Abstract
OBJECTIVE
Our previous findings have shown that systemic administration of IL-1β induces up-regulation of NF-κB in mouse prostate tissue that may be responsible for leukocyte extravasation into prostate stroma. It has been hypothesized that NF-κB plays a role in the development of prostatitis, and that NF-κB activation might provide chemoattractive signals for leukocyte extravasation in the prostate.
METHODS
IL-1β was administrated intravenously, alone or with dexamethasone, to separate groups of C57BL/6J mice. Expression of NF-κB, chemoattractant receptors, and IL-17F in the two groups of mouse prostates were evaluated and compared.
RESULTS
IL-1β administration up-regulated NF-κB/p65 activity in the mouse prostate. IL-1β administration promoted extravasation and accumulation of CD45+ mononuclear cells but not neutrophils in the mouse prostate stroma. IL-1β administration provided earlier (4 h) CXCR1/IL-8RA receptor expression in mouse prostate as a first signal, inducing capillary homing, adhesion and initial extravasation of mononuclear cells into the prostate tissue. IL-1β administration also induced relatively late (24 h) up-regulation of VCAM1 in the endothelial cells of microvessels and of IL-17F in prostate epithelium and in stromal infiltrating leukocytes. Concomitant administration of dexamethasone, a NF-κB inhibitor, resulted in significantly down-regulated IL-1β-induced NF-κB/p65 activity, as well as reduced expression of chemokine receptors and IL-17F in mouse prostate.
CONCLUSION
Systemic IL-1β administration induces aberrant NF-κB/p65 activity, which may be critical in the development of prostatitis through its role in the production of chemoattractant signals promoting extravasation and stromal accumulation of mononuclear cells and initiation of a new wave of pro-inflammatory signals favorable to chronic inflammation.
Keywords: NF-κB, Prostate, IL-1β, CXCR4, IL-17F, Mouse
INTRODUCTION
Leukocyte infiltration and accumulation at the site of specific lesions is a characteristic of any type of inflammation. The recruitment, retention, and survival of inflammatory cells is influenced by a balance between the functional chemokine receptor expression by inflammatory leukocytes and locally expressed chemokines within the lesions. It is now recognized that pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-15, IL-17, and interferon-γ exhibit constitutive secretion by immune and non-immune cells at high levels at the site of prostatic inflammation [1-3]. The control of cytokine production and associated signal transductions may play important roles in the development of chronic inflammation and neoplastic conditions. However, the mechanisms and biologic effects of cytokines in the development and maintenance of chronic inflammation and in the development and progression of cancer are currently not well understood [4].
Nuclear-Factor kappaB (NF-κB) is an inducible transcription factor of the Rel family, sequestered and inactivated in the cytoplasm by the IκB family of proteins. Ultraviolet radiation, bacterial lipopolysaccharide, viral gene products, and inflammatory cytokines promote IκB degradation in cytoplasm, thereby allowing NF-κB to enter the nucleus and induce gene transcription. It has been shown that activation of NF-κB is one of the earliest events in the genesis of chronic inflammation [5] and human prostate cancers [6]. In this connection, it is worth noting that pro-inflammatory cytokines such as TNFα, IL-1, IL-6 and IL-8, all of which are encoded by target genes of the IKK-β-dependent NF-κB-activation pathway, are associated with tumor development and progression in humans and mice [7]. Thus, it appears probable that NF-κB provides a mechanistic link between inflammation and cancer [8].
Previously we have demonstrated that IL-1β causes NF-κB activation in the mouse prostate in vivo and ex vivo, resulting in leukocyte traffic from prostate microvessels into the stroma, followed by the stromal accumulation of mononuclear cells at 8 and 24 h after IL-1β administration in transgenic NF-κB-Luciferase Tag mice coupled to the luciferase reporter gene [9]. Based on these findings, we hypothesized that NF-κB plays a role in the development of prostatic inflammation, and that NF-κB activation may induce a cascade of unknown chemoattractive signals for leukocyte emigration from capillaries into prostate stroma. In the current study, we repeated the protocol used in our previous study on transgenic NF-κB-Luciferase Tag mice, but instead used wild type C57BL/6J mice. Levels of intraprostatic NF-κB activation, expression of IL-17F, levels of chemokine receptors, and leukocyte phenotype in the prostate tissue were measured at 4, 8, and 24 h after i.v. injection of mouse recombinant IL-1β.
MATERIAL AND METHODS
Animals
Forty nine C57BL/6J male mice 24−26 weeks old with body weights of 28−30 g were subjected to a time-course study and were sacrificed at 4, 8, and 24 h after cytokine administration. The animals were bred and maintained at the AAALAC-accredited Animal Resource Facility of Case Western Reserve University.
Cytokine
Based on our previous findings that IL-1β causes high levels of NF-κB activation in the mouse prostate [9], the mouse rIL-1β (10 μg/kg), (R&D System, Minneapolis, MN) was used to induce NF-κB activity by 30 μL i.v. injections in the tail vein. In some experiments mice were pretreated with dexamethasone (Dex) (10 mg/kg in PBS) by i.p. injection.
Immunohistochemistry
The intraprostatic expression of chemokine receptors, adhesion molecules, surface cell markers, and interleukin in formalin-fixed paraffin-embedded prostate tissue was evaluated using the following markers: for chemokine receptors - CXCR1/IL-8RA (Santa Cruz Biotechnology, Santa Cruz, CA) and CXCR4 (R&D System, Minneapolis, MN); for adhesion molecules - VCAM1 (Santa Cruz); for neutrophils - NIMP-R14 (Santa Cruz); for mononuclear cells (MNC) - CD45 (R&D System); and for IL-17F - (R&D System). Immuno-histochemical staining for the markers mentioned above was performed using reagents and protocols from Biocare Medical (Concord, CA). Briefly, 4-μm thick prostate sections were deparaffinized, rehydrated, immersed in target Reveal™ solution (Biocare Medical, Concord, CA), and underwent heating antigen retrieval in a presser cooker chamber. The sections were blocked with PEROXIdazed 1 for endogenous peroxidase activity and BACKGROUNDsniper (both from Biocare Medical) to reduce nonspecific background staining, and incubated in primary monoclonal antibodies for 1 h at room temperature. Control sections were incubated with anti-sera in the presence of tenfold excess of these antibodies or with isotype-matched IgG normal goat serum. After washing three times in TBS, sections were incubated for 30 min at room temperature with biotinylated secondary antibody. Immunoreactive complexes were detected using ABC System (Santa Cruz). Slides were then counterstained in Gill's #3 hematoxylin, mounted in crystal mount media, and dried overnight.
NF-κB activity
The freshly frozen mouse prostates were melted and homogenized using FastPrep-24 Instrument (BIO101Systems FastPrep® homogenizer, MP Biomedicals, Solon, OH) with complete lysis buffer (Active Motif, Carlbad, CA) with two cycles for 20 sec at 6 m/sec at 40C. After centrifuging at 10,000g × 10 min at 40C the levels of NF-κB p65 activity were detected by ELISA in supernatants adjusted to levels of total protein. The commercially available Trans-AM™ NF-κB assay kit was obtained from Active Motif North America (Carlsbad, CA) and used for assay of NF-κB/p65 activity according to the vendor's protocol.
Prostate histology
Routine H&E-stained histological sections of prostatic tissues were evaluated for the presence of intra-prostatic leukocyte traffic as a marker of inflammation in these mice. Histological findings were correlated with NF-κB activation status.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) with 95% confidence limits. Data are presented in the figures as mean ± SD.
RESULTS
Systemic administration of IL-1β leads to extravasation of mononuclear cells into mouse prostate tissue
In the routine H/E staining analysis of leukocyte trafficking in prostate tissue, we noted abnormally high numbers of mononuclear cells in microvessels 4 h after a single systemic administration of 10 μg/kg of IL-1β (Figure 1). The cells were positioned along the endothelium of capillaries and stained positive for the CD45 lymphocyte marker but negatively for neutrophil NIMP-R14 antigen.
Figure 1.
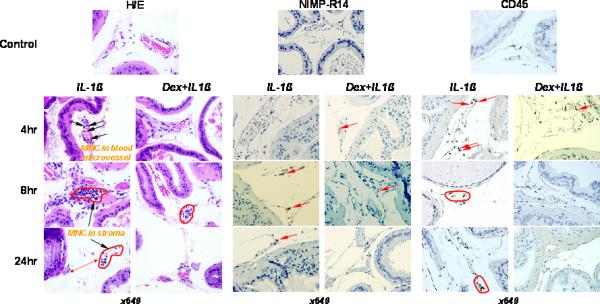
Routine histology (H&E-staining) to detect mononucler cells (MNC) and immunostaining for NIMP-R14 (neutrophils) and CD45 (lymphocytes) in representative samples of prostates from C57BL/6J mice. IL-1β (10 μg/kg) was i.v. injected in the tail vein. To proof pro-inflammatory ability of IL-1β along with NF-κB activation in prostate tissue, some animals were i.p. injected with dexamethasone (Dex) (10 mg/kg) one hour before IL-1β treatment. Animals were sacrificed at 4, 8, and 24 h after cytokine administration. There was accumulation and adhesion of MNC in prostatic microvessels after IL-1β treatment at 4 h (arrowhead). Extravasation and different degrees of stromal accumulation of mononuclear cells (MNC) near capillaries was found at 8 h of study (red circles). Pretreatment with Dex was able to abrogate the distant scattered stromal accumulation of MNC in IL-1β treated mice at 24 h (red circle and dotted double head arrow). Scanty extravasated neutrophils were detected in prostatic stroma at 4, 8 and 24 h after cytokine administration (red arrowhead). Pretreatment with Dex was able completely reduce neutrophil homing and extravasation. Predominance of CD45+ lymphocytes among intra-prostatic leukocytes was observed at all three time points of the study. Majority of lymphocytes were localized inside of the capillaries at 4 h after IL-1β administration (red arrowhead). The predominance of CD45+ cells in prostate stroma was a characteristic of the leukocyte extravasation at 8 and 24 h after IL-1β treatment (red zones). Dex had significant negative impact to the IL-1β-inducible extravasation and prostate stromal accumulation but not homing of the CD45+ lymphocytes (red arrowhead).
Following 8 h of IL-1β administration, perivascular stromal mononuclear cell accumulation was clearly visible in H/E slides. Stromal aggregates of mononuclear cells distant from microvessels were observed 24 h later in mouse prostates. These aggregates of cells were positive for the CD45 lymphocyte marker. In contrast, only rare isolated NIMP-R14-positive neutrophils were identified in the prostatic stroma 8 and 24 h after IL-1β administration (Figure 1).
Concomitant administration of dexamethasone, a well known NF-κB inhibitor [10], resulted in significantly reduced leukocyte extravasation in IL-1β treated mice (Figure 1). Therefore, it seems likely that there is a mechanistic relationship between the elevated levels of pro-inflammatory IL-1β in the systemic circulation and blood mononuclear cell extravasation into prostate tissue. This hypothesis is supported by our observation of prominent adhesion of CD45+ lymphocytes to microvessel endothelium 4 h after IL-1β injection, followed by their accumulation in the prostatic stoma 24 h after this injection (Figure 1).
Systemic administration of IL-1β up-regulates activity of cytosolic NF-κB/p65 in mouse prostate
In order to determine whether systemic IL-1β administration has a direct impact on intraprostatic NF-κB activity in the mouse prostate, we measured total levels of NF-κB/p65 in prostate tissue of IL-1β treated mice. Figure 2a shows that IL-1β administration doubled the total amount of NF-κB/p65 in the prostate tissue. The highest levels of NF-κB/p65 were detected 4 h after IL-1β administration, results that were concordant with the findings in our previous studies on NF-κB Luciferase mice [9].
Figure 2.
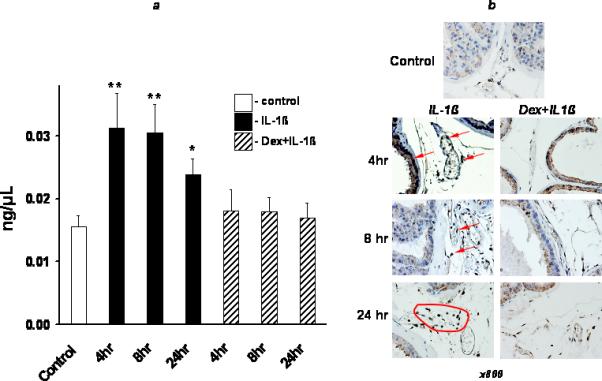
Assessment of prostate tissue NF-κB activity after single IL-1β administration in C57BL/6J mice. IL-1β (10 μg/kg) was i.v. injected in the tail vein. To proof pro-inflammatory ability of IL-1β along with NF-κB activation in prostate tissue, one hour before IL-1β administration some animals were i.p. injected with dexamethasone (Dex) (10 mg/kg). Animals were sacrificed at 4, 8, and 24 h after cytokine administration. a) Data represent the mean value of NF-κB concentration in whole extract of prostate tissue. White bar – control (no treatment); black bars – IL-1β only; strip bars – animals were pretreated with Dex one hour before IL-1β administration. The SD is indicated. * - p<0.05; ** - p<0.01 compared with controls. b) Immunostaining for NF-κB/p65 in representative samples from C57BL/6J mice. The strongest nuclear expression of NF-κB (brown color) was observed in prostate epithelium at 4 h (red arrowheads) and in stromal scattered mononuclear cells (red zone) at 24 h after IL-1β administration. The staining was significantly diminished by Dex. See Material & Methods for details.
In parallel experiment the prostate tissue of IL-1β treated mice was subjected to immunostaining for NF-κB/p65 to evaluate its localization in prostate cells. The most studied form of the NF-κB transcription factor is the heterodimer formed by the p50 and RelA (p65) proteins [11, 12]; it is expressed in most cells but is kept inactive and is maintained in the cytoplasm by interaction with its inhibitor IκB-α. Normally, activation of NF-κB requires signals that lead to the phosphorylation-, ubiquitination- and proteasome-dependent degradation of IκBα. NF-κB then translocates to the nucleus and activates the expression of various genes which may be involved with various pathological conditions including toxic/septic shock, graft versus host disease, acute inflammatory conditions, acute phase response, viral replication, radiation damage, atherosclerosis, and cancer [13]. Figure 2b shows that basic intraprostatic levels of NF-κB/p65 (control) were consistent with a non-functionally active cytosolic fraction. The levels NF-κB/p65 were drastically increased after IL-1β administration. The strongest nuclear expression of functionally active NF-κB/p65 was observed in prostatic epithelial cells, blood and extravasated leukocytes 4 h after IL-1β administration. There was no NF-κB activity in the nuclei of the prostate epithelial cells after 24 h. In contrast, the nuclear expression of NF-κB/p65 was still easily observed in stromal infiltrating leukocytes 8 h and 24 h after IL-1β administration (Figure 2b).
Furthermore, pretreatment with dexamethasone significantly reduced the activity of IL-1β-induced NF-κB/p65 both in prostate tissue and blood-originated mononuclear cells. Indeed, the activity of NF-κB/p65 was almost normal 4 hr after cytokine treatment (Figure 2).
Systemic administration of IL-1β induces multitype time-dependent behavior of intraprostatic chemoattractant receptors and IL-17F
To determine possible intraprostatic mechanistic relationships between IL-1β-induced NF-κB activity and signals that attract immune-competent cells into peripheral tissues and set cellular immune responses in motion, we studied levels of intra-prostatic expression of CXCR4, CXCR1/IL-8RA, VCAM1, and IL-17F molecules in IL-1β treated mice. In prostate tissue of untreated mice (Figure 3), no anti-CXCR1/IL-8RA or anti-IL-17F reactivity was detected. Both stromal and epithelial cells revealed a complete lack of signal. The prostatic epithelium CXCR1/IL-8RA expression was already significant 4 h after cytokine administration but it completely vanished 24 h after injection of IL-1β. Figure 3 shows that CXCR1/IL-8RA receptor was expressed in prostate epithelium at the highest levels 8 h after IL-1β administration.
Figure 3.
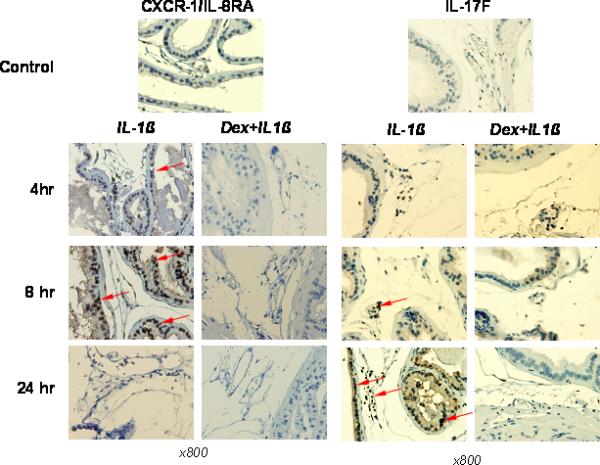
Immunostaining for chemoattractant receptor CXCR1/IL-8RA and IL-17F in representative samples of prostates from C57BL/6J mice. IL-1β (10 μg/kg) was i.v. injected in the tail vein. To proof pro-inflammatory ability of IL-1β along with NF-κB activation in prostate tissue, some animals were i.p. injected with dexamethasone (Dex) (10 mg/kg) one hour before IL-1β administration. The animals were sacrificed at 4, 8, and 24 h after cytokine administration. The strongest staining pattern for CXCR1/IL-8RA (red arrowheads) was observed in prostate epithelium and leukocytes at 4 h and especially 8 h after L-1β treatment. Dex was able almost completely abolish of IL-1β-dependent up-regulation of CXCR1/IL-8RA in both prostate tissue and haematopoietic cells. In contrast with CXCR1/IL-8RA, up-regulation of IL-17F expression in prostate tissue and leukocytes was the most strong at 24 h after IL-1β treatment (arrowhead). Dex pretreatment was able completely abrogate IL-1β-dependent IL-17F expression in mouse prostate.
In contrast with prostate tissue, only blood-originated mononuclear cells showed IL-17F reactivity 4 h after IL-1β administration (Figure 3). The IL-17F protein expression increased gradually with time and was strongest 24 h after cytokine administration. Strong signals were detected from both intravascular and stromal mononuclear cells as well as from prostatic epithelial cells at this time (Figure 3). Pretreatment with dexamethasone resulted in complete inhibition of expression of CXCR1/IL-8RA receptor in mouse prostate at any time point studied. The effect of dexamethasone administration on IL-17F expression was less pronounced, but was sufficient to abolish IL-17F protein expression both from blood-originated mononuclear cells and prostatic epithelial cells 24 h after cytokine administration (Figure 3).
Next we determined the expression of VCAM1 after IL-1β administration in the prostate tissue. No anti-VCAM1 reactivity was detected in the prostates at 4 h and 8 h after IL-1β administration (Figure 4). Weak expression of VCAM1 was observed in microvessel endothelial cells and prostatic epithelial cells 24 h after cytokine treatment. As Figure 4 shows, dexamethasone pretreatment completely eliminated intraprostatic expression of these molecules at any time point studied after cytokine administration.
Figure 4.
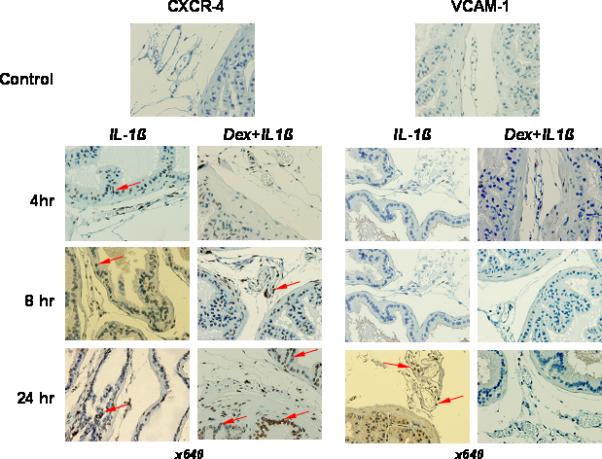
Immunostaining for chemoattractant receptor CXCR4 and adhesion molecules (VCAM1) in representative samples of prostates from C57BL/6J mice. IL-1β (10 μg/kg) was i.v. injected in the tail vein. To proof pro-inflammatory ability of IL-1β along with NF-κB activation in prostate tissue, some animals were i.p. injected with dexamethasone (Dex) (10 mg/kg) one hour before IL-1β administration. The animals were sacrificed at 4, 8, and 24 h after cytokine administration. Up-regulation of CXCR4 expression in prostate tissue and leukocytes was detected at all three time points after IL-1β treatment and was super-induced by Dex (arrowhead). Focal VCAM1 stained capillary's endothelium was observed only at 24 h (red arrowhead). Dex pretreatment was able significantly reduce IL-1β-dependent VCAM1 expression in mouse prostate.
The expression pattern of CXCR4 in prostate was similarly evaluated in mice pretreated with dexamethasone before IL-1β administration and in mice injected with IL-1β alone. Intraprostatic expression of CXCR4 was not detected in untreated mice. Minimal time-independent CXCR4 expression was observed in IL-1β treated mice (Figure 4). However, the pattern of CXCR4 receptor expression in IL-1β treated mice was unusual in that pretreatment with dexamethasone was associated with overexpression of CXCR4 with strong intracellular staining in prostatic epithelial cells as well as in mononuclear cells in the prostatic stroma and lumens of prostate microvessels (Figure 4). This phenomenon has been observed and reported by other investigators [14, 15, 16].
Data concerning levels of intraprostatic protein expression are summarized in Table 1, which allow some insights regarding signals that may possibly be responsible for the extravasation of leukocytes with high NF-κB activity into prostate tissue. The highest IL-1β-induced up-regulation of NF-κB activity at 4 h after cytokine administration correlated with the strongest signal of CXCR1/IL-8RA receptor at 8 h after IL-1β treatment. Subsequently, IL-17F and VCAM1 expression was highest at the 24 h time point (Table 1). In contrast with all other marker studied dexamethasone pretreatment induced overexpression of CXCR4, especially at 24 h time point, a finding noted by other investigators as well.
TABLE-1.
Levels of expression of nuclear factor-κappaB (NF-κB), interleukin (IL)-17F, chemoattractant and adhesion molecules in mouse prostate tissue after systemic IL-1β administration
| NF-κB/p65 | CXCR1/IL-8RA | IL-17F | VCAM1 | CXCR4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | IL-1β + DEXb | IL-1β | IL-1β + DEX | IL-1β | IL-1β + DEX | IL-1β | IL-1β + DEX | IL-1β | IL-1β + DEX | |
| 4 hra | +++ | + | ++ | − | + | + | − | − | + | + |
| 8 hr | ++ | + | +++ | − | ++ | + | − | − | + | ++ |
| 24 hr | + | − | − | − | +++ | − | + | − | ++ | +++ |
Note:
time after mouse rIL-1β i.v. administration (10 μg/kg)
dexamethasone (10 mg/kg) was injected i.p. one hour before IL-1β administration
DEX – dexamethasone; + − low; ++ − mild; and +++ − high expression of markers studied in prostate tissue and/or intraprostatic mononuclear cells. VCAM1 – vascular cell adhesion molecule – 1; CXCR1 and 4 - chemokine (C-X-C motif) receptor – 1 and 4; IL-8RA – IL-8 receptor alpha.
Taken together, our studies indicate that NF-κB may play a central role in the genesis of prostatic inflammation. Our findings suggest that IL-1β-induced NF-κB activity may be responsible for a series of events that are favorable for the intraprostatic accumulation of stromal mononuclear cells.
DISCUSSION
Recent investigations have advanced the concept that inflammation is an important component in carcinogenesis, and have supported the notion that anti-inflammatory therapies may be of adjunctive value in the prevention and treatment of various malignancies. In this regard, the NF-κB pathway has gained attention as an attractive avenue for drug discovery and development [17]. NF-κB is a family of ubiquitously expressed transcription factors that are widely believed to trigger both the onset and the resolution of inflammation. NF-κB also governs the expression of genes encoding proteins essential in control of stress response, maintenance of intercellular communications, and regulation of cellular proliferation and apoptosis. Our previous in vivo study showed that elevated levels of IL-1β in the systemic circulation may contribute to direct NF-κB activation in the prostate of NF-κB luciferase transgenic mice [9]. This intraprostatic up-regulation of NF-κB activity was accompanied by traffic of leukocytes from intraprostatic microvessels into the prostate stroma (unpublished data). In the present study, the phenomenon of rapid extravasation of blood leukocytes followed by their accumulation in the prostate stroma after IL-1β administration was easily reproduced in C57BL/6J wild type mice (Figure 1).
Previous studies have shown that NF-κB is a central regulator of the epithelial cell innate immune response to infection with entero-invasive bacteria [18]. Leukocyte mobilization is one of the phylogenetically oldest, most rapid and most consistent innate immune responses after activation of pattern-recognition Toll-like receptors by bacteria and other invading organisms [19]. Our current study documents the IL-1β-induced traffic of CD45+ mononuclear cells, which rapidly extravasated from prostate capillaries and accumulated in prostatic stromal tissue. After IL-1β injection, we found only rare isolated NIMP-R14-positive neutrophils in the prostatic stroma; the predominant inflammatory cells in this setting were, as noted, CD45+ mononuclear cells.
In concordance with our previously reported data, [9] NF-κB/p65 up-regulation was highest 4 h after IL-1β injection. In prostatic epithelial cells, IL-1β-induced transcription of NF-κB/p65 and nuclear staining for NF-κB/p65 appeared to be rapid, intensive, but transient, having disappeared 24 h post-injection. However, staining for NF-κB/p65 in the nuclei of prostatic mononuclear cells was intense and steady up to 24 h post-injection of IL-1β.
IL-8 has diverse functions in immune surveillance, inflammation, and angiogenesis. It has been reported that NF-κB stimulates IL-8 production [21]. IL-8 has been documented to modulate trans-endothelial T cell migration into certain inflammatory skin lesions in vivo [22-24] and monocyte-endothelial adhesion in vitro [25, 26]. IL-8 is predominantly recognized as a chemoattractant of neutrophils and/or lymphocytes [27]. IL-8–mediated cellular responses are affected by its two high-affinity cell surface receptors, CXCR1 and CXCR2, to which IL-8 cross-links, with resultant mediation of leukocyte recruitment and activation [28, 29, 30]. CXCR1 is selective for IL-8, whereas CXCR2 also binds other chemokines, including growth-related oncogene (CXCL1), epithelial cell-derived neutrophil attractant 78 (CXCL5), and neutrophil-activating peptide 2 (CXCL7) [31, 32]. IL-8 (and its CXCR1 receptor) is a well known pro-inflammatory chemokine that participates in chronic prostatic inflammation and is a marker thereof [33, 34]. Currently, there are no published studies on the involvement of NF-κB in IL-1β-induced chemokine release from prostate cells or traffic of blood leukocytes into prostate stroma. We found that the highest expression of CXCR1/IL-8RA receptors, 8 h following IL-1β injection, occurred after the peak in NF-κB/p65 activity, which was observed 4 h after IL-1β injection. Our data documenting abnormal IL-1β-induced NF-κB activity in prostatic epithelial cells and stromal mononuclear cells, followed by up-regulation of IL-8 chemokine receptor CXCR1/IL-8RA, suggests that IL-8 plays a significant role in leukocyte trafficking from prostatic microvessels into the prostatic stroma.
IL-17-producing cells are a newly recognized subset of CD4+ (helper) T cells, designated TH17 cells. Whereas all CD4+ T cell subtypes are known to play a role in immune-mediated defense against intracellular or extracellular pathogens, TH17 cells are unique in that they are the key mediators in a number of autoimmune diseases, and may play a role in inflammation-associated cancers [35, 36]. It has been shown, that in contrast with normal healthy prostate tissue, where almost no anti-IL-17 reactivity is usually detected, [3] human prostate tissue with evidence of benign prostatic hyperplasia (BPH) exhibits high expression of IL-17. Especially strong signals are found in the apical portions of prostate epithelium. In addition, IL-17 positive lymphocytes are accumulated in smooth muscle and stromal prostate tissues of patients with BPH [3].
Interestingly, administration of vitamin D receptor agonist Elocalcitol to NOD mice with established prostatitis resulted in significant reductions in both the intraprostatic cell infiltrates and ex vivo production of IL-17 in prostate-draining lymph node T cells [37]. Our data are concordant with these previous observations in that intraprostatic expression of IL-17F was detected only after IL-1β administration. Moreover, the IL-17F signals increased in a time-dependent manner and both stromal infiltrating mononuclear cells and prostatic epithelial cells exhibited the strongest expression of this marker up to 24 h after IL-1β administration. In short, the IL-17F activity of mononuclear cells and prostatic epithelial cells occurred latest in response to IL-1β-induced NF-κB/p65 activity in the prostate.
Naive cells do not express VCAM1; however, exposure to inflammatory cytokines IL-1β and TNF-α, lipopolysaccharides, and to the synthetic double-stranded RNA, poly(I:C) results in rapid up-regulation of the gene responsible for its production [38]. VCAM1 is an adhesion molecule whose expression by endothelial, smooth muscle, and some nonvascular cells is inducible by inflammatory cytokines. It is responsible for the attraction and accumulation of leukocytes in sites of inflammation [39]. In the current study, significant intraprostatic expression of VCAM1 was only evident 24 h after IL-1β administration at which time mononuclear cells had already extravasated and accumulated in the stroma. Our data indicate that VCAM1 and IL-17F protein expression may not be involved in the initial phase of IL-1β–induced NF-κB dependent leukocyte extravasation.
The role of the CXCR4 molecule in prostatic inflammation, if any, is unclear. It has been shown that the chemokine stromal-derived factor-1A (SDF-1A/CXCL-12) and its receptor, CXCR4, play a crucial role in adhesion and trans-endothelium migration of prostate cancer cells [40, 41] and CXCR4 significantly contributes in CD4+ T cell immune responses [42, 43]. In the present study the expression of the CXCR4 receptor was observed at all time points after IL-1β cytokine administration. These findings are not surprising since SDF-1α-induced expression of CXCR4 in prostate PC-3 cells have been shown to be dependent on the MEK/ERK signaling cascade and NF-κB activation, [44] and NF-κB has been shown to regulate the motility of breast cancer cells by directly up-regulating the expression of CXCR4 [45].
To clarify the effects of IL-1β-induced NF-κB/p65 activation on expression of different signaling molecules in prostatic tissue, some animals were pretreated with dexamethasone one hour before IL-1β administration. Dexamethasone is a well established NF-κB inhibitor [46] and has been widely used in the treatment of a number of allergic and autoimmune inflammatory disorders. Our current findings clearly indicate that dexamethasone pretreatment inhibits IL-1β-induced NF-κB/p65 activation in the prostate (Fig. 2a) and stops the translocation of NF-κB subunits to the nucleus (Fig. 2b). In animals pretreated with dexamethasone, we observed profound reductions in all protein inflammatory markers measured, except CXCR4, as well as marked reductions in both extravasation and accumulation of leukocytes in the prostatic stroma. The tissue receptor to IL-8 (CXCR-1/IL-8RA) chemokine was the most significantly down-regulated marker. At 24 h post IL-1β injection in mice pretreated with dexamethasone, only overexpression of CXCR4 was observed in stromal leukocytes and in prostatic epithelial cells. In normal prostatic epithelial cells, CXCR4 protein expression is weak or absent [47]. However, cytoplasmic expression of the CXCR4 receptor is markedly increased in areas of prostatic inflammation associated with BPH, and has also been reported to be strongly expressed in localized prostate cancer samples [48]. As noted previously, enhanced expression of CXCR4 in response to dexamethasone pretreatment was not unexpected: dexamethasone administration has previously been reported to induce up-regulation of the expression of CXCR4 in human CD4+ T lymphocytes and monocytes [15, 49]. Our findings indicate that dexamethasone pretreatment of mice injected with IL-1β reduces NF-κB/p65 activity, expression of IL-17F, and completely abolishes expression of CXCR1/IL-8RA and VCAM1. The effects of dexamethasone administration on the expression of IL-17F, CXCR1/IL-8RA and VCAM1 suggest that a common mechanism is involved in the activation of these molecules.
IL-1β–induced NF-κB activation in the prostate, resulting in a predominant CD45+ lymphocyte extravasation into the prostatic stroma and their stromal accumulation, suggests a possible role of NF-κB in the genesis of intraprostatic inflammation. The schematic representation has been shown in Figure 5. We propose that IL-8 may be the intermediary chemoattractant messenger that accounts for the mechanistic relationships between leukocyte extravasation and IL-1β–induced NF-κB activation in the prostate. The role of IL-17 is not well understood. It has been shown that IL-17 receptor is ubiquitously expressed in the prostate [3]. It is known that IL-17 stimulates transcriptional NF-κB activity [50], induces IL-8 [51], IL-1β [52], and receptor activator of nuclear factor-κB ligand (RANKL) expression at sites of inflammation [53] that might lead to nuclear NF-κB activation promoting metastatic phenotype in prostate cancer cells in vivo [54]. It is possible that the enhanced expression of IL-17F that we observed following CXCR1/IL-8RA up-regulation may set into motion a cytokine auto-inflammatory loop that favors long-term persistence of prostate inflammation, as illustrated in Figure 5. Our data also indicate that the SDF-1/CXCR4 axis may be regulated by an NF-κB-independent mechanism.
Figure 5.
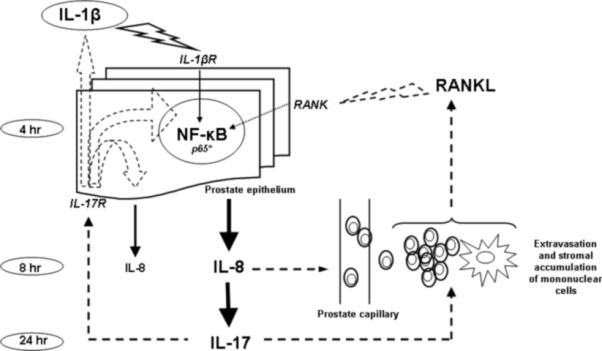
Hypothetical cytokine auto-inflammatory loop in prostate tissue after initial pro-inflammatory signal induced by IL-1β administration. Note: NF-κB - nuclear factor-κB; RANK - receptor activator of nuclear factor-κB; RANKL - receptor activator of nuclear factor-κB ligand; IL – interleukin.
Taken together, the present data may suggest that IL-1β-induced NF-κB activity contributes in chemoattractant urge for initial phase of prostatic inflammation. The IL-1β-induced NF-κB pattern of intraprostatic chemoattractive signals might have a capability for maintaining the chronic inflammation and proliferative inflammatory atrophy (PIA) in the prostate, which are recognized as putative precursor lesions in the development of prostate cancer.
Financial Support
Supported by the grants from United States Public Health Services RO1 CA108512, Sullivan Foundation for the Study of Prostatitis and The James & Eilleen Dicke Research Endowment funds.
Abbreviations
- NF-κB
nuclear factor-kappa B
- IL
interleukin
- RANK
receptor activator of nuclear factor-κB
- RANKL
receptor activator of nuclear factor-κB ligand
- IL-8RA
IL-8 receptor alpha
- VCAM1
vascular cell adhesion molecule – 1
- CXCR1
chemokine (C-X-C motif) receptor –1
- CXCR4
chemokine (C-X-C motif) receptor – 4
- MNC
mononuclear cells
REFERENCES
- 1.Handisurya A, Steiner GE, Stix U, Ecker RC, Pfaffeneder-Mantai S, Langer D, Kramer G, Memaran-Dadgar N, Marberger M. Differential expression of interleukin-15, a pro-inflammatory cytokine and T-cell growth factor, and its receptor in human prostate. Prostate. 2001;49(4):251–262. doi: 10.1002/pros.10020. [DOI] [PubMed] [Google Scholar]
- 2.Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52(1):43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 3.Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56(3):171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 4.Steiner GE, Djavan B, Kramer G, Handisurya A, Newman M, Lee C, Marberger M. The picture of the prostatic lymphokine network is becoming increasingly complex. Rev Urol. 2002;4(4):171–177. [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 6.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6(4):390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 9.Vykhovanets EV, Shukla S, MacLennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 2008;68(1):34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 10.Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, Dana MR, Kuwano M, Ono M, Ishibashi T, Hafezi-Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171(3):1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 13.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91(4):417–420. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 14.Nagase H, Miyamasu M, Yamaguchi M, Kawasaki H, Ohta K, Yamamoto K, Morita Y, Hirai K. Glucocorticoids preferentially upregulate functional CXCR4 expression in eosinophils. J Allergy Clin Immunol. 2000;106(6):1132–1139. doi: 10.1067/mai.2000.110923. [DOI] [PubMed] [Google Scholar]
- 15.Caulfield J, Fernandez M, Snetkov V, Lee T, Hawrylowicz C. CXCR4 expression on monocytes is up-regulated by dexamethasone and is modulated by autologous CD3+ T cells. Immunology. 2002;105(2):155–162. doi: 10.1046/j.0019-2805.2001.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engl T, Relja B, Marian D, Blumenberg C, Müller I, Beecken WD, Jones J, Ringel EM, Bereiter-Hahn J, Jonas D, Blaheta RA. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8(4):290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5(5):325–344. doi: 10.2174/1568009054629645. [DOI] [PubMed] [Google Scholar]
- 18.Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163(3):1457–1466. [PubMed] [Google Scholar]
- 19.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol. 2004;75(3):400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 20.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 21.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 22.Qin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Buthcher EC, Mackay CR. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26(3):640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 23.Santamaria Babi LF, Moser B, Perez Soler MT, Moser R, Loetscher P, Villiger B, Blaser K, Hauser C. The interleukin-8 receptor B and CXC chemokines can mediate transendothelial migration of human skin homing T cells. Eur J Immunol. 1996;26(9):2056–2061. doi: 10.1002/eji.1830260914. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156(3):932–938. [PubMed] [Google Scholar]
- 25.Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156(3):932–938. [PubMed] [Google Scholar]
- 26.Lundahl J, Sköld CM, Halldén G, Hallgren M, Eklund A. Monocyte and neutrophil adhesion to matrix proteins is selectively enhanced in the presence of inflammatory mediators. Scand J Immunol. 1996;44(2):143–149. doi: 10.1046/j.1365-3083.1996.d01-296.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoch RC, Schraufstatter IU, Cochrane CG. In vivo, in vitro, and molecular aspects of interleukin-8 and the interleukin-8 receptors. J Lab Clin Med. 1996;128(2):134–145. doi: 10.1016/s0022-2143(96)90005-0. [DOI] [PubMed] [Google Scholar]
- 28.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253(5025):1278–1380. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 31.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 33.Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56(6):1025–1029. doi: 10.1016/s0090-4295(00)00844-x. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 37.Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Sanvito F, Doglioni C, Adorini L. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177(12):8504–8511. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 38.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-,induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 39.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9(10):899–909. [PubMed] [Google Scholar]
- 40.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 41.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66(1):32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 42.Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165(11):6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- 43.Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pène J, Yssel H. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165(2):716–724. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- 44.Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65(21):9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- 45.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 46.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 47.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 48.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 49.Curnow SJ, Wloka K, Faint JM, Amft N, Cheung CM, Savant V, Lord J, Akbar AN, Buckley CD, Murray PI, Salmon M. Topical glucocorticoid therapy directly induces up-regulation of functional CXCR4 on primed T lymphocytes in the aqueous humor of patients with uveitis. J Immunol. 2004;172(11):7154–7161. doi: 10.4049/jimmunol.172.11.7154. [DOI] [PubMed] [Google Scholar]
- 50.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 51.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a ”fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44(9):2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 53.Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 2005;52(8):2307–2312. doi: 10.1002/art.21211. [DOI] [PubMed] [Google Scholar]
- 54.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446(7136):690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]


