Abstract
AIM: The association of hepatitis C virus (HCV) infection with type II mixed cryoglobulinemia is well established, but the role of HCV in B-cell lymphoma remains controversial. In patients with HCV infection, B-cell clonal expansions have been detected in peripheral blood and bone marrow, and a high prevalence of B-cell non-Hodgkin’s lymphomas has been documented. Liver biopsies in chronic HCV infection frequently show portal lymphoid infiltrates with features of B follicles, whose clonality has not yet been investigated. The object of this study was to determine the frequency of liver-infiltrating monoclonal B-cells in 40 patients with HCV infection.
METHODS: Eight hundred and forty-eight patients were studied prospectively, including 40 HCV-positive patients and 808 patients with chronic hepatitis B virus (HBV) infection. Immunohistochemical study for B- and T-cell markers was performed on the paraffin-embedded liver tissue sections. The clonality of lymphoid B-cells was tested using a polymerase chain reaction (PCR) approach designed to identify immunoglobulin heavy chain gene (IgH) rearrangements.
RESULTS: Liver-infiltrating monoclonal B-cells were detected in the liver for 4 (10%) of 40 HCV-positive patients but were present in only 3 (0.37%) of 808 liver biopsy specimens with chronic HBV infection. Chi-square testing showed that the monoclonal B-cells infiltration in the liver was more frequent in the HCV-infected patients (P = 0.000). A clonal IgH rearrangement was detected in 5 (71.4%) of 7 liver biopsy specimens with monoclonal B-cells infiltration. In 2 of 5 patients with both a clonal B-cell expansion and monoclonal B-cells infiltration in the liver, a definite B-cell malignancy was finally diagnosed.
CONCLUSION: Liver-infiltrating monoclonal B-cells are detected in the liver of patients with chronic HCV and HBV infection. A high percentage of patients with monoclonal B-cells infiltration and B-cell clonality in the liver were finally diagnosed as having a definite B-cell malignancy.
Keywords: Hepatitis, Hepatitis C virus, B-lymphocytes, Polymerase chain reaction, Gene rearrangement, Clonality
INTRODUCTION
The relationship between lymphoproliferative disorders and infectious agents has been recognised and studied for many decades. A causative association between hepatitis C virus (HCV) and non-Hodgkin’s lymphoma (NHL) was postulated relatively recently and has been the subject both of intense investigation and of some debate[1–5]. On the strength of epidemiological data, emerging biological investigations and clinical observations, HCV appears to be involved in the pathogenesis of at least a proportion of patients with NHL[6,7]. This hypothesis is supported by the evidence that HCV is not only hepatotropic, but also a lymphotropic virus[8]. In vitro, HCV is able to replicate in human T-cell lines[9] and in normal peripheral blood mononuclear cells from healthy subjects[10]. Moreover, viral genomic sequences have been found in T- and B-cell populations, as well as in monocyte-derived cells from peripheral blood and liver tissue in patients with HCV-related chronic hepatitis[10–12]. Most studies on HCV-associated B-cell proliferations have focused on peripheral blood and bone marrow lymphocytes. During HCV infection, liver tissue is frequently characterized by prominent lymphoid aggregates in portal tracts that show histological and immunophenotypical features of B follicles[13,14]. The nature of these aggregates has not yet been investigated in detail; in particular, the clonality of B-cells within lymphoid infiltrates in the liver of HCV-infected patients has not been analyzed at the molecular level. In the present study, we analyzed the frequency of liver-infiltrating monoclonal B-cells from the paraffin-embedded liver biopsies of 40 patients with HCV infection. In 7 patients with monoclonal B-cells infiltration in the liver who were followed up, B-cell clonality was tested using a polymerase chain reaction (PCR) approach designed to identify immunoglobulin heavy chain gene (IgH) rearrangements.
MATERIALS AND METHODS
Ethics
All patients were notified of the risk of the liver puncturation and provided informed written consent.
Selection of cases
From June 2003 to December 2005, 848 patients were enrolled in a prospective study and were followed up as outpatients at the Department of Infectious Disease at our NanFang Hospital. In 40 HCV-positive patients (anti-HCV antibody and HCV RNA positive), anti-hepatitis B virus (HBV) and anti-human immunodeficiency virus antibodies were negative. The patients had not received anti-HCV therapy for at least 6 mo. For each patient, a sample of liver biopsy was performed. A control group, consisting of 808 HBV-infected patients with other non-immune chronic liver diseases, was followed up at the same department. All the patients were diagnosed as having chronic hepatitis according to the criteria formulated by the Chinese Society of Infectious disease and Parasitology and Chinese Society of Hepatology, Chinese Medical Association.
Immunohistochemical characterization of lymphoid aggregates
The 848 liver biopsies contained 7 monoclonal B-cell infiltrations. The composition of the 7 infiltration cell specimens was investigated by immunohistochemistry, using antibodies against B-cell (L26/CD20) markers, following the streptavidin–biotin-complex immunoperoxidase technique.
Polymerase chain reaction of IgH rearrangements
The DNA from the 7 monoclonal B-cells infiltration liver specimens successfully amplified for the β-actin gene was then subjected to clonality assessment by PCR amplification of rearranged IgH genes. After heating for 10 min at 95°C, 2 μL from each sample was added to the PCR mixture containing 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 25 pmol/L of each primer, 200 μmol/L of each dNTP, 1.25 units of Taq polymerase, and 4.5 mmol/L MgCl2 in a final volume of 50 μL.
We amplified the hypervariable complementary region (CDR-II and CDR-III), included between the third and joining regions (FR-III and JH) of IgH genes, with a 5'-primer homologous to the FR-III region, and JH as 3'-primers, using a seminested protocol. The primers were FR3, CTGTCGACACGGCCGTGTATTACTG; JH, AACTGCAGAGGAGACGGTGACC. The first PCR cycle consisted of denaturation of the sample DNA at 93°C for five minutes, annealing of the primers at 55°C for one minute, then extension of the DNA at 73°C for two minutes. Each experiment was duplicated and accompanied by a negative control containing no template DNA. Ten microlitres of the PCR products were analyzed by electrophoresis on 3% agarose gels, stained by ethidium bromide, and viewed under UV light.
The histologic features of liver biopsy specimens were analyzed
Liver biopsy specimens (> 10 mm in length) were fixed, paraffin-embedded, and stained with hematoxylin-eosin-saffron, and Warthin-Starry stained for collagen. For each liver biopsy specimen, a stage of fibrosis and a grade of activity were established according to the criteria formulated by the Chinese Society of Infectious Disease and Parasitology and Chinese Society of Hepatology, Chinese Medical Association.
Data analysis
Categorical variables were compared by chi-square testing, and continuous variables were compared by the two-sided Student t test.
RESULTS
Among the 848 patients prospectively included in this study, 40 were HCV-positive (anti-HCV antibody and HCV RNA positive), and 808 were HBV-infected patients. The main characteristics of patients with, and those without, chronic HCV infection are detailed in Table 1.
Table 1.
Baseline characteristics of patients with, and those without, chronic HCV infection
| Characteristic | HCV positive (n = 40) | HCV negative (n = 808) | P |
| Age, (mean ± SD, yr) | 50 ± 14 | 51 ± 15 | > 0.05 |
| Male | 28/40 | 550/808 | > 0.05 |
| ALT, (mean ± SD, upper limit of normal value) | 136 ± 7.8 | 132 ± 6.9 | > 0.05 |
| Liver histologic activity | |||
| None or mild (G0-G1) | 7/40 | 142/808 | > 0.05 |
| Moderate or severe (G2-G4) | 33/40 | 666/808 | > 0.05 |
| Liver histologic fibrosis | |||
| None or portal fibrosis (S0-S1) | 8/40 | 17/808 | > 0.05 |
| Few or many septa or cirrhosis (S2-S4) | 32/40 | 791/808 | > 0.05 |
| Liver lymphoid infiltrate | |||
| None | 0/40 | 0/808 | > 0.05 |
| Mild | 32/40 | 646/808 | > 0.05 |
| Severe | 8/40 (65) | 162/808 | > 0.05 |
| Liver lymphoid aggregate | |||
| None | 36/40 | 805/808 | |
| Yes | 4/40 | 3/808 | 0.000 |
The histological appearance of a B-cell lymphoma is shown in Figure 1.
Figure 1.
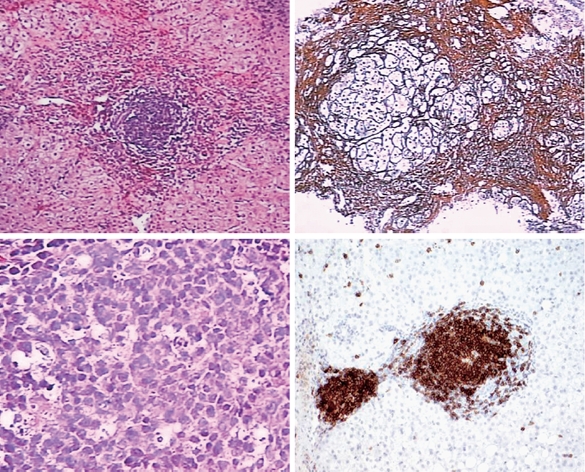
Histological appearances of a B-cell lymphoma, best classified as a marginal zone lymphoma involving the liver. A, B: Low power view of liver core biopsy shows chronic hepatitis and marked portal lymphoid infiltrates. C: High power view of liver core biopsy shows a monotonous portal lymphoid infiltrate composed of small lymphocytes with moderate amount of clear cytoplasm (so-called monocytoid appearance). Note that the infiltrate does not involve the biliary epithelium. D: CD20 immunostain shows that virtually all of the lymphoid cells are B-cells.
A clonal B-cell expansion was detected in 5 (71.4%) of 7 of the livers with monoclonal B-cells infiltration. In 2 of 5 patients with both a clonal B-cell expansion and monoclonal B-cells infiltration in the liver, a definite B-cell malignancy was finally diagnosed.
DISCUSSION
In this study, we addressed the question of whether lymphoid aggregates in the liver of patients with chronic hepatitis C are clonal B-cell proliferations.
Seven of the 848 patients have monoclonal B-cells infiltrating in the livers, including 4 of 40 chronic HCV-infected patients and 3 of 808 chronic HBV-infected patients. Five of the 7 patients with monoclonal B-cells infiltration showed a single band, suggesting that they were formed by a single B-cell clone. We were able to observe progression in patients who continued to be followed up after the end of this study. Lymphoma developed in 2 HCV-infected patients out of 5 patients who had monoclonal B-cells infiltration and B-cell proliferation in the liver.
Although epidemiological data link HCV infection and NHL, the pathobiological processes leading to clonal B-cell expansion and subsequent malignant transformation are only recently becoming better understood. CD81 has emerged as a potentially key mediator of B-cell/HCV interaction, in light of the finding that CD81 can bind to at least two sites on the HCV envelope protein, E2. CD81/E2 interaction does not apparently promote viral entry into B-cells; however, B-cells with specific anti-HCV surface immunoglobulins can simultaneously interact with viral E2 protein via CD81, resulting in dual activation signals leading to B-cell proliferation. Furthermore, clonal immunoglobulin gene rearrangements from HCV-positive lymphomas often share a similar restricted gene segment usage pattern, as seen in B-cells from patients with mixed cryoglobulinemia, and also show somatic hypermutation, emphasising the link between chronic viral antigenic stimulation and NHL pathogenesis[15–20].
A multistep process has also been documented in a lymphoproliferative disorder in an HCV-infected patient, in whom the bcl-2 translocation was followed by myc translocation during the clinical progression of the disease[21]. However, the wide spectrum of lymphomas that have been described in patients with HCV infection, ranging from lymphoplasmacytoid[2], to MALT-type[22],to follicle-centre cell lymphomas[23], seem to indicate that more heterogeneous and complex processes are probably involved in the lymphomagenesis associated with HCV.
In conclusion, in patients with chronic HCV infection, the presence of a B-cell clonality and monoclonal B-cells infiltration in the liver may be useful for detecting patients at high risk for developing malignant lymphoproliferative disease. The importance of B-cell clonality analysis in the course of chronic HCV disease needs to be further evaluated, as do the indications for, and the efficacy of, antiviral treatment in patients at risk for B-cell malignancy.
COMMENTS
Background
The association of hepatitis C virus (HCV) infection with type II mixed cryoglobulinemia is well established, but the role of HCV in B-cell lymphoma remains controversial. The incidence of B-cell lymphoma is currently rising in line with the progression of hepatitis C though the cause of this increase is largely unknown.
Research frontiers
Investigating the clonality of B-cells in the liver by analyzing the IgH gene rearrangement has been shown to correlate the development of B-cell lymphoma with HCV-infected patients. Liver biopsies in chronic hepatitis C frequently show portal lymphoid infiltrates with features of B follicles, whose clonality has not yet been investigated. In this study, the authors demonstrate that the clonality of B-cells in the liver may represent a low-grade lymphoma.
Innovations and breakthroughs
Recent reports have highlighted the importance of antiviral treatment in the HCV-infected patient with B-cell clonality in the liver. This is the first study to analyze the association of monoclonal B-cells infiltration in the liver with the B-cell clonality. Furthermore, our follow up study showed that the lymphoma developed more frequently in the patients who had monoclonal B-cells infiltration and B-cell proliferation in liver.
Applications
The presence of a B-cell clonality and monoclonal B-cells infiltration in the liver may be useful for detecting patients at high risk for developing malignant lymphoproliferative disease. The study results suggest a strategy for antiviral treatment in patients at risk for B-cell malignancy.
Terminology
Polymerase chain reaction (PCR) amplification is a method currently in widespread use for detection of clonal IgH rearrangements. In PCR, rearranged DNA is amplified with a series of consensus primers that are complementary to sequences of variable regions; framework 1, framework 2, and framework 3 and to joining regions of the IgH gene.
Peer review
The authors investigate the association of HCV infection and liver B-cell clonality in a prospective clinical trial. Liver biopsy specimens from 40 HCV-positive patients were analyzed, and specimens from hepatitis B virus (HBV)-positive patients served as a control. This is the first study to describe B-cell clonality in the liver of HCV-infected patients, and the results of their study are of interest.
Supported by National Natural Science Foundation of China, 30271181
Peer reviewer: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., 107031, Moscow, Russia
S- Editor Li LF L- Editor Logan S E- Editor Zheng XM
References
- 1.Franzin F, Efremov DG, Pozzato G, Tulissi P, Batista F, Burrone OR. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–552. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–4301. [PubMed] [Google Scholar]
- 3.Paydas S, Kilic B, Yavuz S, Disel U, Tanriverdi K, Sahin B, Burgut R. Anti-HCV and HCV-RNA prevalence and clinical correlations in cases with non-Hodgkin's lymphoma. Am J Hematol. 2003;74:89–93. doi: 10.1002/ajh.10386. [DOI] [PubMed] [Google Scholar]
- 4.Takeshita M, Sakai H, Okamura S, Oshiro Y, Higaki K, Nakashima O, Uike N, Yamamoto I, Kinjo M, Matsubara F. Splenic large B-cell lymphoma in patients with hepatitis C virus infection. Hum Pathol. 2005;36:878–885. doi: 10.1016/j.humpath.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Landau DA, Saadoun D, Calabrese LH, Cacoub P. The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev. 2007;6:581–587. doi: 10.1016/j.autrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Cocco P, Piras G, Monne M, Uras A, Gabbas A, Ennas MG, Palmas A, Murineddu M, Collu S, Melis M, et al. Risk of malignant lymphoma following viral hepatitis infection. Int J Hematol. 2008;87:474–483. doi: 10.1007/s12185-008-0086-3. [DOI] [PubMed] [Google Scholar]
- 7.Schöllkopf C, Smedby KE, Hjalgrim H, Rostgaard K, Panum I, Vinner L, Chang ET, Glimelius B, Porwit A, Sundström C, et al. Hepatitis C infection and risk of malignant lymphoma. Int J Cancer. 2008;122:1885–1890. doi: 10.1002/ijc.23416. [DOI] [PubMed] [Google Scholar]
- 8.Vallat L, Benhamou Y, Gutierrez M, Ghillani P, Hercher C, Thibault V, Charlotte F, Piette JC, Poynard T, Merle-Béral H, et al. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–3678. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu YK, Iwamoto A, Hijikata M, Purcell RH, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc Natl Acad Sci USA. 1992;89:5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller HM, Pfaff E, Goeser T, Kallinowski B, Solbach C, Theilmann L. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J Gen Virol. 1993;74(Pt 4):669–676. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- 11.Zignego AL, De Carli M, Monti M, Careccia G, La Villa G, Giannini C, D'Elios MM, Del Prete G, Gentilini P. Hepatitis C virus infection of mononuclear cells from peripheral blood and liver infiltrates in chronically infected patients. J Med Virol. 1995;47:58–64. doi: 10.1002/jmv.1890470112. [DOI] [PubMed] [Google Scholar]
- 12.Ferri C, Monti M, La Civita L, Longombardo G, Greco F, Pasero G, Gentilini P, Bombardieri S, Zignego AL. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701–3704. [PubMed] [Google Scholar]
- 13.Hino K, Okuda M, Konishi T, Yamashita A, Kayano K, Kubota M, Yasunaga M, Fukumoto Y, Okita K. Analysis of lymphoid follicles in liver of patients with chronic hepatitis C. Liver. 1992;12:387–391. doi: 10.1111/j.1600-0676.1992.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 14.Mosnier JF, Degott C, Marcellin P, Hénin D, Erlinger S, Benhamou JP. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993;17:366–371. [PubMed] [Google Scholar]
- 15.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. [PubMed] [Google Scholar]
- 16.Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, Burgio V, Di Stasio E, Giardina B, Houghton M, et al. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:4824–4830. doi: 10.1128/jvi.74.10.4824-4830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sayed GM, Mohamed WS, Nouh MA, Moneer MM, El-Mahallawy HA. Viral genomes and antigen detection of hepatitis B and C viruses in involved lymph nodes of Egyptian non-Hodgkin's lymphoma patients. Egypt J Immunol. 2006;13:105–114. [PubMed] [Google Scholar]
- 18.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D'Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G, et al. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanovski M, Silvestri F, Pozzato G, Anand S, Mazzaro C, Burrone OR, Efremov DG. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–2442. [PubMed] [Google Scholar]
- 20.De Re V, De Vita S, Marzotto A, Rupolo M, Gloghini A, Pivetta B, Gasparotto D, Carbone A, Boiocchi M. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood. 2000;96:3578–3584. [PubMed] [Google Scholar]
- 21.Ellis M, Rathaus M, Amiel A, Manor Y, Klein A, Lishner M. Monoclonal lymphocyte proliferation and bcl-2 rearrangement in essential mixed cryoglobulinaemia. Eur J Clin Invest. 1995;25:833–837. doi: 10.1111/j.1365-2362.1995.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 22.Luppi M, Grazia Ferrari M, Bonaccorsi G, Longo G, Narni F, Barozzi P, Marasca R, Mussini C, Torelli G. Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia. 1996;10:351–355. [PubMed] [Google Scholar]
- 23.Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E, Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994;88:392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]


