Abstract
Background
Schizophrenia is a chronic, severe mental illness with profound emotional and economic burdens for those afflicted and their families. An increasing number of studies have found that schizophrenia is marked by dysregulation of glutamatergic neurotransmission. While numerous studies have found alterations of postsynaptic molecules in schizophrenia, a growing body of evidence implicates presynaptic factors. Vesicular glutamate transporters (VGLUTs) have been identified and are known to package glutamate into vesicles in the presynaptic terminal for subsequent release into the synaptic cleft. Recent studies have shown that VGLUTs regulate synaptic activity via the amount of glutamate released. Accordingly, we hypothesized that VGLUTs are altered in schizophrenia, contributing to dysfunction of presynaptic activity.
Methods
Using in situ hybridization and Western blot analysis, we investigated alterations in VGLUT1 and VGLUT2 transcript and protein expression in the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) of subjects with schizophrenia and a comparison group.
Results
We found increased VGLUT1 transcript and reduced VGLUT1 protein expression in the ACC, but not DLPFC, in schizophrenia. VGLUT2 was unchanged at both levels of gene expression. We did not find changes in VGLUT1 mRNA or protein levels following 28 day treatment of rats with haloperidol (2 mg/kg/day), suggesting that our findings in schizophrenia are not due to an effect of antipsychotic treatment.
Conclusions
Overall, our data suggest decreased glutamate release in the ACC, as well as discordant regulation of VGLUT1 expression at different levels of gene expression.
Keywords: VGLUT1, VGLUT2, prefrontal cortex, haloperidol, postmortem, Western blot, in situ hybridization
Introduction
The glutamate hypothesis of schizophrenia is based, in part, on the observation that phencyclidine (PCP) and other antagonists of the NMDA subtype of glutamate receptor can induce both positive and negative symptoms resembling schizophrenia in healthy subjects and exacerbate these symptoms in patients with this illness [1, 2]. Evidence of brain glutamate receptor dysfunction in schizophrenia itself has been deduced from in vivo studies finding altered glutamate and glutamate metabolite levels, from postmortem studies finding altered levels of glutamatergic enzymes, transporters, and receptors, and from clinical studies demonstrating attenuation of some schizophrenic symptoms following treatment with NMDA receptor co-agonists [3].
The predominantly postsynaptic localization of NMDA receptors led to the refinement of the glutamate hypothesis to include abnormalities of postsynaptic neurotransmission and signaling. Another refinement of the hypothesis was introduced with the discovery that alterations in glutamatergic transmission were not only post-synaptic, but pre-synaptic. For example, abnormalities of several proteins involved in fusion of synaptic vesicles to the plasma membrane and exocytosis have been found in postmortem studies[4–9]. These studies indicate that the presynaptic component of glutamatergic synapses may be abnormal in schizophrenia and suggest deficiencies in the molecular machinery that facilitates glutamate release into the synaptic cleft. To explore this hypothesis, we evaluated the expression of critical components of the presynaptic terminal, the vesicular glutamate transporters (VGLUT), in schizophrenia.
Three distinct vesicular transporters (VGLUT1–3) have been cloned and characterized [10–14]. VGLUT3 expression is restricted to some cholinergic neurons in the striatum, serotoninergic neurons in the raphe nuclei, and scattered glutamatergic neurons throughout the brain and will not be considered further [15]. VGLUT1–2 are heterogenously expressed in the brain, and are associated with molecular correlates of synaptic plasticity such as long-term potentiation (LTP) [12]. Alterations in synaptic activity can be induced by the modulation of the amount of glutamate released from synaptic vesicles and VGLUT-mediated transport of glutamate into vesicles for their subsequent release into the synaptic cleft is a pivotal control point for normal synaptic activity [16–20]. Thus, the expression levels of VGLUT mRNA and protein may approximate the relative strength of presynaptic innervation for a given brain region. The essential role of VGLUTs in the presynaptic terminal, combined with postmortem data in schizophrenia indicating alterations in the molecular machinery that facilitates glutamate release suggest that the VGLUTs are a high yield target for study in this illness. Accordingly, we hypothesized that there are region and gene specific decreases in VGLUT expression in schizophrenia, suggesting decreased presynaptic innervation. To test this hypothesis, we measured mRNA and protein expression for VGLUT1 and VGLUT2 in the ACC and DLPFC in schizophrenia.
Materials and Methods
Tissue acquisition and preparation
Postmortem brain tissue from patients with schizophrenia and a non-psychiatrically ill comparison group was obtained from the Mount Sinai Medical Center Brain Bank. Subjects were matched for age, postmortem interval (PMI) and pH. The medical records of the subjects designated as controls were examined using a formal blinded medical chart review instrument as well as in person interviews with the subjects and/or their caregivers. The subjects were evaluated for NINCDS-AIREN criteria for a diagnosis of vascular dementia; NINCDS, DSMIV and CERAD diagnosis of dementia; Consensus criteria for a clinical diagnosis of Probable or Possible diffuse Lewy body disease; UPDRS for Parkinson’s disease; clinical criteria for diagnosis of Frontotemporal dementia; medical history of psychiatric disease; history of drug or alcohol abuse; and other tests of cognitive function including the MMSE and CDR. In addition, each brain tissue specimen was examined neuropathologically using systematized macro- and microscopic evaluation using CERAD guidelines. Since the patients in our cohort were elderly at the time of death, many of the subjects have the cognitive impairment associated with aged subjects with schizophrenia [21–23]. All samples were derived from the left side of the brain. Subjects with schizophrenia were diagnosed with this illness for at least 30 years. Characteristics of the subjects are summarized in Tables 1 and 2. 8 subjects with schizophrenia and 5 control subjects were in both sets of experiments. Brains were obtained after autopsy and cut coronally into 10 mm slabs and frozen until further dissection. ACC (Brodmann areas 24 and 32) and DLPFC (Brodmann areas 9 and 46) were dissected from coronal slabs, snap frozen and kept at −80°C until further processing.
Table 1.
Subject characteristics of in situ hybridization studies
| Comparison group | Schizophrenia | |
|---|---|---|
| n | 11 | 18 |
| Age | 84.5 ± 12.9 | 76.8 ± 9.3 |
| Sex | 3M/8F | 10M/8F |
| PMI (hours) | 8.5 ± 6.8 | 11.5 ± 5.5 |
| Tissue pH | 6.4 ± 0.2 | 6.4 ± 0.3 |
Abbreviations: number (n), male (M), female (F), postmortem interval (PMI)
Table 2.
Subject characteristics for western studies
| Comparison group | Schizophrenia | |
|---|---|---|
| n | 27 | 23 |
| Age | 79.0 ± 12.7 | 72.1 ± 11.5 |
| Sex | 14M/13F | 16M/7F |
| PMI (hours) | 7.7 ± 7.2 | 14.6 ± 8.9 |
| Tissue pH | 6.4 ± 0.2 | 6.4 ± 0.3 |
Abbreviations: number (n), male (M), female (F), postmortem interval (PMI)
In situ hybridization
In situ hybridization was performed as previously described [14, 24]. Since our human VGLUT2 probe did not specifically label rat tissue a rat VGLUT2 subclone (U07609; 210–800) was prepared from a rat brain library. Sections were exposed to film for 4 days for VGLUT1 in both the ACC and DLPFC, 23 days for VGLUT2 in the DLPFC, and 21 days for VGLUT2 in the ACC. In the rodent studies, films were developed after 5 days for VGLUT1 and 6 days for VGLUT2.
Western blot analysis
Antibodies
We used mouse monoclonal anti-human VGLUT1 (1:2,000 dilution) and VGLUT2 (1:1,000) antibodies from Synaptic Systems and B-tubulin (1:4,000) antibody from Upstate for our western blot studies [25, 26]. We normalized VGLUT1 and VGLUT2 expression to B-tubulin protein levels for each sample.
Tissue acquisition and preparation
Approximately 1 cm3 of frozen tissue was first pulverized, then homogenized (10% W/V) in 50mM Tris-HCl (pH 7.0) for 30 sec with a polytron homogenizer and stored at −80°C in 0.5 ml aliquots. Dissection of cortical tissue for our western blot studies was performed with the aim of including grey matter only. However, because the dissections are done from tissue blocks and not from cryostat sections, the inclusion of some small amount of white matter cannot be completely excluded. Samples were prepared and Western blot analyses were performed as previously described [27]. Prior to examining VGLUT protein expression in schizophrenia, we first tested our VGLUT1, VGLUT2, and B-tubulin western blot assays using varying concentrations of total protein of human cortical tissue homogenate. These control studies demonstrated that our assay is linear for the protein concentrations used in our ACC (20 ug) and DLPFC (25 ug) protein studies.
Animal studies
The Institutional Animal Care and Use Committee approved all animal procedures. Adult male rats (Sprague-Dawley, 250 g) received daily subcutaneous injections of haloperidol (2 mg/kg) or vehicle (acidified DMSO) for 28 consecutive days (n = 10 per group for in situ hybridization, n = 8 per group for Western blot analysis). Twenty-four hours following the last injection, animals were sacrificed, brains were rapidly removed, snap frozen in isopentane, and cryostat sectioned (15 um) for in situ hybridization or dissected for Western blot analysis. For the rat in situ hybridization studies we measured gray scale values across the full thickness of all cortical layers and analyzed the data as described below. For the rat Western blot studies we used the same antibodies and assay conditions as described above.
Data analysis
Images of each slide were captured with a CCD based imaging system using Scion Image 4.0.2 software. For in situ hybridization, we analyzed the data in two ways; 1) we measured gray scale values across the full thickness of all cortical layers (II–VI), and 2) we obtained gray scale values of individual isodense bands for each subject (4 isodense bands for VGLUT1 and 3 for VGLUT2). Gray scale values, corrected for tissue background, were averaged to generate one value for each region per subject or animal and converted to optical density (OD). For Western blot studies, gray scale values were measured for protein bands at the expected molecular weight. The membrane background was subtracted and the adjusted gray scale values were converted to OD, and multiplied by the area of each band. Duplicate samples in adjacent lanes were averaged. The OD × area values for VGLUT1 or VGLUT2 were normalized to β-tubulin.
Statistical analysis
All statistical analyses were performed using Statistica (StatSoft, Tulsa, OK). Correlation analysis was performed for both in situ and Western blot studies to analyze associations between gene expression and age, postmortem interval (PMI), and pH. We analyzed mRNA expression using ANOVA with diagnosis and isodense bands as independent variables and optical density as the dependent variable. For analysis of mRNA expression across the full cortical thickness, we analyzed mRNA expression using ANOVA with diagnosis as an independent variable and optical density as the dependent variable. We analyzed protein expression using ANOVA with diagnosis as an independent variable and VGLUT/tubulin ratio as the dependent variable. When significant associations were found by regression analysis, analysis of covariance (ANCOVA) was used instead of ANOVA. For all animal studies, ANOVA was performed for each region for each gene product with drug treatment (haloperidol or vehicle) as the independent variable. Alpha = 0.05 for all analyses.
Results
VGLUT transcript expression in schizophrenia
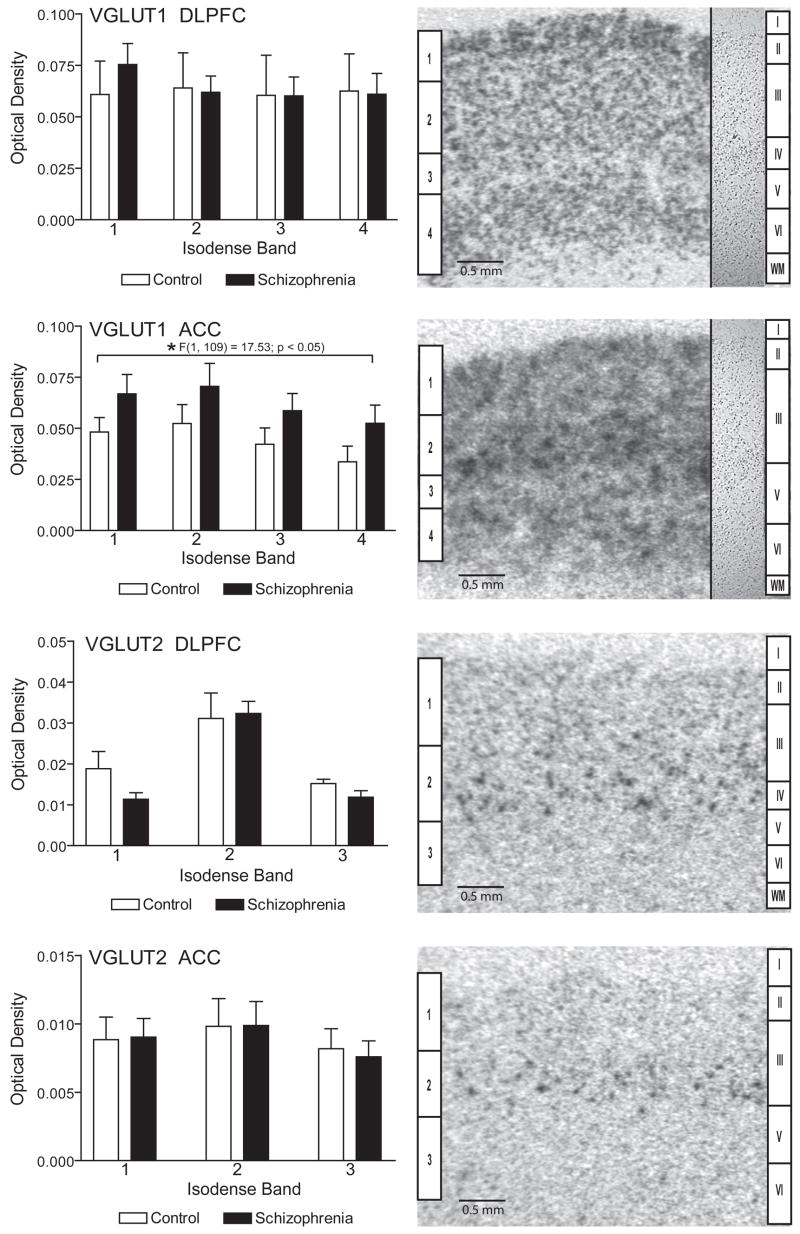
VGLUT1 transcripts were seen in four distinct isodense bands in the ACC and DLPFC, spanning cortical layers II–VI (Figure 1), while VGLUT2 expression was localized in three distinct isodense bands. Isodense band 1 for VGLUT1 mRNA expression corresponds to cortical layers II and III, isodense band 2 to layer III and V in the ACC and III and IV in the DLPFC, isodense band 3 to layer(s) V in the ACC and IV and V in the DLPFC, and isodense band 4 to layers V and VI. For VGLUT2 mRNA expression, isodense band 1 corresponds to layers II–III, isodense band 2 to layer III in the ACC and III and IV in the DLPFC, and isodense band 3 to layers V and VI.
Figure 1.
In situ hybridization analysis of VGLUT1 and VGLUT2 mRNA expression in the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) in subjects with schizophrenia and a comparison group. The inset panels on the right side of the VGLUT1 images are from nissl stained tissue sections. Isodense bands (1–4 for VGLUT1 and 1–3 for VGLUT2) are shown on the left side of each image and corresponding cortical lamina (I–VI) and white matter (wm) on the right. VGLUT1 mRNA is increased in ACC in schizophrenia. Data are expressed as mean ± SEM optical density values.
Regression analysis showed no association between VGLUT1 transcript expression in the DLPFC and age or pH, but a significant association with postmortem interval (r = 0.27; p < 0.05). Using ANCOVA with PMI as a covariate, we did not find an effect of diagnosis on VGLUT1 transcript expression in the DLPFC (Figure 1).
In the ACC, we did not detect significant associations between VGLUT1 transcript expression and PMI or pH, but we did find a significant association with age (r = 0.27; p < 0.05). Using ANCOVA with age as a covariate, and diagnosis and isodense bands as independent variables, there was a main effect for diagnosis (F(1,109) = 17.53; p < 0.05) showing increased VGLUT1 transcript expression in the ACC (Figure 1). There was no diagnosis by isodense band interaction, suggesting that changes in VGLUT1 mRNA expression in this region are not isodense band specific. Since we found no isodense band specific changes in VGLUT1 mRNA expression, we also analyzed mRNA expression across the full thickness of the cortex, which also indicated increased VGLUT1 transcript expression (F(1, 23) = 6.36, p < 0.02) in the ACC in schizophrenia.
In the DLPFC there were no significant associations between VGLUT2 and age, PMI, or pH. There were no differences between diagnostic groups for VGLUT2 transcript expression in the DLPFC.
In the ACC, we did not find a significant correlation between VGLUT2 transcript expression and age, but we did for PMI (r = 0.30, p < 0.05) and pH (r = 0.27, p < 0.05). When ANCOVA was performed using PMI and pH as covariates, no main effect for diagnosis for VGLUT2 expression was found (Figure 1).
VGLUT transcript expression in rats treated with antipsychotics
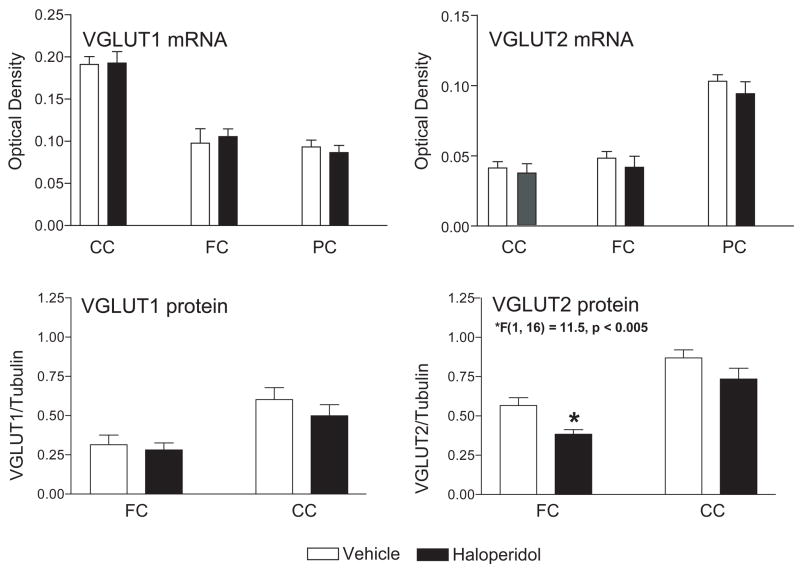
Since many of the patients with schizophrenia were taking typical antipsychotic medications at the time of death, we measured the expression of VGLUT transcripts in the cingulate cortex (CC), frontal cortex (FC), and parietal cortex (PC) from rats treated with haloperidol (Figure 2). We did not detect an effect of drug treatment on expression of either mRNA in these regions (Figure 2).
Figure 2.
VGLUT 1 and VGLUT2 mRNA (n = 10) and protein (n = 8) expression in rat cortex following 28 days treatment with haloperidol 2/mg/kg/day or vehicle. Haloperidol decreased VGLUT2 protein in FC but had no other effects. Abbreviations: frontal cortex (FC), parietal cortex (PC), cingulate cortex (CC). mRNA data are expressed as mean ± SEM optical density values. Protein data are expressed as the mean ± SEM of VGLUT optical density/tubulin optical density.
VGLUT protein expression in schizophrenia
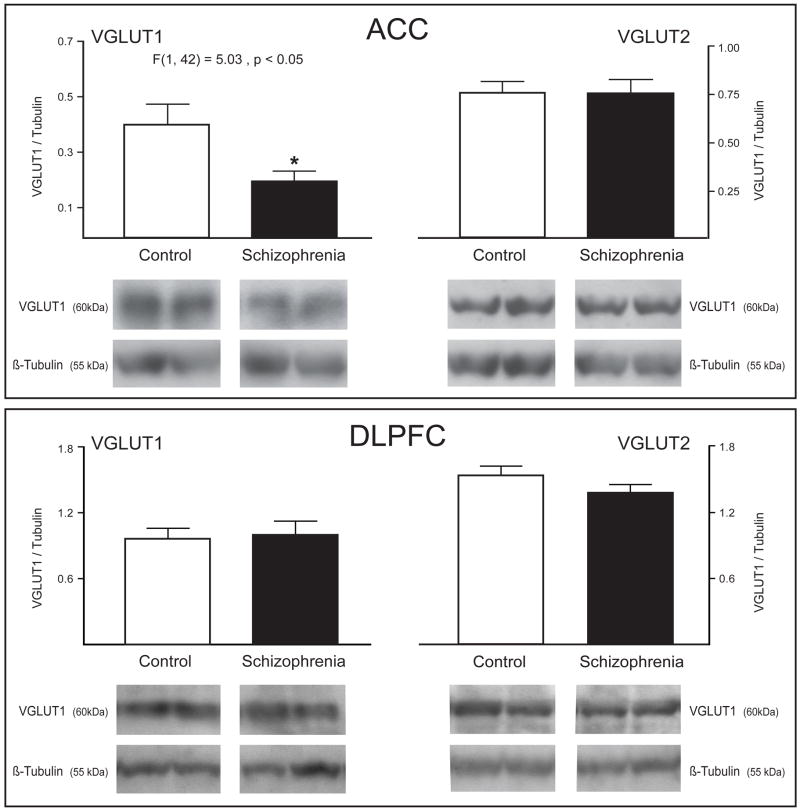
For both VGLUT1 and VGLUT2, we analyzed a single band at the predicted molecular weight for each protein. Regression analysis of dependent variables indicated that there were no associations between VGLUT1 or VGLUT2 protein expression and PMI, pH, or age in the ACC or DLPFC. We found decreased VGLUT1, but not VGLUT2, protein expression in the ACC in schizophrenia (F(1, 42) = 5.0321, p < 0.05) (Figure 3). No differences were detected in either VGLUT1 or VGLUT2 protein expression in DLPFC.
Figure 3.
Western blot analysis of VGLUT 1 and VGLUT2 protein expression in the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) in subjects with schizophrenia and a comparison group. VGLUT1 protein is decreased in ACC in schizophrenia. Data are expressed as the mean ± SEM of VGLUT optical density/tubulin optical density.
VGLUT protein expression in rats treated with antipsychotics
Using Western blot analysis, we measured the expression of VGLUT1 and VGLUT2 protein in the frontal (FC) and cingulate cortices (CC) of haloperidol treated rats (Figure 2). We did not detect an effect of drug treatment on expression of VGLUT1 in FC or CC or VGLUT2 in CC (Figure 2). We detected a significant decrease in VGLUT2 in FC (F(1, 16) = 11.5, p < 0.005) following haloperidol treatment (Figure 2).
Discussion
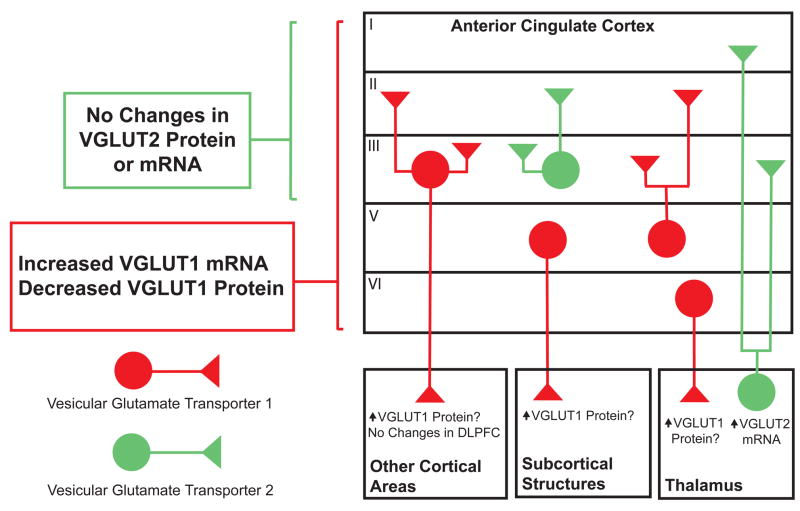
Our findings suggest that there are alterations in the presynaptic release of glutamate in the ACC in this illness (Summarized in Figure 4). Furthermore, the disparate changes in VGLUT1 mRNA versus protein expression suggest that coordination of VGLUT1 gene expression is altered in this area as well. Numerous reports have implicated the ACC in schizophrenia. The ACC had the second most abnormally expressed genes in a study using microarray analyses that examined 15 discrete brain regions [28]. Several other postmortem studies have found changes in the number of neurons and glia in this region [29–31]. The ACC has also been implicated in schizophrenia by studies employing functional magnetic resonance imaging (MRI), high resolution MRI and regional cerebral blood flow [32–35]. Altered glutamate transmission in this brain area is suggested by the low levels of glutamate and glutamine found in chronic schizophrenia cases [36]. Our data add to this growing body of evidence implicating abnormalities of the ACC in the pathophysiology of schizophrenia.
Figure 4.
Summary of changes and schematic of VGLUT1 (red) and VGLUT2 (green) expression in the anterior cingulate cortex in schizophrenia, based on data from the present study and earlier reports. In a previous study, increased VGLUT2 mRNA was found in the thalamus in schizophrenia. Question marked statements in the boxes labeled Other Cortical Areas, Subcortical Structures, and Thalamus are putative explanations to account for the observed changes in the ACC in schizophrenia.
In contrast to our findings in the ACC, we did not detect any changes in VGLUT expression in the DLPFC. While our study was not sufficiently powered to conclude with statistical certainty that there were in fact no changes in the DLPFC, our findings are generally consistent with other studies that have found few changes in presynaptic markers in the frontal cortex [37, 38]. Expression of synaptosomal-associated protein-25 (SNAP-25), syntaxin, synaptophysin and growth-associated protein-43 (GAP-43) was unchanged, while synaptobrevin/vesicle-associated membrane protein (VAMP) expression was increased, in the PFC in schizophrenia [37]. Another group found no changes in SNAP25 or syntaxin expression in subjects with schizophrenia who did not commit suicide [38], while expression of synaptophysin, syntaxin and SNAP25 was unchanged in the PFC in two other studies [39, 40]. Taken together these data suggest that there is not a global change in presynaptic function in schizophrenia, and that alterations in presynaptic innervation may be found in discrete cortical regions.
Interpretation of our data is dependent upon the cellular and subcellular distribution of the VGLUTs in glutamatergic circuits, which has been well characterized. VGLUT1 and VGLUT2 protein expression is generally restricted to synaptic puncta in brain and spinal cord regions receiving glutamatergic input [41]. VGLUT1 and VGLUT2 are colocalized with the presynaptic vesicular marker synaptophysin and are clearly segregated from the postsynaptic dendritic marker MAP2 [41]. The complementary pattern of VGLUT1 and VGLUT2 expression suggests that there are functionally discrete subsets of glutamatergic neurons [42]. In the DLPFC, VGLUT1 transcript and protein are expressed throughout the pyramidal neurons of layers II–VI, while expression of VGLUT2 mRNA is localized predominantly to layer IV [42].
In the ACC, we found increased VGLUT1 mRNA expression across the full thickness of the cortex. Since VGLUT1 mRNA expression in this region in the primate is predominately found in the soma of pyramidal cells and in some non-pyramidal excitatory neurons (unpublished observation), our results might indicate a transcript–level compensatory response to decreased presynaptic glutamate release that is suggested by our finding of decreased VGLUT1 protein expression in this region. Increased VGLUT1 mRNA could also be secondary to decreased cortical inhibitory tone, or a reaction to abnormal subcortical input to the ACC. Several studies have demonstrated abnormalities in GABAergic interneurons in the PFC, while others have found changes in regions with dense reciprocal innervation from the PFC, such as the thalamus [24, 31, 43–47]. For example, transcript expression for VGLUT2 and glutaminase was increased in the dorsal thalamus in schizophrenia in a study using a different sample from the same brain bank we used in this study [24, 46]. These data suggest increased excitatory thalamocortical innervation and/or increased release of glutamate. However, in the present study, we did not detect any alterations in VGLUT2 protein expression in the ACC or DLPFC, suggesting that changes in thalamic VGLUT2 transcript expression may not be apparent at the protein level in the regions receiving these thalamic afferent projections.
We also found decreased VGLUT1 protein expression in the ACC in schizophrenia. This result is discrepant from our finding of increased VGLUT1 mRNA in a similar sample from the same brain bank. There are several possible explanations for these potentially disparate findings. Changes in VGLUT1 protein expression could originate from intrinsic excitatory neurons of the ACC, from extrinsic presynaptic terminals expressing VGLUT1 protein, or both. If the change in protein expression is in the same population of neurons with increased VGLUT1 mRNA, this would suggest a loss of coordination of mRNA and protein expression in these cells. This could result from abnormalities of protein synthesis or from an increased rate of VGLUT1 protein degradation. Alternatively, the loss of VGLUT1 protein could be due to diminished excitatory input to the ACC from other cortical regions. We did not detect changes in VGLUT1 transcript or protein expression in the DLPFC, consistent with findings from a recent study that also found no changes in VGLUT1 protein expression in the PFC (BA 10) [48]. However, another study has reported decreases in VGLUT1 mRNA expression in the DLPFC, a region with excitatory projections to the ACC [49]. This study also found decreased VGLUT1 mRNA in the hippocampal formation, which is part of the efferent leg of limbic circuitry, that also includes the amygdala and the entorhinal cortex, which projects to the ACC [49, 50]. VGLUT1 mRNA expression has not been evaluated in the amygdala or entorhinal cortex in schizophrenia. Another source of excitatory input to the ACC is the thalamus. However, VGLUT1 mRNA expression is very low in most subcortical structures including the thalamus [24].
Another possibility for our divergent VGLUT1 findings is a process involving riboswitch mRNA [51, 52]. These specialized RNA molecules containing structures called aptamers have been found to regulate mRNA expression by sensing the need for their protein product [51, 52]. If present, riboswitch mRNA in anterior cingulate cortex neurons might detect a decrease in VGLUT1 protein, and initiate increased VGLUT1 transcription.
In must also be considered that changes in VGLUT1 mRNA or protein expression could be in non-neuronal cells. While VGLUT expression was originally reported in neurons, astrocytic expression of VGLUTs has not been evaluated in postmortem tissue, but several studies have found astrocytic expression of VGLUTs and vesicular release of glutamate from astrocytes in culture and in organotypic slices [53–62]. Interestingly, decreases in astrocytes have been reported in the ACC and DLPFC in schizophrenia, which could be consistent with decreased VGLUT1 protein expression, but not an increase in VGLUT1 transcripts [29, 63–65]. However, cell-level studies suggest that a majority of VGLUT1 mRNA and protein expression in the PFC is localized to neurons and presynaptic terminals forming asymmetric synapses, respectively, suggesting that the changes we have observed in the present study are likely limited to excitatory neurons [49, 66–70].
In contrast to the homogenously distributed VGLUT1, we detected the highest levels of VGLUT2 mRNA expression in an isodense band that encompasses layer III in the ACC and layers III and IV in the DLPFC (Figure 1). Intrinsic excitatory neurons in this cortical lamina generally project within layer III (and IV in the DLPFC) and to the other superficial layers (Figure 4) [71–73]. We did not detect changes in VGLUT2 transcript expression in the ACC or DLPFC in schizophrenia, suggesting that presynaptic function in the population of neurons expressing VGLUT2 in these regions is not affected. We also did not detect changes in VGLUT2 protein expression. In the DLPFC, VGLUT2 protein expression is highest in layer IV and is found at lower levels in layers I, II, III and VI [41, 47, 70, 74, 75]. Thus, our results suggest that presynaptic innervation arising from intrinsic PFC neurons expressing VGLUT2 is not altered in schizophrenia. Another study has suggested that decreased spine density in the DLPFC is secondary to a reduction in excitatory thalamocortical projections [76], which primarily express VGLUT2 and not VGLUT1 [24]. However, we did not detect changes in VGLUT2 protein expression in the DLPFC, suggesting that thalamocortical projections from this region are unchanged, or that if there is a reduction in projections there is more VGLUT2 protein expression per terminal. Alternatively, there could be a reduction in extrinsic VGLUT2 protein expression coupled with an increase in intrinsic VGLUT2 protein expression yielding a net level of expression that is apparently unchanged.
There are several potential limitations to this study. Many of the subjects with schizophrenia were taking typical antipsychotics at the time of death, raising the concern that changes in VGLUT1 expression in the ACC could be secondary to a medication effect. However, we found no changes in VGLUT1 mRNA and protein expression in several cortical regions of the rat brain following 4 weeks of treatment with haloperidol. Consistent with our findings, another group reported no changes in VGLUT1 mRNA or protein expression following 15 day treatment with a similar dose of haloperidol (1 mg/kg/day) [77] and another found no changes following 21 day treatment with 1.5 mg/kg/day [78]. While these studies do not model a lifetime of antipsychotic treatment in schizophrenia, they do suggest that our findings of altered VGLUT1 may not be due to a medication effect.
In contrast to VGLUT1 expression, we did find decreased VGLUT2 protein expression in the FC in rats treated for 28 days with haloperidol. This result raises the possibility that we did not detect an increase in VGLUT2 protein expression in schizophrenia due to a masking effect of treatment with haloperidol. The effects of haloperidol treatment on VGLUT2, but not VGLUT1, protein expression also suggest that these genes are differentially regulated by antipsychotic medications.
Another limitation of our study is that we are unable to measure protein levels in specific cortical lamina. It is possible that the decrease in VGLUT1 protein expression is limited to either the superficial layers (II–III), which generally receive cortico-cortical projections, or the deep layers (V–VI), which generally receive subcortical projections. Another concern is that the cortical ribbon may be thinner in samples from patients with schizophrenia versus control subjects, possibly leading to the differential inclusion of small amounts of white matter in the dissected samples, and thus affecting our western blot results due to disease-specific differences in the grey:white matter ratio. In addition, our identification of VGLUT1–2 protein was based on molecular weight, leaving the possibility that apparent changes in expression levels could be due to a change in protein structure. Finally, our studies were performed in elderly subjects. Using linear regression we examined our data set for associations between age and gene expression and with the exception of a positive correlation between VGLUT1 transcript expression and age in the ACC, we did not find any significant associations. In this instance, we utilized analysis of covariance to test for effects of diagnosis with age as a covariate. Another study found a negative correlation between VGLUT1 mRNA expression and age in the HPC in a younger cohort, but only in schizophrenia [49]. Significant associations between VGLUT1 protein and age have not been reported. In summary, while antipsychotic treatment and advanced age are potential limitations of this study, our data from antipsychotic treated rats, as well as our statistical analyses of the effects of age, suggest that these issues had minimal impact on our findings of altered VGLUT1 expression in the ACC.
While we did not find an effect of age on VGLUT1 protein expression, we have examined an aged cohort, and thus the question remains whether our findings are related to the end stage of the disease process or instead reflect an early pathological change. This question would best be answered by examining VGLUT expression in the ACC in a younger cohort. If our findings are only related to the latter stages of the illness, targeting changes in presynaptic function would not be a useful strategy for early pharmacological intervention. On the other hand, enhancing or reversing a loss of presynaptic function in aged patients with schizophrenia might diminish the loss of cognitive functioning associated with the end stage of this disease.
Regardless of the timing, VGLUT expression levels determine presynaptic vesicle filling and thus impact synaptic glutamate release [17–20, 26, 79]. Thus, reduced VGLUT1 expression is consistent with a loss of excitatory neurotransmission in a region that integrates myriad cognitive functions, many of which are impaired in schizophrenia [80, 81].
Acknowledgments
This work was supported by a Civitan Emerging Scholar Award (LVK), MH074016 (REM), VA Merit Review (VH), MH064673 (VH), and MH53327 (JMW).
Footnotes
Financial Disclosure Statements
Akin Oni-Orisan reported no biomedical financial interests or potential conflicts of interest. Lars V. Kristiansen reported no biomedical financial interests or potential conflicts of interest. Vahram Haroutunian reported no biomedical financial interests or potential conflicts of interest. Robert E. McCullumsmith reported no biomedical financial interests or potential conflicts of interest. James H. Meador-Woodruff reports that his laboratory receives grant and other financial support from NIH, Stanley Foundation, NARSAD, and American Psychiatric Institute for Education and Research and that he receives compensation from the ACNP as the Editor-in-Chief of Neuropsychopharmacology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 3.McCullumsmith RE, Clinton SM, Meador-Woodruff JH. Schizophrenia as a disorder of neuroplasticity. Int Rev Neurobiol. 2004;59:19–45. doi: 10.1016/S0074-7742(04)59002-5. [DOI] [PubMed] [Google Scholar]
- 4.Davidsson P, Gottfries J, Bogdanovic N, Ekman R, Karlsson I, Gottfries CG, et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr Res. 1999;40:23–9. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 5.Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci U S A. 1996;93:14182–7. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PM, Sower AC, Perrone-Bizzozero NI. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biological Psychiatry. 1998;43:239–43. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, et al. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Archives of General Psychiatry. 1997;54:559–66. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- 8.Honer WG, Falkai P, Young C, Wang T, Xie J, Bonner J, et al. Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience. 1997;78:99–110. doi: 10.1016/s0306-4522(96)00489-7. [DOI] [PubMed] [Google Scholar]
- 9.Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Molecular Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- 10.Hisano S. Vesicular glutamate transporters in the brain. Anat Sci Int. 2003;78:191–204. doi: 10.1046/j.0022-7722.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 11.Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, et al. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–5. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- 12.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 13.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Research - Brain Research Reviews. 2004;45:250–65. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.McCullumsmith RE, Meador-Woodruff JH. Expression of transcripts for the vesicular glutamate transporters in the human medial temporal lobe. Ann N Y Acad Sci. 2003;1003:438–42. doi: 10.1196/annals.1300.046. [DOI] [PubMed] [Google Scholar]
- 15.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. Journal of Neuroscience. 2005;25:6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, et al. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, et al. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–74. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–6. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, et al. Postmortem studies in schizophrenia. Schizophr Bull. 1998;24:325–41. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- 22.Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, et al. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006;66:1399–404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, et al. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993;33:255–60. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- 24.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport. 2001;12:2885–7. doi: 10.1097/00001756-200109170-00026. [DOI] [PubMed] [Google Scholar]
- 25.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- 26.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–47. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 28.Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77:241–52. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry. 2004;161:882–8. doi: 10.1176/appi.ajp.161.5.882. [DOI] [PubMed] [Google Scholar]
- 30.Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 32.Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63:356–65. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 33.Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–9. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 34.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–30. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi T, Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Iida H, et al. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129:399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 36.Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–3. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 37.Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- 38.Honer WG, Falkai P, Bayer TA, Xie J, Hu L, Li HY, et al. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb Cortex. 2002;12:349–56. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, et al. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Arch Gen Psychiatry. 1997;54:559–66. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- 40.Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–43. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 41.Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–45. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 44.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–9. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 45.Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59:394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- 46.Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res. 2005;75:27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Vogt BA, Rosene DL, Peters A. Synaptic termination of thalamic and callosal afferents in cingulate cortex of the rat. J Comp Neurol. 1981;201:265–83. doi: 10.1002/cne.902010210. [DOI] [PubMed] [Google Scholar]
- 48.Corti C, Crepaldi L, Mion S, Roth AL, Xuereb JH, Ferraguti F. Altered Dimerization of Metabotropic Glutamate Receptor 3 in Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–72. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, Braak E, Yilmazer D, Bohl J. Functional anatomy of human hippocampal formation and related structures. J Child Neurol. 1996;11:265–75. doi: 10.1177/088307389601100402. [DOI] [PubMed] [Google Scholar]
- 51.Blencowe BJ, Khanna M. Molecular biology: RNA in control. Nature. 2007;447:391–3. doi: 10.1038/447391a. [DOI] [PubMed] [Google Scholar]
- 52.Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- 53.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–20. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 54.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–42. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, et al. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–33. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- 56.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–73. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paterson DS, Thompson EG, Kinney HC. Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: Observations relevant to central chemosensitivity in early human life. Auton Neurosci. 2006;124:112–24. doi: 10.1016/j.autneu.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 58.Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study. Glia. 2005;49:96–106. doi: 10.1002/glia.20094. [DOI] [PubMed] [Google Scholar]
- 59.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–43. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem. 2006;281:4274–84. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- 62.Carmignoto G, Fellin T. Glutamate release from astrocytes as a non-synaptic mechanism for neuronal synchronization in the hippocampus. J Physiol Paris. 2006;99:98–102. doi: 10.1016/j.jphysparis.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Archives of General Psychiatry. 1986;43:31–5. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 64.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 65.Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–95. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 66.Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–60. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 67.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–59. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem. 2001;276:36764–9. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- 69.Alonso-Nanclares L, De Felipe J. Vesicular glutamate transporter 1 immunostaining in the normal and epileptic human cerebral cortex. Neuroscience. 2005;134:59–68. doi: 10.1016/j.neuroscience.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 70.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981;195:603–25. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- 72.Schubert D, Kotter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci. 2003;23:2961–70. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones EG. Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol. 1975;160:205–67. doi: 10.1002/cne.901600204. [DOI] [PubMed] [Google Scholar]
- 74.Sakata-Haga H, Kanemoto M, Maruyama D, Hoshi K, Mogi K, Narita M, et al. Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotransporters in rat forebrain. Brain Res. 2001;902:143–55. doi: 10.1016/s0006-8993(01)02290-9. [DOI] [PubMed] [Google Scholar]
- 75.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–73. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 76.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia.[see comment] Archives of General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 77.Moutsimilli L, Farley S, Dumas S, El Mestikawy S, Giros B, Tzavara ET. Selective cortical VGLUT1 increase as a marker for antidepressant activity. Neuropharmacology. 2005;49:890–900. doi: 10.1016/j.neuropharm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 78.Nudmamud-Thanoi S, Piyabhan P, Harte MK, Cahir M, Reynolds GP. Deficits of neuronal glutamatergic markers in the caudate nucleus in schizophrenia. J Neural Transm. 2007 doi: 10.1007/978-3-211-73574-9_34. In press. [DOI] [PubMed] [Google Scholar]
- 79.Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochem Int. 2006;48:643–9. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 80.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 81.Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry. 2004;161:707–15. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]