Abstract
In order to test the hypothesis that alteration of cell cycle proteins are involved in the neuronal damage caused by human immunodeficiency virus (HIV), the authors have been studying the effect of chemokines on the CDK/Rb/E2F-1 pathway—which is involved in neuronal apoptosis and differentiation.
First, they have asked whether CXCR4, the specific receptor for the chemokine SDF-1 and X4-using gp120s, can regulate Rb and E2F-1 activity in cultures of differentiated rat neurons. Although CCR3 and CCR5 are known to mediate infection of microglia by HIV-1, recent evidence indicate that CXCR4 also play important roles in HIV-induced neuronal injury, and dual-tropic isolates that use CXCR4 to infect macrophages have recently been reported. The authors have focused on two specific brain areas in which CXCR4 is physiologically relevant, i.e., the cerebellum and the hippocampus. So far, the data indicate that changes in the nuclear and cytosolic levels of Rb, which result in the functional loss of this protein, are associated with apoptosis in these neurons, and that SDF-1α and gp120IIIB affect this pathway. A summary of the findings are presented.
Keywords: E2F-1, gp120, HIV neuropathology, neuronal differentiation and apoptosis, Rb, SDF-1α
The neurological manifestations of acquired immunodeficiency syndrome (AIDS) remain an important complication of human immunodeficiency virus (HIV) infection, as they not only interfere with patients’ daily activities, but also affect therapeutic decisions and represent an independent risk factor for mortality. Though in the last few years progresses have been made regarding the understanding of the development of HIV infection in the central nervous system (CNS), the basic mechanisms of HIV neuropathogenesis are still a matter of investigation (Bensalem and Berger, 2002; Mamidi et al, 2002). The combined effort of scientists interested in different aspects of neuroAIDS has provided evidence that neuronal damage and loss may derive from a complex series of events implicating both neuronal and non-neuronal cells, and that, in analogy to the immune system, chemokine receptors act as HIV coreceptors in the CNS (Berger et al, 1999). According to current models, CNS damage is caused by the release of neurotoxic factors by immune-activated and HIV-infected macrophages and microglia. These toxins may include the HIV envelope proteins, gp120 and gp41, other viral proteins (e.g., tat, nef), as well as various cellular products (i.e., cytokines, excitatory amino acids, free radicals, and amines), which are able to induce neuronal injury, dendritic and synaptic damages, and neuronal and glial apoptoses (Bezzi et al, 2001; Kaul et al, 2001; Nath, 2002). Astrocytes activation and infection may also contribute to the neuronal damage, as their function is critical for neuronal survival and they can produce neurotoxins as well (Lawrence and Major, 2002). One well-studied viral protein with reported neurotoxic effects in vitro and in vivo is the HIV-1 envelope protein gp120, which normally mediates binding of HIV to target cells.
Recently, we have focused our attention on the effect of chemokine receptors on cell cycle proteins involved in neuronal apoptosis and differentiation, namely the retinoblastoma gene product, Rb, and the transcription factor, E2F-1 (Harbour and Dean, 2000a), with the intent of evaluating their potential involvement in HIV-induced neurodegeneration.
In the mammalian CNS, neurons withdraw from the cell cycle immediately after their differentiation. However, they still express components of the cell cycle machinery. The cell cycle is tightly regulated by sequential activation of cyclin-dependent kinases (CDKs), which then regulate the activity of target substrates involved in specific transitional steps. For instance, the CDK4/6 and cyclin D1 complex is thought to control the G0 to G1 transition. One of the best characterized substrates of CDK4/6 is the tumor suppressor protein Rb. Rb is normally hypophosphorylated in quiescent cells and binds and inhibits members of the transcription factor family E2F, such as E2F-1. Phosphorylation of Rb induces release of E2F-1, which dimerizes with its transcription partner DP1/2 and activates genes required for S phase transition. However, Rb may also associate with other transcription factors, which probably enable this protein to regulate gene expression in an E2F-independent manner. In addition to cell cycle progression, the Rb/E2F pathway has also been shown to integrate with pathways that control differentiation and programmed cell death (Harbour and Dean, 2000a, 2000b).
Interestingly, overexpression of phosphorylated Rb (pRb) and E2F-1 have been reported in the brain (i.e., hippocampus, cortex, and basal ganglia) of monkeys with simian immunodeficiency virus (SIV) encephalitis (Jordan-Sciutto et al, 2000) and HIV-demented patients (Jordan-Sciutto et al, 2002), suggesting their involvement in HIV neuropathogenesis. Also, initial observations about the up-regulation of the cyclin D3 gene (which is upstream of Rb phosphorylation and E2F-1 deregulation) in HIV encephalopathy (HIVE) and SIV encephalopathy (SIVE) have recently been presented (H. Fox, this meeting). Some neurotrophins, such as nerve growth factor (NGF) and brain-derived growth factor (BDNF), may also alter the subcellular localization of Rb, E2F, and p53 in human neuroglia cultures (Jordan-Sciutto et al, 2001).
In order to study whether CXCR4 is able to affect the CDK/Rb/E2F1 pathway, we have analyzed the changes in expression, localization, and activation of Rb and E2F-1 induced by short-term (10 to 30 min) and long-term (1 to 24 h) treatments with SDF-1α (and gp120IIIB) in primary cultures of postmitotic neurons and in a human cell line (HOS) transfected with human recombinant CXCR4. Immunocytochemistry, immunoblotting, and gel retardation analyses (EMSA) showed that SDF-1α affects the subcellular localization of Rb and E2F-1 in a time-dependent manner. Changes in the expression levels of the two proteins were observed in cells treated with the chemokine under different experimental conditions. Preliminary data indicate that gp120IIIB does not mimic the action of SDF-1α on Rb and E2F-1 expression and has opposite effects at the nuclear level.
Initial evidence about the coupling of CXCR4 with the cell cycle proteins were obtained in the HOS cells, which offer the advantage of homogenous CXCR4 expression in the absence of other chemokine receptors. In analogy to primary neurons (Meucci et al, 1998; Miller and Meucci, 1999), both SDF-1α (0.2 to 20 nM) and gp120IIIB (0.2 to 20 nM) activate CXCR4 in these cells, and induce Ca2+ responses sensitive to PTX and to anti-CXCR4 antibodies, as well as ERK phosphorylation. However, similarly to what we had found in neurons treated with the chemokine fractalkine and/or gp120IIIB (Meucci et al, 2000), SDF-1α, but not gp120IIIB, was able to activate prosurvival pathways, such as Akt, in the HOS cells.
Treatment with SDF-1α (20 nM) increased Rb levels in HOS cells as demonstrated by Western blot analysis and immunocytochemistry. Despite its activity as a nuclear protein, Rb was also detectable in the cytosol of HOS cells. Although Rb staining in the cytosol was quite low in control conditions, a significant increase in the protein levels was observed after a 3-h treatment with SDF-1α. Conversely, gp120IIIB (200 pM) induced an inhibition of the nuclear Rb content. The increase in Rb induced by SDF-1α was blocked by the protein synthesis inhibitor cycloheximide (CHX; 1 μM). The effect of SDF-1α on Rb was also observed when cells were deprived of serum (6 to 24 h), a condition that caused a dramatic decrease in Rb cytosolic levels. The addition of SDF-1α to serum-free medium prevented the cytosolic loss of Rb induced by the lack of trophic factors.
Experiments with different antibodies that recognize Rb phosphorylated at Ser795 and Ser780 (pRb), respectively, as well as in vitro kinase assays using a glutathione S-transferase (GST) fusion protein containing the COOH-terminal region of Rb (amino acids 769 to 921) indicate that SDF-1α treatment (20 nM, up to 60 min) does not directly induce Rb phosphorylation in these cells. Although a slight increase in the Rb phosphorylated band was observed after SDF-1α treatment, this effect was reverted by CHX, suggesting that it was correlated to the overall increase in total Rb expression. Further studies with normal populations of dividing cells, such as glia, are currently in progress to confirm these findings in other cell types. Initial results indicate that SDF-1α is able to affect total Rb expression in primary culture of mixed cortical glia as well.
We next studied the effects of SDF-1α and gp120IIIB in differentiated neurons. Immunostaining and immunoblotting of hippocampal and cortical neurons with anti-Rb antibodies showed that Rb is homogenously distributed to the nucleus and the cell body, whereas pRb staining is mostly localized to the cytoplasm in neurons maintained in the presence of glial trophic factors, which support their survival. Conversely, when neurons are kept in a saline solution in the absence of the glia support, an increase in the phosphorylated level of Rb is observed, particularly in the nucleus. Preliminary experiments indicate that treatment of neurons with SDF-1α (20 nM) reduced the Rb phosphorylation induced by glia deprivation. On the contrary, treatment of neurons with gp120IIIB (200 pM) seemed to increase the level of phosphorylated Rb and to enhance the DNA binding activity of E2F-1. These data suggest that changes in the expression and subcellular localization of Rb that translate in loss of Rb function are associated with apoptosis in differentiated neurons, and that SDF-1α is able to affect the expression and the phosphorylation of Rb. Further experiments are in progress to support this conclusion and correlate these observations with the action of SDF-1α on the survival of hippocampal neurons and with gp120 neurotoxicity.
Meanwhile, we have gathered interesting results in a different model of neuronal apoptosis that is known to involve Rb deregulation (Padmanabhan et al, 1999), i.e., cerebellar granule neurons deprived of depolarizing concentrations of potassium (Galli et al, 1995). When maintained in a serum-free low-potassium medium (KCl 5 mM), granule neurons undergo apoptosis, a process that involves several changes of cell cycle regulators (Park et al, 2000), including (i) accumulation of cyclin D1 and increased CDK4 activity; (ii) phosphorylation and subsequent loss of the Rb protein; (iii) up-regulation of E2F-1. These alterations are implicated in apoptosis of granule neurons. This, along with the reported role of SDF-1α in cerebellar development (Lu et al, 2002; Zhu et al, 2002), made this system very attractive to investigate the effect of SDF-1α on neuronal cell cycle proteins.
In line with previous reports, total Rb levels decreased when neurons were deprived of extracellular potassium, an effect observed after a relatively short incubation in K5 (1 h). The addition of SDF-1α (20 nM) to the K5 medium inhibited Rb reduction within the first 2 to 6 h. A modest increase in Rb levels was also observed when neurons were exposed to SDF-1α in a medium containing 25 nM KCl, whereas SDF-1α was unable to affect Rb levels when added to neurons that were deprived of potassium 5 h earlier. There were no significant changes in the expression levels of CXCR4 between K5 and K25 neurons, as determined by immunocytochemistry. These data suggest that the chemokine interferes with the earliest steps of the death signal cascade triggered by K5.
The ability of Rb to inhibit E2F-1 depends on its phosphorylation, which also affects Rb localization and degradation. Although several different sites of phosphorylation have been identified on Rb, four residues COOH-terminal to the pocket domain (Ser 975/807/811/780) are critical for its interaction with E2F proteins and are targets of CDK4/6. According to previous reports on cerebellar granule neurons, phosphorylation of Rb by CDK4 is one of the earliest events in the K5-induced apoptotic cascade. Thus, to evaluate whether the effect of SDF-1α on Rb may affect E2F-1 function, we probed the neuronal extracts with antibodies against Rb phosphorylated at Ser795 or Ser780. As in the case of total Rb levels, we found that SDF-1α treatment prevents the phosphorylation of Rb caused by potassium deprivation, which is generally associated to the degradation of the protein and activation of apoptotic genes by E2F-1. SDF-1α did not alter expression of structural proteins, such as actin.
In agreement with our findings on Rb levels and phosphorylation, cell survival experiments showed that SDF-1α was able to reduce neuronal cell death induced by potassium deprivation—an effect prevented by the CXCR4 antagonist AMD3100 (100 ng/ml). The neuronal death induced by K5 after 20 to 22 h was reduced by 50% in the presence of SDF1-α (20 nM). A similar degree of protection (60%) was observed by incubating neurons with PD0183812 (0.5 μM), a specific and potent inhibitor of CDK4/6 (Fry et al, 2001), suggesting that SDF-1α might inhibit Rb phosphorylation and its subsequent degradation. As expected, PD0183812 was also able to reduce phosphorylation and loss of Rb in these neurons. Thus, in cerebellar granule neurons, SDF-1α increases total Rb expression, prevents the phosphorylation of Rb induced by KCl deprivation (which seems to precede Rb degradation in these neurons), and promotes neuronal survival. These findings indicate that Rb function is important in preventing neuronal death, and may be involved in the regulation of neuronal survival by chemokines.
One of the functions of Rb is to bind and inhibit the activity of the transcription factor E2F-1 (Harbour and Dean, 2000a), which is implicated in apoptosis of the CNS. E2F-1 protein levels are a critical parameter to the apoptotic property of this transcription factor, and ectopic expression of E2F-1 in quiescent cells leads to entry into DNA synthesis and apoptosis. Thus, we studied the effect of SDF-1α on E2F-1 expression, localization, and DNA-binding activity by Western blot analysis and EMSA. Experiments in HOS cells have shown that when cells are treated with SDF-1α in the presence of serum, a time-dependent increase of E2F-1 in the cytosol (peak at 3 h), associated with a slower decrease in the nucleus (peak at 24 h), is observed. The DNA-binding activity of E2F-1 in nuclear extracts of HOS cells treated with SDF-1α for 3 h was reduced, and, in some experiments, a band of “protein-bound” E2F-1 appears in SDF-1α–treated cells, suggesting that SDF-1α may favor the binding of the transcription factor to regulatory proteins. Additional experiments are required to characterize the nature of the E2F-1 complex and determine the DNA-binding activity in different experimental conditions (i.e., longer treatments, total cell extracts).
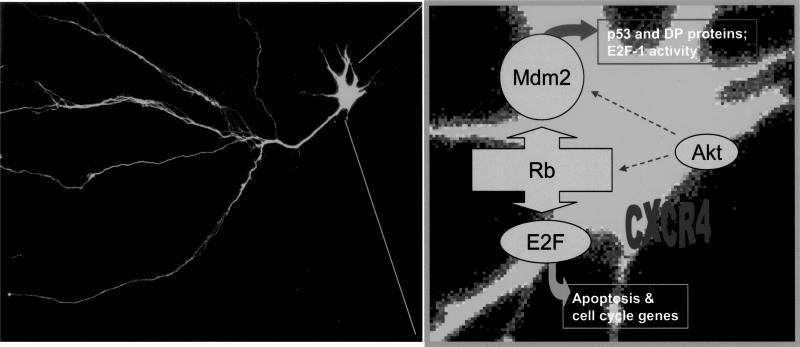
However, we found that protein levels of E2F-1 and its DNA-binding activity were higher than normal in preapoptotic cerebellar granule neurons. Both effects were inhibited by SDF-1α, suggesting that the chemokine may interfere with the apoptotic action of E2F-1. This is in agreement with experiments with hippocampal neurons showing that the HIV envelope protein increases E2F-1 DNA-binding activity. This is a very interesting observation, considering that in the same cultures (as well as in cortical neurons) gp120 activates the p38 kinase, which is able to block Rb-mediated inhibition of E2F-1. As the transcriptional potential of the E2F family members is dependent upon their nuclear localization, these data suggest that SDF-1α could reduce E2F-1 proapoptotic activity by “retaining” it into the cytosol and/or favoring its binding to inhibitory proteins. To further support this possibility, we are currently testing the effects of SDF-1α on additional proteins that are known to interact with Rb and E2F-1 and regulate their actions, such as mdm2 and p53. Although the data related to p53 are still unclear, we have found that SDF-1α increases total mdm2 levels in HOS cells and granule neurons. Mdm2 is thought to contribute to neuronal survival in several ways: by repressing p53 transcriptional activity and targeting it for degradation, by regulating E2F-1 accumulation and activity, as well as protecting Rb from degradation. On the other hand, loss of Rb also leads to degradation of mdm2, whereas phosphorylation of mdm2 by Akt facilitates targeting of p53 for destruction. Our current working model, based on these and other observations, is reported in Figure 1.
Figure 1.
Simplified model for the involvement of cell cycle proteins in the regulation of survival and differentiation by neuronal chemokine receptors: SDF-1α may affect expression of survival genes by regulating Rb and E2F-1 function. Also, SDF-1α could affect the interaction of Rb and/or E2F-1 with additional proteins involved in the regulation of cell survival (i.e., mdm2, Akt and MAP kinases). Thus, alterations of the CDK/Rb/E2F pathway—induced by activation of chemokine receptors under pathological conditions—may be implicated in HIV-induced neuropathogenesis.
In conclusion, activation of the chemokine receptor CXCR4 modulates the activity of two important regulators of cell survival and apoptosis, the tumor suppressor protein Rb and the transcription factor E2F-1, in dividing cells as well as differentiated neurons. This suggests that chemokines may be directly involved in the regulation of glia and neuronal survival and differentiation, and supports the potential involvement of cell cycle deregulation in HAD.
The ability of CXCR4 to regulate cell cycle progression and survival/apoptosis is not restricted to the nervous system, but has long been recognized as part of the important regulatory function of SDF-1 in hematopoiesis (Furukawa, 1998). The chemokine is implicated in homing and survival of progenitor hematopietic cells as well as in their differentiation. Recent reports showed that SDF-1 suppresses apoptosis, promotes survival and cell cycle progression in CD34+ stem cells, while inhibiting cycling of quiescent hematopoietic cells (Cashman et al, 2002; Lataillade et al, 2002). As cell cycle arrest in hematopietic cells is mediated through inhibition of pRb and E2F activity (as well as induction of CDKs) (Furukawa, 1998), it is expected that SDF-1 regulates the CDK/Rb/E2F-1 pathway in these cells. Should this be the case, the effect of SDF-1 (or other chemokines) on this pathway would have more general implications in HIV pathogenesis, beyond HIV-associated dimentia (HAD). At this regard, an interesting report just showed that G1 cycle arrest in T lymphocytes results in increased levels of secreted β-chemokines, which inhibited R5 HIV-1 replication of infected lymphocytes (Heredia et al, 2003). Furthermore, antiretroviral agents have been reported to have different activity against HIV reverse transcription, depending on the state of cell cycle of the infected cells (Davis et al, 2001).
Acknowledgments
This work was supported by grants from the NIH/NIDA (R01 DA15014-01) and the American Foundation of AIDS Research (amfAR 02816-30-RG) to O.M. Some of the data presented at this meeting are now in press as full-length paper. The authors wish to thank Dr. Peter Toogood (Pfizer Inc, Ann Arbor, MI) for kindly providing the PD0183812 compound, Dr. Todd Pappas (University of Texas Medical Branch, Galveston, TX) for generously sharing AMD3100, and Brian J. Musser for his valuable technical assistance.
References
- Bensalem MK, Berger JR. HIV and the central nervous system. Compr Ther. 2002;28:23–33. doi: 10.1007/s12019-002-0039-3. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–799. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- Davis C, Heredia A, Le N, Dominique JK, Redfield RR. Differential human immunodeficiency virus-suppressive activity of reverse transcription inhibitors in resting and activated peripheral blood lymphocytes: implications for therapy. J Hum Virol. 2001;4:113–122. [PubMed] [Google Scholar]
- Fry DW, Bedford DC, Harvey PH, Fritsch A, Keller PR, Wu Z, Dobrusin E, Leopold WR, Fattaey A, Garrett MD. Cell cycle and biochemical effects of PD 0183812. A potent inhibitor of the cyclin D-dependent kinases CDK4 and CDK6. J Biol Chem. 2001;276:16617–16623. doi: 10.1074/jbc.M008867200. [DOI] [PubMed] [Google Scholar]
- Furukawa Y. Cell cycle regulation of hematopoietic stem cells. Hum Cell. 1998;11:81–92. [PubMed] [Google Scholar]
- Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci. 1995;15:1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000a;2:E65–E67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000b;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- Heredia A, Davis C, Amoroso A, Dominique JK, Le N, Klingebiel E, Reardon E, Zella D, Redfield RR. Induction of G1 cycle arrest in T lymphocytes results in increased extracellular levels of beta-chemokines: A strategy to inhibit R5 HIV-1. Proc Natl Acad Sci U S A. 2003;100:4179–4184. doi: 10.1073/pnas.0630584100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Murray Fenner BA, Wiley CA, Achim CL. Response of cell cycle proteins to neurotrophic factor and chemokine stimulation in human neuroglia. Exp Neurol. 2001;167:205–214. doi: 10.1006/exnr.2000.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphy-Corb M, Wiley CA. Induction of cell-cycle regulators in simian immunodeficiency virus encephalitis. Am J Pathol. 2000;157:497–507. doi: 10.1016/S0002-9440(10)64561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22:2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, Bousse-Kerdiles MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–1129. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Major EO. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/s1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002 doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. J Neuro Virol. 2002;8:158–167. doi: 10.1080/13550280290049723. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Padmanabhan J, Park DS, Greene LA, Shelanski ML. Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J Neurosci. 1999;19:8747–8756. doi: 10.1523/JNEUROSCI.19-20-08747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Obeidat A, Giovanni A, Greene LA. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiol Aging. 2000;21:771–781. doi: 10.1016/s0197-4580(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]