Abstract
Objectives
Imaging agents capable of providing cell compartment-specific information will facilitate studies of pathophysiological mechanisms, natural history of diseases, and therapeutic development. To demonstrate the effects of liver injury on the disposal of the organic anion mebrofenin, we performed animal studies.
Methods
Acute liver injury was induced in Fischer 344 rats with 0.25–1 ml/kg single doses of carbon tetrachloride followed by studies of animals over 4 weeks. The liver injury was analyzed by blood tests and histological grading. Additional rats were treated with lipopolysaccharide, interleukin-6 or tumor necrosis factor-α to activate inflammatory events. Hepatic clearance of 99mTc-mebrofenin was studied with dynamic imaging and fractional retention after 60 min of peak hepatic mebrofenin activity was determined.
Results
In healthy rats, only 24 ± 2% of peak mebrofenin activity was retained in the liver after 60 min. By contrast, 24 h after carbon tetrachloride, virtually all mebrofenin activity was retained in the liver (P < 0.001). Three weeks were required for mebrofenin excretion to become normal after carbon tetrachloride administration. In this situation, we found that Kupffer cell activity was increased. In addition, the abnormality in mebrofenin excretion was reproduced by lipopolysaccharide, which activates Kupffer cells. Moreover, mebrofenin excretion was highly sensitive to interleukin-6 and/or tumor necrosis factor-α, which help mediate the Kupffer cell response.
Conclusion
Hepatobiliary excretion of mebrofenin was affected rapidly and over an extended period by inflammatory cytokines released after liver injury. The remarkable sensitivity of mebrofenin excretion to cytokines suggests that 99mTc-mebrofenin imaging will be helpful for assessing cytokine-mediated liver inflammation.
Keywords: cytokine, inflammation, liver, mebrofenin, transport
Introduction
The ability to identify liver inflammation in a noninvasive manner will help in the assessment of disease activity and therapeutic development. Current modalities for demonstrating liver inflammation, for example, blood tests, such as serum aminotransferases, need to be supplemented with additional assays, particularly to elicit information concerning underlying pathophysiologic mechanisms. Although efforts are ongoing to develop novel tests through the application of genomic–proteomic approaches [1], we considered that use of radioligands capable of demonstrating gene-specific or pathway-specific processes offers noninvasive means for assessing the integrity of cell compartments [2].
In this regard, mebrofenin – N-(3-bromo-2,4,6-trimethyl-acetanilide)iminodiacetic acid – and related compounds possess exclusive affinity for hepatocytes followed by transport into bile [3-13]. Although relevant hepatobiliary transporters have been well studied [14,15], gaps remain in critical areas and the identity of specific mebrofenin transporter(s) is unknown. Recently, hepatic handling of mebrofenin was found to be altered in the setting of spontaneous liver injury, for example, Long-Evans Cinnamon rats with copper toxicosis [7] as well as in the setting of induced liver disease, for example, acute toxic injury with D-galactosamine [8], chronic injury with carbon tetrachloride (CCl4) [9], or diet-induced steatosis [10]. Similarly, alterations in mebrofenin handling have been recognized in people with cirrhosis and following liver transplantation [11-13]. In these studies, underlying mechanisms responsible for altering mebrofenin handling were not established, although the inflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), impaired mebrofenin release from primary rat hepatocytes under culture conditions [9].
Here, to understand mechanisms of inflammation-associated changes in hepatic excretion of 99mTc-mebrofenin, we studied rats with CCl4-induced acute liver injury, which produces dose-dependent and reversible injury with recovery over 2–3 days, although persistent exposure to CCl4 produces chronic liver injury and death [16,17]. To demonstrate the effects of minor liver injury on mebrofenin excretion, we administered CCl4 in relatively small amounts. We considered that this model would reproduce aspects of hepatitis in humans, where the spectrum of liver injury ranges from near-normal to mild, moderate, or severe changes in liver tests and liver histology. Moreover, we administered inflammatory cytokines to rats for directly eliciting their effects on mebrofenin excretion.
Materials and methods
Cytokines
Recombinant mouse TNF-α, IL-6, and lipopolysaccharide (LPS) were obtained commercially (T7539, I9646, and L-2758, respectively; Sigma Chemical Company, St Louis, Missouri, USA). Cytokines were solubilized in water and stored at −20°C followed by reconstitution in normal saline when ready for use.
Animals and surgical procedures
A total of 140 Fischer 344 (F344) rats were used at 6–8 weeks of age and body weight of 120–180 g. Rats were divided into multiple experimental groups of 3–6 animals as described below. Animals were anesthetized with ketamine and xylazine when indicated. The Animal Care and Use Committees at Albert Einstein College of Medicine and Long Island Jewish Medical Center approved all animal protocols, in accordance with the regulations of the institutions and National Institutes of Health.
To induce liver injury, CCl4 in mineral oil (1 : 1, v/v) (Sigma) was given intramuscularly in single doses of 0.25, 0.5 or 1 ml/kg body weight. LPS was used in a dose of 7.4 × 106 EU/kg as described previously [18]. LPS and cytokines were administered through the tail vein in 0.1 ml normal saline. Control animals received vehicle alone. To analyze mebrofenin handling with imaging, the spleen was isolated through left subcostal laparotomy. Mebrofenin was mixed with 185–222 MBq 99mTc-sodium pertechnetate in 3 ml of normal saline according to the manufacturer's instructions (Choletec, Bracco Diagnostics, Princeton, New Jersey, USA). A 23-gauge butterfly needle was used to inject 7.4 MBq 99mTc-mebrofenin in 1 ml volume over 9–12 s into the splenic pulp followed by flushing with 0.5 ml normal saline. Hemostasis was secured with a ligature around the splenic pole. The radiotracer was injected into the spleen for convenience as the abdomen was opened to move the spleen away from the liver for gathering superior images.
Global Kupffer cell activity was demonstrated in tissue samples from animals treated with India ink (Pelican #17, Hannover, Germany), which was prepared as described previously and carbon contained in 0.5 ml ink was injected into the tail vein after centrifugation and dilution in 5 volumes of gelatin [19]. Tissue samples were obtained 60 min after carbon injection to demonstrate the phagocytotic capacity of Kupffer cells, which was graded as described previously. Animals were killed immediately after the completion of the studies and blood and liver samples were collected as appropriate.
Animal imaging
Immediately after mebrofenin injection, image acquisition commenced on a γ camera equipped with low-energy, high-resolution, parallel-hole collimator (Argus, ADAC Laboratories, Milpitas, California, USA). A 20% window was centered on 140 keV for energy discrimination. Ten-second dorsal images were acquired for 60 min, using 64 × 64 × 16 matrix at a zoom factor of 2. The data were analyzed with a commercially available nuclear medicine workstation (Pegasys, ADAC Laboratories). Regions of interest were drawn over the liver to obtain time–activity curves. The time at which maximal hepatic activity of mebrofenin occurred – Tpeak – was determined. The fraction of Tpeak mebrofenin activity remaining in the liver after intervals of up to 60 min following mebrofenin injection was obtained.
Histological analysis
Liver samples were fixed in 10% buffered formalin and paraffin-embedded sections were prepared for hematoxylin and eosin staining using standard procedures. The severity of liver injury was independently graded by two experienced investigators, according to Batts and Ludwig [20], by analyzing portal or lobular inflammation and necrosis as follows: grade 0, no disease activity; grade 1, minimal portal inflammation with minimal or patchy lobular inflammation and necrosis; grade 2, mild portal inflammation with mild lobular inflammation and little hepatocellular change; grade 3, moderate portal inflammation, moderate lobular inflammation and necrosis with noticeable hepatocellular damage; and grade 4, severe portal and lobular inflammation, necrosis with prominent diffuse hepatocellular damage. The observers were blinded to sample identities.
Blood tests
Sera stored at −20°C were analyzed for bilirubin, alanine aminotransferase (ALT), and alkaline phosphatase with an automated clinical system.
Cytokine assays
Serum IL-6 and TNF-α were assayed with rat cytokine/chemokine LINCOplex kit, which uses fluorescently labeled microsphere beads for the assay, according to the manufacturer's instructions (RCYTO-80K; Luminex Corporation, Austin, Texas, USA). Each serum sample was run in duplicate. The assay included samples from six normal control rats as well as internal negative and positive controls and cytokine quantitation standards.
Statistical analysis
Data are shown as mean ± SD. The significances were analyzed with Sigma Stat 3.0 software (Jandel Scientific, Carpenteria, California, USA) by Student's t-test for intergroup comparisons and analysis of variance (ANOVA) using the Holm–Sidak pairwise multiple comparison post-hoc procedure. P < 0.05 was considered significant.
Results
Experimental design
Typically, each animal group used six rats. Initial studies of mebrofenin handling utilized CCl4 in doses of 0.25, 0.5, and 1 ml/kg (n = 6 each) with analysis of serum ALT and total bilirubin, liver histology, and mebrofenin handling (Fig. 1a). Long-term perturbations in animals were studied with 1 ml/kg dose of CCl4 (Fig. 1b). These studies used groups of animals 1, 3, 7, 14, 21, and 28 days after CCl4 (n = 6 each). In further studies, we demonstrated Kupffer cell activity at these times and examined the effect of LPS on mebrofenin excretion (n = 3–6 rats each). Finally, we studied dose-specific and duration-dependent effects on mebrofenin handling of IL-6 alone, TNF-α alone, and in combination (n = 3 rats each) (Fig. 1c). The studies were repeated on two to three separate occasions.
Fig. 1.
General experimental design. The experiments were aimed at initially analyzing the sensitivity of mebrofenin handling to CCl4-induced liver injury. (a) CCl4 was administered in doses of 0.25, 0.5, and 1 ml/kg 1 day before analysis of liver tests, histology and mebrofenin handling. (b) Plan for testing whether acute inflammation induced by CCl4 resulted in perturbations of mebrofenin handling in the long term, up to 28 days. These studies used groups of six rats per time-point. (c) Treatment of animals with LPS and cytokines followed by mebrofenin analysis after various intervals. LPS, lipopolysaccharide.
Effect of acute liver injury on mebrofenin handling
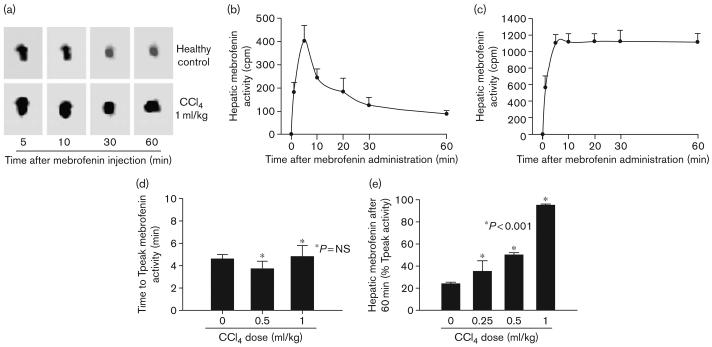
In healthy control rats, mebrofenin promptly appeared in the liver and was excreted rapidly (Fig. 2a and b). The mean Tpeak of mebrofenin incorporation in the liver was 276 ± 24 s (Fig. 2d). Only 24 ± 1% of Tpeak mebrofenin activity remained in the liver after 60 min (Fig. 2e). In CCl4-treated rats, mebrofenin accumulated in the liver at greater levels than in normal rats, although the time to Tpeak remained essentially unchanged; for example, this was 288 ± 59 s 24 h after 1 ml/kg CCl4 had been given (Fig. 2d). Hepatic excretion of mebrofenin, however, was progressively impaired 24 h after CCl4-treatment, such that 35 ± 10, 50 ± 2, and 95 ± 8% of Tpeak mebrofenin activity was still in the liver after 60 min after 0.25, 0.5, and 1 ml/kg CCl4, respectively (P < 0.001, ANOVA with the Holm–Sidak method; Fig. 2e).
Fig. 2.
Scintigraphy showing hepatic mebrofenin uptake and excretion. (a) Representative sequential γ images of 99mTc-mebrofenin in the liver of a healthy rat and a rat treated 24 h previously with 1 ml/kg CCl4. In the healthy control, mebrofenin excretion resulted in less 99mTc-mebrofenin activity over time, whereas this decline was not obvious in the CCl4-treated rat. The activity in proximal intestine is seen in the area below the liver 5 and 10 min after mebrofenin injection. Subsequently, mebrofenin migrated into distal intestine, which is not visible. (b) Hepatic time–activity curves from several healthy control rats to demonstrate the accumulation and loss of 99mTc-mebrofenin. (c) Corresponding hepatic time–activity curves of hepatic 99mTc-mebrofenin in animals treated 24 h previously with 1 ml/kg CCl4. (d) The time required for maximal incorporation of mebrofenin in the liver (Tpeak), which was similar in animals with or without CCl4-treatment (n = 3 each); NS, not significant. (e) The extent of mebrofenin retention after 60 min in animals (n = 6 each). Hepatic mebrofenin excretion was significantly impaired in CCl4-treated rats as indicated by asterisks.
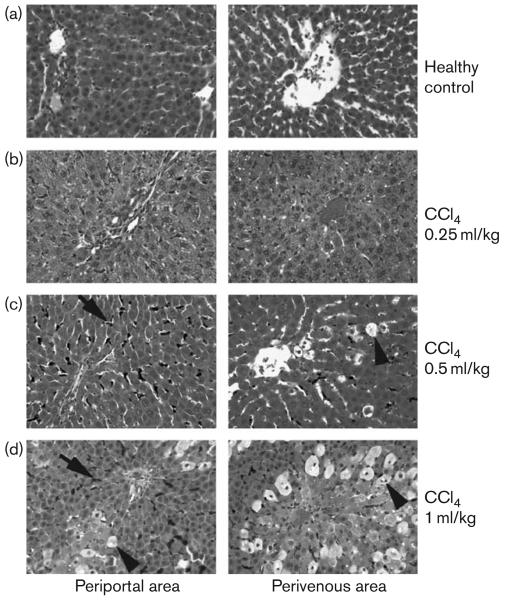
The extent of mebrofenin retention was related to severity of liver injury. For instance, serum ALT levels were highest in animals 24 h after 1 ml/kg CCl4 (Table 1). It was noteworthy that rats treated with only 0.25 ml/kg CCl4 showed mild abnormalities in liver tests and limited histological injury (Fig. 3 and Table 2). The liver of healthy control rats showed no inflammatory changes and low-grade accumulation of carbon in Kuppfer cells (Fig. 3a). In contrast, CCl4 produced progressive dose-dependent increases in liver necrosis, particularly in perivenous areas of the liver lobule (Fig. 3b–d), which are more susceptible to CCl4-induced injury [16]. Increased carbon uptake was observed in Kupffer cells, which reflected activation of this cell compartment.
Table 1.
Liver test abnormalities
| CCl4-treated rats |
||||
|---|---|---|---|---|
| Parameter | Healthy controls |
0.25ml/kg | 0.5 ml/kg | 1 ml/kg |
| Total serum bilirubin (mg/dl) |
0.2± 0.1 | 0.2± 0.0 | 0.3± 0.1 | 0.4± 0.1 |
| Serum ALT (U/l) | 42 ± 3 | 50± 3 | 59± 6a | 1721 ± 297a |
P values of less than 0.05 versus sham-treated controls; analysis of variance with Holm–Sidak test, n = 3–6 each.
Fig. 3.
Histological extent of liver injury in rats. Periportal areas are shown in panels on the left and perivenous areas in panels on the right. Tissues shown were from healthy rats or CCl4-treated rats as indicated. In CCl4-treated rats, hepatic steatosis and hydropic changes were more pronounced (arrowheads in c and d) along with more activated Kupffer cells containing carbon (arrows). Moreover, CCl4-treated rats showed inflammatory infiltrates with polymorphonuclear and mononuclear cells, although these changes were limited in rats treated with 0.25 ml/kg CCl4 (b). Hematoxylin and eosin stain; Orig. mag., × 100.
Table 2.
Histological grading of liver lesions
| Animal groups | Disease activity (grade 0–4) |
|---|---|
| Sham controls (n=6) | 0±0 |
| CCl4 0.25 ml/kg (n=6) | 1±0 |
| CCl4 0.5 ml/kg (n=6) | 2±1 |
| CCl4 1 ml/kg (n=6) | 3±0 |
Alterations in mebrofenin excretion persisted in the long term
To determine the duration of hepatic necroinflammation after CCl4, we examined rats treated with 1 ml/kg CCl4 for up to 4 weeks. Liver histology, serum ALT, and total serum bilirubin reverted to near-normal within 1 week after CCl4. Serum ALT after 3, 7, 14, and 21 days was 250 ± 168, 59 ± 6, 81 ± 13, and 70 ± 1 U/l, respectively.
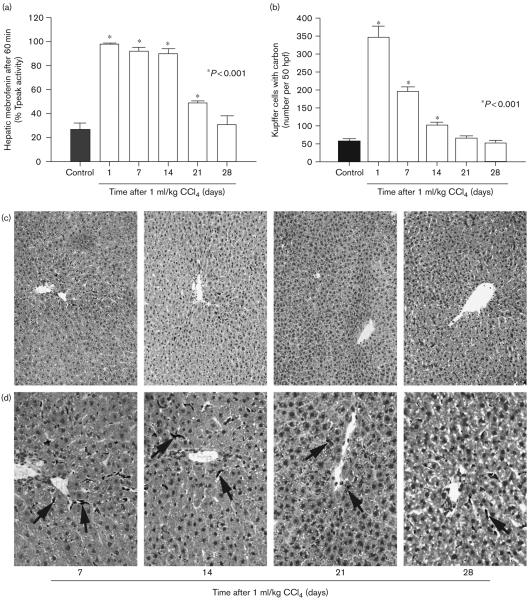
Hepatic mebrofenin excretion was perturbed for at least 3 weeks after a single dose of CCl4 (Fig. 4a). This abnormality was particularly pronounced for 2 weeks, with the retention after 60 min of 98 ± 1, 92 ± 3, and 90 ± 4% Tpeak mebrofenin after 1, 7, and 14 days, respectively. After 3 weeks, 49 ± 2% of Tpeak mebrofenin was still retained in the liver, versus 27 ± 5% in healthy controls (P < 0.001, ANOVA and the Holm–Sidak test). This suggested that despite transient or nonobvious liver injury after CCl4, hepatic inflammation lasted for a considerable period.
Fig. 4.
Mebrofenin excretion was perturbed in the long term after liver injury. (a) Analysis of mebrofenin excretion over 28 days in rats treated once with 1 ml/kg CCl4 (n = 6 each). Mebrofenin excretion was impaired for 21 days. (b) Global Kupffer cell activity after single dose of 1 ml/kg CCl4 (n = 6 each) with the numbers of Kupffer cells containing carbon per 50 high power fields at various intervals. (c) Absence of histological damage in the long term in CCl4-treated rats. (d) Presence of activated Kupffer cells containing carbon (arrows). Orig. mag., C, × 100; D × 400; toluidine blue counterstain.
As Kupffer cells are major mediators of hepatic inflammation, we measured the number of Kupffer cells containing carbon particles as described previously [19]. In CCl4-treated animals, the number of carbon-containing Kupffer cells was significantly greater and this Kupffer cell activation persisted for at least 2 weeks (Fig. 4b). Analysis of tissues at later times indicated that liver histology rapidly reverted to normal after CCl4 (Fig. 4c), in agreement with normalization of serum ALT levels, whereas Kupffer cells containing carbon were present in the liver over the long term (Fig. 4d).
The findings were in agreement with the possibility of inflammatory cytokine release from Kupffer cells during the perturbation of mebrofenin excretion. To verify this, we measured serum TNF-α and IL-6 with a fluorescent assay reported by the manufacturer to be sensitive to 9.8 pg/ml rtIL-6 and 4 pg/ml rtTNF-α. In our own assay, cytokine levels of less than 24 pg/ml were considered negative. In CCl4-treated rats, TNF-α levels were below assay detection limit (Fig. 5). Serum IL-6, however, was elevated in several animals at early and late intervals after CCl4. Increased expression of IL-6, far removed from the initial CCl4-induced injury, was again in agreement with prolonged activation of Kupffer cells.
Fig. 5.
Serum IL-6 levels. Shows data from rats treated with 1 ml/kg CCl4 followed by time-course analysis of serum IL-6 levels (n = 3 each). Serum IL-6 was detected in 5 of 18 rats shown, ranging from 43 to 201 pg/ml, with the assay threshold of 24.9 pg/ml.
Effect of inflammatory cytokines on mebrofenin excretion
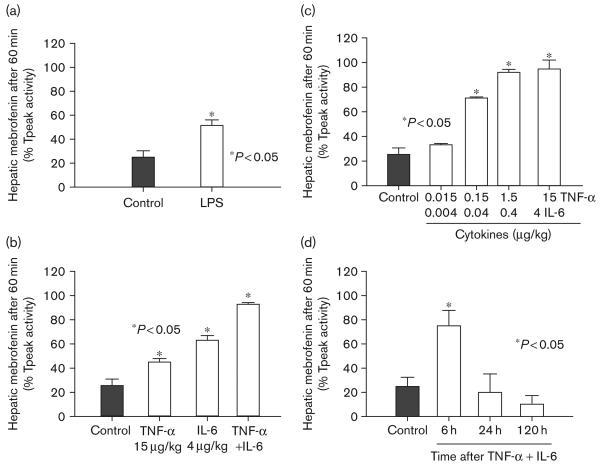
To verify that CCl4-induced hepatic perturbations were not simply because of hepatic necrosis or other non-inflammatory mechanisms, we studied the effect of LPS, which is well known to activate Kupffer cells [18]. In these studies, we administered a single dose of LPS intravenously followed by analysis of mebrofenin excretion. In LPS-treated rats, mebrofenin excretion rapidly became abnormal (Fig. 6a). This further suggested that soluble cytokines regulated mebrofenin transport. Therefore, to determine whether IL-6 and TNF-α affected mebrofenin transport in vivo, we treated rats with 15 μg/kg TNF-α alone, 4 μg/kg IL-6 alone, and both cytokines together in these doses, followed by analysis of mebrofenin excretion after 6 h (Fig. 6b). IL-6 was more effective than TNF-α in perturbing mebrofenin excretion. Simultaneous administration of IL-6 and TNF-α was even more effective in perturbing mebrofenin excretion. The combination of IL-6 and TNF-α reproduced suppression of mebrofenin excretion to 93 ± 1%, which was similar to rats treated with 1 ml/kg CCl4. Further studies using IL-6 and TNF-α revealed that nanogram amounts of these cytokines were sufficient for perturbing mebrofenin excretion (Fig. 6c). We also examined the duration of perturbation in mebrofenin excretion following a single dose of cytokines to determine whether persistent cytokine release was necessary after CCl4. As the effect of cytokines on mebrofenin transport was short lived (Fig. 6d), these studies verified that persistent release of cytokines from inflammatory cells, that is, Kupffer cells, was necessary for perturbing mebrofenin excretion over the long term.
Fig. 6.
Perturbation of mebrofenin excretion by administered cytokines. (a) The effect of LPS on mebrofenin excretion 6 h after LPS was administered to animals compared with control rats (P<0.05, t-test). (b) Data from animals treated with single doses of IL-6, TNF-α, and both cytokines, after 6 h later by analysis of mebrofenin excretion, compared with controls. Note that mebrofenin retention significantly increased in cytokine-treated rats, particularly after IL-6 and TNF-α (P<0.05, ANOVA). (c) Dose–response analysis of the effect of IL-6 and TNF-α on mebrofenin excretion after 6 h. The data show that hepatobiliary transport of mebrofenin was inhibited by extremely small amounts of cytokines. (d) Time-course analysis of the cytokine effect on mebrofenin excretion using 4 μg IL-6 and 15 μg TNF-α. These studies demonstrated rapid dissipation of the effects of cytokines on mebrofenin handling. LPS, lipopolysaccharide; ANOVA, analysis of variance.
Discussion
These studies further established mechanisms in hepatobiliary mebrofenin excretion. First, acute hepatic necroinflammation rapidly and profoundly perturbed mebrofenin transport, resulting in marked impairment of the capacity to excrete mebrofenin. Second, prolonged Kupffer cell activation was observed after liver injury and the abnormality in mebrofenin excretion was reproduced by LPS, which activates Kupffer cells, implicating these cells in the inflammatory response. Third, mebrofenin excretion was exquisitely sensitive to systemically administered IL-6 and TNF-α, implicating central roles of these cytokines in affecting mebrofenin transport.
The mechanism by which 99mTc-mebrofenin enters into hepatocytes is unknown. Our studies, this and earlier, indicated that mebrofenin accumulated in the liver despite acute or chronic liver injury [7,9]. This should suggest that the uptake mechanism, whatever that may be, was not blocked by the prevalent mechanisms of hepatic injury. In contrast, hepatic excretion of 99mTc-mebrofenin was inhibited by acute liver injury with hepatocellular necrosis and also by persistent hepatic inflammation long after the subsidence of liver injury as well as by systemic administration of LPS and inflammatory cytokines. It should be noted that neither LPS nor inflammatory cytokines produce hepatocellular necrosis. We did not address the issue of hepatic mebrofenin extraction in our studies. The measure of Tpeak activity should not be equated with the extraction of 99mTc-mebrofenin as Tpeak only reflected net hepatic accumulation of mebrofenin over time. Our studies in healthy rats using the T1/2 measurement of mebrofenin excretion over 60 min reproduced the finding that mebrofenin was rapidly excreted from the liver [7,9], which was in agreement with long-standing observations in people [21]. By contrast, excretion of 99mTc-mebrofenin was rapidly and dose-dependently impaired after CCl4-induced liver injury, despite recovery from overt hepatocellular necrosis and/or damage. In CCl4-treated animals, greater accumulation of 99mTc-mebrofenin activity compared with healthy animals (see counts in Fig. 2b and c) should have largely resulted from the failure to excrete this into bile.
We were particularly impressed by the inhibition of mebrofenin excretion in rats treated with only 0.25 ml/kg CCl4, which caused minimal liver injury. Furthermore, liver injury induced by CCl4 rapidly resolved, although abnormality in mebrofenin excretion persisted over long periods, which was explained by persistent activation of Kupffer cells or by other mechanisms. After liver injury, inflammatory cytokines are produced locally, largely by Kupffer cells, and cytokine levels in the peripheral blood need not be significantly elevated, as in our studies, where serum TNF-α was undetectable and increases in serum IL-6 levels were limited to only some animals. Therefore, even as measurement of inflammatory cytokines or other local changes poses practical difficulties, analysis of mebrofenin excretion offers an alternative for demonstrating hepatic inflammation in pathophysiological states.
Our studies further substantiated this possibility by analyzing the effects of cytokine administration, where remarkably small amounts of IL-6 and TNF-α impaired mebrofenin excretion. As shown in Fig. 6, administration of IL-6 in as little as 40 ng/kg dose was sufficient for perturbing mebrofenin excretion. This actually translates to only 6 ng IL-6 in a rat weighing 150 g. As water constitutes 60% of the body weight, that is, 90 ml in a 150-g rat, if IL-6 were distributed uniformly in the aqueous compartment of the body, 6 ng IL-6 would have produced a circulating level of not more than 67 pg/ml. It should be noted that in our study serum IL-6 levels ranged between 40 and 200 pg/ml, when IL-6 was detected. Of course, for mebrofenin excretion to be perturbed in the long term, persistent cytokine release should have been necessary. The demonstration of prolonged Kupffer cell activation following CCl4-induced liver injury offers such a mechanism for cytokine release over long periods.
Our studies indicated that administration of cytokines in peripheral circulation was sufficient for perturbing hepatobiliary transport, although circulatory cytokines must obviously enter the liver for exerting this effect. These findings are in agreement with current concepts regarding molecular regulation of hepatic organic anion transporters by cytokines, notwithstanding that the specific transporter(s) for mebrofenin has yet to be identified definitively. We consider it unlikely that hepatic uptake mechanisms, for example, Na+/taurocholate cotransporting polypeptide (ntcp) or other potential organic anion importers [14,15,22], were incriminated because hepatic accumulation of mebrofenin was unperturbed after either CCl4 or cytokines. Transporters regulating bile canalicular anion export should be more relevant, for example, the bile salt export pump (BSEP), multidrug resistance-related proteins (MRPs), organic anion transporters (OATPs), etc. Recent mouse studies showed that TNF-α decreased mRNA expression in the liver of MRP2, MRP3, and OATP2 but not of OATP1 or BSEP, whereas IL-6 decreased mRNA expression of MRP2, OATP1, OATP2, and BSEP [23]. In another study, administration of TNF-α to mice resulted in downregulation of BSEP, OATP, and MRP2 mRNA expression [24]. Similarly, inflammation induced in the mouse liver by turpentine or LPS was associated with decreased BSEP and MRP2 mRNAs [25]. By contrast, in IL-6-deficient mice, turpentine no longer altered mRNA expression of these bile canalicular transporters, whereas LPS-mediated regulation of bile canalicular transporter activity was short-lived. Our results, showing that LPS, as well as IL-6 and TNF-α-inhibited mebrofenin excretion, both directly and synergistically, suggest that inflammatory cytokines regulate mebrofenin transport. IL-6 likely played more effective roles in regulating bile canalicular transport, particularly in the long term, corresponding to studies using LPS injection, which activated Kupffer cells in IL-6-deficient mice [25].
The remarkable sensitivity of mebrofenin excretion to cytokine regulation of biliary transport suggests that study of intracellular mechanisms in this process, for example, role of nuclear receptors [26-29], and other signaling networks [30], will be informative. In particular, IL-6 and TNF-α can perturb intracellular signaling mechanisms, including nuclear receptors, for example, pregnane X-, farnesoid X-, and constitutively activated receptors, through their regulatory partners, while rapidly perturbing expression of bile canalicular transporters.
Conclusion
In an animal model of acute liver injury, mebrofenin excretion was perturbed rapidly and over several weeks. The underlying mechanism involved activation of inflammatory processes in the liver with cytokine release. The findings will be helpful in developing mebrofenin imaging for assessing liver inflammation.
Acknowledgements
This study was supported in part by National Institutes of Health grants R01 DK46952, P30 DK41296, P30 CA13330, and M01 RR12248.
References
- 1.Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–434. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 2.Joseph B, Bhargava KK, Malhi H, Schilsky ML, Jain D, Palestro CJ, Gupta S. Sestamibi is a substrate for MDR1 and MDR2 P-glycoprotein genes. Eur J Nucl Med. 2003;30:1024–1031. doi: 10.1007/s00259-002-1111-z. [DOI] [PubMed] [Google Scholar]
- 3.Daniel GB, Bahr A, Dykes JA, DeNovo R, Young K, Smith GT. Hepatic extraction efficiency and excretion rate of technetiun-99m-mebrofenin in dogs. J Nucl Med. 1996;37:1846–1849. [PubMed] [Google Scholar]
- 4.Newell SM, Selcer BA, Roberts RE, Cornelius LM, Mahaffey EA. Hepatobiliary scintigraphy in the evaluation of feline liver disease. J Veter Intern Med. 1996;10:308–315. doi: 10.1111/j.1939-1676.1996.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 5.Chavez-Cartaya R, Ramirez P, Fuente T, DeSola GP, Marin J, Pinero A, et al. Blood clearance of 99mTc-trimethyl-Br-IDA discriminates between different degrees of severe liver ischaemia-reperfusion injury in the rat. Eur Surg Res. 1997;29:346–355. doi: 10.1159/000129542. [DOI] [PubMed] [Google Scholar]
- 6.Daniel GB, DeNovo RC, Schultze AE, Schmidt D, Smith GT. Hepatic extraction efficiency of technetium-99m-Mebrofenin in the dog with toxic-induced acute liver disease. J Nucl Med. 1998;39:1286–1292. [PubMed] [Google Scholar]
- 7.Malhi H, Bhargava KK, Afriyie M, Volenberg I, Schilsky ML, Palestro CJ, Gupta S. 99mTc-mebrofenin scintiscanning for evaluating liver disease in a rat model of Wilson's disease. J Nucl Med. 2002;43:246–252. [PubMed] [Google Scholar]
- 8.Deshpande UR, Joseph LJ, Samuel AM. Hepatobiliary clearance of labelled mebrofenin in normal and D-galactosamine HCl-induced hepatitis rats and the protective effect of turmeric extract. Indian J Physiol Pharmacol. 2003;47:332–336. [PubMed] [Google Scholar]
- 9.Joseph B, Bhargava KK, Trunco G, Kumaran V, Palestro CJ, Gupta S. Regulation of hepatobiliary transport activity and noninvasive identification of cytokine-dependent liver inflammation. J Nucl Med. 2005;46:146–152. [PubMed] [Google Scholar]
- 10.Vetelainen RL, Bennink RJ, de Bruin K, van Vliet A, van Gulik TM. Hepatobiliary function assessed by (99m)Tc-mebrofenin cholescintigraphy in the evaluation of severity of steatosis in a rat model. Eur J Nucl Med Mol Imaging. 2006;33:1107–1114. doi: 10.1007/s00259-006-0125-3. [DOI] [PubMed] [Google Scholar]
- 11.Keeffe EB, Lieberman DA, Krishnamurthy S, Krishnamurthy GT, Gilbert S. Scintigraphic features of primary biliary cirrhosis: comparison with sclerosing cholangitis. Radiology. 1988;166:143–148. doi: 10.1148/radiology.166.1.3257301. [DOI] [PubMed] [Google Scholar]
- 12.Koruk M, Ozkilic S, Savas MC, Celen Z, Kadayifci A, Ozkilic C. Evaluation of hepatic functions and biliary dynamics in patients with liver cirrhosis by quantitative scintigraphy. Hepatogastroenterology. 2003;50:1803–1805. [PubMed] [Google Scholar]
- 13.Aktas A, Koyuncu A, Dalgic A, Haberal M. Comparison of early postoperative function of liver and renal allografts with radionuclide imaging. Transplant Proc. 2005;37:355–358. doi: 10.1016/j.transproceed.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Jansen PLM, Sturm E. Genetic cholestasis, causes and consequences for hepatobiliary transport. Liver Int. 2003;23:315–322. doi: 10.1034/j.1478-3231.2003.00856.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner M, Trauner M. Transcriptional regulation of hepatobiliary transport systems in health and disease: implications for a rationale approach to the treatment of intrahepatic cholestasis. Ann Hepatol. 2005;4:77–99. [PubMed] [Google Scholar]
- 16.Gupta S, Rajvanshi P, Aragona E, Yerneni PR, Lee C-D, Burk RD. Transplanted hepatocytes proliferate differently after CCl4 treatment and hepatocyte growth factor infusion. Am J Physiol. 1999;276:G629–G638. doi: 10.1152/ajpgi.1999.276.3.G629. [DOI] [PubMed] [Google Scholar]
- 17.Cai J, Ito M, Nagata H, Westerman KA, Lafleur D, Chowdhury JR, et al. Treatment of liver failure in rats with end-stage cirrhosis by transplantation of immortalized hepatocytes. Hepatology. 2002;36:386–394. doi: 10.1053/jhep.2002.34614. [DOI] [PubMed] [Google Scholar]
- 18.Yee SB, Ganey PE, Roth RA. The role of Kupffer cells and TNFα in monocrotaline and bacterial lipopolysaccharide-induced liver injury. Toxicol Sci. 2002;17:124–132. doi: 10.1093/toxsci/71.1.124. [DOI] [PubMed] [Google Scholar]
- 19.Joseph B, Malhi H, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology. 2002;123:1677–1685. doi: 10.1053/gast.2002.36592. [DOI] [PubMed] [Google Scholar]
- 20.Batts KP, Ludwig J. Chronic hepatitis, and update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert S, Brown PH, Krishnamurthy GT. Quantitative nuclear hepatology. J Nucl Med Technol. 1987;15:38–43. [Google Scholar]
- 22.Krishnamurthy GT, Krishnamurthy S. Cholescintigraphic measurement of liver function: how is it different from other methods? Eur J Nucl Med Mol Imaging. 2006;33:1103–1106. doi: 10.1007/s00259-006-0182-7. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303:273–281. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 24.Geier A, Dietrich CG, Voigt S, Ananthanarayanan M, Lammert F, Schmitz A, et al. Cytokine-dependent regulation of hepatic organic anion transporter gene transactivators in mouse liver. Am J Physiol Gastrointest Liver Physiol. 2005;289:831–841. doi: 10.1152/ajpgi.00307.2004. [DOI] [PubMed] [Google Scholar]
- 25.Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem Biophys Res Commun. 2004;322:232–238. doi: 10.1016/j.bbrc.2004.07.102. [DOI] [PubMed] [Google Scholar]
- 26.Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, Daujat M, Fabre JM, Maurel P, Vilarem MJ. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000;274:707–713. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- 27.Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–8995. doi: 10.1074/jbc.M212633200. [DOI] [PubMed] [Google Scholar]
- 28.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312:841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem. 2006;281:15434–15440. doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
- 30.Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]