Abstract
Children with narrow phenotype bipolar disorder (NP-BD; i.e., history of at least one hypomanic or manic episode with euphoric mood) are deficient when labeling face emotions. It is unknown if this deficit is specific to particular emotions, or if it extends to children with severe mood dysregulation (SMD; i.e., chronic irritability and hyperarousal without episodes of mania). Thirty-nine NP-BD, 31 SMD, and 36 control subjects completed the emotional expression multimorph task, which presents gradations of facial emotions from 100% neutrality to 100% emotional expression (happiness, surprise, fear, sadness, anger, and disgust). Groups were compared in terms of intensity of emotion required before identification occurred and accuracy. Both NP-BD and SMD youth required significantly more morphs than controls to label correctly disgusted, surprised, fearful, and happy faces. Impaired face labeling correlated with deficient social reciprocity skills in NP-BD youth and dysfunctional family relationships in SMD youth. Compared to controls, patients with NP-BD or SMD require significantly more intense facial emotion before they are able to label the emotion correctly. These deficits are associated with psychosocial impairments. Understanding the neural circuitry associated with face-labeling deficits has the potential to clarify the pathophysiology of these disorders.
The diagnosis of bipolar disorder (BD) in children and adolescents is controversial, in part because of disagreement on the nosological status of youth with severe, chronic irritability and symptoms like those seen in attention-deficit/hyperactivity disorder (ADHD). To facilitate research on these youth, Leibenluft, Charney, Towbin, Bhangoo, and Pine (2003) operationalized criteria for a syndrome called severe mood dysregulation (SMD); the SMD criteria were designed to capture the children at the center of the BD diagnosis controversy. SMD youth have chronic irritability, including a persistently angry mood and overreactivity to negative emotional stimuli, as well as hyperarousal symptoms (Figure 1; Leibenluft et al., 2003). Thus, they typically meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994) criteria for ADHD and oppositional defiant disorder (ODD), although, because the SMD criteria require extreme irritability, SMD youth are among the most severely impaired of those meeting criteria for comorbid ADHD and ODD. Because SMD children do not meet DSM-IV criteria for a distinct manic episode, they fail to meet BD diagnostic criteria. Despite this, youth with SMD symptoms are often diagnosed in the community with BD (Pogge et al., 2001). In comparison, children with narrow phenotype BD (NP-BD), as defined by Leibenluft et al., clearly meet DSM-IV criteria for BD, because they have a lifetime history of at least one distinct episode of mania or hypomania meeting DSM-IV duration criteria, with the additional proviso that their mania is defined by the presence of euphoria and/or grandiosity (Leibenluft et al., 2003).
Figure 1.
Research diagnostic criteria for severe mood dysregulation.
One way to address the diagnostic controversy surrounding pediatric BD is to compare SMD and NP-BD youth on a task that assesses a domain known to be impaired in one of these groups, to determine whether comparable deficits are found in the other. Such a study may also clarify the extent to which the pathophysiologies of SMD and BD are shared or distinct. One such domain is emotional face processing. The ability to accurately discern different emotional facial expressions is an integral component of competent human social interaction (Taylor, Batty, & Itier, 2004). Studies indicate that individuals with BD are impaired in their ability to process facial expressions. Adults with BD are deficient in facial affect perception (Addington & Addington, 1998; Bozikas, Tonia, Fokas, Karavatos, & Kosmidis, 2006; Getz, Shear, & Strakowski, 2003; Harmer, Grayson, & Goodwin, 2002; Lembke & Ketter, 2002), and two recent studies also find face processing deficits in youth with BD. Specifically, compared to controls, BD youth make more errors when labeling emotional faces (McClure et al., 2005) and misinterpret neutral facial expressions as being more hostile and fear-producing (Rich et al., 2006).
Results of these studies in BD youth generated three questions. Are face-labeling deficits specific to children with strictly defined manic episodes (e.g., NP-BD), or are such impairments also characteristic of children with chronic irritability and hyperarousal (e.g., SMD)? Are face-processing deficits in BD youth specific to certain emotional expressions? Are face-labeling deficits related to social deficits in youth with NP-BD or SMD?
The first question is relevant to the controversy surrounding the diagnosis of BD in children and the appropriate nosological status of SMD youth. By comparing face-labeling deficits in SMD and NP-BD youth, researchers can begin to identify common and divergent impairments. Researchers can then use these data to determine the extent to which these two disorders represent the same or different diagnostic entities.
Our second question was whether face-labeling deficits in BD youth were specific to certain emotions. We did not have the power to examine this issue in our previous studies documenting face emotion misidentification and misinterpretation in BD youth (McClure et al., 2005; Rich et al., 2006). The identification of emotion-specific face-labeling deficits in NP-BD and SMD youth might differentiate these syndromes while indicating targets for treatment. Further, because different emotional expressions are processed by distinct neural circuits (Adolphs, 2002), specifying the relationship between particular psychopathologies and misidentification of specific face emotions may begin to differentiate the pathophysiology of these disorders.
Our third and final question concerned potential associations between face-labeling deficits and social deficits in NP-BD and SMD youth. There is increasing evidence that BD children are socially impaired (Geller et al., 2000; Goldstein, Miklowitz, & Mullen, 2006; Lewinsohn, Seeley, & Klein, 2003; Rucklidge, 2006; Towbin, Pradella, Gorrindo, Pine, & Leibenluft, 2005). Prior work indicates an association between social deficits and face-labeling deficits in healthy and psychologically impaired individuals, because accurate interpretation of facial expressions is related to social competence, interpersonal conflict resolution, and reduced aggression (Cicchetti, Ackerman, & Izard, 1995; De Sonneville et al., 2002; Dodge et al., 2003; Klinnert, Emde, Butterfield, & Campos, 1987; Penn, Mueser, Spaulding, Hope, & Reed, 1995). However, the direction of a possible causal relationship remains unclear. Further, it is unclear whether such associations exist in BD or SMD youth. If so, this might suggest the utility of psychosocial treatment interventions.
In this study, we compared NP-BD and SMD youth on their ability to label emotional facial expressions using a multimorph task that allows a fine-grained assessment of the intensity required before specific facial emotions can be identified (Adolphs & Tranel, 2004; Blair, Colledge, Murray, & Mitchell, 2001). We then examined if impaired face labeling was associated with psychosocial dysfunction. We predicted that, compared to controls, both NP-BD and SMD youth would make more errors and require greater face emotion intensity before responding. We also predicted the deficits would differ between the two patient groups based upon the face emotion. Specifically, given that NP-BD youth have experienced manic episodes and SMD youth have not, we predicted that the NP-BD sample would respond more quickly than control and SMD subjects to happy faces. Finally, SMD youth display severe behavioral dysregulation and irritability comparable to that seen in youths with psychopathy. Because the latter display aberrant identification of distressed faces (i.e., fearful and sad; Blair & Coles, 2000; Blair et al., 2001; Blair, Jones, Clark, & Smith, 1997; Stevens, Charman, & Blair, 2001), we predicted that, compared to NP-BD and control subjects, SMD youth would require greater face emotion intensity to identify fearful and sad faces.
Methods and Materials
Participants
Inclusion/exclusion criteria
NP-BD (N = 39), SMD (N = 31), and controls (N = 36) were enrolled in an institutional review board-approved study at the National Institute of Mental Health. Parents and children gave written informed consent/assent. NP-BD subjects met DSM-IV criteria for BD, with the strict requirements of a history of at least one full duration hypomanic or manic episode, that is, lasting ≥4 days for hypomania or ≥7 days for mania, with abnormally elevated or expansive mood and/or grandiosity, and at least three DSM-IV criterion “B” mania symptoms (Leibenluft, Charney, Towin, Bhangoo, & Pine, 2003). Although youth with irritability but without elation met NP-BD inclusion criteria provided they had grandiosity and three other B criteria, in actuality all NP-BD youth reported here presented with euphoric mood. Of our current NP-BD subjects, 25 (64%) were included in the McClure et al. (2005) study of face emotion processing and 18 (46%) were included in the Rich et al. (2006) study.
Inclusion criteria for SMD were chronic irritability, characterized by angry mood and overreactivity to negative emotional stimuli (i.e., explosive tantrums at least three times weekly), and hyperarousal symptoms (including at least three of insomnia, intrusiveness, pressured speech, flight of ideas/racing thoughts, distractibility, psychomotor agitation; Leibenluft et al., 2003; Figure 1). Symptoms had to begin prior to age 12, and be present for at least 1 year without remission ≥2 months. Symptoms had to cause severe impairment in at least one setting (home, school, peers), and mild impairment in another. Euphoric mood or distinct episodes lasting ≥4 days were exclusionary (Leibenluft et al., 2003).
Measures
Diagnoses were made using the Kiddie Schedule for Affective Disorders—Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997), a semistructured diagnostic interview administered to parents and children separately by graduate level clinicians with established reliability (i.e., κ ≥ 0.9, agreement regarding the specific type of “BD spectrum” diagnosis, i.e., SMD vs. NP-BD, based on blinded video-tape review). Based on criteria established by Leibenluft et al. (2003), SMD was assessed using a KSADS supplementary module developed in collaboration with Joan Kaufman. Items in the SMD module specified that there had been no symptom-free period longer than 2 months in the past year, consistent with SMD criteria. Items drawn from the depression and mania sections were adapted so that, rather than ascertaining episodic symptoms, they assessed for the nonepisodic presentation of symptoms. This was done because the SMD criteria explicitly select for youth with chronic, nonepisodic illness; indeed, the presence of a DSM-IV episode of mania or hypomania is exclusionary for SMD. For all questions, symptoms were scored as not present, subthreshold (e.g., presence was of insufficient impairment, intensity, frequency, or duration), or meeting threshold.
The SMD module assesses the presence and severity of eight symptoms. Questions pertaining to seven of these symptoms (irritable mood, insomnia, racing thoughts, distractibility, physical restlessness, intrusiveness, and pressured speech) are drawn from the mania, depression, and ADHD sections of the KSADS. Specifically, the “racing thoughts” and “pressured speech” items are copied from the mania section. From the KSADS depression section, “irritable mood” is drawn from the irritability and anger question (i.e., copied with minor modifications to better map onto the clinical presentation of SMD youth), and “insomnia” is a composite of the initial, middle, and terminal insomnia questions in the depression section (i.e., those three questions that assess similar constructs were collapsed into a single question). Finally, from the KSADS ADHD section, “distractibility” is a composite of the difficulty sustaining attention and easily distracted questions, “physical restlessness” is drawn from the fidget question, and “intrusiveness” is drawn from the interrupts or intrudes item. The eighth and final SMD criteria, markedly excessive reactivity, is not assessed in the KSADS. Thus, using a format identical to that of other KSADS questions, this question assesses the subject’s tendency to become very angry, explode, scream, call people names, destroy things, and threaten or actually hurt another person in response to a negative emotional stimulus such as being told “no,” or other frustrating events. Reliability of the SMD module was established by comparing consistency of ratings for the SMD diagnosis between raters, and by differentiation of the SMD diagnosis from NP-BD and other KSADS diagnoses (all κs ≥ 0.9).
Exclusion criteria for both patient groups included IQ< 70, pervasive developmental disorder, unstable medical illness, or substance abuse within 2 months. Controls had normal physical and neurological examinations. They and a parent completed the KSADS to ensure that there was no mood disorder in the subject, and an interview established that there was no history of a mood disorder in any of the child’s first-degree relatives.
IQ was measured with the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). To evaluate mood, clinicians with interrater reliability administered the Children’s Depression Rating Scale (CDRS; Poznanski et al., 1984), and the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978). Patients completed the Manifest Anxiety Scale for Children (MASC; March, Parker, Sullivan, Stallings, & Conners, 1997). The Children’s Global Assessment Scale (CGAS; Shaffer et al., 1983) measured general level of function.
Social function was assessed using two measures: the Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987) and the Social Responsiveness Scale (SRS; Constantino et al., 2004). The LIFE is a clinician-administered interview given to parents. It evaluates functioning over the previous 6 months in domains including interpersonal relations with family and interpersonal relations with peers, among others. For family function, open-ended questions ask about general family relations, chores, rules, arguments, and problem solving. Peer relationship questions ask about school and neighborhood friendships and frequency of get togethers. Clinicians assess the quality of interpersonal relations, based on a 1-5 scale, where 1 = very good (e.g., experiences close emotional relationships, arguments are rare and quickly resolved, feels only minor need to improve quality of relations) and 5 = very poor (e.g., constantly argues with friends/family members or withdraws from them most of the time). The SRS is a parent-completed measure of social behaviors with an emphasis on social reciprocity (e.g., social awareness, social information processing, and capacity for reciprocal social responses). Studies support the validity and reliability of the SRS and find that it has a normal distribution in the population (Constantino & Todd, 2003).
Finally, because face-processing deficits have been identified in youth with pervasive developmental disorders (PDD; Baron-Cohen et al., 1999; Dalton et al., 2005), and social deficits similar to those in youth with SMD and NP-BD have been identified in PDD youth (Towbin et al., 2005), we examined the relationship between face-processing deficits and PDD traits in our patients. Specifically, parents of NP-BD and SMD youth completed the Social Communication Questionnaire (SCQ; Berument, Rutter, Lord, Pickles, & Bailey, 1999) and the Children’s Communication Checklist (CCC-2; Bishop, 1998). The SCQ, previously termed the Autism Screening Questionnaire, was designed to screen for a broad range of DSM-IV and International Classification of Diseases-10 PDD symptoms. Parents rate their child’s normative communication and social behaviors, such as directing another person’s attention, nodding one’s head, or initiating a social gesture, as well as deviant communication or social behaviors, such as using odd phrases over and over or asking strangers embarrassing questions. The CCC is a qualitative measure of pragmatic language and social communication. Parents rate their child’s speech, syntax, inappropriate initiation of conversation, coherence, stereotyped language, use of context, rapport, social ability, and interests. Although the CCC is not a screening measure for PDD, it does assess domains (e.g., pragmatic language and social communication) that are, by diagnostic definition, impaired in PDD youth (Bishop & Baird, 2001).
Procedure
The emotional expression multimorph task
The emotional expression multimorph task (morph task) is a variation of a task by Blair et al. (2001). Face stimuli were taken from the valid and reliable pictures of facial affect (Ekman & Friesen, 1976). Visual stimuli were created by blending a picture of a prototypical emotional expression (i.e., 100% intensity) with a picture of neutral facial expression (i.e., 0% intensity). Gradual blending created a continua of 39 morphed images of each emotional expression.
During the task, participants viewed each facial expression as it morphed through the 39 incremental stages, from neutral to the full emotional expression (Figure 2). The task included six different expressions: happiness, surprise, fear, sadness, anger, and disgust (Figure 3). Participants were told that the expression would begin as neutral, but would slowly change to reveal one of the six emotions, all of which were listed on the computer screen beneath the face stimulus. Subjects were instructed to carefully watch the face and, once they thought they knew the emotional expression being revealed, to press the “stop” button on the computer. This froze the morph, at which time the subject selected the emotional expression from the six options listed on the screen. Once the subject selected the emotion, the face continued through the remaining morph iterations. Subjects were told that they could stop the morph at any time to change their response. Finally, when the face reached the final morph, subjects were asked to provide their final response. Each morph iteration was presented for 100 ms, and each emotional expression was presented six times, in randomized order, for a total of 36 trials.
Figure 2.
Gradations of multimorph emotional expression. Depicted are examples of disgust facial expressions across the 39 increment stages from 0% intensity (i.e., neutral) to 100% intensity (i.e., prototypical emotional expression).
Figure 3.

Multimorph face emotions. Examples of the six types of facial emotions displayed during the emotional expression multimorph task are depicted.
Scoring of the emotional expression multimorph task
The primary outcome variables involved the response point, that is, the morph along the 1-39 continua at which the subject provided his/her response. Response point indicates the facial intensity required before recognition occurred. Two response point variables were created: first response point (i.e., the number of morphs required before first responding, regardless of accuracy), and correct response point (i.e., the number of morphs required before successfully identifying the emotional expression, where the final response was correct). Trials on which the final answer of the subject was incorrect were not used to calculate the correct response point; this was done so that subjects were not given credit for trials on which they may have at some point correctly identified the face expression, but later changed and remained with an erroneous answer. A higher response point score indicated better performance, that is, a 39 would indicate that the subject responded immediately at absolute neutral expression, whereas a 1 would indicate that the subject responded at the final morph iteration.
Other outcome variables were percentage of trials on which subjects changed their responses, total number of changes per trial, percentage correct on first response, percentage correct at final response (i.e., accuracy), and type of incorrect response.
Statistical procedures
Data analysis used repeated-measures analysis of covariance (ANCOVAs), where group (NP-BD, SMD, control) was the between-group factor, the measures described above were the dependent variables, and age, IQ, and gender served as covariates. Age and IQ were covariates due to group differences in these variables (see below). We controlled for gender based on previous work showing gender differences in facial affect processing in school-age children (Gross & Ballif, 1991; McClure, 2000; Brody, 1985; De Sonneville et al., 2002). Results were considered significant based on a two-tailed p ≤ .05. To minimize Type I errors, the Greenhouse-Geisser procedure was applied when appropriate for within-subject and repeated measures comparisons. All subsequent between-subject post hoc comparisons employed the Tukey honestly significant difference test.
To examine associations between impaired social function and face emotion labeling deficits, we conducted bivariate correlational analyses between correct response point and social function as measured by the LIFE and the SRS. Correct response was the variable of interest because, as presented below, both euthymic NP-BDs and SMDs differed from controls on this variable. To correct for multiple comparisons, p < .01 was required for significance. All data were analyzed using SPSS version 14.0 (SPSS, 2005).
Results
Participant demographics and clinical characteristics
Analysis of variance (ANOVA) comparison found significant group differences for age, F (2, 105) = 6.96, p = .001, and IQ, F (2, 105) = 6.82, p = .002. SMDs were significantly younger than both NP-BDs (p = .01) and controls (p = .002), and SMDs had significantly lower IQ than controls (p = .001), with a trend compared to NP-BDs (p = .08). There were no significant age or IQ differences between NP-BDs and controls. Because of these results, all comparisons controlled for age and IQ.
Among NP-BDs, 92.3% (N = 36) met criteria for BP-I. 66.7% (N = 26) of NP-BDs had comorbid diagnoses, the most common being ADHD, ODD, and social phobia. The most common diagnoses among the SMD children were ADHD (77.4%) and ODD (74.2%); anxiety disorders were also common (45.2%), and 87.1% had more than one DSM-IV diagnosis (Table 1).
Table 1. Demographic data.
| NP-BD | SMD | Control | p Value | |
|---|---|---|---|---|
| N | 39 | 31 | 36 | |
| Age (years) | 13.99 ± 2.63 | 12.29 ± 2.12 | 14.34 ± 2.28 | .001 |
| Gender (male), % (N) | 53.80 (21) | 61.30 (19) | 55.60 (20) | .81 |
| IQ | 110.08 ± 14.15 | 102.74 ± 14.36 | 115.61 ± 14.21 | .002 |
| CGAS | 48.95 ± 12.41 | 47.50 ± 7.76 | — | .60 |
| No. of diagnoses | 2.33 ± 1.30 | 2.55 ± 1.23 | — | .49 |
| Diagnoses, % (N) | ||||
| BP I | 92.30 (36) | — | — | — |
| BP II | 7.70 (3) | — | — | — |
| ADHD | 46.20 (18) | 77.40 (24) | — | .02 |
| ODD | 23.10 (9) | 74.20 (23) | — | .001 |
| Any anxiety | 35.90 (14) | 45.20 (14) | — | .59 |
| GAD | 15.40 (6) | 29.00 (9) | — | .28 |
| Separation anxiety | 15.40 (6) | 25.80 (8) | — | .43 |
| Social phobia | 20.50 (8) | 9.70 (3) | — | .37 |
| MDD | — | 9.70 (3) | — | — |
| No. of medications when medicated | 2.88 ± 1.16 | 2.29 ± 1.14 | .12 | |
| Medications | 82.10 (32) | 45.20 (14) | — | .003 |
| Antiepileptics | 64.10 (25) | 19.40 (6) | — | .001 |
| Atypical antipsychotics | 51.30 (20) | 16.10 (5) | — | .005 |
| Lithium | 35.90 (14) | 22.60 (7) | — | .35 |
| Stimulants | 35.90 (14) | 29.00 (9) | — | .73 |
| Antidepressants | 23.10 (9) | 12.90 (4) | — | .44 |
| Mood rating scores | ||||
| CDRS | 30.05 ± 9.09 | 26.45 ± 5.24 | — | .06 |
| YMRS | 10.84 ± 6.73 | 9.83 ± 7.25 | — | .56 |
| MASC t score | 43.63 ± 11.35 | 44.75 ± 10.41 | — | .77 |
| Social function scores | ||||
| SRS | 80.39 ± 17.30 | 83.27 ± 25.32 | — | .94 |
| LIFE: family | 2.70 ± 0.77 | 2.71 ± 0.87 | — | .96 |
| LIFE: friends | 3.03 ± 1.12 | 2.72 ± 1.02 | — | .35 |
Note: NP-BD, narrow phenotype bipolar disorder; SMD, severe mood dysregulation; CGAS, Children’s Global Assessment Scale; BP, bipolar disorder; ADHD, attention-deficit/hyperactivity disorder; GAD, generalized anxiety disorder; MDD, major depressive disorder; ODD, oppositional defiant disorder; CDRS, Children’s Depression Rating Scale; YMRS, Young Mania Rating Scale; MASC, Manifest Anxiety Scale for Children; SRS, Social Reciprocity Scale; LIFE, Longitudinal Interval Follow-up Evaluation. All diagnoses are current.
ANOVA comparisons of CDRS, F (1, 67) = 3.82, p = .06, YMRS, F (1, 67) = .35, p = .56, and MASC, F (1, 46) = .09, p = .77, scores showed that NP-BDs did not differ from SMDs on mood ratings. Scores showed that 48.7% (N = 19) of NP-BDs and 58.1% (N = 18) of SMDs were euthymic at testing (i.e., CDRS ≤40 and YMRS ≤12). Of the 20 noneuthymic NP-BDs, 13 were hypomanic, 6 had mixed hypomania, and 1 was depressed. None of the SMD were currently depressed, and because, by definition, no SMD child had a definable episode of mania, YMRS scores >12 in 13 SMD patients reflected hyperarousal symptoms, rather than manic symptoms per se. CGAS, LIFE, and SRS scores, comparable between patient groups, indicated severe overall impairment and moderately impaired social function (Table 1). Among NP-BDs, 82.1% (N = 32) were medicated, with a mean of 2.9 ± 1.2 medications per subject. Among SMDs, 45.2% (N = 14) were medicated, with a mean of 2.6 ± 1.2 medications (see Table 1 for specific medications).
Faces morph behavioral data
The repeated-measures ANCOVA for first response point revealed a significant main effect of group, F (2, 100) = 6.08, p = .003, with both NP-BDs (p = .001) and SMDs (p = .02) requiring more morphs before responding than did controls. The Group × Emotion interaction was nonsignificant, F (10, 192) = 1.62, p = .10, indicating that this group difference did not differ based on the facial emotion. The main effect for all three covariates was nonsignificant: age, F (5, 96) = .61, p = .70; IQ, F (5, 96) = .77, p = .58; gender, F (5, 96) = 2.13, p = .07.
The repeated-measures ANCOVA for correct response point yielded a significant main effect of group, F (2, 98) = 8.33, p, < .001; compared to controls, both NP-BDs (p, < .001) and SMDs (p = .005) required more morphs before responding correctly. The main effect for all three covariates was nonsignificant: age, F (5, 94) = 1.05, p = .40; IQ, F (5, 94) = 1.08, p = .38; gender, F (5, 94) = 1.10, p = .37. The Group × Emotion interaction was significant, F (10, 188) = 1.91, p = .04. Post hoc analyses revealed significant between-group differences for disgusted, F (2, 98) = 8.85, p, < .001, surprised, F (2, 98) = 5.73, p = .004, fearful, F (2, 98) = 4.36, p = .02, and happy, F (2, 98) = 8.05, p = 0.001 faces, with a trend for angry, F (2, 98) = 2.88, p = .06. The group-level contrast for sad was nonsignificant, F (2, 98) = .96, p = .39. For all emotions on which group differences were found, both NP-BDs and SMDs had significantly later correct response points than controls, indicating that they required more intense facial expressions before they responded correctly (Table 2 and Figure 4). NP-BDs did not differ from SMDs on any of the correct response point measures.
Table 2. Group differences in response points on faces multimorph task.
| Response Point | NP-BD | SMD | Control | p Value |
|---|---|---|---|---|
| First overall | 12.24 ± 4.28 | 12.13 ± 5.34 | 16.32 ± 5.02 | .003 |
| Correct: overall | 10.68 ± 3.78 | 10.14 ± 3.99 | 14.52 ± 4.44 | <.001 |
| Correct: disgusted | 7.20 ± 4.13 | 7.04 ± 5.42 | 13.03 ± 7.23 | <.001 |
| Correct: surprised | 11.48 ± 4.90 | 9.79 ± 4.95 | 15.31 ± 5.44 | .004 |
| Correct: fearful | 8.45 ± 4.22 | 7.99 ± 3.91 | 11.38 ± 4.68 | .02 |
| Correct: happy | 17.66 ± 6.15 | 18.52 ± 6.46 | 23.36 ± 4.60 | <.001 |
| Correct: angry | 10.90 ± 4.86 | 10.48 ± 6.04 | 14.22 ± 6.81 | .06 |
| Correct: sad | 8.32 ± 4.23 | 7.05 ± 3.91 | 9.84 ± 5.42 | .39 |
Note: NP-BD, narrow phenotype bipolar disorder; SMD, severe mood dysregulation. Responses indicate points along expression morph at which subjects responded, where higher scores indicate better performance, that is, fewer morphs required before successful emotion recognition. Results of repeated measures analyses of covariance, age, IQ, and gender as covariates. For all post hoc comparisons, both NP-BD and SMD samples required significantly more morphs before responding, but the two patient samples did not differ from each other.
Figure 4.
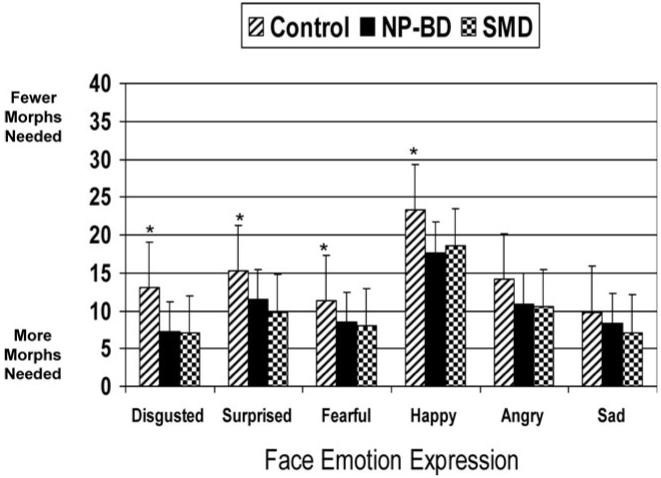
The correct response point. NP-BD, narrow phenotype-bipolar disorder; SMD, severe mood dysregulation. *p < .05 for both controls versus NP-BDs and controls versus SMDs. Shown is the response point along the 1-39 continua at which subjects correctly identified the facial expression. Scores are adjusted after controlling for age, IQ, and gender. Compared to controls (N = 36), NP-BDs (N = 39) and SMDs (N = 31) required significantly greater face emotion intensity before correctly labeling the expression for disgusted, F (2, 98) = 8.85, p < .001, surprised, F (2, 98) = 5.73, p = .004, fearful, F (2, 98) = 4.36, p = .02, and happy, F (2, 98) = 8.05, P = .001, p = .001 faces. NP-BDs and SMDs did not differ from each other.
We conducted repeated-measures ANCOVAs for our other outcome variables: percentage of trials with changed responses, total number of changes per trial, percentage correct on first response, percentage correct on final response, and type of incorrect response. All group, Group × Emotion interactions, and covariate effects were nonsignificant.
Post hoc analyses
Post hoc analyses focused on associations between correct response point and clinical and demographic variables. Age, IQ, and gender were again used as covariates. Caution should be used when interpreting these post hoc analyses because of the risk of Type I error due to multiple comparisons and risk of Type II error due to small sample sizes and limited power.
Demographic variables
Although age, gender, and IQ showed nonsignificant main effects when entered as covariates in our repeated measures ANCOVAs, we further examined their relationship to face processing. For each sample we conducted bivariate correlational analyses between correct response point and age and IQ, separately, as well as repeated-measures ANOVAs with gender as the between-group variable. For all subjects, age and IQ did not significantly correlate with correct response point. There was a significant main effect of gender in both controls, F(1, 34) = 7.24, p = .01, and NP-BD subjects, F (1, 35) = 4.27, p = .04, with females responding correctly at a significantly earlier morph point than males. The main effect of gender was nonsignificant in SMD subjects, F (1, 29) = .04, p = .84. In all three samples, the Gender × Face Emotion interaction was nonsignificant.
Mood state
Because only one NP-BD subject was depressed, in our analyses of the impact of mood state, we removed that subject from the data set and divided the NP-BD sample into euthymic (N = 19) and hypomanic/mixed (N = 19). We then compared these two NP-BD samples to SMDs and controls using an ANCOVA, with age, IQ, and gender as covariates. We did not divide the SMD sample because none were acutely depressed and, by definition, none were currently manic/hypomanic.
With correct response point, we found a significant main effect for group, F (3, 96) = 6.81, p, < .001, a significant Group × Emotion interaction, F (15, 480) = 1.78, p = .04, but non-significant main effects for the covariates. With the group effect, all three patient groups required significantly more morphs than did controls before responding correctly: versus controls, euthymic NP-BDs (p = .04), hypomanic/mixed NP-BDs (p, = .001), and SMDs (p = .004). The three patient groups did not differ from each other. Post hoc analyses of the Group × Emotion interaction revealed that, compared to controls, hypomanic/mixed NP-BDs and SMDs required significantly more morphs before responding correctly on disgusted (hypomanic/mixed NP-BDs: p = .002; SMDs: p = .004), surprised (hypomanic/mixed NP-BDs: p < .001; SMDs: p = .004), fearful (hypomanic/mixed NP-BDs: p = .002; SMDs: p = .04), and happy (hypomanic/mixed NP-BDs: p < .001; SMDs: p = .04) faces. Euthymic NP-BDs required significantly more morphs than controls before correctly responding to disgusted (p = .001) and happy faces (p = .03). Finally, before responding correctly, hypomanic/mixed NP-BDs required significantly more morphs than did euthymic NP-BDs on surprised faces (p = .02), and more than did SMDs on happy faces (p = .04). In sum, hypomanic/mixed NP-BDs display face-labeling deficits across all emotions, whereas euthymic NP-BDs have face-labeling deficits on disgusted and happy faces.
Comorbid diagnoses
Using correct response point as the outcome variable, we conducted a series of ANCOVAs comparing NP-BD with versus without comorbid anxiety, SMD with versus without comorbid anxiety, NP-BD with versus without ADHD, and NP-BD with versus without ODD. Analyses examining the impact of ADHD and ODD diagnoses could not be conducted in the SMD sample because most subjects had these diagnoses. For all ANCOVAs, we found no significant differences in response point scores based on the presence or absence of any comorbidity. Finally, we conducted bivariate correlational analyses, Bonferroni corrected for multiple comparisons, between correct response point and scores on two measures of PDD symptomatology: the SCQ (Berument et al., 1999) and the CCC-2 (Bishop, 1998). SCQ and CCC scores did not correlate significantly with face-processing ability in either the NP-BD or SMD sample.
Social function
Bivariate correlational analyses compared face labeling in the SMD and NP-BD samples, analyzed separately, and social function as measured by LIFE scores in the family and friends domains and total SRS scores. Face-labeling ability was measured by correct response point overall and correct response point for those emotions on which NP-BD and SMD subjects differed from controls (i.e., disgusted, surprised, fearful, and happy faces). In the SMD sample, there was a significant relationship between worse family function and requiring greater emotion intensity before responding correctly for all emotions (r = -.76, p = .002), and between worse family function and requiring greater emotion intensity before responding correctly for fearful (r = -.79, p = .001) and happy faces (r = -.71, p = .004; Table 3). Interpersonal relationships with friends was not significantly correlated with any response point measures in SMD youth. In the NP-BD sample, family and friend social function was not correlated significantly with any response point measures. SRS scores were unrelated to face-processing deficits in SMD youth, but in NP-BD youth, poorer social reciprocity correlated significantly with requiring more intense emotion displays for a correct response across emotion type (r = -.48, p = .006; Table 3).
Table 3. Correlations between correct response point on faces multimorph task and measures of social functioning.
| SMD |
NP-BD |
|||||
|---|---|---|---|---|---|---|
| Correct Response Point | LIFE: Family | LIFE: Friends | SRS | LIFE: Family | LIFE: Friends | SRS |
| Overall | -.76** | -.47 | -.15 | -.04 | -.16 | -.48** |
| Fearful faces | -.79** | -.34 | -.17 | -.06 | -.21 | -.28 |
| Happy faces | -.71** | -.36 | -.15 | -.10 | -.25 | -.39 |
| Disgusted faces | -.53 | -.02 | -.20 | -.04 | -.06 | -.25 |
| Surprised faces | -.58 | -.47 | -.12 | -.10 | -.11 | -.42 |
Note: NP-BD, narrow phenotype bipolar disorder; SMD, severe mood dysregulation; LIFE, Longitudinal Interval Follow-up Evaluation; SRS, Social Reciprocity Scale.
p < .01.
Discussion
In this study we used a face emotion labeling task to compare children with NP-BD (i.e., episodic mania with elevated and/or grandiose mood), to those with SMD (i.e., chronic irritability, marked reactivity, and hyperarousal, but no episodic mania; Leibenluft et al., 2003), and controls. We sought to determine if children with NP-BD and SMD differ in their ability to label emotional facial expressions, if face-processing deficits in these patients were specific to particular emotions, and if impaired face labeling was associated with psychosocial dysfunction.
Regarding our first research aim, we found that, compared to controls, both NP-BD and SMD youth displayed face-processing deficits, but the patient samples did not differ from each other. Specifically, both NP-BD and SMD subjects required significantly greater intensity of emotional expression than did controls before the first response and before correctly identifying the facial expression. The finding that our patient samples require more intense displays of face emotion is important because subtle displays of emotion are common in social interactions, and facial expressions change rapidly during social interactions (Addington & Addington, 1998; De Sonneville et al., 2002). These results extend previous work with NP-BD children (McClure et al., 2005; Rich et al., 2006) by documenting that face-processing deficits are also present in SMD youth. These results are also consistent with another study that used a task of face emotion labeling and found that NP-BD and SMD youth were equally deficient in labeling face emotions compared to controls and youth with ADHD and anxiety/depression, who themselves were not impaired (Guyer et al., 2007). Although methodological differences between that study and our current study may limit the complete integration of these results, it is notable that two different paradigms found NP-BD and SMD youth to be equally impaired when processing faces.
Because the rate of ADHD in both patient samples is high, one might have predicted that NP-BD and SMD youth would have responded more quickly (i.e., impulsively) than controls to the faces. We found just the opposite, in that the time to first response was longer in both patient samples than in controls. This result, coupled with the similar accuracy rates among our three samples, suggests that NP-BD and SMD youth were in fact attending to the faces. In addition, it is possible that the group differences in response point reflect slowed motor responsivity in patients, rather than a face-labeling deficit per se. Although we cannot rule this out, the fact that the subjects’ later responding was not found across all emotion types (i.e., subjects were deficient on happy, surprised, fearful, and disgusted faces, but not sad faces) does not seem to indicate an overall slowness to respond. In sum, we believe that our results support the conclusions that, (a) compared to controls, NP-BD and SMD youth require significantly greater intensity of emotional expression before they can identify a facial emotion, and (b) our findings do not simply reflect inattention or motor slowness in patients.
Our data here should be interpreted in light of other research comparing SMD and NP-BD youth. Previous studies, coupled with the data presented here, demonstrate shared and divergent affective, behavioral, cognitive, and psychophysiological impairments in NP-BD versus SMD youth. Indeed, the question of the nosological relationship between SMD and NP-BD may have a dimensional, rather than categorical, answer, because BD is a multigenic illness with many affective and behavioral diagnostic symptoms (Althoff, Faraone, Rettew, Morley, & Hudziak, 2005; Kowatch, Youngstrom, Danielyan, & Findling, 2005). Overall, research to date largely supports the notion that SMD is distinct from NP-BD: children with SMD have an elevated risk for MDD at age 18 (Brotman et al., 2006); youth with NP-BD are more likely than those with SMD to have a family history of BD (Brotman et al., 2007); and SMD and NP-BD youth differ in response flexibility deficits and in the brain mechanisms mediating response to frustration (Dickstein et al., 2007; Rich et al., 2007). Nonetheless, the fact that both SMD and NP-BD exhibit face-labeling deficits, and that those deficits are not present in other psychopathological groups (Guyer et al., 2007), indicates that SMD and NP-BD may ultimately be found to be on the same pathophysiological continuum.
Our results may also suggest avenues for beginning to distinguish the neural substrates of SMD and NP-BD. Pathophysiological differences may be apparent even when behavioral differences are absent, because the same behavioral deficit may be mediated by different brain mechanisms (Wilkinson & Halligan, 2004). Thus, despite the comparable performance between SMD and NP-BD youth in this study, face-processing tasks may identify different neural correlates of these two disorders. There is substantial overlap between the neural regions implicated in the pathophysiology of pediatric BD and those involved in processing face emotions. For example, in BD youth, volumetric and functional aberrations have been documented in the amygdala (Blumberg et al., 2005; Blumberg, Kaufman et al., 2003; Chang et al., 2005; Chen et al., 2004; Delbello, Zimmerman, Mills, Getz, & Strakowski, 2004; Dickstein et al., 2005; Rich et al., 2006), orbitofrontal cortex (Rich et al., 2006; Wilke, Kowatch, Delbello, Mills, & Holland, 2004), cingulate (Chang et al., 2004; Kaur et al., 2005; Wilke et al., 2004), insula (Chang et al., 2004), putamen (Blumberg, Martin, et al., 2003; Chang et al., 2004; Delbello et al., 2004), and basal ganglia (Wilke et al., 2004; Leibenluft et al., 2007). These same neural regions have been shown to assist in the processing of emotional facial expressions (Adolphs et al., 1999; Blair, Morris, Frith, Perrett, & Dolan, 1999; Gorno-Tempini et al., 2001; Killgore & Yurgelun-Todd, 2004; Morris et al., 1996; Phillips et al., 1997, 1998; Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998; Yang et al., 2002; Whalen et al., 1998). Functional magnetic resonance imaging studies utilizing face-processing tasks may identify the neural correlates of NP-BD and SMD and clarify the extent to which the pathophysiology of these two disorders overlaps or differs.
A second goal of this study was to determine if face-labeling deficits were specific to certain emotional expressions. We predicted that, given their history of mania, NP-BD youth might show particular sensitivity to happy faces. We also predicted that, because SMD youth display severe behavioral dysregulation and irritability similar to that seen in psychopathic youths, SMD youth would display aberrant identification of fearful and sad faces similar to psychopathic youths (Blair et al., 1997, 2000, 2001; Stevens et al., 2001). In general, these predictions were not supported. Of the six emotional expressions used in this task, both samples of patients were significantly impaired on four (happy, surprised, fearful, and disgusted), and there was a strong trend for impairment on a fifth (anger). Only sad faces failed to elicit NP-BD or SMD deficits. Our results indicate that the difficulty identifying facial emotions in NP-BD and SMD youth is evident across multiple emotions, and is best characterized as a general face emotion identification impairment, as opposed to being specific to certain emotions.
As previously noted, another study from our group found no face emotion identification deficits in youth with ADHD or comorbid depression and anxiety (Guyer et al., 2007). However, the literature has not been entirely consistent on this point. That is, some studies find that ADHD youth display a generalized face-labeling deficit across emotions when face stimuli are paired with voice recordings (Cadesky, Mota, & Schachar, 2000; Corbett & Glidden, 2000). Similarly, other studies suggest that some childhood psychopathologies may be associated with deficits recognizing specific emotions; for example, threatening faces (i.e., fearful and angry) in depressed children (Lenti, Giacobbe, & Pegna, 2000), angry faces with maltreated (Pollak & Kistler, 2002; Pollak, Cicchetti, Hornung, & Reed, 2000) and physically abused children (Pollak & Tolley-Schell, 2003), and distressed faces (i.e., fearful and sad) in children with psychopathy (Blair et al., 1997, 2000, 2001; Stevens et al., 2001). Clearly, direct comparisons of different childhood psychopathologies (e.g., youth with anxiety, depression, ADHD, ODD, BD, and SMD), using an identical face-processing task, are needed to clarify the specificity of face-processing deficits between diagnoses.
Elucidating face-processing impairments in BD may also be achieved using developmental studies that compare children and adults on identical tasks. Comparable to the face emotion labeling difficulties we identified in NP-BD youth, prior work finds that adults with BD have face emotion labeling deficits that do not appear to be emotion specific (Bozikas et al., 2006; Getz et al., 2003; Lembke et al., 2002; Yurgelun-Todd et al., 2000). For example, whereas two studies suggest that adults with BD may show enhanced recognition of disgust (Harmer et al., 2002) and sad faces (Lennox, Jacob, Calder, Lupson, & Bullmore, 2004), other studies find BD adults to be deficient in their identification of these specific face emotions (Bozikas et al., 2006; Getz et al., 2003; Lembke et al., 2002). Further, when identifying happy faces, studies have found BD adults both to be deficient (Bozikas et al., 2006; Getz et al., 2003) and comparable to controls (Yurgelun-Todd et al., 2000).
The inconsistency of the data in BD adults, coupled with minimal data on BD youth, makes it difficult to draw conclusions regarding the developmental progression of face-processing deficits in individuals with BD. In contrast, there may be differences in face processing between youth and adults with BD. These may reflect a variety of developmental factors, including adults’ more extended exposure to social interactions (which may improve face-processing skills), longer use of psychotropic medications (which may lessen the neurochemical or neurophysiological dysfunction associated with impaired face processing), or developmental changes in brain function. At the same time, in this study we did not find an age effect on our results, and a prior study with BD adults found that impaired face affect identification was not related to age of onset or duration of illness (Bozikas et al., 2006). The lack of age effects may reflect the fact that by 6 years of age, the recognition of basic emotions such as happy, angry, scared, and sad, appears to be fully developed (Markham & Adams, 1992; McClure, 2000), and there is not substantial improvement in face emotion identification accuracy after age 10 (Harrigan, 1984). A longitudinal study of children at risk for BD, or a comparison of subjects with early-onset BD to those with adult-onset BD, would begin to elucidate the developmental progression of deficient face emotion processing in individuals with BD.
An additional line of future research may explore the role of gender in BD youth when processing faces. Consistent with a prior review in healthy youth (McClure, 2000), we found that, in NP-BD youth and controls, males may require more intense displays of facial expression for accurate emotion identification than do females. Gender-related differences were not identified in SMD youth. Studies with large samples are necessary to explore further the role of gender in NP-BD face-labeling deficits.
Our third goal in this study was to examine associations between social impairment and face-labeling deficits. Two aspects of social function were measured: social reciprocity and a general measure of social interactions with peers and family. In the NP-BD sample, we found a negative correlation between delayed face emotion identification and parentreported social reciprocity (e.g., social awareness, social information processing, and capacity for reciprocal social responses). These results expand upon a prior study of parent-reported poor social skills in BD youth (Goldstein et al., 2006).
In contrast, in the SMD sample we found an association between delayed face emotion identification, in particular of fearful and happy faces, and impaired family function. The relationship between impaired face processing and family dysfunction may be bidirectional. Consistent with prior work (Cicchetti & Curtis, 2005; Pollak & Kistler, 2002; Pollak, Klorman, Thatcher, & Cicchetti, 2001; Marshall & Fox, 2004; Parker & Nelson, 2005), adverse environmental conditions, such as family dysfunction, may result in the impaired face emotion processing seen in SMD youth. Indeed, recent work suggests that aberrant social environments may impact adversely the development of neuropeptide systems central to social and emotional development (Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). Conversely, impaired identification of facial emotions may lead to problematic family interactions in SMD children. Our results suggest that psychotherapeutic interventions might target NP-BD and SMD children’s face-labeling deficits, perhaps in the form of psychoeducation and practicing face emotion identification, in an effort to improve social function.
One potential limitation of our study is the varied mood states of our NP-BD subjects. However, consistent with previous data (McClure et al., 2005; Rich et al., 2006), we found that euthymic NP-BD patients were impaired in face emotion recognition when compared to controls. Another limitation is the high rates of co-occurring diagnoses, particularly ADHD and ODD, in our NP-BD sample, although this rate is typical of that seen in other samples of BD children (Biederman, Faraone, Chu, & Wozniak, 1999). When we compared NP-BD subjects with and without comorbid ADHD or ODD, we did not find between-group differences in response point. However, small sample sizes limit the interpretation of these negative results. Comparable examinations in the SMD sample were not possible due to the high rates of ADHD and ODD in this group; indeed, SMD, ODD, and ADHD can be viewed as different approaches to describing closely related clinical phenomena. Future studies should compare BD children with and without comorbid conditions (e.g., ADHD, ODD, anxiety) to children with ADHD, ODD, and anxiety.
In conclusion, the current study extends prior work on face-processing deficits in bipolar youth by documenting face-labeling deficits in SMD children, that is, those in whom the BD diagnosis is controversial. Our results indicate that NP-BD and SMD youth require significantly greater intensity of facial expression before correctly identifying an emotion, in particular disgust, surprise, fear, and happiness. Finally, we provide evidence of an association between impaired face labeling and impaired social reciprocity in NP-BD youth and impaired family function in SMD youth.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIMH. We gratefully acknowledge the children and families of patients and controls without whose participation this research would not have been possible. We also thank the staff of the Section on Bipolar Spectrum Disorders at the NIMH.
References
- Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophrenia Research. x1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Currrent Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. Journal of Cognitive Neuroscience. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Althoff RR, Faraone SV, Rettew DC, Morley CP, Hudziak JJ. Family, twin, adoption, and molecular genetic studies of juvenile bipolar disorder. Bipolar Disorder. 2005;7:598–609. doi: 10.1111/j.1399-5618.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism Screening Questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Chu MP, Wozniak J. Further evidence of a bidirectional overlap between juvenile mania and conduct disorder in children. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:468–476. doi: 10.1097/00004583-199904000-00021. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Development of the Children’s Communication Checklist (CCC): A method for assessing qualitative aspects of communicative impairment in children. Journal of Child Psychology and Psychiatry. 1998;39:879–891. [PubMed] [Google Scholar]
- Bishop DV, Baird G. Parent and teacher report of pragmatic aspects of communication: Use of the children’s communication checklist in a clinical setting. Developmental Medicine and Child Neurology. 2001;43:809–818. doi: 10.1017/s0012162201001475. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Coles M. Expression recognition and behavioural problems in early adolescence. Cognitive Development. 2000;15:421–434. [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: A lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disorders. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: Preliminary observations from functional MRI. American Journal of Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Tonia T, Fokas K, Karavatos A, Kosmidis MH. Impaired emotion processing in remitted patients with bipolar disorder. Journal of Affective Disorders. 2006;91:53–56. doi: 10.1016/j.jad.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Brody L. Gender differences in emotional development: A review of theories and research. Journal of Personality. 1985;53:102–149. [Google Scholar]
- Brotman MA, Kassem L, Reising M, Guyer AE, Dickstein DP, Rich BA, et al. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. American Journal of Psychiatry. 2007;164:1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello AJ, et al. Prevalence, clinical correlates, and longitudinal course of severe mood and behavioral dysregulation in children. Biological Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Cadesky EB, Mota VL, Schachar RJ. Beyond words: How do children with ADHD and/or conduct problems process nonverbal information about affect? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1160–1167. doi: 10.1097/00004583-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Ackerman B, Izard C. Emotions and emotion regulation in developmental psychopathology. Development and Psychopathology. 1995;7:1–10. [Google Scholar]
- Cicchetti D, Curtis WJ. An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Developmental Psychopathology. 2005;17:641–677. doi: 10.1017/S0954579405050315. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Corbett B, Glidden H. Processing affective stimuli in children with attention-deficit hyperactivity disorder. Child Neuropsychology. 2000;6:144–155. doi: 10.1076/chin.6.2.144.7056. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sonneville LM, Verschoor CA, Njiokiktjien C, Op het Veld V, Toorenaar N, Vranken M. Facial identity and facial emotions: Speed, accuracy, and processing strategies in children and adults. Journal of Clinical and Experimental Neuropsychology. 2002;24:200–213. doi: 10.1076/jcen.24.2.200.989. [DOI] [PubMed] [Google Scholar]
- Delbello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al. Frontotemporal alterations in pediatric bipolar disorder: Results of a voxel-based morphometry study. Archives of General Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Lansford JE, Burks VS, Bates JE, Pettit GS, Fontaine R, et al. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Development. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Bolhofner K, Craney JL, Williams M, Delbello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- Getz GE, Shear PK, Strakowski SM. Facial affect recognition deficits in bipolar disorder. Journal of the International Neuropsychological Society. 2003;9:623–632. doi: 10.1017/S1355617703940021. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Miklowitz DJ, Mullen KL. Social skills knowledge and performance among adolescents with bipolar disorder. Bipolar Disorders. 2006;8:350–361. doi: 10.1111/j.1399-5618.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: An fMRI study. NeuroImage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Gross AL, Ballif B. Children’s understanding of emotion from facial expression and situations: A review. Developmental Review. 1991;11:368–398. [Google Scholar]
- Guyer AE, McClure EB, Adler A, Brotman MA, Rich BA, Pine DS, et al. Specificity of facial expression labeling deficits in childhood psychopathology. Journal of Clinical Psychology and Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Grayson L, Goodwin GM. Enhanced recognition of disgust in bipolar illness. Biological Psychiatry. 2002;51:298–304. doi: 10.1016/s0006-3223(01)01249-5. [DOI] [PubMed] [Google Scholar]
- Harrigan J. The effects of task order on children’s identification of facial expressions. Motivation and Emotion. 1984;8:157–169. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. American Journal of Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. NeuroImage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Emde RN, Butterfield P, Campos JJ. Social referencing: The infant’s use of emtoional signals from a friendly adult with mother present. Annual Progress in Child Psychiatry and Child Development. 1987;22:427–432. [Google Scholar]
- Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disorders. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. American Journal of Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Lenti C, Giacobbe A, Pegna C. Recognition of emotional facial expressions in depressed children and adolescents. Perceptual and Motor Skills. 2000;91:227–236. doi: 10.2466/pms.2000.91.1.227. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Klein DN. Bipolar disorders during adolescence. Acta Psychiatrica Scandinavica Supplementum. 2003:47–50. doi: 10.1034/j.1600-0447.108.s418.10.x. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Markham R, Adams K. The effect of type of task on childrens’ identification of facial expressions. Journal of Nonverbal Behavior. 1992;16:21–39. [Google Scholar]
- Marshall PJ, Fox NA. A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: An event-related potential study. Child Development. 2005;76:54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Penn DL, Mueser KT, Spaulding W, Hope DA, Reed D. Information processing and social competence in chronic schizophrenia. Schizophrenia Bulletin. 1995;21:269–281. doi: 10.1093/schbul/21.2.269. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, et al. Investigation of facial recognition memory and happy and sad facial expression perception: An fMRI study. Psychiatry Research. 1998;83:127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pogge DL, Wayland-Smith D, Zaccario M, Borgaro S, Stokes J, Harvey PD. Diagnosis of manic episodes in adolescent inpatients: Structured diagnostic procedures compared to clinical chart diagnoses. Psychiatry Research. 2001;101:47–54. doi: 10.1016/s0165-1781(00)00248-1. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. American Journal of Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton D, Roberson-Nay R, Hommer R, Berghorst L, McClure E, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ. Psychosocial functioning of adolescents with and without paediatric bipolar disorder. Journal of Affective Disorders. 2006;91:181–188. doi: 10.1016/j.jad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children’s Global Assessment Scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings in Biological Sciences/ The Royal Society. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair RJ. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. The Journal of Genetic Psychology. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: A review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Towbin KE, Pradella A, Gorrindo T, Pine DS, Leibenluft E. Autism spectrum traits in children with mood and anxiety disorders. Journal of Child and Adolescent Psychopharmacology. 2005;15:452–464. doi: 10.1089/cap.2005.15.452. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, Delbello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: First results. Psychiatry Research. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nature Reviews. Neuroscience. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. NeuroReport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Britsh Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]



