004 BRFSS | 59.3(40–99)† | White 75.2%; black 7.3%; Hispanic 9.7%; others 7.8%‡ | <40 years of age, missing BMI, mammography response, or any confounding variable |
| Cohen et al. 2007 (36) | 25,060 participants in the Southern Community Cohort Study | NR (42–70+)† | White 25.2%; black 74.8% | <42 or >79 years of age, BMI <18.5 kg/m2, not black or white, diagnosis of breast cancer, treatment for cancer in last year, missing BMI or mammography use, not English-speaking |
| Coughlin et al. 200429 | 49,564 respondents to the 1999 BRFSS | NR | NR | <40 years of age |
| Ferrante et al. 200640 | 1,809 patients in 3 urban New Jersey academic family medicine practices from 2000–2003 | 53.4(40–74)† | Hispanic 50%; black 36%‡ | <40 or ≥75 years of age, breast or cervical cancer, pregnant, missing weight, no visit in 12 months before index visit, new patient |
| Ferrante et al. 200737 | 8,289 respondents to the 2000 NHIS | NR(40–74)† | White 31.3%; black 26%; Hispanic 28.7%; other 14%‡ | <40 or ≥75 years of age, BMI <18.5 kg/m2 |
| Fontaine et al. 199841 | 3,105 respondents to the 1992 NHIS | 46.2(18–97)† | White 79.9%‡ | NR |
| Fontaine et al. 2001§30 | 38,682 respondents to the 1998 BRFSS | 47.7† (NR) | White 84.4%; non-white 15.6% | <40 years of age |
| Gorin et al. 200142 | 408 respondents to Harlem Survey from 46 blocks in Central Harlem in 1991 | NR | NR║ | <40 or >65 years of age, not English-speaking, unable to answer questions |
| Ostbye et al. 2005§31 | 8,449 participants in the Health and Retirement Study (1996, 2000 waves) | NR(50–64)¶ | White 82%; black 18%¶ | Lack of response to 1996 and/or 2000 waves of HRS |
| Rosenberg et al. 200539 | 14,706 participants in the Black Women’s Health Study 1995–2001 | NR(40–69)† | Black 100% | <40 years of age, not African American, lack of valid address, lack of completion of survey |
| Satia et al. 200743 | 405 enrollees in cancer risk behavior surveillance study in North Carolina in 2003 | NR(41–70) | Black 100% | <40 years of age, not African American, not on Department of Motor Vehicles roster in one six counties in North Carolina |
| Wee et al. 2000§33 | 3,077 respondents to the 1994 NHIS | 62 | White 81%; black 10% | <50 or >75 years of age |
| Wee et al. 2004§32 | 5,277 respondents to 1998 NHIS Sample Adult and Prevention questionnaires | 61(50–75) | White 80%; black 10%; Hispanic/Asian/other 10% | <50 or >70 years of age |
| Winkleby et al. 200334 | 169 women responding to a community random-digit-dial survey in Monterey California | NR(18–64)† | Latino 100% | <40 years of age, not Latino, not living in Monterey County, California |
| Zhu et al. 2006§35 | 9,188 respondents to the 2000 NHIS | NR(40–80)† | White 83.7%; black 16.3% | <40 or >80 years of age, not white or black, history of breast cancer, mammography for reason other than screening |
*Characteristics of participants included in the main analysis unless otherwise noted
†Mean age and range from overall study
‡Race from overall study
§Studies included in the main, unstratified meta-analysis
║Authors stated, “…majority of women in the survey were non-Hispanic blacks.”
¶From 1996 wave of Health and Retirement Study
BRFSS, Behavioral Risk Factor Surveillance System; NR, not reported; BMI, body mass index; NHIS, National Health Interview Survey; HRS, Health and Retirement Study
Table 2.
Results of Studies Included in Qualitative and Quantitative Analyses
| Author, year | BMI (kg/m2)* | Outcome assessment | Outcome measure | Outcome estimate (95% CI)† | Adjustments |
|---|---|---|---|---|---|
| Amonkar et al. 200227 | Self-report, standard 2 categories | Self-report of mammogram in last year | OR | 0.81 (0.69 to 0.95) | Age, race, education, marital status, residential status, smoking, health status, health-care utilization |
| Amy et al. 200628 | Self-report, standard 5 categories‡ | Self-report of mammogram in last 2 years | Proportion | Overweight 94%, class I 82%, class II 80%, class III 78% P = 0.24§ | None |
| Berz et al. 2008║38 | Self report, standard 5 categories | Self-report of screening mammogram in last 2 years | OR | Normal 1.00, overweight 1.08 (1.01 to 1.15), class I 1.08 (0.99 to 1.18), class II 1.10 (0.98 to 1.25), class III 0.97 (0.84 to 1.13) | Age, race, education, income, smoking, general health perception |
| Cohen et al. 200736 | Self-report, standard 5 categories | Self-report of mammogram in last 2 years | OR | Whites: normal 1.00, overweight 0.89 (0.76 to 1.05), class I 0.99 (0.83 to 1.18), class II 0.96 (0.78 to 1.18), class III 0.70 (0.56 to 0.87) | Age, education, income, smoking status, number of live births, co-morbid conditions, family history of breast cancer, time since last physician visit, type of insurance |
| Blacks: normal 1.00, overweight 1.12 (1.00 to 1.25), class I 1.25 (1.12 to 1.40), class II 1.22 (1.07 to 1.38), class III 1.06 (0.93 to 1.21) | |||||
| Coughlin et al. 200429 | Self-report, BMI categories: >18.5-<25, 25–30, >30 | Self-report of mammogram in last 2 years | Adjusted proportion | >18.5-<25: 76.0% (75.1 to 76.8), 25–29: 76.6% (75.7 to 77.5), >30: 74.6% (73.5 to 75.8) P <0.001¶ | Age, race, education, marital status, income, employment, smoking, physical activity, alcohol, use of preventive services, number of children, number of persons in household, health status, diabetes, physician visit in last year, insurance |
| Ferrante et al. 200640 | Chart review, standard 5 categories‡ | Mammogram in last 2 years recorded in chart | OR | Normal 1.00, overweight 1.61 (1.03 to 2.54), class I 1.32 (0.84 to 2.07), class II 1.92 (1.12 to 3.28), class III 1.53 (0.88 to 2.65) | Age, race, marital status, smoking, co-morbid conditions, physician visits, insurance |
| Ferrante et al. 200737 | Self-report, standard 5 categories | Self-report of mammogram in last 2 years | OR | Normal 1.00, overweight 0.95 (0.81 to 1.10), class I 1.01 (0.83 to 1.23), class II 0.79 (0.60 to 1.05), class III 0.50 (0.37 to 0.68) | Age, race/ethnicity, education, marital status, smoking, vitamin use, number of visits, contact with primary care doctor, family history of breast cancer, insurance |
| Fontaine et al. 199841 | Self-report, BMI groups: 25 (reference), 35, and 40 | Self-report of no mammogram in last 3 years# | OR | 25: 1.0, 35: 0.81 (0.59 to 1.12), 45: 0.73 (0.45 to 1.19) | Age, race, education, income, smoking status, insurance status |
| Fontaine et al. 2001║30 | Self-report, standard 5 categories | Self-report of no mammogram in last 2 years# | OR | Normal 1.00, overweight 1.00 (0.94 to 1.07), class I 1.12 (1.02 to 1.23), class II 1.13 (0.98 to 1.30), class III 1.32 (1.09 to 1.59) | Age, race, smoking, insurance |
| Gorin et al. 200142 | Self-report, BMI categories: ≤27.3 and >27.3 | Self-report of mammogram in last 2 years | OR | Not overweight: 1.00, overweight: 3.60 (0.57 to 22.64) | Age, marital status, employment, fruit/vegetable intake, insurance |
| Ostbye et al. 2005║31 | Self-report, standard 5 categories | Self-report of mammogram in last 2 years | OR | Whites: normal 1.00, overweight 0.90 (0.78 to 1.05), class I 0.73 (0.60 to 0.88), class II 0.69 (0.51 to 0.93), class III 0.59 (0.40 to 0.88) | Age, education, marital status, income, smoking, physical activity, health status, co-morbid conditions, physician visits, hospitalization, insurance |
| Blacks: normal 1.00, overweight 1.13 (0.79 to 1.62), class I 0.97 (0.65 to 1.45), class II 1.03 (0.61 to 1.76), class III 1.07 (0.60 to 1.92) | |||||
| Rosenberg et al. 200539 | Self-report, standard 5 categories‡ | Self-report of mammogram every 2 years from 1995–2001 | OR | Normal 1.00, overweight 1.09 (0.98 to 1.22), class I 1.08 (0.95 to 1.23), class II 1.13 (0.95 to 1.34), class III 0.96 (0.79 to 1.16) | Age, education, region, income, neighborhood SES score, childcare responsibilities, smoking, multivitamins, Pap smear, cystic breast disease, breast self exam, hormone use, family history of breast cancer, insurance |
| Satia et al. 200743 | Self-report, BMI categories: normal 18.5–24.9, overweight 25–29.9, obese >30 | Self-report of mammogram in last 2 years | OR | Normal 1.00, overweight 1.5 (0.6 to 3.6), obese 0.5 (0.2 to 1.3) P = 0.39** | Age, education, BMI |
| Wee et al. 2000║33 | Self-report, standard 5 categories | Self-report of mammogram in last 2 years | Adjusted difference in proportion | Normal 0, overweight -2.8 (-6.7 to 0.9), class I -5.3 (-11.1 to 0.5), class II -4.5 (-12.5 to 3.4), class III -8.8 (-22.9 to 5.3) | Age, race, education, marital status, region of country, health status, health-care use, hospitalization, days in bed, insurance type, physician specialty |
| Wee et al. 2004║32 | Self report, standard 5 categories | Self-report of mammogram in last 2 years | RR | Normal 1.00, overweight 1.01 (0.95 to 1.06), class I 0.99 (0.91 to 1.05), class II 0.89 (0.77 to 1.01), class III 0.88 (0.71 to 1.01) | Age, race, education, marital status, region of country, health-care access, health status, co-morbid conditions, mobility, hospitalization |
| Winkleby et al. 200334 | Self-report, standard 5 categories‡ | Self-report of mammogram in last year | OR | Normal 1.00, overweight 1.03 (0.41 to 2.62), class I 0.85 (0.25 to 2.89), class II 2.94 (0.42 to 20.61), class III 0.59 (0.06 to 5.79) | Age, education, marital status, years in US |
| Zhu et al. 2006║35 | Self-report, standard 5 categories | Self-report of no screening mammogram in last 2 years** | OR | Normal 1.00, overweight 0.9 (0.8 to 1.1), class I 0.9 (0.8 to 1.1), class II 1.0 (0.8 to 1.3), class III 1.3 (1.0 to 1.8) | Age, race, education, marital status, income, employment, smoking, alcohol, skin cancer exam, health status, co-morbid conditions, days in bed, need for special equipment, functional limitations, home health-care, recent surgery, status of walking, moving, lifting, and carrying, medical care visits, insurance |
*Standard two categories of BMI: non-obese <30 kg/m2 and obese ≥30 kg/m2; standard five categories of BMI: normal 18–24.9 kg/m2, overweight 25–29.9 kg/m2, class I obesity 30–34.9 kg/m2, class II obesity 35–39.9 kg/m2, class III obesity ≥ 40 kg/m2
†Adjusted results reported with the exception of Amy et al.28
‡Obtained data in standard five categories upon request from author
§Result of chi-square test
║Studies included in main, unstratified meta-analysis
¶Unclear which statistical test used by authors to obtain reported P value
#Study used lack of mammogram as an outcome
**P value for trend
BMI, body mass index; CI, confidence interval; OR, odds ratio; SES, socioeconomic status; RR, relative risk
Sixteen of the 17 studies27–39,41–43 (94%) relied on self-reported BMI and mammography. Fourteen studies accounted for confounding adequately27,29–41, and one study did not adjust for any confounding factors28. Reported survey response rates ranged from 55% to 88%. Eight studies did not report missing data27,31,32,35,37,39,41,43, seven had <10% missing data28–30,32,34,36,42, and two reported >20% missing data38,40. All studies provided an adequate exposure description, and all but one27 provided an adequate outcome description. Ten studies used nationally representative surveys27,29–33,35,37,38,41, and 14 did not report the validity of the surveys used27,29–33,35–39,41–43. See Table 3.
Table 3.
Quality Review of Included Studies*
| Author | Missing data | Exposure description | Outcome description | Confounding | Validity | Response rate |
|---|---|---|---|---|---|---|
| Amonkar et al. 200227 | NR | Adequate | Fair | Adequate | NR† | NR |
| Amy et al. 200628 | <10% | Adequate | Adequate | Inadequate | Fair | NR |
| Berz et al. 200838 | >20% | Adequate | Adequate | Adequate | NR† | NR |
| Cohen et al. 200736 | <10% | Adequate | Adequate | Adequate | NR | NR |
| Coughlin et al. 200429 | None | Adequate | Adequate | Adequate | NR† | 55.2% |
| Ferrante et al. 200640 | >20% | Adequate | Adequate | Adequate | N/a | N/a |
| Ferrante et al. 200737 | NR | Adequate | Adequate | Adequate | NR‡ | 72% |
| Fontaine et al. 199841 | NR | Adequate | Adequate | Adequate | NR‡ | 87% |
| Fontaine et al. 200130 | <10% | Adequate | Adequate | Adequate | NR† | NR |
| Gorin et al. 200142 | None | Adequate | Adequate | Fair | Referred to other reference for details of Harlem Survey used | 72% |
| Ostbye et al. 200531 | NR | Adequate | Adequate | Adequate | NR§ | 84.7% |
| Rosenberg et al. 200539 | NR | Adequate | Adequate | Adequate | NR | 61.7% |
| Satia et al. 200743 | NR | Adequate | Adequate | Fair | NR | 17.5% |
| Wee et al. 200033 | NR | Adequate | Adequate | Adequate | NR‡ | 94% for NHIS overall; 88% for supplement║ |
| Wee et al. 200432 | <10% | Adequate | Adequate | Adequate | NR‡ | 90% for NHIS overall; 73% for Family Core and supplement¶ |
| Winkleby et al. 200334 | <10% | Adequate | Adequate | Adequate | Fair | 87% |
| Zhu et al. 200635 | NR | Adequate | Adequate | Adequate | NR‡ | 72% |
*Quality rating based on scale: inadequate, fair, adequate
†Study based on the Behavioral Risk Factor Surveillance System
‡Study based on the National Health Interview Survey
§Study based on the Health and Retirement Study
║Participants given an additional questionnaire regarding preventive health-care service use
¶Participants given additional questionnaires inquiring about height, weight, medical conditions, sociodemographics, health status, health-care utilization, health habits, tobacco use, physical activity, functional status, and cancer screening
NR, not reported; NHIS, National Health Interview Survey
Quantitative Assessment of Mammography by BMI
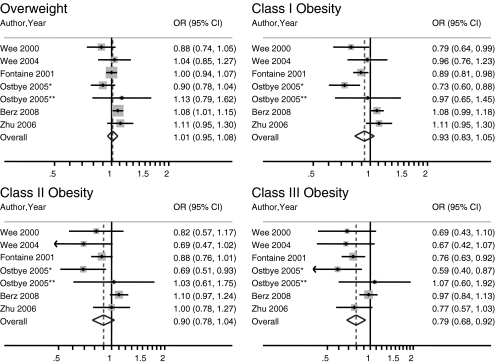
Fourteen27–39,43 of 17 studies reported an inverse association between recent mammography and increasing BMI that was statistically significant in five27,29,31,36,37. Seven studies30–33,35,37,38 used nationally representative surveys with BMI in five standard categories. Using the six studies based on unique data, class III obesity was inversely associated with the likelihood of having recently undergone mammography compared to women with a normal BMI. The seventh study by Ferrante et al.37 was excluded from the main analysis because it was based on the same data as the study by Zhu et al.35 Combined odds ratios for mammography (95% confidence interval) by BMI category were 1.01 (0.95 to 1.08), 0.93 (0.83 to 1.05), 0.90 (0.78 to 1.04), and 0.79 (0.68 to 0.92) for overweight, class I, class II, and class III obese women, respectively, compared to women with a normal BMI (Fig. 2). We found statistical evidence of heterogeneity for the class I and II obesity categories; I2 statistics were 41%, 74%, 59%, and 42% for the overweight, and class I, II, and III obesity categories, respectively. The exclusion of any one study did not change the results of the meta-analyses substantially (data not shown). No statistically significant publication bias was found, although evaluation was limited by the relatively small number of studies.
Figure 2.
Meta-analyses of nationally representative studies with BMI in five categories. Note: Included studies: 30–33,35,38; BMI categories: overweight 25–29.9 kg/m2, class I obesity 30–34.9 kg/m2, class II obesity 35–39.9 kg/m2, class III obesity ≥40 kg/m2. *Data from analysis of white women. **Data from analysis of black women. BMI, body mass index; OR, odds ratio; CI, confidence interval.
Sensitivity Analyses
We obtained similar results when we excluded the article by Zhu et al.35 and instead included the article by Ferrante et al.37, which used the same data. Results were also similar when we included all nine studies with BMI in five categories including three that were not based on nationally representative surveys (data not shown)30–36,38,40.
Effect of Race
Five nationally representative studies30–33,35 evaluated the effect of race on the relationship between BMI and recent mammography. Compared to women with a normal BMI, meta-analyses of the three race-stratified studies using five categories of BMI31,32,35 revealed an inverse association between class II and III obesity and recent mammography for white women, but a positive association between overweight and recent mammography among black women (Table 4). We found statistical evidence of heterogeneity for class I obesity in the analyses for white women and for class I and II obesity in the analyses for black women. There was no statistical evidence of publication bias.
Table 4.
Combined Odds Ratios for Mammography by BMI for Race-Stratified Analyses*†
| BMI category | Combined odds ratios (95% CI) | I2 (%)‡ |
|---|---|---|
| White women | ||
| Normal | 1.00 (reference) | |
| Overweight | 0.98 (0.85 to 1.13) | 49 |
| Class I obesity | 0.84 (0.69 to 1.02) | 60 |
| Class II obesity | 0.73 (0.56 to 0.95) | 47 |
| Class III obesity | 0.67 (0.53 to 0.84) | 0 |
| Black women | ||
| Normal | 1.00 (reference) | |
| Overweight | 1.28 (1.03 to 1.60) | 0 |
| Class I obesity | 1.38 (0.90 to 2.12) | 54 |
| Class II obesity | 1.46 (0.76 to 2.80) | 66 |
| Class III obesity | 0.91 (0.62 to 1.33) | 0 |
*Studies included:31,32,35. Additional studies30,33 evaluated the interaction between race and BMI, but did not provide the quantitative results necessary for inclusion in our meta-analyses. Fontaine et al. in 200130 provided a P value (P = 0.908) for the interaction between race and mammography, and Wee et al.33 reported adjusted rate differences, suggesting a possible decline in screening with BMI among white women, but not among black women. We contacted the authors, but were unable to obtain further results
†Adjusted odds ratios used in analysis
‡I2 Statistic is a measure of heterogeneity with an I2 >50% signifying “substantial heterogeneity”51
BMI, body mass index
Four studies conducted in primarily non-white populations did not find a statistically significant association between BMI and recent mammography34,39,42,43. One study based on a chart review of patients (86% non-white) of urban family practices reported an increased odds of recent mammography among overweight and class II obese patients compared to patients with a normal BMI40. A study of baseline data from the Southern Community Cohort Study found that compared to women with a normal BMI, white women with class III obesity were less likely to report recent mammography, but overweight and class I and II obese black women were more likely to report recent mammography36.
DISCUSSION
This systematic review demonstrates an inverse relationship between class I, II, and III obesity and recent mammography that was statistically significant for class III obesity. Compared to their lean counterparts, women with class III obesity were 20% less likely to report recent mammography. In white women, we found a statistically significant negative association between class II and III obesity and being up-to-date with mammography. We did not find this association between BMI and mammography among black women.
Two of the three studies that did not report an inverse association between recent mammography and increasing BMI were not nationally representative. One was a chart review from family practices in New Jersey with primarily non-white patients40, and the other was a Harlem survey among mostly non-Hispanic blacks42. The findings of these two studies are consistent with the results of our meta-analyses in which we observed no significant inverse relationship between obesity and mammography in non-whites. The third negative study41 included women <40 years of age. These results may be confounded by age since younger women are more likely to have a lower BMI54 and to report a lower prevalence of mammography since it is not routinely recommended for them.
Obese women may experience several possible barriers to mammography. Prior data show that obese women may delay medical care55 because of poor self-esteem and body image, embarrassment29,30,55,56, a perceived lack of respect from health-care providers, or to avoid unwanted weight loss advice28. Obesity may be a marker for sub-optimal health behavior in general, of which lack of mammography is simply one facet30,33. Also, beliefs regarding cancer screening may vary by BMI33. There could be physical limitations to obtaining mammography for obese women, but obesity is associated with a higher content of fat in the breast tissue that actually increases the sensitivity of mammography for detecting breast cancer57,58. Finally, obesity is associated with lower socioeconomic status59, which may decrease access to preventive care.
There are also many physician-related factors that may decrease screening mammography among obese women. Obesity-related co-morbid conditions may hinder referral for purely preventive services41,60,61. In addition, providers have reported difficulty and inadequate resources and education in providing care for obese women28. Finally, physicians may have biases against obese women, resulting in less screening62–64.
Obesity did not appear to affect the report of recent mammography in black women. This may be due to racial differences in obesity-related body image65–67. In particular, it has been reported that overweight or obese white, but not black, women were more likely to feel worthless, which may impact willingness to undergo mammography32. Black women may have a similar risk of developing breast cancer68,69, but higher breast cancer mortality21,68–71. They tend to present with a higher stage of breast cancer69,71, which has been linked to (1) less follow-up for abnormal exams72, (2) higher rates of obesity72–75, (3) socioeconomic factors76, (4) cultural beliefs (e.g., belief in herbal treatments)76, and possibly, lower likelihood of screening77–79, although this is controversial68,80–82. Our findings, the first meta-analyses by race, suggest that rates of mammography in black women do not vary significantly by BMI.
We included only 6 of 17 studies in our meta-analyses based on the provision of unique nationally representative data and BMI in five standard categories. However, 14 of the 17 studies reported a negative association between BMI and report of mammography. Also, we obtained similar results when we included all nine studies that reported BMI in five standard categories.
Most of the included studies were cross-sectional and cannot establish causality, but it is unlikely that failure to undergo mammography would contribute to weight gain. Also, we relied on the use of observational studies, which are susceptible to residual and unmeasured confounding. In particular, socioeconomic factors and health behaviors may confound the relationship between obesity and breast cancer and are difficult to account for fully. Although we did not find publication bias, we had limited power with a small number of studies. However, our search also included articles in which body weight was not the primary exposure, and thus, the potential for publication bias should be low.
The included studies used self-report of BMI as the measure of body weight, which has several limitations: It may underestimate obesity, especially in women83, but may also overestimate obesity, especially in blacks83. Self-report of height and weight may differ by survey type (telephone versus in-person), age, and BMI84. Overall, the included studies may have placed more obese participants into less obese categories, which would bias our results toward the null or result in finding an inverse association in overweight or milder obesity. However, the overall qualitative association between body weight and mammography would be unchanged.
Most of the included studies also relied upon self-report of mammography. A recent meta-analysis found that self-report of mammography had a sensitivity of 93% and specificity of 62%85. While this study reported similar sensitivities for self-reported mammography in blacks and whites, the specificity of self-reported mammography was only 49% among blacks85. Thus, mammography results are likely inflated above their actual rates with the degree of inflation higher for blacks. There is no evidence that the accuracy of self-report of mammography varies by BMI, but if it does, our results would also be biased.
The included studies did not stratify on menopausal status, but only one study included women under the age of 40 years41. It seems unlikely that menopausal status would affect willingness to be screened in women over age 40. While the relationship between obesity and premenopausal breast cancer risk and mortality is unclear10–12, obesity increases postmenopausal breast cancer risk10,11,13–15 and mortality16–19.
Finally, our search strategy may have been susceptible to selection bias given that we included a small number of full articles from the total citations reviewed, we manually searched only 11 key journals, and we had limited success obtaining full results from contacted authors. However, the qualitative results matched our meta-analytic results, we included no new articles from the manual search of 11 journals, and we were very sensitive in promoting a title or abstract to full article review (i.e., if an article discussed risk factors associated with mammography, we promoted that to full article review). Additionally, we re-reviewed a random sample of 2.5% of the full articles excluded at title review and 5% of the full articles excluded at abstract review and did not find any additional articles that satisfied our inclusion criteria.
Our study also has several strengths. This is the first systematic review with meta-analyses exploring the relationship between obesity and mammography and the only one to examine the effect of race on this association. We comprehensively searched multiple electronic databases in addition to manual searching. Also, we contacted authors for data leading to additional results from four studies. Finally, the meta-analyses were based on nationally representative surveys and thus are generalizable to the US population.
The main implication of our study is that a lack of routine screening mammography may explain some of the increased breast cancer mortality in obese postmenopausal women. Clinicians should be aware of this disparity in evaluating their own practices. Future research should determine why obese women are less likely to report recent mammography, including the investigation of a lack of health care access due to perceived bias or lack of insurance as a possible cause and explore whether there are consistent differences by race.
Acknowledgments
We thank the following individuals for contributions to the study and/or providing access to their data: Eliseo Guallar, MD, DrPH (Johns Hopkins University, Baltimore, MD); Nancy K. Amy, PhD (University of California, Berkeley, CA); Jeanne Ferrante, MD (University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School and New Jersey Medical School, Newark, NJ); Marilyn Winkleby, PhD (Stanford School of Medicine, Stanford, CA); Lynn Rosenberg, ScD (Slone Epidemiology Center at Boston University, Boston, MA). No compensation was given to those acknowledged.
Funding There was no project-specific support. Dr. Maruthur was supported by a training grant (5 T32 HL007024–31) from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH). Dr. Brancati was supported by a mid-career investigator award (5 K24 DK062222–05) from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Conflicts of Interest Dr. Brancati declares the following conflicts: Healthways (disease management), Kidd and Company (venture capital), Klinger Advanced Aesthetics (cosmetics), and law firms (Burg Simpson Law Firm; Garrettson Law Firm; Richardson, Patrick, Westbrook & Brickman, LLC)—Zyprexa litigation. He donates all fees to Johns Hopkins University. Drs. Bolen and Clark had unrestricted grants from Pfizer, Glaxo-Smith-Kline, and Johnson & Johnson for analyses from several large Blue Cross Blue Shield Plans. Dr. Bolen received an honorarium from Laboratorios Faltrex in February 2007 to give a talk to health care providers in the Dominican Republic on the comparative effectiveness of oral diabetes medications. Dr. Maruthur has no conflicts to disclose.
Appendix
Appendix A
Obesity and Cancer Screening
Quality Assessment Form
Reviewer: __________
Author/Year: ___________
Ref ID: _____
*Please check one answer for each question.
INTRODUCTION
- Were objectives and pre-specified hypotheses reported?
- _ adequate (objectives and pre-specified hypotheses were reported)
- _ fair (objectives specified but hypotheses not clearly stated)
- _ inadequate (minimal or no description)
METHODS
- Was the study setting described?
- _ adequate (setting, location, and dates of data collection stated)
- _ fair (setting, location, and dates of data collection stated incompletely)
- _ inadequate (minimal or no description)
- Was the study population described?
- _ adequate (There was a complete description of methods of selection and exclusion criteria OR statement that all eligible patients enrolled.)
- _ fair (There was an incomplete description of methods of selection and exclusion criteria. Would be difficult to replicate with the information provided)
- _ inadequate (minimal or no description)
- How was the study population selected?
- _ random sampling
- _ convenience sampling
- _ consecutive selection
- _ other purposive sampling
- _ other (please specify.): ____________
- _ not described
- Was there information on excluded or non-participating subjects?
- _ adequate (All reasons for exclusion or lack of participation noted OR no exclusions.)
- _ fair (There was some discussion of this topic, but not sufficient to allow replication.)
- _ inadequate (no description)
- Was the exposure well-described?
- _ adequate (exposure explicitly defined, and method of measurement described)
- _ fair (exposure described incompletely)
- _ inadequate (no description)
- Was the outcome well-described?
- _ adequate (outcome explicitly defined, and method of measurement described)
- _ fair (outcome described incompletely)
- _ inadequate (no description)
- If the study involved medical record review, was there standardized data abstraction?
- _ yes (please specify.) __________________________________
- _ no
- _ not described
- _ other (please specify) _________________________________
- _ not applicable
- If the study involved medical record review, was there blinding of abstractors to the study question?
- _ yes
- _ no
- _ not described
- _ not applicable
- _ other (please specify) _________________________________
- If the study involved medical record review, was there a description of handling of disagreements?
- _ not applicable
- _ adequate (method for handling of disagreements described completely)
- _ fair (method for handling of disagreements described incompletely)
- _ poor (method for handing of disagreements not described)
- If data abstracted from medical records, was inter- and intra-rater reliability described?
- _ not applicable
- _ inter-rater reliability
- _ yes
- _ kappa (please list) _______
- _ other (please list) ________
- _ no
- _ other (please specify) ___________
- _ intra-rater reliability
- _ yes
- _ kappa (please list) _______
- _ other (please list) ________
- _ no
- _ other (please specify) ___________
- If the study used a survey, was the survey response rate reported?
- _ not applicable
- _ not reported
- _ rate reported (please list) _____________________________
- Were key baseline characteristics ascertained?
- age
- sex
- comorbidity
- socioeconomic factors
- family history
- race
- smoking status
- _ adequate (0–2 applicable categories not described)
- _ fair (2–3 applicable categories not described)
- _ inadequate (>3 applicable categories not described)
- How did the study report the numbers of individuals at each stage of the study? (e.g., number of potentially eligible, examined for eligibility, confirmed eligible, included in the study, completed follow-up, and analyzed)
- _ adequate
- _ fair (one of the above not described)
- _ inadequate (>1 not described)
- For what percentage of participants were there missing data?
- _ none
- _ <10%
- _ 10–20%
- _ >20%
- _ not reported
- _ n/a
- Was there a discussion of sample size rationalization?
- _ adequate (Practical and statistical considerations were described.)
- _ fair (Rationale for sample size was discussed incompletely.)
- _ inadequate (Rationale for sample size not discussed.)
- Were statistical analyses clearly described?
- _ adequate (described for all analyses)
- _ fair (described for some analyses)
- _ inadequate (not described)
- For main analyses, were numbers of individuals experiencing the outcome reported?
- _ adequate (numbers provided or can be calculated for outcomes)
- _ fair (proportions but not numbers provided for outcomes)
- _ inadequate (no enumeration of outcome provided)
- For main analyses, are there estimates and a measure of variability (e.g., standard error, standard deviation, confidence intervals) reported?
- _ adequate (estimates and variability reported)
- _ fair (estimates and p-value or test statistic reported)
- _ inadequate (estimate only reported)
- Were confounding factors treated adequately?
- _ adequate (Adjustments were made for most or all potential confounders.)
- _ fair (Adjustments were made for most confounders.)
- _ inadequate (There were minimal or no adjustments for confounding.)
- Were methods for use of quantitative variables explained?
- _ adequate (description of covariates present)
- _ inadequate (description of covariates not present)
CONFLICTS OF INTEREST
- Were sources of funding identified?
- _ adequate (source of funding or no funding specified)
- _ poor (funding not described)
Other comments on study quality:
_________________________________________________________
_________________________________________________________
_________________________________________________________
Footnotes
Preliminary results from this project were presented in a poster at the 2007 Society of General Internal Medicine national meeting in Toronto on April 26, 2007.
Dr. Maruthur was supported by a training grant (5 T32 HL007024–31) from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH). Dr. Brancati was supported by a mid-career investigator award (5 K24 DK062222–05) from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2004 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. Available at: http://www.cdc.gov/uscs. Accessed February 6, 2009.
- 2.Miller AB, Baines CJ, To T, Wall C. Canadian national breast screening study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. Can Med Assoc J. 1992;147:1477–88. [PMC free article] [PubMed]
- 3.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. Can Med Assoc J. 1992;147:1459–98. [PMC free article] [PubMed]
- 4.Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from the Edinburgh Randomised Trial of Breast-Cancer Screening. Lancet. 1999;353(9168):1903–8. [DOI] [PubMed]
- 5.Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am. 2000;38(4):625–51. [DOI] [PubMed]
- 6.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–19. [DOI] [PubMed]
- 7.U.S. Preventive Services Task Force. Screening for breast cancer. Available at: http://www.ahrq.gov/clinic/uspstf/uspsbrca.htm Accessed February 6, 2009.
- 8.Smith RA, Cokkinides V, Eyre HJ. American cancer society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56(1):11–25. quiz 49–50. [DOI] [PubMed]
- 9.Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nccdphp/dnpa/obesity/trend/index.htm. Accessed February 6, 2009.
- 10.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet;371(9612):569–78. [DOI] [PubMed]
- 11.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335(7630):1134. [DOI] [PMC free article] [PubMed]
- 12.Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev.. 2005;14(7):1686–91. [DOI] [PubMed]
- 13.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23(38):6365–78. [DOI] [PubMed]
- 14.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–27. [DOI] [PubMed]
- 15.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–11. [DOI] [PubMed]
- 16.Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7(4):333–40. [DOI] [PubMed]
- 17.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13(4):325–32. [DOI] [PubMed]
- 18.Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med. 1994;120(1):18–25. [DOI] [PubMed]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. [DOI] [PubMed]
- 20.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–8. [DOI] [PubMed]
- 21.Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–54. [DOI] [PubMed]
- 22.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg. 2007;245(5):665–71. [DOI] [PMC free article] [PubMed]
- 23.Werner RS, McCormick B, Petrek J, et al. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology. 1991;180(1):177–84. [DOI] [PubMed]
- 24.van der Veen P, De Voogdt N, Lievens P, Duquet W, Lamote J, Sacre R. Lymphedema development following breast cancer surgery with full axillary resection. Lymphology. 2004;37(4):206–8. [PubMed]
- 25.Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer. 2002;98(2):279–83. [DOI] [PubMed]
- 26.Chagpar AB, Mcmasters KM, Saul J, et al. Body mass index influences palpability but not stage of breast cancer at diagnosis. Am Surg. 2007;73(6):555–60. [PubMed]
- 27.Amonkar MM, Madhavan S. Compliance rates and predictors of cancer screening recommendations among Appalachian women. J Health Care Poor Underserved. 2002;13(4):443–60. [DOI] [PubMed]
- 28.Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond). 2006;30(1):147–55. [DOI] [PubMed]
- 29.Coughlin SS, Uhler RJ, Hall HI, Briss PA. Nonadherence to breast and cervical cancer screening: what are the linkages to chronic disease risk? Prev Chronic Dis. 2004;1(1):A04. [PMC free article] [PubMed]
- 30.Fontaine KR, Heo M, Allison DB. Body weight and cancer screening among women. J Womens Health Gend Based Med. 2001;10(5):463–70. [DOI] [PubMed]
- 31.Ostbye T, Taylor DH Jr, Yancy WS Jr, Krause KM. Associations between obesity and receipt of screening mammography, Papanicolaou tests, and influenza vaccination: results from the Health and Retirement Study (HRS) and the Asset and Health Dynamics among the Oldest Old (AHEAD) study. Am J Public Health. 2005;95(9):1623–30. [DOI] [PMC free article] [PubMed]
- 32.Wee CC, McCarthy EP, Davis RB, Phillips RS. Obesity and breast cancer screening. J Gen Intern Med. 2004;19(4):324–31. [DOI] [PMC free article] [PubMed]
- 33.Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med. 2000;132(9):697–704. [DOI] [PubMed]
- 34.Winkleby MA, Snider J, Davis B, Jennings MG, Ahn DK. Cancer-related health behaviors and screening practices among Latinos: findings from a community and agricultural labor camp survey. Ethn Dis. 2003;13(3):376–86. [PubMed]
- 35.Zhu K, Wu H, Jatoi I, Potter J, Shriver C. Body mass index and use of mammography screening in the United States. Prev Med. 2006;42(5):381–5. [DOI] [PubMed]
- 36.Cohen SS, Signorello LB, Gammon MD, Blot WJ. Obesity and recent mammography use among black and white women in the Southern Community Cohort Study (United States). Cancer Causes Control. 2007;18(7):765–73. [DOI] [PubMed]
- 37.Ferrante JM, Chen PH, Crabtree BF, Wartenberg D. Cancer screening in women: body mass index and adherence to physician recommendations. Am J Prev Med. 2007;32(6):525–31. [DOI] [PMC free article] [PubMed]
- 38.Berz D, Sikov W, Colvin G, Weitzen S. ‘Weighing in’ on screening mammography. Breast Cancer Res Treat. 2008. [DOI] [PubMed]
- 39.Rosenberg L, Wise LA, Palmer JR, Horton NJ, Adams-Campbell LL. A multilevel study of socioeconomic predictors of regular mammography use among African-American women. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2628–33. [DOI] [PubMed]
- 40.Ferrante JM, Chen PH, Jacobs A. Breast and cervical cancer screening in obese minority women. J Womens Health (Larchmt). 2006;15(5):531–41. [DOI] [PubMed]
- 41.Fontaine KR, Faith MS, Allison DB, Cheskin LJ. Body weight and health care among women in the general population. Arch Fam Med. 1998;7(4):381–4. [DOI] [PubMed]
- 42.Gorin SS, Jacobson J. Diet and breast cancer surveillance behaviors among Harlem women. Ann N Y Acad Sci. 2001;952:153–60. [DOI] [PubMed]
- 43.Satia JA, Galanko JA. Demographic, behavioral, psychosocial, and dietary correlates of cancer screening in African Americans. J Health Care Poor Underserved. 2007;18(4 Suppl):146–64. [DOI] [PubMed]
- 44.STROBE Group. STROBE statement: Checklist of essential items version 3 (September 2005). Available at: http://www.strobe-statement.org/PDF%20hochladen/STROBE-Checklist-Version3.pdf. Accessed February 6, 2009.
- 45.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. The Lancet. ;370(9596):1453–7. [DOI] [PubMed]
- 46.World Health Organization. Obesity: Preventing and managing the global epidemic. Part 1: The problem of overweight and obesity. 2004;894:1–33. [PubMed]
- 47.NHLBI Obesity Education Intiative Panel on the Identification, Treatment, and Management of Overweight and Obesity in Adults. Clinical guidelines on the identification, treatment, and management of overweight and obesity in adults. The evidence report. 1998;98–4083:1–126.
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed]
- 49.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1. [DOI] [PubMed]
- 50.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed]
- 51.Deeks JJ, Higgins JPT, Altman DG, editors. Analyzing and presenting results. In: Higgins JPT, Green S, editors, editors. Cochrane handbook for systematic reviews of interventions 4.2.5 [updated May 2005]; Section 8; 2005.
- 52.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [DOI] [PubMed]
- 53.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed]
- 54.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. [DOI] [PubMed]
- 55.Olson CL, Schumaker HD, Yawn BP. Overweight women delay medical care. Arch Fam Med. 1994;3(10):888–92. [DOI] [PubMed]
- 56.Adams CH, Smith NJ, Wilbur DC, Grady KE. The relationship of obesity to the frequency of pelvic examination: do physician and patient attitudes make a difference? Women & health. 1993;20:45–57. [DOI] [PubMed]
- 57.Hunt KA, Sickles EA. Effect of obesity on screening mammography: outcomes analysis of 88,346 consecutive examinations. AJR Am J Roentgenol. 2000;174(5):1251–5. [DOI] [PubMed]
- 58.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996;276(1):33–8. [DOI] [PubMed]
- 59.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29(1):29–48. [DOI] [PubMed]
- 60.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209–14. [DOI] [PMC free article] [PubMed]
- 61.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. [DOI] [PMC free article] [PubMed]
- 62.Hebl MR, Xu J. Weighing the care: Physicians’ reactions to the size of a patient. Int J Obes Relat Metab Disord. 2001;25(8):1246–52. [DOI] [PubMed]
- 63.Price JH, Desmond SM, Krol RA, Snyder FF, O'Connell JK. Family practice physicians’ beliefs, attitudes, and practices regarding obesity. Am J Prev Med. 1987;3(6):339–45. [PubMed]
- 64.Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord. 2001;25(10):1525–31. [DOI] [PubMed]
- 65.Stevens J, Kumanyika SK, Keil JE. Attitudes toward body size and dieting: differences between elderly black and white women. Am J Public Health. 1994;84(8):1322–5. [DOI] [PMC free article] [PubMed]
- 66.Anderson LA, Eyler AA, Galuska DA, Brown DR, Brownson RC. Relationship of satisfaction with body size and trying to lose weight in a national survey of overweight and obese women aged 40 and older, United States. Prev Med. 2002;35(4):390–6. [DOI] [PubMed]
- 67.Flynn KJ, Fitzgibbon M. Body images and obesity risk among black females: a review of the literature. Ann Behav Med. 1998;20(1):13–24. [DOI] [PubMed]
- 68.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56(3):168–83. [DOI] [PubMed]
- 69.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: Factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–48. [DOI] [PubMed]
- 70.Polednak AP. Racial differences in mortality from obesity-related chronic diseases in US women diagnosed with breast cancer. Ethn Dis. 2004;14(4):463–8. [PubMed]
- 71.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. [DOI] [PubMed]
- 72.Jones BA, Patterson EA, Calvocoressi L. Mammography screening in African American women: Evaluating the research. Cancer. 2003;97(1 Suppl):258–72. [DOI] [PubMed]
- 73.Cui Y, Whiteman MK, Langenberg P, et al. Can obesity explain the racial difference in stage of breast cancer at diagnosis between black and white women? J Womens Health Gend Based Med. 2002;11(6):527–36. [DOI] [PubMed]
- 74.Moorman PG, Jones BA, Millikan RC, Hall IJ, Newman B. Race, anthropometric factors, and stage at diagnosis of breast cancer. Am J Epidemiol. 2001;153(3):284–91. [DOI] [PubMed]
- 75.Zhu K, Caulfield J, Hunter S, Roland CL, Payne-Wilks K, Texter L. Body mass index and breast cancer risk in African American women. Ann Epidemiol. 2005;15(2):123–8. [DOI] [PubMed]
- 76.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279(22):1801–7. [DOI] [PubMed]
- 77.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–8. [DOI] [PMC free article] [PubMed]
- 78.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–53. [DOI] [PubMed]
- 79.McCarthy EP, Burns RB, Coughlin SS, et al. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128(9):729–36. [DOI] [PubMed]
- 80.Jones BA, Kasl SV, Curnen MG, Owens PH, Dubrow R. Can mammography screening explain the race difference in stage at diagnosis of breast cancer? Cancer. 1995;75(8):2103–13. [DOI] [PubMed]
- 81.Jacobellis J, Cutter G. Mammography screening and differences in stage of disease by race/ethnicity. Am J Public Health. 2002;92(7):1144–50. [DOI] [PMC free article] [PubMed]
- 82.Gill KS, Yankaskas BC. Screening mammography performance and cancer detection among black women and white women in community practice. Cancer. 2004;100(1):139–48. [DOI] [PubMed]
- 83.Burkhauser RV, Cawley J. Beyond BMI: The value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008 3;27(2):519–29. [DOI] [PubMed]
- 84.Rowland ML. Self-reported weight and height. Am J Clin Nutr. 1990;52:1125–33. [DOI] [PubMed]
- 85.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748–57. [DOI] [PubMed]