Abstract
Acetaminophen is an analgesic and antipyretic drug believed to exert its effect through interruption of nociceptive processing. In order to determine whether this effect is due to peripheral or central activity, we studied the efficacy of systemic (oral) and intrathecal (IT) application of acetaminophen in preventing the development of hyperalgesia induced through the direct activation of pro-algogenic spinal receptors. Spinal administration of substance P (SP, 30 nmol, IT) in rats produced a decreased thermal threshold, indicating centrally-mediated hyperalgesia. Pretreatment of rats with oral acetaminophen (300 mg/kg), but not vehicle, significantly attenuated IT SP-induced hyperalgesia. Acetaminophen given IT also produced a dose-dependent (10–200 μg) antinociceptive effect. In addition, oral acetaminophen suppressed spinal PGE2 release evoked by IT SP in an in vivo IT dialysis model. The ability of IT as well as oral acetaminophen to reverse this spinally initiated hyperalgesia emphasizes the likely central action and bioavailability of the systemically delivered drug. Jointly, these data argue for an important central antihyperalgesic action of acetaminophen.
Keywords: acetaminophen, intrathecal, hyperalgesia, substance P
Acetaminophen is one of the most widely used pharmaceutical formulations in the United States. Discovered in 1878, it has been in human clinical use for over a century, although its mechanism of action is still not completely understood. It has been shown to act with modest efficacy against cyclooxygenase (prostacyclin endoperoxidase H2 synthase) in vitro prompting its loose affiliation with the non-steroidal anti-inflammatory drugs (NSAIDs) [11, 14]. Preclinically, this agent does not exert an effect upon normal thermal escape latencies but reverses hyperalgesic states that develop as a result of local tissue injury[5]. NSAIDs such as acetylsalicylic acid and ibuprofen have similar properties that have been ascribed to their ability to reduce inflammation by inhibiting cycloxygenase activity [14]. However, acetaminophen does not produce parallel anti-inflammatory effects in the periphery and is unable to limit cyclooxygenase activity to the same degree as the other NSAIDs [5]. This observation implies a possible central mechanism of acetaminophen that is responsible for mediating its antihyperalgesic action. Such a central action has additionally been attributed to the NSAIDS [15]. We note that a variety of other central mechanisms may also be relevant, including the generation of endocannabinoids, inhibition of nitric oxide generation and alteration in the activity in bulbospinal projections [1, 2, 8].
In the present studies we sought to evaluate a potential spinal action of acetaminophen upon the development of a centrally-evoked hyperalgesia. It is known that subsequent to tissue injury, spinal facilitation evolves secondary to the release of substance P (SP) and its action upon local dorsal horn neurokinin-1 (NK1) receptors [12, 17]. It is possible to create such a state of hyperalgesia in the absence of peripheral injury with direct administration of SP into the intrathecal (IT) space and subsequent activation of NK1 receptors, as though a peripheral signal was being transmitted. Here we examined the effects of oral and IT acetaminophen pretreatment on the development of SP-evoked hyperalgesia and on the central release of prostaglandins customarily initiated by a similar IT injection of SP [9, 16].
All experiments were carried out according to protocols approved by the Institutional Animal Care Committee of the University of California, San Diego. Male Holtzman Sprague-Dawley rats (300–350 g, Harlan Industries, Indianapolis, IN, USA) were used in the study. Animals were housed individually and maintained on a 12-hour light/dark cycle with free access to food and water. Animals were implanted with intrathecal catheters under isoflurane anesthesia according to the method described [18]. In this procedure, the animal’s head was stabilized with a stereotactic frame, and following skin incision and dissection, the cisternal membrane was exposed. A polyethylene catheter (PE-5, 8.5 cm) was inserted through a dural incision and advanced caudally, down the length of the intrathecal space adjacent to the spinal cord. The catheter was plugged and secured, and the subjects were observed for behavioral status, motor coordination and function, and muscle tone for a period of at least five days postoperatively.
Thermal-evoked paw withdrawal responses were assessed using a Hargreaves-type testing device with modification [4, 7]. The device consists of a glass surface (maintained at 25°C) on which the rats are placed individually in Plexiglas cubicles. The thermal nociceptive stimulus originates from a focused projection bulb positioned below the glass surface. A timer is actuated by the light source, and latency was defined as time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminate the stimulus. Basal paw withdrawal latencies were assessed at time −30 min. At time −10 min the animals received either oral or IT administration of vehicle (5% DMSO) or acetaminophen (Sigma Chemicals, St. Louis, MO, USA). SP (30 nmol, Sigma) was administered IT at time = 0. Paw withdrawal latencies were measured at 10, 20, 30, 45 and 60 minutes following SP injection. The hyperalgesic index, which defines the magnitude of IT SP-induced sensitization, was calculated for each treatment. It represents the area under the time effect curve (AUC) after SP in which the ‘percent reduction from baseline response latency’ is plotted against time. The resulting metric is % change × min. The formula for calculating the percent change is (baseline latency – post drug latency) × 100 (baseline latency)−1, where latency is expressed in seconds. Increasing values indicate increasing hyperalgesia.
To define the spinal release of PGE2, dialysis experiments were conducted in non-anesthetized rats three or four days after implantation of a three-lumen dialysis catheter: two for dialysis and one for injection [10]. A syringe pump (Harvard Apparatus Inc., Holliston, MA) was connected to the catheter, and one arm of the dialysis tubing was infused with artificial cerebrospinal fluid (CSF) at a rate of 10 μl/min. The artificial cerebrospinal fluid contained 151.1 mM Na+, 2.6 mM K+, 0.9 mM Mg2+, 1.3 mM Ca2+, 122.7 mM Cl−, 21.0 mM HCO3, 2.5 mM HPO4, and 3.5 mM dextrose and was bubbled with 95% O2/5% CO2 before each experiment to adjust the final pH to 7.2. The dialysate (15 min/fraction) was collected in an automatic fraction collector (Eicom, Kyoto, Japan) at 4°C. Two baseline samples were collected following a 30-minute washout and four additional samples were taken after IT injection of SP (30 nmol). Acetaminophen (300 mg/kg, oral) or the vehicle (0.5% methylcellulose, 0.025% Tween 20, 3 ml/kg, oral) was given 60 min prior to IT SP. The concentration of PGE2 in spinal dialysates was measured by enzyme-linked immunosorbent assay using a commercially available kit (Assay Designs 90001; Assay Designs, Ann Arbor, MI). The antibody is selective for PGE2 with less than 2.0% cross-reactivity to PGF1, PGF2, 6-ketoPGF1, PGA2, or PGB2 but does cross-react with PGE1 and PGE3.
All the data are presented as mean ± SEM. Differences were assessed by one-way ANOVA (for independent measurements), two-way ANOVA analysis (for repeated measurements over time) followed by Bonferroni post-hoc test for multiple groups, or a student t-tests for two groups, with a criterion of p < 0.05 for significance.
RESULTS
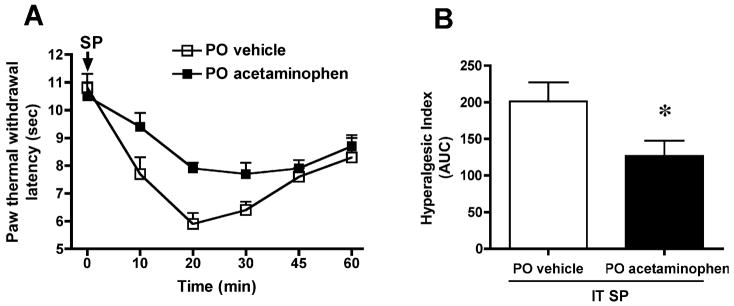
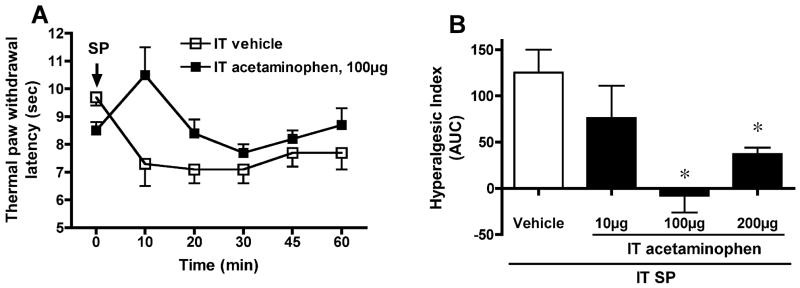
IT injection of SP (30 nmol) resulted in a significant decrease in paw withdrawal latency to thermal stimulation, indicating thermal hyperalgesia. In the oral vehicle group, the latency before IT SP was 10.8±0.5, and 20 min after IT SP 5.8 ± 0.4 sec (N=8, p < 0.05, Figure 1A). PO acetaminophen (300 mg/kg; N=10) had no effect upon either basal thermal latency (10.5±0.5 sec) or motor function, but attenuated the magnitude of the SP-induced hyperalgesic state. As seen in Figure 1, thermal response latency diminished to a significantly lesser degree (AUC of hyperalgesic index: vehicle, 201±26, vs. acetaminophen 127±21, p < 0.05) following oral acetaminophen administration when compared with vehicle administration. In addition, IT acetaminophen dose-dependently (10–200 μg) suppressed SP-induced thermal hyperalgesia when compared with the vehicle group (AUC: vehicle 125 ± 25, vs. acetaminophen 100 μg, −8 ± 18; 200 μg, 37 ± 9; p < 0.05, N=5–10) (Figure 2). Acetaminophen at any given IT dose did not alter the general behavior or motor function.
Figure 1. Effect of oral administration of acetaminophen on substance P (SP)-evoked thermal hyperalgesia.
(A) Paw withdrawal latency (sec) to thermal stimulation plotted vs. time after intrathecal (IT) injection of SP (30 nmol). Acetaminophen (300 mg/kg, N=10) or vehicle (3 ml/kg, N=8) was delivered orally (PO) 30 min prior to IT SP. (B) The antihyperalgesic effect of PO acetaminophen is presented as hyperalgesic index (area under the curve, AUC, calculated from 0 to 60 min after IT SP injection). The data are presented as mean ± SEM. * p < 0.05, Student t-test.
Fig. 2. Effect of intrathecal (IT) administration of acetaminophen on substance P (SP)-evoked thermal hyperalgesia.
(A) Paw withdrawal latency (sec) to thermal stimulation plotted vs. time after IT injection of SP (30 nmol). Acetaminophen (10 μg, N=5; 100 μg, N=10 and 200 μg, N=9) or vehicle (10 μl, N=6) was delivered IT 10 min prior to SP. (B) The antihyperalgesic effect of acetaminophen is presented as hyperalgesic index (area under the curve, AUC, calculated from 0 to 60 min after the SP injection). * p < 0.05, vs. vehicle, one-way ANOVA.
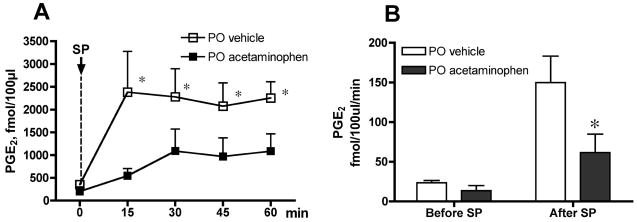
In the in vivo spinal dialysis model, SP given IT at the same dose (30 nmol) as in the behavioral study evoked a profound increase in PGE2 dialysate concentration. Baseline PGE2 concentrations in spinal dialysates was 409 ± 71 fmol/100 μl in the vehicle group (N=7). Fifteen minutes after SP, the PGE2 level increased to 2295 ± 761 fmol/100 μl. This elevation was significantly different from the pre IT-SP baseline at 15, 30, 45 and 60 min (p < 0.05; Figure 3A). In the rats receiving PO acetaminophen (300 mg/kg; N=6), baseline PGE2 concentration was 205 ± 96 fmol/100 μl. Though the baseline of the acetaminophen group was numerically reduced compared to the vehicle group, this difference was not statistically significant (p > 0.05). More importantly, acetaminophen did significantly attenuate IT SP-evoked PGE2 release. Thus, comparison of PGE2 concentrations over time revealed that SP-evoked PGE2 release in the acetaminophen-treated group was not significantly different from the pre IT-SP baseline. Similarly, plotting the release as a function of the level of PGE2 released per min in the samples pre IT-SP (time = −30 min to 0 min) versus release after IT-SP (0 min to 60 min) showed a significant reduction in the acetaminophen versus vehicle treated group (PGE2 fmol/100μl/min: vehicle 150±33; Acetaminophen 62±23, p < 0.05. Figure 3B).
Figure 3.
Oral administration of acetaminophen attenuates substance P (SP)-evoked PGE2 release. (A) PGE2 concentration measured in cerebrospinal fluid (fmol/100μl) collected by in vivo spinal dialysis of conscious rats before (Time = 0) and after intrathecal (IT) injection of SP. Acetaminophen (300 mg/kg, N=6) or vehicle (3 ml/kg, N=6) was delivered orally (PO) 60 min before SP. Two-way ANOVA analysis (repeated measurements) showed that SP evoked a significant increase in PGE2 release in vehicle-, but not acetaminophen-treated group (*p < 0.05, all time points). (B) The data are expressed as PGE2 release (fmol/100μl) per minute during baseline collection (Before SP: −30 to 0 min) and following IT injection of SP (After SP: 0–60 min) with oral administration of acetaminophen or vehicle. *p < 0.05 (Student t-test).
DISCUSSION
Acetaminophen has been shown to be effective in a variety of preclinical models of hyperalgesia. An essential question is, to what degree is this effect mediated by a peripheral or a central action? We sought to address this question using two combined strategies. First, we examined the effects of intrathecal verses oral delivery of acetaminophen, and secondly we examined the effects of these two treatments on the hyperalgesia induced by the central activation of spinal neurokinin 1 (NK1) receptors. Previous work has shown that activation of spinal NK1 receptors by intrathecal delivery of SP initiates a hyperalgesic state. This effect is believed to be mediated in part by activation of phospholipase A2 which releases arachidonic acid to be converted by constitutively expressed spinal cyclooxygenase. Thus, the hyperalgesic actions of IT SP are indeed blocked by intrathecal COX inhibitors [12]. Using this system to examine the effects of systemic and intrathecally-delivered acetaminophen, we observed that after intrathecal or systemic delivery, there was a prominent reduction in the spinally-initiated hyperalgesia. For the systemic route of delivery, the observation that acetaminophen reversed that centrally-mediated hyperalgesia is consistent with its known ability to penetrate into the brain [3] at a dose which failed to alter the acute thermal threshold. This emphasizes that the site of systemic drug action was within the neuraxis on mechanisms that mediate the spinal sensitization. Similarly, the oral dose of acetaminophen required to produce a central antihyperalgesic effect was 1000 times than the dose required when administered intrathecally. It is therefore unlikely that the spinal effect of the intrathecal drug effect was due to redistribution of the drug into the periphery. These observations thus support the assertion that acetaminophen given systemically can produce a potent effect on the spinal facilitatory system. Indeed, the anti-hyperalgesic effect of intrathecal acetaminophen at systemically inactive doses also points to a direct action on the spinal cord. The observation that systemic acetaminophen can reduce hyperalgesia evoked by a spinal system does not exclude the possibility that systemic drugs may act through unexpected pathways secondary to a metabolite. It is notable that a metabolite of acetaminophen via liver conversion acts as a centrally bioavailable fatty acid amide hydrolase inhibitor and increases the production of endocannabinoids, that could regulate spinal excitability through local cannabinoid receptors [8].
An important question relates to the systems altered by the spinal acetaminophen. In previous work we showed that activation of spinal NK1 receptors leads to spinal release of PGE2, and that the systemic delivery of antihyperalgesic doses of cyclooxygenase inhibitors diminish that PGE2 release [9, 16]. In the present work, we found that at systemically-active analgesic doses, acetaminophen indeed was able to reduce the release of PGE2. While these observations indicate that acetaminophen can regulate an NK1-receptor-evoked activation of the cyclooxygenase cascade (as evidenced by PGE2 release), such observations do not provide specific insights into the component of the cascade which is the target for this centrally mediated antihyperalgesic effect. Thus, while the results are consistent with an inhibition of a component enzyme, such as cyclooxygenase, it is possible that the effects may reflect any of the several intervening links. For example, we have previously shown that the afferent-evoked release of PGE2 from the spinal cord is similarly reduced by analgesic doses of morphine [13]. As noted above, one speculative hypothesis points to the potential role of a fatty acid amide hydrolase inhibitor. There is a large and complex literature suggesting that acetaminophen may have only modest effects upon cyclooxygenase activity [5]. Thus, acetaminophen does not possess significant anti-inflammatory effects and is unable to limit cyclooxygenase activity to the same degree as the other NSAIDs in a variety of assays [5]. Acetaminophen has been shown to act in intact cells to limit the cellular production of prostaglandins [11] and this may happen as a result of its interference with intracellular cyclooxygenases at an active site separate from the one utilized by most NSAIDs [5]. Similarly, it has been argued that non-neuronal cells may be responsible for the bulk of prostaglandin production within the central nervous system, and acetaminophen affects them at a dose much lower than what is necessary to block peripheral prostaglandin production [6]. It is also known that acetaminophen acts with greater efficacy in environments with low peroxide tone and low arachidonic acid levels, such as what exists within the central nervous system [5]. Thus, the possibility that there is a direct effect of acetaminophen on spinal cyclooxygenase cannot be excluded.
We conclude, therefore, that acetaminophen affects the development of sensitization by a spinal action after systemic and intrathecal delivery. These effects on spinal pro-facilitatory cascades are reflected in part by its ability to reduce the centrally evoked PGE2 release. Whether the effect on eicosanoid production is via an effect on cyclooxygenase or an intervening step remains to be determined.
Acknowledgments
We wish to thank Alan Moore and Damon McCumber for technical assistance. This work was supported by NIH NS 16541 and DA 02110 (TLY).
References
- 1.Bjorkman R, Hallman KM, Hedner J, Hedner T, Henning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994;57:259–64. doi: 10.1016/0304-3959(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefont J, Alloui A, Chapuy E, Clottes E, Eschalier A. Orally administered paracetamol does not act locally in the rat formalin test: evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiology. 2003;99:976–81. doi: 10.1097/00000542-200310000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Courad JP, Besse D, Delchambre C, Hanoun N, Hamon M, Eschalier A, Caussade F, Cloarec A. Acetaminophen distribution in the rat central nervous system. Life Sci. 2001;69:1455–64. doi: 10.1016/s0024-3205(01)01228-0. [DOI] [PubMed] [Google Scholar]
- 4.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–91. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 5.Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Greco A, Ajmone-Cat MA, Nicolini A, Sciulli MG, Minghetti L. Paracetamol effectively reduces prostaglandin E2 synthesis in brain macrophages by inhibiting enzymatic activity of cyclooxygenase but not phospholipase and prostaglandin E synthase. J Neurosci Res. 2003;71:844–52. doi: 10.1002/jnr.10543. [DOI] [PubMed] [Google Scholar]
- 7.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 8.Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–12. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 9.Hua XY, Chen P, Marsala M, Yaksh TL. Intrathecal substance P-induced thermal hyperalgesia and spinal release of prostaglandin E2 and amino acids. Neuroscience. 1999;89:525–34. doi: 10.1016/s0306-4522(98)00488-6. [DOI] [PubMed] [Google Scholar]
- 10.Koetzner L, Hua XY, Lai J, Porreca F, Yaksh T. Nonopioid actions of intrathecal dynorphin evoke spinal excitatory amino acid and prostaglandin E2 release mediated by cyclooxygenase-1 and -2. J Neurosci. 2004;24:1451–8. doi: 10.1523/JNEUROSCI.1517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas R, Warner TD, Vojnovic I, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. Faseb J. 2005;19:635–7. doi: 10.1096/fj.04-2437fje. [DOI] [PubMed] [Google Scholar]
- 12.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–9. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg AB, Yaksh TL. The effect of morphine on formalin-evoked behaviour and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br J Pharmacol. 1995;114:1069–75. doi: 10.1111/j.1476-5381.1995.tb13315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–83. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 16.Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci. 2001;21:5847–53. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–7. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- 18.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]