Abstract
Neoplastic needle track seeding following percutaneous radiofrequency ablation (RFA) of secondary liver tumors is exceedingly rare. Reports on cutaneous tumor seeding after percutaneous RFA for colorectal liver metastasis are even rarer in the literature. Here we report a case of a 46-year-old female who developed an ulcerating skin lesion along the needle track of a previous percutaneous RFA site around 6 mo after the procedure. The previous RFA was performed by the LeVeen® needle for a secondary liver tumor from a primary rectal cancer. The diagnosis of secondary skin metastasis was confirmed by fine needle aspiration cytology. The lesion was successfully treated with wide local excision. We believe that tumor seeding after percutaneous RFA in our patient was possibly related to its unfavorable subcapsular location and the use of an expansion-type needle. Hence, prophylactic ablation of the needle track should be performed whenever possible. Otherwise, alternative routes of tumor ablation such as laparoscopic or open RFA should be considered.
Keywords: Radiofrequency catheter ablation, Needles, Neoplasm seeding, Liver neoplasms, Skin neoplasms, Neoplasm metastasis
INTRODUCTION
Radiofrequency ablation (RFA) is a well established local ablative treatment for primary or secondary hepatic neoplasms. Despite a relatively low complication rate, the percutaneous application of RFA carries a potential risk of needle track seeding. As reported by a recent systematic review[1], the seeding risk after percutaneous RFA for hepatocellular carcinoma (HCC) is around 0.6%. However, such a seeding risk in secondary liver tumors is less well defined. Reports on tumor seeding after percutaneous RFA for colorectal liver metastasis are rare. The following report illustrates a patient with cutaneous tumor seeding after percutaneous RFA for a small colorectal liver metastasis.
CASE REPORT
A 46-year-old female, who had no other major medical illness, was referred to our unit for stage IV rectal cancer with liver metastasis after receiving laparoscopic anterior resection of the primary rectal tumor in the private sector. Preoperative staging computed tomography (CT) of the abdomen showed no distant metastasis. The operation for the primary rectal tumor was uneventful. However, a suspicious liver lesion was incidentally found on the surface of the liver intra-operatively and was biopsied. Histopathology revealed a T3N2 well-differentiated adenocarcinoma of the rectum and a metastatic adenocarcinoma of the liver. A postoperative re-staging CT scan identified a 1.7 cm solitary subcapsular liver metastasis at segment 4 of the liver (Figure 1). A subsequent positron emission tomography scan excluded additional distant metastasis. Unfortunately, the patient refused curative hepatic resection. Hence, a single session of CT-guided percutaneous RFA of the liver lesion using a 3 cm 17-gauge LeVeen® needle electrode (Super-slim, Boston Scientific, United States) with a single puncture was performed (Figure 2). Thermal ablation of the needle tract was not done in this case. She was subsequently put on a complete course of oxaliplatin-based chemotherapy.
Figure 1.
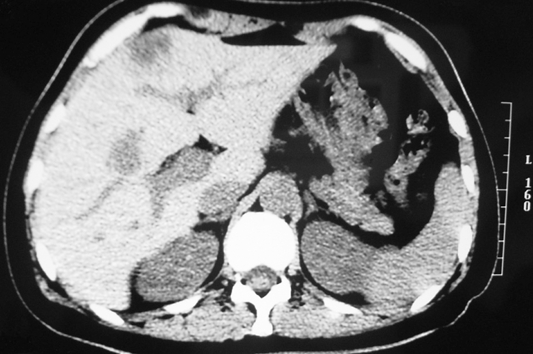
Subcapsular liver metastasis at segment 4 of liver.
Figure 2.
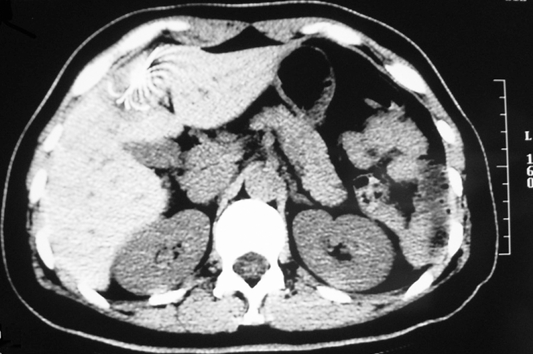
Percutaneous radiofrequency ablation by LeVeen® needle.
Six months after RFA, she presented with a 2 cm ulcerating skin nodule at the previous RFA puncture site (Figure 3). Fine needle aspiration cytology of the nodule confirmed a metastatic adenocarcinoma of primary colorectal origin. At the same time, a CT scan revealed multiple simultaneous recurrences at the left liver. Left hepatectomy for the liver lesions and wide local excision of the cutaneous tumor were performed. Multiple suspicious peritoneal deposits were incidentally identified intra-operatively and they were all resected. Final histopathology revealed metastatic disease in the liver, skin and peritoneum. She then received further courses of palliative chemotherapy in view of likely recurrence. In the subsequent follow-up period, no cutaneous recurrence was identified at the RFA needle track. However, she developed progressive disease with lung metastasis and carcinomatosis. The patient finally died around 5 mo after the second operation.
Figure 3.
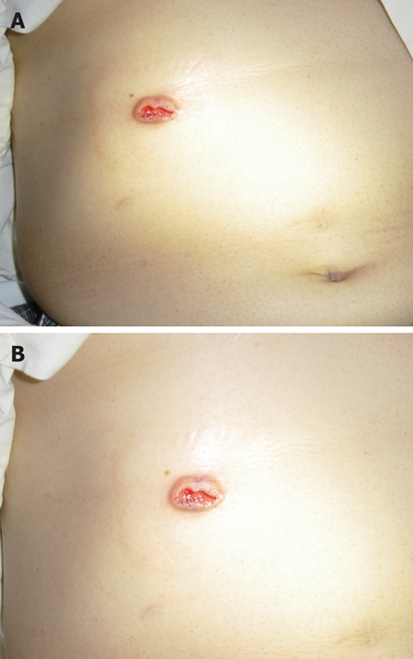
Ulcerating skin nodule. A: Cutaneous neoplastic seeding 6 mo after RFA. B: A closer view.
DISCUSSION
Neoplastic seeding is an uncommon but well-recognized complication following percutaneous diagnostic and therapeutic procedures for primary liver cancer. For diagnostic percutaneous biopsy, the risk of neoplastic seeding was approximately 2.2%[1]. As for therapeutic RFA of HCC, initial results from a small-scale Spanish study suggested an alarmingly high risk of 12.5%[2]. A recent large-scale multicenter study by Livraghi et al[3] in contrast identified a substantially lower risk of only 0.9%. As highlighted by a recent systematic review[1], such a seeding risk for HCC was definitely lower with an overall median risk of only 0.6%. For secondary liver tumors, objective evidence on the seeding risk following percutaneous RFA is lacking. In the English literature, there were only two related case reports identified[4,5] (Table 1).
Table 1.
Literature review on neoplastic seeding after RFA for colorectal liver metastasis
| Author (yr) | Age; gender | Size of liver metastasis (cm) | Subcapsular location of liver lesion | Number of RFA sessions | Size of seeding nodule (cm) | Time lag after RFA |
| Bonatti et al[4] (2003) | 56; male | Not stated | Not stated | 2 | 2 | 6 wk |
| Charalampopoulos et al[5] (2007) | 64; male | 3 | Yes | 2 | Not stated | 10 mo |
| Present case-2008 | 46; female | 1.7 | Yes | 1 | 2 | 6 mo |
Several associated risk factors have been identified for neoplastic seeding following RFA for HCC, notably subcapsular tumor location[2,6], poor tumor differentiation grade[2], multiple RFA sessions[6], multiple electrode placements[6] and history of previous biopsy[6]. In an Italian study by Latteri et al[7], the risk of neoplastic seeding after open RFA was virtually zero but the risk was as high as 1.4% after percutaneous RFA. Remarkably, most of these identified factors were based on seeding risk for HCC. As for our patient, tumor seeding in the RFA needle track was possibly related to its unfavorable subcapsular location and the use of an expansion-type electrode. The previous use of laparoscopic biopsy in such a subcapsular tumor was probably a major detrimental factor related to its peritoneal dissemination. Nevertheless, potential risk factors of neoplastic seeding solely for secondary liver tumors were still undefined. To evaluate the seeding risk, a larger-scale prospective cohort study for secondary liver tumors is required. However, these sorts of studies are practically difficult to conduct because of the rare occurrence.
To prevent neoplastic seeding, some investigators advocated the application of thermocoagulative ablation along the needle track while withdrawing the RFA needle[8]. However, this ablative technique may not be technically feasible in cases of subcapsular lesions as in our case. With regard to treatment, surgical excision with a wide margin seems to be the most justifiable option. Alternatively, different novel techniques had been described. Shibata et al[9] successfully treated a chest wall neoplastic seeding from HCC by transarterial embolization of the feeding vessel. Espinoza et al[10] suggested the use of RFA again for ablating the metastatic seeding tract as treatment.
To conclude, despite its rare occurrence, needle track seeding is a real hazard following percutaneous RFA for secondary liver tumors. Prophylactic ablation of the needle track should be performed whenever possible for high risk patients. Otherwise, alternative routes of tumor ablation like laparoscopic or open RFA should be considered.
Peer reviewers: Professor Walter E Longo, Department of Surgery, Yale University School of Medicine, 205 Cedar Street, New Haven 06510, United States; Dr Takayuki Yamamoto, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan; Professor Francis Seow-Choen, MBBS, FRCSEd, FAMS, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, 3 Mt Elizabeth Medical Centre #09-10, 228510, Singapore
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 3.Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856–858. doi: 10.1002/bjs.4986. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti H, Bodner G, Obrist P, Bechter O, Wetscher G, Oefner D. Skin implant metastasis after percutaneous radio-frequency therapy of liver metastasis of a colorectal carcinoma. Am Surg. 2003;69:763–765. [PubMed] [Google Scholar]
- 5.Charalampopoulos A, Macheras A, Misiakos E, Batistatou A, Peschos D, Fotiadis K, Charalabopoulos K. Thoracoabdominal wall tumour seeding after percutaneous radiofrequency ablation for recurrent colorectal liver metastatic lesion: a case report with a brief literature review. Acta Gastroenterol Belg. 2007;70:239–242. [PubMed] [Google Scholar]
- 6.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 7.Latteri F, Sandonato L, Di Marco V, Parisi P, Cabibbo G, Lombardo G, Galia M, Midiri M, Latteri MA, Craxì A. Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis. 2008;40:684–689. doi: 10.1016/j.dld.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281–289. doi: 10.1245/aso.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Shibata T, Shibata T, Maetani Y, Kubo T, Nishida N, Itoh K. Transcatheter arterial embolization for tumor seeding in the chest wall after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2006;29:479–481. doi: 10.1007/s00270-004-0107-4. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza S, Briggs P, Duret JS, Lapeyre M, de Baère T. Radiofrequency ablation of needle tract seeding in hepatocellular carcinoma. J Vasc Interv Radiol. 2005;16:743–746. doi: 10.1097/01.RVI.0000153109.56827.70. [DOI] [PubMed] [Google Scholar]


