Abstract
AIM: To compare the treatment modalities for patients with massive pancreaticojejunal anastomotic hemorrhage after pancreatoduodenectomy (PDT).
METHODS: A retrospective study was undertaken to compare the outcomes of two major treatment modalities: transcatheter arterial embolization (TAE) and open surgical hemostasis. Seventeen patients with acute massive hemorrhage after PDT were recruited in this study. A comparison of two treatment modalities was based upon the clinicopathological characteristics and hospitalization stay, complications, and patient prognosis of the patients after surgery.
RESULTS: Of the 11 patients with massive hemorrhage after PDT treated with TAE, one died after discontinuing treatment, the other 10 stopped bleeding completely without recurrence of hemorrhage. All the 10 patients recovered well and were discharged, with a mean hospital stay of 10.45 d after hemostasis. The patients who underwent TAE had a re-operation rate of 18.2% and a mortality rate of 9.1%. Among the six patients who received open surgical hemostasis, two underwent another round of open surgical hemostasis. The mortality was 50%, and the recurrence of hemorrhage was 16.67%, with a mean hospital stay of 39.5 d.
CONCLUSION: TAE is a safe and effective treatment modality for patients with acute hemorrhage after PDT. Vasography should be performed to locate the bleeding site.
Keywords: Pancreatoduodenectomy, Massive hemorrhage, Transcatheter artery embolization, Complication, Treatment
INTRODUCTION
Massive pancreaticojejunal anastomotic hemorrhage is the second most common complication of pancreatoduodenectomy (PDT). Blind open surgical hemostasis, however, poses additional risks and complications, which can be prevented by transcatheter arterial embolization (TAE). The present study was to evaluate the effectiveness of TAE and open surgical hemostasis. Between June 2005 and August 2008, a total of 308 patients underwent PDT in our hospital. Of these patients, 17 had massive pancreaticojejunal anastomotic hemorrhage following PDT. In this retrospective study, we summarized our clinical experiences with these patients in order to compare the safety and efficacy of TAE and open surgical hemostasis. The results may help determine the therapeutic approaches to massive pancreaticojejunal anastomotic hemorrhage after PDT.
MATERIALS AND METHODS
Patient information
A total of 17 subjects were enrolled in our study, including 13 men and five women, aged 42-68 years (mean 60 ± 2.45 years). There were 10 cases of lower common bile duct carcinoma, three cases of pancreatic head carcinoma and four cases of ampullary carcinoma. Obstructive jaundice was found in 12 patients. A catheter was inserted into the pancreatic duct in four cases, pancreatic duct exterior drainage was placed in two cases, and T-tube external drainage was placed in six cases, respectively. No T-tube or pancreatic duct drainage was placed in the remaining five patients. Post-surgical hemorrhage occurred in 10 cases from the gastroduodenal artery, from the posterior edge of the pancreatic stump in one case, and from the inferior pancreaticoduodenal artery in one case. Patients with upper gastrointestinal hemorrhage after PDT could be divided into early- and late-stage groups depending on the occurrence of hemorrhage within 5 d after PDT[1]. In our study, there were two cases of early-stage hemorrhage and 15 cases of late-stage hemorrhage. Pancreatic leakage was confirmed in five cases. One patient withdrew from the study because his family gave up the treatment. All patients enrolled in this study signed the informed consent.
Inclusion criteria
Patients who were diagnosed as lower common bile duct cancer, pancreatic head cancer, or digestive tract tumors such as ampullary carcinoma, with the need of pancreatoduodenectomy. Modified Child procedures were adopted in each patient, were included in this study. Digestive tract reconstruction was performed in order of pancreas-intestine, bile duct-intestine and stomach-intestine. An internal support tube was placed in the pancreatic duct and a cross-section of the pancreas was sutured to stop bleeding. Patients with massive pancreaticojejunal anastomotic hemorrhage after PDT and specific criteria are listed below.
Exclusion criteria
Patients not meeting the above diagnostic criteria or with accompanying mental disorders or severe primary diseases in cardiovascular, liver, kidney and hematopoietic systems, or those giving up treatment and withdrawing from the study were excluded, except for women in pregnancy or breast-feeding, or those going to be pregnant.
Diagnosis of massive pancreaticojejunal anastomotic hemorrhage after PDT
In our study, a modified Child’s procedure was adopted for all subjects, namely digestive tract reconstruction was performed in order of pancreas-intestine, bile duct-intestine and stomach-intestine. An internal support tube was placed in the pancreatic duct and a cross-section of the pancreas was sutured to stop bleeding as previously described[2–4]. The diagnosis of massive pancreaticojejunal anastomotic hemorrhage after PDT was based on the literature[3]. The main diagnostic criteria for patients in our study were as follows. (1) Hemorrhage occurred within 1 mo of PDT (the last hemorrhage in our study occurred on day 23 post-surgery) and the presence of a massive hemorrhage impacting vital signs was confirmed by angiography or gastroscopy (subjects whose origin of hemorrhage could not be found by angiography or those with acute ulcer hemorrhage were confirmed by gastroscopy). (2) Massive hemorrhage manifested as fresh blood effusing suddenly from the abdominal drainage tube or T-tube, or massive hematemesis or blood drainage from the gastric tube (> 200 mL). (3) Patients experienced hypovolemic shock accompanying a simultaneous decrease in hemoglobin (hemoglobin decreased more than 30 g/L in 24 h). Massive pancreaticojejunal anastomotic hemorrhage after PDT was diagnosed when the patients had hemorrhage and any other manifestations.
Treatment of massive hemorrhage
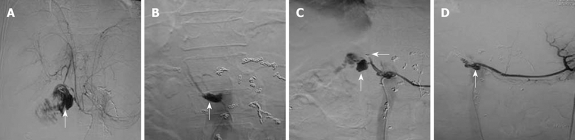
TAE: Of the 11 patients treated with TAE, eight underwent hepatic artery embolization, two underwent embolization of hepatic artery proper, and one refused any treatment upon initiating TAE. Hemorrhage was stopped in six cases after a single embolization procedure. Two patients underwent TAE twice for hemostasis (Figure 1). Emergency arteriography showed that the two patients had pancreatic stump artery bleeding into the jejunum. Hepatic artery was embolized during the first TAE, but recurrence of bleeding was found 4 h later. Arteriography showed that the pancreatic stump artery bled again, and microcoils embolizing the hepatic artery were displaced into the right hepatic artery. TAE was performed again with the common hepatic artery embolized using five microcoils, which stopped postoperative hemorrhage. Arteriography showed no obvious origin of hemorrhage in patient 3 during the first TAE. However, 7 h later, the patient received another arteriogram because of recurrence of bleeding. Duodenal stump bleeding was detected, and the common hepatic artery was then embolized to stop the hemorrhage. Arteriography revealed a crude edge on hepatic artery in patient 5, and the bleeding was stopped after embolization of the hepatic artery. A “vascular pool” image was observed in the inferior pancreaticoduodenal artery of patient 6, which was considered the origin of bleeding stopped by embolizing the common hepatic artery.
Figure 1.
Images from patient 2 (treated using TAE). A: Angiogram of the gastroduodenal stump bleeding before the first embolization. Arrow: Angiogram of the gastroduodenal stump bleeding; B: Angiogram of the gastroduodenal stump, which stopped bleeding after the first TAE. Arrow: Angiogram of the gastroduodenal stump); C: Angiogram of the stump prior to the second embolization. Angiography indicates that microcoils have entered the hepatic artery, causing renewed bleeding from the stump. Arrows: The second angiogram of the gastroduodenal stump bleeding; D: After the second embolization, angiography confirmed that the bleeding was stopped. Arrow: Angiogram of the gastroduodenal stump.
Complications such as hepatophyma and spleen necrosis may occur after TAE. In this study, patient 2 suffered from fever 2 mo after operation, with its peak at 39°C. Hepatophyma larger than 5 cm in diameter was seen in the right liver lobe. The patient recovered after paracentesis and anti-infective therapy. No such complications were found in the other patients.
Open surgery: A total of six patients received direct open surgical hemostasis for their hemorrhage.
RESULTS
Efficacy of TAE
A total of 11 cases of massive hemorrhage after PDT underwent TAE which completely stopped their bleeding, except for one patient who died after refusing further treatment. Bleeding was stopped in eight patients after a single TAE procedure and in two patients after two treatments with TAE. All 10 patients recovered well and were discharged.
Total bilirubin (TB) exceeded the ULN level in seven out of the 10 patients and was as high as 178 μmol/L in patient 2 after TAE, who was discharged with a full recovery of liver function after treatment. Patient 4 experienced a fever at 39.5°C after TAE and his TB level was 140 μmol/L. Blood bacterial culture showed pyemia, and this patient abandoned treatment 5 d after TAE, and died 7 d after discharge. The other patients had a good prognosis after anti-infective and supportive treatment. Recurrence of hemorrhage was not found during a follow-up period of 2 mo and all 10 patients reported having an acceptable quality of life. The mean hospital stay was 10.45 d after hemostasis. Patients requiring a second operation accounted for 18.2%, and the overall mortality rate was 9.1% (Table 1).
Table 1.
Overview of 11 patients with massive hemorrhage after PDT treated with TAE
| Case | Time until bleeding | Symptom | Bleeding site | Embolization site | TAE | Hospital stay | Complications after TAE | Prognosis |
| 1 | 21 | T-tube bleeding | Pancreatic stump artery | Proper hepatic artery | 1 | 9 | High TB | Good |
| 2 | 23 | T-tube bleeding, hematemesis | Gastroduodenal stump | Common hepatic artery | 2 | 14 | High TB | Good |
| 3 | 12 | Double catheterization cannula, T-tube bleeding | Gastroduodenal stump | Common hepatic artery | 2 | 14 | High TB | Good |
| 4 | 14 | Hematemesis | Gastroduodenal stump | Common hepaticaArtery | 1 | 5 | Hyperpyrexia, pyemia | Dead |
| 5 | 3 | Double catheterization cannula bleeding | Proper hepatic artery | Proper hepatic artery | 1 | 11 | None | Good |
| 6 | 5 | Double catheterization cannula bleeding | Inferior pancreaticoduodenal artery | Common hepatic artery | 1 | 8 | High TB | Good |
| 7 | 7 | Single lumen cannula bleeding | Gastroduodenal Stump | Common hepatic artery | 1 | 7 | None | Good |
| 8 | 9 | Hematemesis | Gastroduodenal stump | Common hepatic artery | 1 | 11 | High TB | Good |
| 9 | 10 | Hematemesis | Gastroduodenal stump | Common hepatic artery | 1 | 13 | High TB | Good |
| 10 | 7 | Double catheterization cannula bleeding | Gastroduodenal stump | Common hepatic artery | 1 | 13 | High TB | Good |
| 11 | 8 | Double catheterization cannula bleeding | Gastroduodenal stump | Common hepatic artery | 1 | 10 | None | Good |
TB: Total bilirubin.
Efficacy of open surgery
Six patients underwent a second open surgical hemostasis for hemorrhage. Patients 1, 3 and 6 recovered and were discharged. However, patient 6 received an additional open surgical hemostasis, and patient 2 received emergency open surgical hemostasis again because of rebleeding 5 and 9 d after surgery, respectively. Patient 2 died of multiple organ failure. Liver and renal failure was observed in patients 4 and 5 after surgery. Both patients abandoned treatment, and one of them died 1 wk after discharge.The other patients had a good prognosis after anti-infective and supportive treatment. The mortality rate for patients undergoing open surgery was 50%, the recurrence rate of hemorrhage was 16.67%, and the mean hospital stay was 39.5 d (Table 2).
Table 2.
Overview of six patients with massive hemorrhage after PDT treated with open surgical hemostasis
| Case | Time until bleeding | Symptom | Bleeding site | Hemostasis surgeries | Duration of hospital stay after hemostasis | Complications after surgery | Prognosis |
| 1 | 17 | T-tube bleeding | Gastroduodenal stump | 1 | 59 | None | Good |
| 2 | 9 | T-tube bleeding, hematemesis | Gastroduodenal stump | 2 | 32 | Multiple organ failure | Dead |
| 3 | 10 | T-tube bleeding | Gastroduodenal stump | 1 | 63 | None | Good |
| 4 | 11 | Hematemesis | Gastroduodenal stump | 1 | 16 | Liver failure | Dead |
| 5 | 8 | T-tube bleeding | Gastroduodenal stump | 1 | 13 | Renal failure | Dead |
| 6 | 8 | T-tube, pancreatic duct bleeding | Gastroduodenal stump | 2 | 54 | None | Good |
DISCUSSION
Pancreatojejunal and choledochojejunal anastomotic internal drainage can effectively prevent biliary and pancreatic leakage. PDT is a common abdominal operation. However, it is associated with the most common complications of hemorrhage and pancreatic leakage, with a relatively high risk[5]. The incidence of postoperative massive hemorrhage is 7.5%-12.4%. From June 2005 to August 2008, we performed 308 PDT, and massive pancreaticojejunal anastomotic hemorrhage occurred in 16 patients, accounting for 5.2% of its overall incidence rate, which is slightly lower than the reported rate[6]. Massive hemorrhage after PDT is difficult to stop, and if it is not stopped immediately, the mortality rate can be as high as 30%-58%[7,8]. Massive pancreaticojejunal anastomotic hemorrhage after PDT, mainly from the gastroduodenal stump, is often caused by transudatory digestive juices or corrosion of peripheral tissues by local fluid infection. Corrosion of the anastomotic vicinal vessels is especially common. In the present study, pancreatic leakage was confirmed in five of the 16 patients, and the number of leukocytes was increased before bleeding in six of them, and the number of WBC was 20.5 × 109/L in one patient. In addition, four of the six patients had a fever. Gastroduodenal arterial bleeding was detected by DSA in five of our patients, suggesting that it is particularly important to prevent intra-abdominal infection after PDT. Abdominal CT and B-ultrasound should be regularly performed to monitor the fever or blood pressure of such patients, to ensure that seroperitoneum is not compromised. If there is any indication of infection, puncture and drainage should be carried out to prevent further infection. During the surgery, arteries should be ligated twice or transfixed, with longer suturing ends as appropriate[9].
Pancreaticojejunal anastomotic hemorrhage after PDT is difficult to treat. TAE with or without surgery might be a more effective procedure to stop bleeding. Since PDT may produce significant surgical trauma, and the site of hemorrhage is often difficult to locate, blind open surgical exploration often ends in failure. In addition, postoperative complications such as pancreatic leakage and intra-abdominal infection can result in more severe consequences than those caused by hemorrhage. Thus, better treatment modalities are needed for pancreaticojejunal hemorrhage after PDT. In our study, six patients underwent open surgical hemostasis again after PDT, and two of them received additional open surgical hemostasis because of recurrence of bleeding, which can be explained as follows. Namely, their overall poor condition following two major operations may have hindered recovery; the exact site of bleeding could not be accurately located during the first operation for hemorrhage; intra-abdominal organ edema such as pancreatic edema was common and pancreatic leakage and intra-abdominal infections were not completely controlled. One patient with an intra-abdominal infection died of multiple organ failure. Liver or renal failure was also found in two patients after surgery. Both patients abandoned treatment, and one of them died 1 wk after discharge. Overall, the mortality rate for open surgery was 50%, the recurrence rate of bleeding was 16.67%, and the mean hospital stay was 39.5 d, suggesting that the prognosis of patients undergoing TAE is rather poor.
However, TAE can prevent re-operation risk and complications. In our study, 18.2% patients had a second TAE with a mortality of only 0.9%. In addition, the mean hospital stay after TAE was 10.45 d. Angiography should be performed as soon as hemorrhage is diagnosed to locate the site of bleeding. Then, embolization can be performed to stop hemorrhage[4,5].
Angiography can diagnose hemorrhage and evaluate the efficacy of its treatment.
Angiography can locate the site of bleeding. TAE after PDT is needed for successful treatment of hemorrhage, and cooperation between surgeons is necessary to maximize the therapeutic efficacy and minimize the risk of TAE.
Since patients will be transported and allergy testing of contrast medium will be carried out during TAE, preoperative and intraoperative anti-hemorrhagic shock should be prevented. Any change in vital signs should be monitored while performing TAE. Larger microcoils should be selected for TAE because the common hepatic artery is large with a high-pressure blood flow.
After TAE, patients should be closely observed, and the outcome of hemostasis should be confirmed by arteriography. Patients without recurrence of bleeding can be sent to the intensive care unit for observation. The site of bleeding was found in eight of our 10 patients who underwent TAE. No recurrence of bleeding was observed at first in the other two patients who required additional surgery, which may have resulted from their poor condition (unresolved shock and low blood pressure), leading to reduced bleeding despite. Spastic contraction of blood vessels and/or obstruction of vessels or blood clots may have prevented accurate angiography. It was reported that gastroduodenal arterial stump bleeding is one of the main sources of pancreaticojejunal anastomotic hemorrhage after PDT[10]. If pancreaticojejunal anastomotic hemorrhage is suspected but not dealt with, massive hemorrhage may occur. Common hepatic arterial embolization may be a better choice of treatment.
Nevertheless, we found that common hepatic artery embolization with TAE was likely to result in liver injury, which was manifested as elevated TB and aminotransferase level. In our study, TB level was higher in five patients than in normal controls after TAE, and the aminotransferase level was three-fold higher in four patients than in normal controls. However, if not accompanied with liver disease, liver failure rarely occurs after TAE. W provided supportive treatment for patients with liver injury by increasing the oxygen flow and concentration, extending oxygen absorption time, maintaining a high oxygen pressure and 100% oxygen saturation, and improving liver support. We also provided low-dose hormone therapy with prostaglandin E, growth hormone, and hepatocyte growth factor to increase the liver blood flow and promote liver cell regeneration. The patients received additional supportive treatment with plasma and human serum albumin when necessary.
In summary, TAE, which can avoid reoperation and complications of surgery, is an effective and safe treatment modality for acute postoperative hemorrhage after PDT. However, TAE may damage liver function due to hepatic artery embolization. Angiography can locate the site of bleeding, and if there are indications for surgery, TAE should be performed in time.
COMMENTS
Background
Massive pancreaticojejunal anastomotic hemorrhage after pancreatoduodenectomy (PDT) is the second most common severe complication, only next to pancreaticojejunal anastomotic dehiscence. Blind open surgical hemostasis has more surgical risks and complications. However, transcatheter arterial embolization (TAE) can prevent reoperation risk and complications.
Research frontiers
An internal support tube was placed in the pancreatic duct and a cross-section of pancreas was sutured to stop bleeding. If the bleeding site was located by selective angiography, embolization could be performed to stop bleeding.
Applications
TAE is an effective and safe treatment modality for acute hemorrhage after PDT. Vasography should be performed in time to locate the site of bleeding. TAE can also prevent reoperation risk and complications.
Peer review
The authors showed that TAE was an effective and safe treatment modality for acute hemorrhage after PDT. The initial observations are interesting.
Peer reviewer: María IT López, Professor, Experimental Biology, University of Jaen, araje de las Lagunillas s/n, Jaén 23071, Spain
S- Editor Li LF L- Editor Wang XL and Kerr C E- Editor Lin YP
References
- 1.Yoon YS, Kim SW, Her KH, Park YC, Ahn YJ, Jang JY, Park SJ, Suh KS, Han JK, Lee KU, et al. Management of postoperative hemorrhage after pancreatoduodenectomy. Hepatogastroenterology. 2003;50:2208–2212. [PubMed] [Google Scholar]
- 2.Mao QS, Zhou XZ, Zan ZZ, Chen SZ. The currelation between pancreatic fistula and the methods of pancreatoenterostomy after pancreatoduodenectomy. Hebei Yixue. 2002;8:797–780. [Google Scholar]
- 3.Du ZG, Li B, Feng X, Yin J, Yan LN, Wen TF, Zeng Y. Cause and treatment of upper gastrointestinal hemorrhage after pancreatoduodenectomy. Zhongguo Puwai Jichu Yu Linchuang Zazhi. 2008;15:511–513. [Google Scholar]
- 4.Wu GZ, Liu HA, Jiang J, Cao XC. Prevention of pancreatic fistula afar pancreaticoduodenectomy using complete external drainage of pancreatic fluid. Zhongguo Xiandai Shoushu Zazhi. 2005;9:225–227. [Google Scholar]
- 5.Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, Sauvanet A. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3–9. doi: 10.1016/j.amjsurg.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 6.Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236–241. doi: 10.1097/00000658-199802000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trede M, Carter DC. The complications of pancreato-duodenectomy and their management. In: M Trede, DC Carter., editors. Surgery of the pancreas[M]. 1st. Churchill Livingstone: Edinburgh; 1993. pp. 629–644. [Google Scholar]
- 8.Cao LP, Chen BW, Peng SY. Pathogenesis,prevention and treatment of upper gastrointestinal hemorrhage after pancreatoduodenectomy. Zhonghua Jizhen Yixue Zazhi. 2005;14:942–944. [Google Scholar]
- 9.Wang QC, Xiao N, Diao YF, Tian KL, Fan XQ, Chen HS. Experimental study of fluid resuscitation in uncontrolled hemorrhagic shock. Zhongguo Weizhongbing Jijiu Yixue. 2002;14:746–748. [Google Scholar]
- 10.Lu L, Hao ZQ, Tan ZG. Clinical experience in 102 patients undergoing pancreoduodenectomy. Linchuang Junyi Zazhi. 2007;35:54–57. [Google Scholar]