Abstract
Grafting of immature mammalian testis tissue to mouse hosts can preserve the male germline. To make this approach applicable to a clinical or field situation, it is imperative that the testis tissue and/or spermatozoa harvested from grafted tissue are preserved successfully. The aim of the present study was to evaluate protocols for the preservation of testis tissue in a porcine model. Testis tissue was stored at 4°C for short-term preservation or cryopreserved by slow-freezing, automated slow-freezing or vitrification for long-term storage. Preserved tissue was transplanted ectopically to mouse hosts and recovered xenografts were analysed histologically. In addition, spermatozoa were harvested from xenografts and cryopreserved. Total cell viability and germ cell viability remained high after tissue preservation. Complete spermatogenesis occurred in xenografts preserved by cooling up to 48 h, whereas spermatogenesis progressed to round spermatids in the xenografts that were frozen–thawed before grafting. Approximately 50% of spermatozoa harvested from xenografts remained viable after freezing and thawing. The in vivo developmental potential of cryopreserved tissue was reduced despite high post-thaw viability. Therefore, it is important to evaluate germ cell differentiation in vivo in addition to cell viability in vitro when optimising freezing protocols for testis tissue.
Keywords: cooling, cryopreservation, grafting, spermatogenesis
Introduction
Grafting of testis tissue into mouse hosts can result in the production of spermatozoa from different mammalian donor species (Honaramooz et al. 2002, 2004; Schlatt et al. 2002, 2003; Ohta and Wakayama 2004; Snedaker et al. 2004; Rathi et al. 2005; Arregui et al. 2008). Testis tissue xenografting is a powerful system for the investigation of spermatogenesis and testis maturation, and it provides a novel system by which to obtain spermatozoa from immature male individuals. In testis tissue xenografts, the donor testis microenvironment remains intact and spermatogenesis in grafts appears representative of spermatogenesis in the donor species (Zeng et al. 2006, 2007). Live progeny were produced from spermatozoa or round spermatids extracted from ectopic allografts of neonatal or fetal testes by intracytoplasmic injection into mouse oocytes and subsequent embryo transfer (Schlatt et al. 2003; Ohta and Wakayama 2004). Similarly, spermatozoa isolated from xenografts of immature rhesus monkey and pig testes supported embryonic development in vitro to the blastocyst stage (Honaramooz et al. 2004, 2008). The successful generation of fertile mouse offspring and monkey and pig embryos indicates that male germ cells recovered from testis grafts are functional to support normal development.
In practice, recipient animals may not be immediately available and/or offspring may be desired at a later time. Therefore, short- or long-term preservation of testis tissue may be required before transplantation. To make grafting of testis tissue for the preservation and propagation of male germ cells applicable to a clinical or field situation, it is imperative to preserve the spermatogenic potential of the testis tissue. Cryopreservation and subsequent grafting of testis tissue could then provide a potentially inexhaustible source of male germ cells for the preservation of genetic resources of experimental, domestic and rare or endangered animals.
In humans, it has been estimated that, by 2010, 0.4% of young adults (aged 20−29 years) will be long-term survivors of childhood cancer (Keros et al. 2005; Geens et al. 2006). Because chemotherapy or radiation therapy frequently causes permanent sterility as a result of the loss of spermatogonial stem cells (Keros et al. 2005), preservation of testis tissue before treatment offers an option for fertility preservation for these patients where spermatozoa cannot be preserved (Bahadur et al. 2000; Hovatta 2003). Testis tissue cryopreservation could also be beneficial for infertile men who are undergoing testis biopsy for diagnostic or treatment purpose (Tuuri et al. 1999; Hovatta 2000). Autologous transplantation of spermatogonial stem cells back to testis may generate spermatozoa and, hence, avoid the need for assisted reproduction later in life (Hovatta 2001; Jahnukainen et al. 2006). However, transplantation of cells collected before cancer treatment may carry the risk of reintroducing cancer cells to the patient (Aslam et al. 2000; Jahnukainen et al. 2001). Maturation of testis tissue in a xenotransplant does not carry this risk; however, ethical and safety considerations will have to be addressed before this approach would be applicable to human patients.
The pig has many similarities in physiology with humans, including size, digestive physiology, dietary habits, kidney structure and function, pulmonary vascular bed structure and propensity to obesity (Tumbleson and Schook 1996). As such, the pig serves as an important model in biomedical research. Previously, we reported the successful development of porcine testis tissue xenografts after cryopreservation by a slow-freezing method routinely used for freezing cultured cells and isolated germ cells (Honaramooz et al. 2002). Tissue cryopreservation is more complex than the cryopreservation of cells. Increased time required for permeation of cryoprotectants results in prolonged exposure of tissue to potentially toxic substances before freezing. Tissue may be damaged by even extremely small amounts of ice formation, which can disrupt critical cell-to-cell relationships that must be maintained intact to allow proper function after thawing. Vitrification is the process of converting a material into a glass-like amorphous solid that is free from crystalline structures, thus providing the benefits of cryopreservation without damage due to ice crystal formation. Vitrification has been applied successfully for the preservation of structures larger than single cells, such as embryos and oocytes (Kuleshova et al. 1999), as well as ovarian tissue (Sugimoto et al. 2000). However, there is no report applying vitrification for the preservation of testis tissue. Automated freezing protocols (Schlatt et al. 2002; Shinohara et al. 2002; Keros et al. 2005; Kvist et al. 2006; Jahnukainen et al. 2007) have been applied in several studies on cryopreservation of testis tissue. Therefore, slow-freezing protocols and vitrification were included in the present study of long-term preservation of testis tissue.
Cryopreservation of testis tissue has been explored in different species with varying degrees of germ cell differentiation achieved after thawing. Mouse spermatozoa were present in testis allografts that were frozen before transplantation (Schlatt et al. 2002; Shinohara et al. 2002; Goossens et al. 2008). Grafts of fresh and cooled rhesus monkey testis tissue showed good survival and spermatogenic differentiation to the spermatocyte stage (Jahnukainen et al. 2007). Cryopreserved rhesus monkey testis tissue also survived and initiated spermatogenesis after xenografting into mouse skin (Jahnukainen et al. 2007). The structural characteristics of human testis tissue were maintained after freezing in a programmable freezer, but no quantification or functional potential for spermatogenesis was reported (Keros et al. 2005; Kvist et al. 2006). Recently, Wyns et al. (2007) reported survival and some proliferative activity of spermatogonial cells and Sertoli cells after cryopreservation of human cryptorchid testis tissue followed by xenografting for only 21 days.
The objective of the present study was to evaluate: (1) the capacity of different protocols to preserve immature testis tissue before grafting to maintain in vivo spermatogenic potential; and (2) cryopreservation of spermatozoa recovered from testis xenografts using the pig as model species.
Materials and methods
Chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA), unless stated otherwise.
Experimental design
In Experiment I, cooling (4°C for 24, 48 and 72 h) and three freezing protocols were applied to preserve porcine testis tissue. Vitrification and an automated slow-freezing protocol were evaluated in addition to a conventional slow-freezing protocol that was found previously (Honaramooz et al. 2002) to yield viability and development after cryopreservation. The preserved testis tissue was transplanted into the back skin of castrated immunodeficient mice (eight grafts per mouse; n = 20 mice per protocol; i.e. 160 pieces of tissue per protocol). In previous work from our laboratory, complete spermatogenesis was observed in xenografts of newborn pig testis tissue 16−18 weeks after transplantation (Honaramooz et al. 2002; W. Zeng and R. Rathi, unpubl. data). Therefore, in the present study, grafts were harvested 16 and 22 weeks after grafting (at least three mice per time point per protocol). The status of testis maturation and the most advanced germ cell types present were assessed by histology.
In Experiment II, we investigated whether spermatozoa recovered from xenografts retain viability after cryopreservation. Previously, we observed full spermatogenesis in most seminiferous tubules 24−35 weeks after transplantation (Zeng et al. 2006, 2007; W. Zeng and R. Rathi, unpubl. data). Spermatozoa were harvested from grafted tissue 24−35 weeks after transplantation, frozen with a pellet technique (Zeng et al. 2001) and assessed for post-thaw viability.
Preservation of testis tissue
Testes were obtained by castration of 1−2-week-old Yorkshire-cross piglets. The testis tissue was cut into small fragments, approximately 1 × 1 × 2 mm, for all preservation protocols. The tissue fragments were placed into Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY, USA) before treatment with cryoprotectants. The protocols outlined below were included in the present study.
Cooling protocol: Testis tissue was prepared and immersed in Dulbecco's phosphate-buffered saline with Ca2+ (DPBS; Invitrogen) at room temperature, then placed at 4°C, resulting in a cooling rate of approximately 0.3°C min−1, before being stored at 4°C for 24, 48 and 72 h. In previous experiments, there was no difference in spermatogenesis between xenografts that were grafted immediately after collection and those that were stored at 4°C for 24 h before transplantation (W. Zeng and R. Rathi, unpubl. data). Therefore, we did not include fresh tissue xenografting as a control group in the present study.
Conventional slow-freezing (CSF): Freezing medium was prepared with fetal bovine serum (FBS; Hyclone, Logan, UT, USA), DMEM and dimethyl sulfoxide (DMSO) at ratio of 1 : 3 : 1. Approximately 10 pieces of testis tissue and 0.5 mL freezing medium was transferred into a 2-mL cryovial (Nalgene-Nunc, Rochester, NY, USA) at room temperature. The vials were placed into a Nalgene freezing container at room temperature, which was designed to provide a controlled cooling rate of 1°C min−1 when put into a −70°C freezer. Tissue fragments were left at −70°C overnight before transfer into liquid nitrogen. For thawing, vials were held at room temperature for 1 min to evaporate any remaining liquid nitrogen and then placed in a 25°C water bath for 1 min. Two millilitres of DMEM was added to each vial and the contents were transferred to a sterile centrifuge tube. Subsequently, tissue fragments were washed twice with DMEM by centrifugation (300g, 2 min) and resuspension to remove the cryoprotectant.
Automated slow-freezing (ASF): Ten testis fragments were placed into a cryovial with 1 mL Leibovitz L-15 medium (Invitrogen) containing 2% FBS and 10% DMSO. The tissue was incubated at room temperature for 15 min and then loaded into a programmable freezer (Kryo II freezer; Planer Products Ltd, Sunbury-on-Thames, England). The testis tissue was cooled at 2°C min−1 from 20°C to 4°C and then manually seeded with forceps immersed in liquid nitrogen. Cooling proceeded at 0.3°C min−1 from 4°C to −30°C and then at 10°C min−1 from −30°C to −130°C. The vials were plunged into liquid nitrogen and then transferred to a storage dewar. For thawing, the vials were held at room temperature for 1 min and then placed in a 25°C water bath for 1 min. One millilitre of Leibovitz L-15 medium was added to each vial and the contents were transferred to a sterile centrifuge tube. Subsequently, tissue fragments were washed twice with Leibovitz L-15 medium to remove cryoprotectant.
Vitrification protocol (VP): The vitrification solution (VS) was ethylene glycol (Fisher Scientific, Fair Lawn, NJ, USA) containing 0.9% NaCl and 0.5 m raffinose. The VS was diluted with DPBS to prepare 12.5%, 25% and 50% (v/v) VS. Fragments of testis tissue were exposed successively to 12.5% VS and 25% VS at room temperature for 5 min. Subsequently, fragments were exposed to 50% VS in ice for 15 min before being transferred to cryovials with enough 100% VS to cover the tissue and then plunged into liquid nitrogen. For thawing, cryovials were warmed up by vigorous agitation in ice water until the medium became liquid. One millilitre of chilled 50% VS was added into each cryovial. The fragments were transferred to a culture dish and incubated successively with 50% VS and 25% VS on ice for 10 min. The tissue was washed successively in 12.5% VS and DPBS at room temperature for 10 min.
Determination of cell viability in vitro
Cell viability was determined by enzymatic digestion of tissue fragments to a single cell suspension and observation of cell viability by Trypan blue exclusion. Briefly, tissue fragments were digested successively at 37°C with collagenase (2 mg mL−1 in DMEM) for 30 min, hyaluronidase (3 mg mL−1 in DMEM) for 15−20 min, Trypsin/EDTA (Invitrogen) for 1 min and deoxyribonuclease (7 mg mL−1 in DMEM) for 1 min. Ten microlitres of cell suspension (5−10 × 106 cells mL−1) was mixed with 10 μL Trypan blue dye. At least 200 cells per sample were counted as either stained (dead) or unstained (live).
Determination of germ cell viability in vitro
After enzymatic digestion of the preserved testis tissue to a single cell suspension, propidium iodide (PI) and Protein Gene Product 9.5 (PGP9.5) immunostaining were applied to assay the viability of germ cells (Luo et al. 2006). Briefly, 1 mL DMEM (with 10% FBS) was used to resuspend cells. One millilitre of cell suspension was stained with 5 μL PI stock solution (2.4 mM in water) at 37°C for 5 min in the dark. After rinsing cells with blocking buffer (DPBS containing 1% bovine serum albumin (BSA)), cells were fixed with 4% paraformaldehyde (PFA) at room temperature for 10 min. After two washes in blocking buffer, non-specific staining was blocked by incubation with blocking buffer for 15 min at room temperature. The monoclonal rabbit anti-PGP antibody (AbD Serotec, Raleigh, NC, USA; diluted 1 : 200 with DPBS containing 1% BSA) was added for 60 min at 37°C, followed by washing in blocking buffer, as in all subsequent washes. The cells were incubated with donkey anti-rabbit IgG (1 : 500) linked to Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) for 60 min at room temperature. Cells were examined under light plus fluorescence microscopy and the percentage of live germ cells (PGP9.5+/PI−) was calculated.
Transplantation of preserved tissue into mice
Testis tissue stored at 4°C for 48 h and frozen–thawed testis tissue from the three freezing protocols was transplanted into immunodeficient NCR Nude mice (Taconic, Germantown, NY, USA). Xenografting was performed as described previously (Honaramooz et al. 2002). Briefly, testis fragments were kept in DMEM on ice until grafting. Recipient mice (6−8 weeks old) were anaesthetised and castrated, and eight pieces of testis fragments were grafted under the back skin of each immune-deficient mouse (n = 80; 20 mice per preservation protocol). Animals were handled and treated in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee.
Collection of grafts and histological evaluation
Grafts were harvested at 16 and 22 weeks after grafting (at least three mice per time point per protocol). Host mice were killed by inhalation of CO2 and grafts were collected for fixation with Bouin's solution. The seminal vesicles of host mice were removed and weighed as an indication of the secretion of bioactive testosterone by the transplanted grafts (Honaramooz et al. 2002, 2004; Zeng et al. 2006). Harvested tissue grafts were embedded in paraffin wax and cut into sections. Sections were stained with haematoxylin and eosin. Slides were coded and the degree of spermatogenesis was evaluated in a blinded manner. Seminiferous tubule cross-sections were classified as described by Schlatt et al. (2002): (1) complete tubular atrophy and Sertoli cell-only tubules; (2) spermatogonia but no other germ cells present; (3) pachytene spermatocytes as the most advanced germ cells; (4) round spermatids as the most advanced germ cells; (5) elongated spermatids as the most advanced germ cells; and (6) mature spermatozoa present in the lumen of the tubule. For immunohistochemistry, slides were deparaffinised and incubated at room temperature in 1% BSA in PBS for 1 h to block the non-specific antibody binding. The monoclonal rabbit anti-PGP9.5 antibody (AbD Serotec, Raleigh, NC, USA) diluted to 1 : 200 in PBS containing 1% BSA was added to samples and incubated overnight at 4°C in a humidified chamber. The samples were rinsed twice in PBS for 5 min each time and treated for 30 min with Cy3-conjugated AffiniPure donkey anti-rabbit IgG (HþL) (Jackson ImmunoResearch, West Grove, PA, USA) as the secondary antibody diluted to 1 : 500 in PBS with 1% BSA. The samples were washed twice in PBS for 5 min each. The sections were mounted in VECTASHIELD mounting medium (VECTOR Laboratories, Burlingame, CA, USA) and analysed under a microscope.
Freezing of spermatozoa recovered from xenografts
Grafts were harvested 24−35 weeks after transplantation of either fresh or preserved (4°C, 48 h) testis tissue. Harvested grafts were cut into small pieces and minced carefully with two fine forceps in a Petri dish with 2 mL DMEM. Subsequently, the minced tissue was held for 60 min at room temperature and then filtered through a 40-μm Falcon Cell Strainer (Fisher Scientific, Pittsburgh, PA, USA) to remove tissue chunks. The spermatozoa harvested from grafts were frozen using a pellet technique (Zeng et al. 2001). Briefly, spermatozoa were suspended with BF5 extender. The spermatozoa were cooled to 5°C in 2 h and then an equal volume of BF5 extender containing 2% glycerol was added. The spermatozoa were frozen in pellets in dry ice and stored in liquid nitrogen. For thawing, two pellets were removed from the liquid nitrogen, placed in a plastic tube and held for 2 min at room temperature, then plunged into another tube containing 1 mL Beltsville thawing solution (BTS; IMV International, Minneapolis, MN, USA) prewarmed to 37°C. The viability of frozen–thawed spermatozoa was assessed using a sperm viability kit (Molecular Probes) under dual-colour fluorescence microscopy. At least 200 spermatozoa were counted for each sample (n = 3). For comparison, testicular spermatozoa recovered from mature porcine testis were frozen and analysed as described above (n = 4).
Statistical analysis
Before statistical analysis, all percentage data were subjected to arcsine transformation. Student's t-test was used to compare two groups and one-way ANOVA followed by Student–Newman– Keuls’ multiple-comparison test was performed to compare data in more than two groups. Data were analysed using Sigma Stat 3.0 (SPSS, Chicago, IL, USA). Data are expressed as the mean ± s.e.m. and P < 0.05 was considered significant.
Results
Total cell viability and germ cell viability after cooling and cryopreservation
Total cell viability was assessed by Trypan blue exclusion in single cell preparations after enzymatic digestion of the preserved testis fragments (Fig. 1). During storage at 4°C, viability remained high for 48 h, but was significantly decreased after 72 h. There was no significant difference in cell viability among the three freezing protocols or between the freezing protocols and cooling up to 48 h.
Fig. 1.
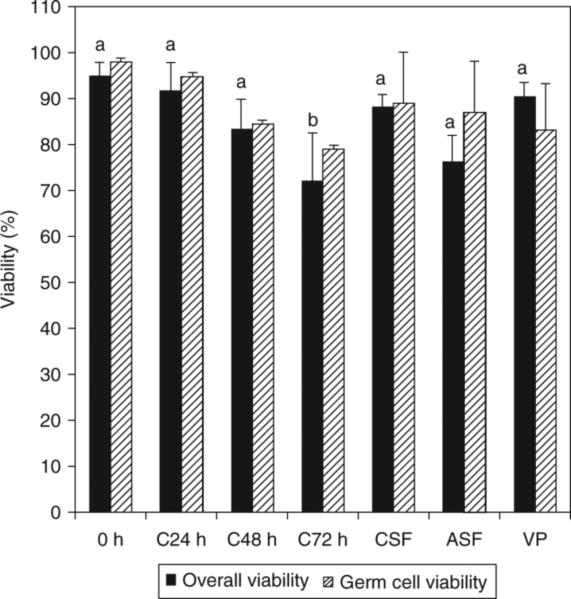
Total cell viability and germ cell viability of testis tissue. 0 h, fresh testis tissue before preservation; C24 h, C48 h, C72 h, testis tissue stored at 4°C for 24, 48 or 72 h, respectively; CSF, testis tissue cryopreserved by conventional slow-freezing; ASF, testis tissue cryopreserved by automated slow-freezing; VP, testis tissue cryopreserved by vitrification. Results are presented as the mean ± s.e.m. of at least three replicates for each treatment. Means with different letters differ significantly (P < 0.05).
Propidium iodide and PGP9.5 immunocytochemistry were used to specifically assay the viability of germ cells after cooling, freezing and thawing. Cells expressing PGP9.5 that did not take up PI were considered live germ cells. The percentage of live germ cells remained high after preservation. No significant difference in post-thaw germ cell viability was observed among the different preservation protocols (Fig. 1).
Based on these results, tissue preserved by cooling for 48 h and by cryopreservation with the three different methods was grafted to host mice to assay its potential to develop and initiate spermatogenesis in vivo.
Evaluation of testis graft survival and growth
Growth of testis tissue grafts was easily observed under the back skin of the recipient mice. Testis graft survival was defined as the percentage of grafts recovered after host mice had been killed. At 16 weeks after transplantation, testis graft survival for cooling, CSF, ASF and vitrification was 75.0 ± 14.4%, 75.0 ± 17.2%, 50.0 ± 22.4% and 80.0 ± 20.0%, respectively. There was no significant difference between the four protocols. At 22 weeks after transplantation, the graft survival rate was significantly lower after ASF (6.3 ± 6.3%) than after cooling (55.0 ± 7.1%). The graft survival rate 22 weeks after transplantation was 50.0 ± 14.4% and 36.0 ± 15.1% after CSF and VP, respectively.
Evaluation of spermatogenesis in xenografts
Histology of the testis tissue at the time of grafting and of recovered grafts is shown in Fig. 2. At the time of grafting, the testis tissue consisted of seminiferous cords that contained Sertoli cells and gonocytes as the only type of germ cell present, as well as interstitial tissue (Fig. 2). At 16 weeks after grafting of testis tissue preserved by cooling for 48 h, complete spermatogenesis and mature spermatozoa were observed in approximately 25% of seminiferous tubules, whereas the remaining tubules contained pachytene spermatocytes or spermatogonia as the most advanced germ cell stage (Fig. 3). The morphology of germ cells from all stages, Sertoli cells and Leydig cells was indistinguishable from the appearance of these cells observed in intact testes, as well as in xenografts of fresh testis tissue at the same time point. At 22 weeks after transplantation, the number of seminiferous tubules with spermatids as the most advanced germ cell stage had increased to 33.7% (range 5.2−71.4%). Spermatozoa were seen in approximately 10% of seminiferous tubules (Fig. 3).
Fig. 2.
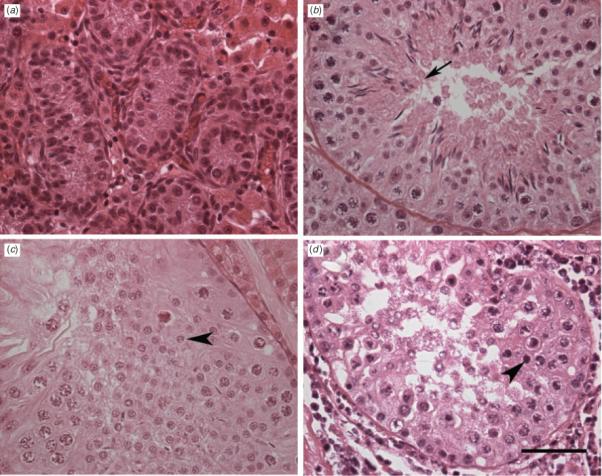
Histological appearance of preserved porcine testis tissue before and after grafting into recipient mice. (a) Testis tissue before grating; (b) 16 weeks after grafting from cooling preserved tissue; (c) 22 weeks after grafting from automated slow-freezing; (d) 22 weeks after grafting from vitrification. The arrow indicates an elongated spermatid, whereas the arrowhead indicates a round spermatid. Scale bar = 50 μm.
Fig. 3.
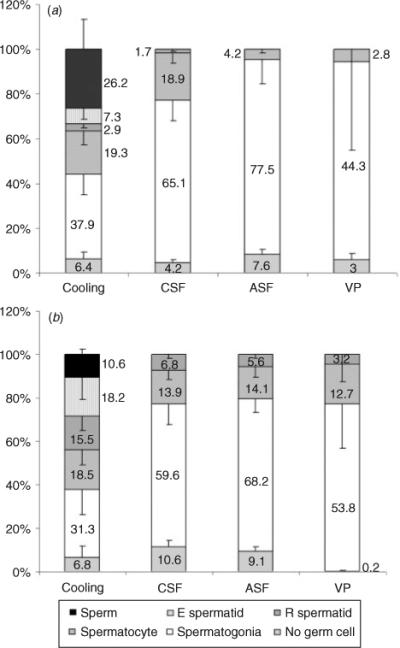
Relative abundance of seminiferous tubules containing the indicated germ cell type as the most advanced stage in xenografts at (a) 16 and (b) 22 weeks after transplantation. Results are presented as the mean ± s.e.m. of at least three mice per preservation protocol.
At 16 weeks after grafting of tissue that had been preserved by CSF, 18.9% (range 0−34.6%; Fig. 3) of seminiferous tubules contained pachytene spermatocytes as the most advanced germ cell stage. Spermatogenesis had progressed beyond meiosis in <5% of seminiferous tubules at 16 weeks and in approximately 7% of seminiferous tubules by 22 weeks (Fig. 3a, b).
For tissue preserved by ASF, 16 weeks after grafting pachytene spermatocytes were the most advanced germ cell stage in a few seminiferous tubules (range 0−12.2%), whereas more than two-thirds of seminiferous tubules contained only spermatogonia. At 22 weeks after grafting, the percentage of seminiferous tubules with pachytene spermatocytes as the most advanced germ cell stage had increased to 14.1 ± 3.8%. Round spermatids were found in approximately 5% of seminiferous tubules (Figs 2, 3).
For tissue preserved by vitrification, 16 weeks after grafting few seminiferous tubules contained pachytene spermatocytes as the most advanced germ cell stage (range 0−16.7%). Seminiferous tubules with pachytene spermatocytes increased to 12.7 ± 5.6% 22 weeks after grafting. Few seminiferous tubules (3.2 ± 3.2%) were found with round spermatids as the most advanced germ cell stage (Figs 2d, 3a, b).
The number of PGP9.5-positive germ cells per seminiferous tubule in the xenografts at 16 weeks after transplantation for cooling, CSF, ASF and VP was 12.2 ± 1.5, 8.4 ± 0.8, 6.8 ± 1.0 and 5.6 ± 0.8, respectively. The number of germ cells was significantly different between cooling and VP, but differences were not significant between cooling and CSF and ASF or between CSF and ASF and VP.
Cryopreservation of spermatozoa recovered from testis xenografts
The viability of frozen–thawed spermatozoa harvested from porcine testis xenografts was 47.4 ± 1.2% (n = 3). In comparison, the viability for testicular spermatozoa recovered from boar testis was 73.2 ± 2.5% (n = 3) after freeze–thawing.
Discussion
Cooling and cryopreservation could maintain the developmental potential of immature porcine testis tissue, but better results were observed after cooling even though the cell viability of testis tissue was equally high in the cryopreserved testis tissue after thawing. Therefore, it is important to evaluate germ cell differentiation in addition to cell viability when optimising freezing protocols for testis tissue.
Preservation of testis tissue has the advantage of providing a potentially inexhaustible source of spermatozoa and may be an option for preserving germ cells from prepubertal males. In practice, short- and long-term storage of testis tissue may be required before transplantation. To critically compare different preservation protocols, we chose cooling and three freezing protocols for the present study. Automated freezing protocols (Schlatt et al. 2002; Shinohara et al. 2002; Keros et al. 2005; Kvist et al. 2006; Jahnukainen et al. 2007; Milazzo et al. 2008) and CSF (Honaramooz et al. 2002; Milazzo et al. 2008) have been used in previous studies on the cryopreservation of testis tissue. Vitrification of testis tissue has not been reported. Therefore, in a preliminary study, we tested different basal media, cryoprotectants and concentrations of cryoprotectants for vitrification (data not shown) to select the best vitrification protocol for the present study. At present, the different approaches to freezing tissues have not been optimised individually, so it may be premature to conclude that conventional cryopreservation is necessarily superior to vitrification, although is seems that conventional protocols tended to yield better results than vitrification.
Testis tissue is composed of germ cells, and different types of somatic cells, including Sertoli cells, Leydig cells, peritubular myoid cells, vasculature and interstitial connective tissue. Because different cell types have different requirements during freezing and thawing (Nogueira et al. 1999) and the cryopreservation of tissue generally requires greater permeation of cryoprotectants than freezing of single cell suspensions (Leibo and Mazur 1971), freezing of testis tissue is more challenging because of its complicated structure (Wyns et al. 2007). In mice, the birth of offspring has been obtained following transplantation of cryopreserved immature testis tissue and in vitro microinsemination using spermatozoa harvested from the grafts (Shinohara et al. 2002). However, because the relationship between the walls of seminiferous tubules, Leydig cells and blood and lymph vessels varies among species (Setchell 1978), the freezing protocol for mouse testis tissue may not be suitable for tissues from other mammals. The structural characteristics of human testis tissue were maintained after being frozen in a programmable freezer (Keros et al. 2005, 2007; Kvist et al. 2006). However, none of these previous studies assessed the in vivo spermatogenic potential of the tissue after thawing. Fresh and preserved rhesus monkey testis tissue survived and initiated spermatogenesis to spermatocytes after xenografting into mouse host (Jahnukainen et al. 2007). Similarly, in the present study, porcine testis tissue that was either preserved at 4°C or cryopreserved survived and germ cells differentiated further to spermatozoa or to the spermatid stage after transplanting into mouse hosts. However, the developmental potential of germ cells was low for the cryopreserved tissue compared with germ cells in tissue preserved by cooling.
There may be several reasons for the limited development of cryopreserved testis tissue after grafting. In order to allow cryoprotectant to penetrate the tissue, small tissue fragments were prepared. This resulted in a limited number of seminiferous tubules in each preserved tissue fragment and may have increased variability between fragments. It is also possible that residual cryoprotectant remained in the frozen–thawed tissue after washing that could have led to tissue toxicity. Some damage to the tissue after freezing and thawing cannot be avoided. As such, the frozen–thawed testis tissue may take longer to become revascularised after grafting and to initiate spermatogenesis compared with fresh or cooled tissue. This may explain why, at the time points analysed in the present study, no spermatozoa were observed in the xenografts that were cryopreserved before transplantation. This observation is in agreement with a previous report that cryopreservation delayed the initiation of spermatogenesis in grafted testis tissue from rhesus monkeys (Jahnukainen et al. 2007).
Interestingly, post-thaw overall viability and germ cell viability were high for all three cryopreservation protocols investigated in the present study. However, the spermatogenic potential of the cryopreserved testis tissue was very limited. Accordingly, Frederickx et al. (2004) reported that the functional capacity of spermatogonial stem cells may be severely damaged during cryopreservation despite an acceptable viability of the cell suspension observed after thawing. Cell viability determined by Trypan blue staining of a single cell suspension, as in the present study, may be higher than that in testis tissue fragments because dead cells may break down during the process of digestion of testis tissue into single cell suspensions and will therefore not be counted. In a recent study, Jahnukainen et al. (2007) did not observe changes in histological morphology in thawed monkey testis tissue at the time of transplantation compared with fresh or cooled grafts. Cell viability before grafting, as evaluated in the present study, or pregrafting tissue morphology could not reveal the later observed difference in the effect of cryoprotectants on germ cell differentiation. Therefore, previous reports and the present study confirm that grafting currently is the only functional assay to assess the developmental potential of testis tissue in vivo.
The present study demonstrates that the spermatozoa harvested from xenografts can be cryopreserved successfully. This technique makes testis tissue xenografting more applicable in the future. Spermatozoa harvested from xenografts can be stored and used at a later date to retain fertilisation potential for valuable or endangered individuals, and potentially even human patients. We reported recently that snap-frozen spermatozoa harvested from porcine testis tissue xenografts supported embryo development after intracytoplasmic sperm injection (ICSI; Honaramooz et al. 2008). However, xenograft-derived spermatozoa resulted in reduced embryo development after ICSI compared with fresh or frozen–thawed porcine testicular spermatozoa (Honaramooz et al. 2008). This lower fertility may be due to the fact that spermatozoa are not removed continuously from the xenografts as they would be from the testis in situ. Therefore, spermatozoa should be harvested from xenografts soon after complete spermatogenesis is present in the grafted tissue (24−35 weeks after grafting of newborn pig testis tissue to a recipient mouse). The pellet technique used in the present study is commonly used for the cryopreservation of porcine spermatozoa (Bwanga 1991) and should be more suitable than snap-freezing for fertility preservation. However, even normal porcine spermatozoa produced endogenously do not freeze well and often exhibit reduced fertility. Further studies should evaluate the fertility of the xenograft-derived spermatozoa frozen–thawed using the pellet technique.
Preservation of genetic material is significant for farm animals, wildlife conservation, valuable research models and also human medicine. Testis tissue grafting provides a new option to preserve the male germline. The preservation of testis tissue and spermatozoa from xenografts would expand the applicability of this novel model of testis tissue grafting.
Because autologous transplantation of germ cells harvested from prepubertal cancer patients before treatment carries a risk of transferring malignant cells back to the patient in case of leukaemia or malignancies with blood-borne metastases, the use of spermatozoa produced in xenografts for assisted fertilisation could theoretically provide an alternative option for the preservation of fertility without possible malignant relapse (Geens et al. 2006). The successful cryopreservation of spermatozoa harvested from xenografts provides a first step towards the practical application of this approach. However, ethical, legal and clinical issues need to be evaluated before the technique of testis tissue xenografting, and the preservation of testis tissue and spermatozoa recovered from xenografts, could be applied clinically in humans. Young children cannot give their consent for the procedure and an intrusive testis biopsy may cause future side effects in the developing testis (Bahadur et al. 2000; Hovatta 2001; Jahnukainen et al. 2006). There are also biological risks, such as the potential for contamination with unknown animal infectious agents, that will have to be addressed if spermatozoa harvested from xenografts are to be used for assisted fertilisation (Aslam et al. 2000; Geens et al. 2006).
Conclusion
In summary, our observations demonstrate that preservation of testis tissue at 4°C for 48 h does not impair cell viability, survival rate and developmental potential after transplanting into mouse host. Therefore, testis tissue can be stored or transported for 2 days without loss of spermatogenic potential. Spermatozoa harvested from xenografts can be cryopreserved for use in assisted reproduction at a later date. Cryopreservation of testis tissue maintained cell viability and supported limited spermatogenic differentiation after thawing; however, current protocols will have to be optimised to make this approach more efficient for practical application. The results of the present study suggest that preservation of testis tissue may serve as a promising technique for fertility preservation for immature males.
Acknowledgements
The authors thank Terry Jordan for animal care. This study was supported by a grant from the National Center for Research Resources (grant no. R01-RR17359-05).
References
- Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ERS, Dobrinski I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008;136:85–93. doi: 10.1530/REP-07-0433. doi:10.1530/REP-07-0433. [DOI] [PubMed] [Google Scholar]
- Aslam I, Fishel S, Moore H, Dowell K, Thornton S. Fertility preservation of boys undergoing anti-cancer therapy: a review of the existing situation and prospects for the future. Hum. Reprod. 2000;15:2154–2159. doi: 10.1093/humrep/15.10.2154. doi:10.1093/HUMREP/15.10.2154. [DOI] [PubMed] [Google Scholar]
- Bahadur G, Chatterjee R, Ralph D. Testicular tissue cryopreservation in boys. Ethical and legal issues: case report. Hum. Reprod. 2000;15:1416–1420. doi: 10.1093/humrep/15.6.1416. doi:10.1093/HUMREP/15.6.1416. [DOI] [PubMed] [Google Scholar]
- Bwanga CO. Cryopreservation of boar semen. I: a literature review. Acta Vet. Scand. 1991;32:431–453. doi: 10.1186/BF03546944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickx V, Michiels A, Goossens E, De Block G, Van Steirteghem AC, Tournaye H. Recovery, survival and functional evaluation by transplantation of frozen–thawed mouse germ cells. Hum. Reprod. 2004;19:948–953. doi: 10.1093/humrep/deh154. doi:10.1093/HUMREP/DEH154. [DOI] [PubMed] [Google Scholar]
- Geens M, De Block G, Goossens E, Frederickx V, Van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum. Reprod. 2006;21:390–396. doi: 10.1093/humrep/dei412. doi:10.1093/HUMREP/DEI412. [DOI] [PubMed] [Google Scholar]
- Goossens E, Frederickx V, Geens M, De Block G, Tournaye H. Cryosurvival and spermatogenesis after allografting prepubertal mouse tissue: comparison of two cryopreservation protocols. Fertil. Steril. 2008;89:725–727. doi: 10.1016/j.fertnstert.2007.03.044. doi:10.1016/J.FERTNSTERT.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. doi:10.1038/NATURE00918. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. doi:10.1095/BIOLREPROD.103.025536. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Cui XS, Kim NH, Dobrinski I. Porcine embryos produced after intracytoplasmic sperm injection using xenogeneic pig sperm from neonatal testis tissue grafted in mice. Reprod. Fertil. Dev. 2008;20:802–807. doi: 10.1071/rd08093. doi:10.1071/RD08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O. Cryopreservation of testicular tissue. Mol. Cell. Endocrinol. 2000;169:113–115. doi: 10.1016/s0303-7207(00)00363-4. doi:10.1016/S0303-7207(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Cryopreservation of testicular tissue in young cancer patients. Hum. Reprod. Update. 2001;7:378–383. doi: 10.1093/humupd/7.4.378. doi:10.1093/HUMUPD/7.4.378. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Cryobiology of ovarian and testicular tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2003;17:331–342. doi: 10.1016/s1521-6934(02)00125-6. doi:10.1016/S1521-6934(02)00125-6. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Schlatt S. Clinical potential and putative risks of fertility preservation in children utilizing gonadal tissue or germline stem cells. Pediatr. Res. 2006;59:40R–47R. doi: 10.1203/01.pdr.0000205153.18494.3b. doi:10.1203/01.PDR.0000205153.18494.3B. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum. Reprod. 2007;22:1060–1067. doi: 10.1093/humrep/del471. doi:10.1093/HUMREP/DEL471. [DOI] [PubMed] [Google Scholar]
- Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum. Reprod. 2005;20:1676–1687. doi: 10.1093/humrep/deh797. doi:10.1093/HUMREP/DEH797. [DOI] [PubMed] [Google Scholar]
- Keros V, Hultenby K, Borgström B, Fridström M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum. Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. doi:10.1093/HUMREP/DEL508. [DOI] [PubMed] [Google Scholar]
- Kuleshova LL, MacFarlane DR, Trounson AO, Shaw JM. Sugars exert a major influence on the vitrification properties of ethylene glycol-based solutions and have low toxicity to embryos and oocytes. Cryobiology. 1999;38:119–130. doi: 10.1006/cryo.1999.2153. doi:10.1006/CRYO.1999.2153. [DOI] [PubMed] [Google Scholar]
- Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum. Reprod. 2006;21:484–491. doi: 10.1093/humrep/dei331. doi:10.1093/HUMREP/DEI331. [DOI] [PubMed] [Google Scholar]
- Leibo SP, Mazur P. The role of cooling rates in low-temperature preservation. Cryobiology. 1971;8:447–452. doi: 10.1016/0011-2240(71)90035-6. doi:10.1016/0011-2240(71)90035-6. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol. Reprod. Dev. 2006;73:1531–1540. doi: 10.1002/mrd.20529. doi:10.1002/MRD.20529. [DOI] [PubMed] [Google Scholar]
- Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Masse L, Mousset-Simeon N, Mace B, Rives N. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum. Reprod. 2008;23:17–28. doi: 10.1093/humrep/dem355. doi:10.1093/HUMREP/DEM355. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Bourgain C, Verheyen G, Van Steirteghem AC. Light and electron microscopic analysis of human testicular spermatozoa and spermatids from frozen and thawed testicular biopsies. Hum. Reprod. 1999;14:2041–2049. doi: 10.1093/humrep/14.8.2041. doi:10.1093/HUMREP/14.8.2041. [DOI] [PubMed] [Google Scholar]
- Ohta H, Wakayama T. Full-term development of offspring using round spermatids produced ectopically from fetal male germ cells. J. Reprod. Dev. 2004;50:429–437. doi: 10.1262/jrd.50.429. doi:10.1262/JRD.50.429. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I. Germ cell fate and seminiferous tubule development in bovine testis xenografts. Reproduction. 2005;130:923–929. doi: 10.1530/rep.1.00912. doi:10.1530/REP.1.00912. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. doi:10.1530/REP.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol. Reprod. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. doi:10.1095/BIOLREPROD.102.014894. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The Mammalian Testis. Cornell University Press; Ithaca, NY: 1978. [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum. Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. doi:10.1093/HUMREP/17.12.3039. [DOI] [PubMed] [Google Scholar]
- Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J. Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology. 2000;53:1093–1103. doi: 10.1016/S0093-691X(00)00255-7. doi:10.1016/S0093-691X(00)00255-7. [DOI] [PubMed] [Google Scholar]
- Tumbleson ME, Schook LB. Advances in swine in biomedical research. In: Tumbleson ME, Schook LB, editors. Advances in Swine in Biomedical Research. Plenum Press; NewYork: 1996. pp. 1–4. [Google Scholar]
- Tuuri T, Moilanen J, Kaukoranta S, Makinen S, Kotola S, Hovatta O. Testicular biopty gun needle biopsy in collecting spermatozoa for intracytoplasmic injection, cryopreservation and histology. Hum. Reprod. 1999;14:1274–1278. doi: 10.1093/humrep/14.5.1274. doi:10.1093/HUMREP/14.5.1274. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum. Reprod. 2007;22:1603–1611. doi: 10.1093/humrep/dem062. doi:10.1093/HUMREP/DEM062. [DOI] [PubMed] [Google Scholar]
- Zeng WX, Shimada M, Isobe N, Terada T. Survival of boar spermatozoa frozen in diluents of varying osmolality. Theriogenology. 2001;56:447–458. doi: 10.1016/s0093-691x(01)00576-3. doi:10.1016/S0093-691X(01)00576-3. [DOI] [PubMed] [Google Scholar]
- Zeng W, Avelar GF, Rathi R, Franca LR, Dobrinski I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J. Androl. 2006;27:527–533. doi: 10.2164/jandrol.05143. doi:10.2164/JANDROL.05143. [DOI] [PubMed] [Google Scholar]
- Zeng W, Rathi R, Pan H, Dobrinski I. Comparison of global gene expression between porcine testis tissue xenografts and porcine testis in situ. Mol. Reprod. Dev. 2007;74:674–679. doi: 10.1002/mrd.20670. doi:10.1002/MRD.20670. [DOI] [PubMed] [Google Scholar]


