Abstract
Taurine is an essential amino acid in some mammals and is conditionally essential in humans. Taurine is an abundant component of meat and fish-based foods and has been used as an oral supplement in the treatment of disorders such as cystic fibrosis and hypertension. The purpose of this investigation was to identity the relative contributions of the solute transporters involved in taurine uptake across the luminal membrane of human enterocytes. Distinct transport characteristics were revealed following expression of the candidate solute transporters in Xenopus laevis oocytes: PAT1 (SLC36A1) is a H+-coupled, pH-dependent, Na+- and Cl−-independent, low-affinity, high-capacity transporter for taurine and β-alanine; TauT (SLC6A6) is a Na+- and Cl−-dependent, high-affinity, low-capacity transporter of taurine and β-alanine; ATB0,+ (SLC6A14) is a Na+- and Cl−-dependent, high-affinity, low-capacity transporter which accepts β-alanine but not taurine. Taurine uptake across the brush-border membrane of human intestinal Caco-2 cell monolayers showed characteristics of both PAT1- and TauT-mediated transport. Under physiological conditions, Cl−-dependent TauT-mediated uptake predominates at low taurine concentrations, whereas at higher concentrations typical of diet, Cl−-independent PAT1-mediated uptake is the major absorptive mechanism. Real-time PCR analysis of human duodenal and ileal biopsy samples demonstrates that PAT1, TauT and ATB0,+ mRNA are expressed in each tissue but to varying degrees. In conclusion, this study is the first to demonstrate both taurine uptake via PAT1 and functional coexpression of PAT1 and TauT at the apical membrane of the human intestinal epithelium. PAT1 may be responsible for bulk taurine uptake during a meal whereas TauT may be important for taurine supply to the intestinal epithelium and for taurine capture between meals.
Taurine is the most abundant free amino acid in the human body. It has a ubiquitous distribution and accounts for approximately 0.1% of total body weight (Huxtable, 1992). The important functions of taurine are too numerous to examine here but are described in detail in a number of reviews (Hayes, 1988; Huxtable, 1992; Sturman, 1993). In short, taurine has many fundamental biological roles such as bile acid conjugation, antioxidation, osmoregulation, membrane stabilization and modulation of Ca2+ signalling, and is essential for cardiovascular function, and development and function of muscle, the retina and the central nervous system. Endogenous taurine is synthesized mainly in the liver and brain but is freely available for utilization from carnivorous and omnivorous diets due to its abundant expression in animal tissues. Indeed, taurine is an essential amino acid in some carnivores (e.g. cats) where, following domestication, a taurine-deficient diet is associated with retinal degeneration, cardiomyopathy, high pregnancy failure, low neonate survival rate and stunted growth (Hayes, 1988; Sturman, 1993). In man, taurine is considered conditionally essential as it is essential during development and is subsequently found at high concentrations in breast milk, for example 0.4 mm in human, 0.8 mm in mouse, 2.8 mm in cats (Sturman, 1993). It is recommended that synthetic infant feeds are supplemented with taurine (Sturman, 1993). However, dietary taurine may also be essential in adults under pathophysiological conditions where surgery, trauma, critical illness and long-term total parenteral nutrition (in both adults and children) are associated with decreased taurine levels (Laidlaw & Kopple, 1987; Paauw & Davis, 1990). The importance of dietary taurine in pathophysiological conditions is emphasized by its successful use as an oral supplement in various clinical disorders including cystic fibrosis (Darling et al. 1985), diabetes (Franconi et al. 1995) and hypertension (Militante & Lombardini, 2002).
There are two intestinal transport mechanisms that mediate taurine movement across the brush-border membrane of the mammalian small intestine. Over recent years, the focus of investigation of intestinal taurine transport has been the high-affinity, low-capacity Na+- and Cl−-dependent transporter TauT (SLC6A6). cDNAs for the TauT carrier have been isolated from a number of species and tissues including MDCK (canine) cells (Uchida et al. 1992), rat (Smith et al. 1992), mouse (Liu et al. 1992) and human (Ramamoorthy et al. 1994). The cloned transporter has identical functional characteristics to the Na+- and Cl−-dependent transport system for the β amino acids taurine and β-alanine characterized using brush-border membrane vesicles (BBMVs) from rat (Barnard et al. 1988) and rabbit (Miyamoto et al. 1989) small intestine. This transport system has been named variously as the β amino acid carrier or taurine transporter and has also been characterized in the intact intestinal epithelium using either flat sheets of intestine from rabbit, guinea pig and rat small intestine (Munck & Munck, 1992, 1994), or monolayers of the human intestinal epithelial cell line Caco-2 (Brandsch et al. 1995; Satsu et al. 1997; Roig-Pérez et al. 2005). Comprehensive investigations of taurine and β-alanine transport demonstrate that this high-affinity, very low-capacity carrier is the only transport mechanism for taurine uptake in the rabbit and guinea pig small intestine (Munck & Munck, 1992, 1994).
Earlier reports of intestinal taurine transport, using intact sheets of rat small intestine, suggested that the major absorptive mechanism for taurine was a low-affinity transporter (Munck, 1977, 1983). Munck and colleagues identified that this taurine uptake in rat small intestine was via the partially Na+-dependent, but Cl−-independent, imino acid carrier, a low-affinity transport system with a broad substrate specificity which is absent from guinea pig and rabbit small intestine (Munck & Munck, 1994; Munck et al. 1994; Anderson et al. 2004). Over the same period, a low-affinity H+-coupled transporter of a broad range of amino acids was identified at the brush-border membrane of human intestinal Caco-2 cell monolayers and named system PAT, for proton-coupled amino acid transporter (Thwaites et al. 1993, 1995). cDNA clones for system PAT have been isolated from rat (Sagnéet al. 2001), mouse (Boll et al. 2002) and human (Chen et al. 2003). This system PAT-related carrier is now known as PAT1 and is the first of four members of solute carrier family 36 (SLC36A1). Recent studies have shown that the rat imino acid carrier and system PAT represent the same transport system and that the partial Na+ dependence of the imino acid carrier observed in rat small intestine is due to a functional coupling between the H+-coupled transporter PAT1 and the Na+/H+ exchanger NHE3 (SLC9A3) such that the driving force (the H+-electrochemical gradient) for PAT1-mediated amino acid movement is maintained during absorption via NHE3-mediated H+ efflux (Thwaites et al. 1995, 1999; Thwaites & Anderson, 2007). Perhaps the earliest evidence for β amino acid transport via this low-affinity transporter were the demonstrations of β-alanine absorption in rat small intestine in vivo and in vitro (Schofield & Lewis, 1947; Randall & Evered, 1964) that were both pH dependent (uptake being stimulated as luminal pH was decreased) and active (Thompson et al. 1970; De la Noüe et al. 1971). PAT1 has been immunolocalized to the brush-border membrane of human intestinal Caco-2 cell monolayers, and both human and rat small intestine (Chen et al. 2003; Anderson et al. 2004). Thus, in some species (e.g. human and rat) an alternative to the well-characterized high-affinity, low-capacity TauT transport system is expressed at the luminal surface of the small intestinal epithelium and this alternative carrier may be important in intestinal absorption when taurine is present at high concentrations such as those found in meat and fish-based foods (Laidlaw et al. 1990; Huxtable, 1992).
The objectives of this study were threefold: to determine whether the two taurine transporters, PAT1 and TauT, are coexpressed functionally in the same membrane surface; to estimate the relative contribution of each to taurine transport across the brush-border surface of confluent monolayers of the human intestinal epithelial cell line Caco-2; to identify the tissue distribution of β amino acid transporters in the human intestine which will aid in predicting the likely role of each transporter (PAT1 (SLC36A1), TauT (SLC6A6) and ATB0,+ (SLC6A14)) in absorptive transport along the length of the human intestine. Part of this study was communicated to the American Physiological Society at the Experimental Biology 2008 meeting and was published in abstract form (Anderson et al. 2008a).
Methods
Materials
[3H]Taurine (specific activity 27–29 Ci mmol−1) and [3H]carnitine (specific activity 84 Ci mmol−1) were from GE Healthcare (Little Chalfont, UK). [3H]β-Alanine (specific activity 50 Ci mmol−1) was from American Radiolabelled Chemicals (St Louis, MO, USA).
Amino acid uptake in Xenopus laevis oocytes
Female Xenopus laevis were killed humanely by cervical dislocation following Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. Oocytes were prepared and injected with 50 nl of either cRNA (1 mg ml−1) or water (as a control), as previously described (Anderson et al. 2004). PAT1 (Anderson et al. 2004), ATB0,+ (Anderson et al. 2008b) and TauT (Ramamoorthy et al. 1994) cRNA were synthesized using either mMessage mMachine T7 or T7 Ultra kits (Ambion, Warrington, UK). Uptake of [3H]taurine or [3H]β-alanine (5 μCi ml−1) were measured 2–5 days after injection and as previously described (Anderson et al. 2004). Briefly, oocytes were washed in uptake solution and then uptake measured at 22°C for 40 min. Uptake solution was either NaCl-containing (100 mm NaCl, 2 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm Hepes adjusted to pH 7.4 with Tris base), Na+ free (NaCl replaced with choline chloride) or Cl− free (chloride replaced with gluconate), as appropriate. pH 5.5 solutions were made by replacing Hepes with Mes (10 mm) and adjusting the pH with Tris base. After uptake, oocytes were washed three times in ice-cold buffer and lysed in 10% SDS. Radioactivity associated with the lysate was measured by scintillation counting.
Cell culture
Human intestinal Caco-2 cells (passage 104–127) were grown as confluent monolayers of polarized cells on Transwell polycarbonate filters (Corning, Schiphol-Rijk, the Netherlands), as previously described (Thwaites et al. 1993). Cell monolayers were used > 14 days post-seeding.
Amino acid uptake in Caco-2 cell monolayers
Caco-2 cell monolayers were washed (4 × 500 ml) and bathed in modified Krebs’ solution. Apical uptake of [3H]taurine or [3H]β-alanine (0.5 μCi ml−1) were measured over 15 min at 37°C in either NaCl-containing (137 mm NaCl, 5.4 mm KCl, 1 mm MgSO4, 0.34 mm KH2PO4, 0.3 mm NaH2PO4, 2.8 mm CaCl2, 10 mm glucose, 10 mm Hepes adjusted to pH 7.4 with Tris base), Na+-free (choline chloride replacing NaCl, and NaH2PO4 omitted) or Cl−-free (gluconate salts replacing chloride salts) modified Krebs’ solutions. The pH of the apical bathing solution was altered to pH 5.5 or pH 6.5 by replacing Hepes with 10 mm Mes and adjusting solution pH with Tris base. The basolateral solution was of the same composition as the apical solution but the pH was always pH 7.4. At the end of the uptake period the monolayers were washed (3 × 500 ml) in ice-cold solution, removed from the plastic inserts and cell monolayer-associated radioactivity measured by scintillation counting.
Real-time PCR: a human gastrointestinal tract cDNA panel and human mucosal biopsy samples
The abundance of PAT1, TauT and ATB0,+ cDNAs in a human intestinal cDNA panel and human mucosal biopsy samples was determined by quantitative real-time PCR on a LightCycler 480 (Roche) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Duodenal (Walters et al. 2006) and ileal (Balesaria et al. 2008) mucosal endoscopic biopsies (where ≥ 3 biopsies were pooled per patient) were obtained separately from two cohorts of control patients at Hammersmith Hospital (London, UK) undergoing investigation for diagnostic purposes and obtained with patient consent and local ethical approval (for full details see Walters et al. 2006; Balesaria et al. 2008). RNA was extracted using the SV Total RNA extraction kit (Promega, Southampton, UK). Concentration and purity of RNA was quantified (Nanodrop 1000, ThermoScientific) and RNA integrity checked by electrophoresis on a 1% agarose formaldehyde gel stained with ethidium bromide. Reverse transcription (RT) was performed using M-MLV RNase H+ Reverse Transcriptase (Promega). A human gastrointestinal tract multiple tissue cDNA panel was purchased from Clontech. For real-time PCR, a standard curve (plot of concentration versus crossing point, with efficiency approaching 2 (> 1.9) and r value of > 0.99) was established for each target gene using appropriate cloned cDNAs (quantified and assessed for purity using a Nanodrop 1000). HPLC-purified oligonucleotide primers were: PAT1 (NM_078483) forward CATAACCCTCAACCTGCCCAAC, reverse GGGACGTAGAACTGGAGTGC; TauT (NM_003043) forward TATCTGTATCCTGACATCACCCG, reverse CCCAGGCAGATGGCATAAGAG; ATB0,+ (NM_007231) forward CTTCTGGCTTGGCTCATAG, reverse TGAAGCACCCTCCAGAGTTGC. GAPDH primers were purchased from PrimerDesign (Southampton, UK). PCR reactions contained 0.5 μm each primer, 1 μl cDNA (panel samples) or 2 μl RT product (biopsy samples) in 1 × LightCycler 480 SYBR Green 1 Master (Roche) in a total reaction volume of 20 μl. PCR was performed over 35–45 cycles and included an initial hot start (95°C, 2 min), denaturation (94°C, 5 s), annealing (57–60°C, 15 s) and extension (72°C, 20 s). Fluorescence was measured at the end of the extension step in each cycle. Following cycling, melt curve analysis was performed over the temperature range 60–90°C to confirm unique amplification of the desired product. Products from preliminary PCR amplifications for each template were characterized by cloning and sequencing. PCR analyses were performed in duplicate for each sample. Results are expressed as mRNA abundance relative to GAPDH (pg/ng GAPDH).
Statistics
Data are expressed as mean ±s.e.m. Statistical comparisons of mean values were made using unpaired Student's two-tailed t test or one-way ANOVA (with Tukey–Kramer or Bonferroni's multiple comparisons post test) as appropriate. Curves were fitted using FigP software or GraphPad Prism 4. Significance was assumed if P < 0.05.
Results
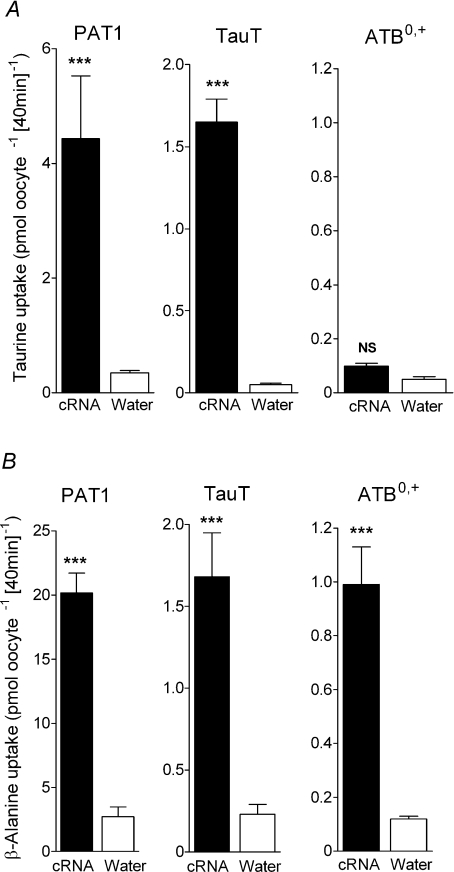
Uptake of the two β amino acids [3H]taurine (Fig. 1A) and [3H]β-alanine (Fig. 1B) is significantly greater (P < 0.001) in both PAT1- and TauT-injected oocytes compared to water-injected oocytes demonstrating that taurine and β-alanine are substrates for both PAT1 and TauT.
Figure 1. β amino acid uptake in PAT1-, TauT- or ATB0,+-expressing oocytes.
Uptake of [3H]taurine (A) and [3H]β-alanine (B) into oocytes injected with PAT1, TauT or ATB0,+ cRNA (filled bars). For PAT1, β-alanine and taurine (10 μm) uptakes were measured at pH 5.5 in the absence of extracellular Na+. For TauT and ATB0,+, β-alanine and taurine (2 μm), uptakes were measured at pH 7.4 in the presence of extracellular Na+. As a control, uptake into water-injected oocytes (open bars) was measured under identical conditions in all cases. Data are mean ±s.e.m. (n= 15–30). NS, P > 0.05; ***, P < 0.001; all versus water-injected oocytes.
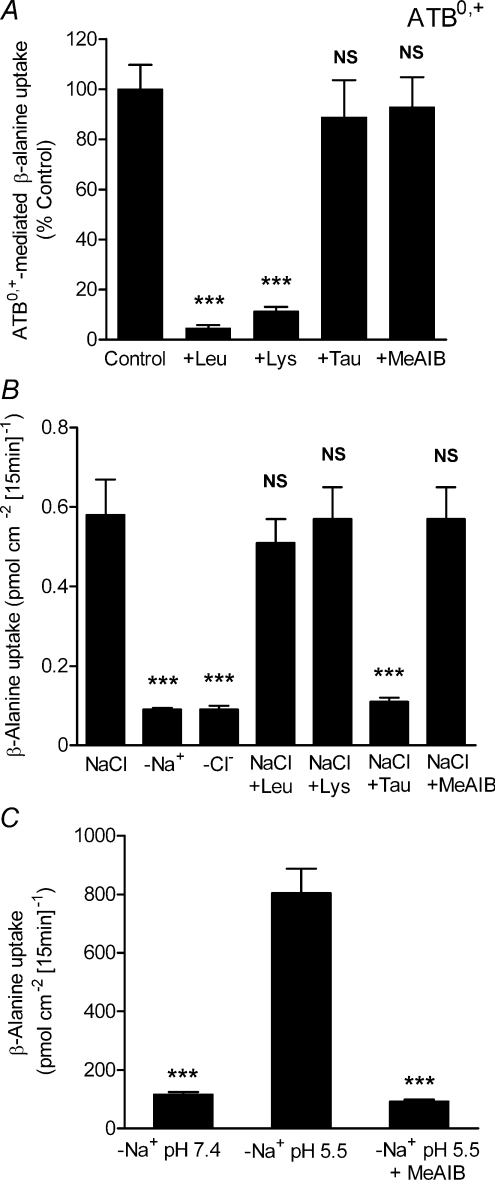
It should be noted that an additional transporter for β-alanine is expressed in rabbit ileum which was originally known as the β-alanine carrier (Munck, 1985; Munck & Munck, 1992). cDNAs for this transporter have been isolated (Sloan & Mager, 1999; Nakanishi et al. 2001) and it is now known as ATB0,+ (SLC6A14), a Na+- and Cl−-dependent transporter of both dipolar and cationic amino acids which has a low affinity for β-alanine (Sloan & Mager, 1999; Nakanishi et al. 2001; Anderson et al. 2008b). We have recently demonstrated that β-alanine transport via ATB0,+ has the same functional characteristics as those described in rabbit ileum for the β-alanine carrier (Anderson et al. 2008b). Although β-alanine is a substrate for ATB0,+ (Fig. 1B), in contrast to PAT1 and TauT, the uptake of taurine into ATB0,+-expressing oocytes is only slightly greater than that observed in water-injected oocytes (Fig. 1A) suggesting that taurine is excluded or is a very poor substrate for this third intestinal transporter of β amino acids.
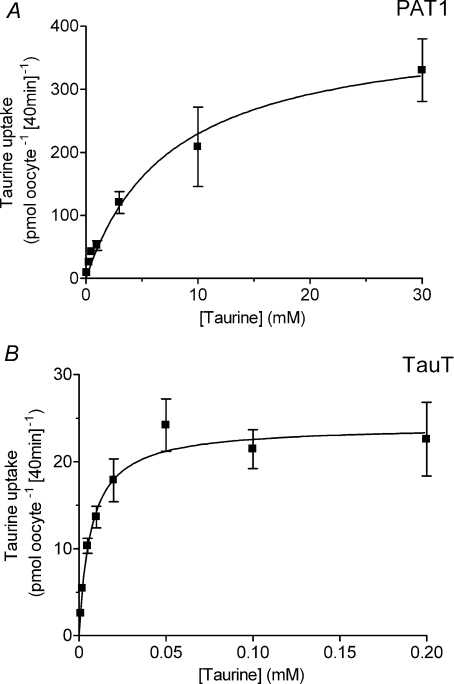
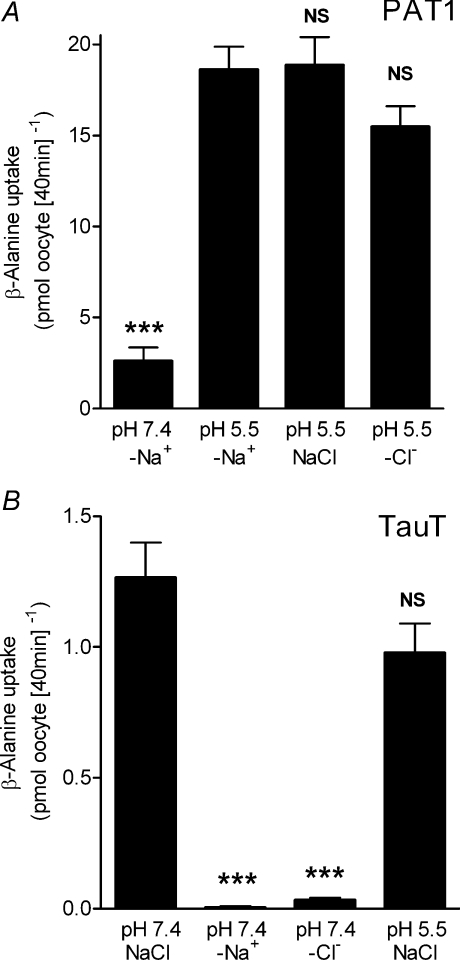
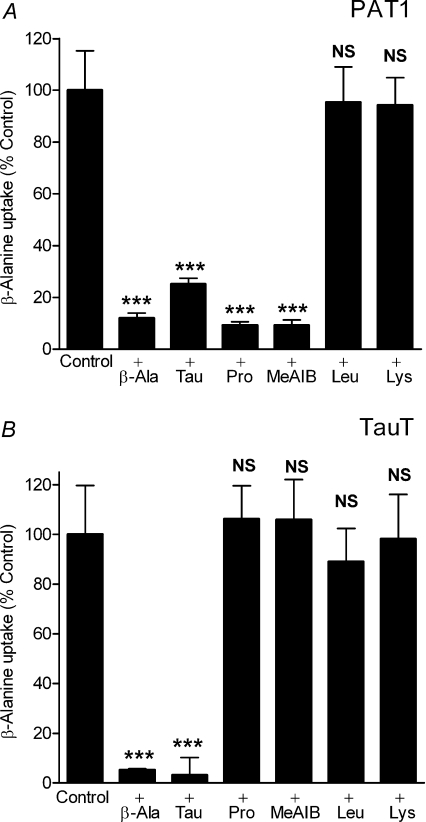
Taurine uptake by both PAT1 and TauT is via saturable carrier-mediated transport processes (Fig. 2) with distinct functional characteristics. Saturable, pH-dependent, Na+-independent taurine uptake via PAT1 demonstrates that PAT1 has a relatively low affinity for taurine (Km 7.5 ± 3.0 mm, Fig. 2A). In contrast, Na+- and Cl−-dependent taurine uptake via TauT confirms that TauT has a high affinity for taurine (Km 6.9 ± 1.8 μm, Fig. 2B) (Liu et al. 1992; Smith et al. 1992; Uchida et al. 1992; Ramamoorthy et al. 1994). At 100 μm taurine, taurine uptake via TauT is essentially saturated (Fig. 2B). The distinct ion-dependency of β amino acid uptake via these two transporters is demonstrated in Fig. 3. β-Alanine uptake via PAT1 is increased by a decrease in extracellular pH (P < 0.001), and is independent of both Na+ and Cl− (Fig. 3A). In contrast, β amino acid uptake via TauT is highly dependent upon both Na+ and Cl− (P < 0.001) (Liu et al. 1992; Smith et al. 1992; Uchida et al. 1992; Ramamoorthy et al. 1994) and is slightly but not significantly (P > 0.05) reduced upon extracellular acidification (Fig. 3B). Under optimal conditions for each transporter, clear differences in substrate selectivity can be observed (Fig. 4). Low-affinity PAT1-mediated [3H]β-alanine uptake (pH 5.5, Na+-free conditions) can be inhibited by the two β amino acids taurine and β-alanine (10 mm) as well as the known PAT1 substrates proline and MeAIB (Thwaites et al. 1995; Chen et al. 2003; Anderson et al. 2004) (Fig. 4A). In contrast, high-affinity TauT-mediated [3H]β-alanine uptake (pH 7.4, in the presence of Na+ and Cl−) is significantly inhibited (P < 0.001) by taurine and β-alanine (500 μm) whereas proline and MeAIB were without effect (500 μm, both P > 0.05, Fig. 4B). In additional experiments, MeAIB had no effect on uptake of either taurine or β-alanine in TauT-expressing oocytes even at concentrations (30 mm MeAIB) that would completely inhibit PAT1-mediated transport (data not shown). These observations demonstrate that TauT has a clear preference for β amino acids. For comparison, the effects of two high-affinity ATB0,+ substrates, leucine (Km 12 μm) and lysine (Km 100 μm) (Sloan & Mager, 1999), were investigated. At concentrations that would typically be saturating for either PAT1 or TauT substrates, leucine and lysine are unable to inhibit either transport system (P > 0.05, Fig. 4A and B).
Figure 2. Concentration-dependent taurine uptake in PAT1- or TauT-expressing oocytes.
Concentration-dependent [3H]taurine uptake was measured into oocytes injected with cRNA for PAT1 (A) and TauT (B). Uptake into PAT1-expressing oocytes was measured in Na+-free solutions at extracellular pH 5.5. Uptake into TauT-expressing oocytes was measured in NaCl-containing solutions at extracellular pH 7.4. Under each condition, uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give either PAT1- or TauT-specific uptake. Michaelis–Menten kinetics are fitted to the data which are mean ±s.e.m. (n= 7–10).
Figure 3. Distinct ion dependency of β amino acid uptake in PAT1- and TauT-expressing oocytes.
A, [3H]β-alanine (10 μm) uptake was measured into PAT1 cRNA-injected oocytes at pH 5.5 in the presence of NaCl or in Na+-free (–Na+) or Cl−-free (–Cl−) conditions, and at pH 7.4 under Na+-free (–Na+) conditions. Uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give PAT1-specific uptake. Data are mean ±s.e.m. (n= 17–18). NS, P > 0.05; ***, P < 0.001; all versus pH 5.5 –Na+. B,[3H]β-alanine (2 μm) uptake was measured into TauT cRNA-injected oocytes at pH 7.4 in the presence of NaCl or in Na+-free (–Na+) or Cl−-free (–Cl−) conditions, or at pH 5.5 in the presence of NaCl. Uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give TauT-specific uptake. Data are mean ±s.e.m. (n= 9–10). NS, P > 0.05; ***, P < 0.001; all versus pH 7.4 NaCl.
Figure 4. Inhibition of PAT1- or TauT-mediated β amino acid uptake by unlabelled amino acids.
A, the ability of various amino acids and the amino acid analogue MeAIB (all 10 mm) to inhibit [3H]β-alanine (10 μm) uptake into PAT1-expressing oocytes was measured in Na+-free pH 5.5 solutions. Uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give PAT1-specific uptake. Data are mean ±s.e.m. (n= 19–20). NS, P > 0.05; ***, P < 0.001; all versus Control. B, the ability of various amino acids and the amino acid analogue MeAIB (all 500 μm) to inhibit [3H]β-alanine (2 μm) uptake into TauT-expressing oocytes was measured in NaCl-containing pH 7.4 solutions. Uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give TauT-specific uptake. Data are mean ±s.e.m. (n= 16–20). NS, P > 0.05; ***, P < 0.001; all versus Control.
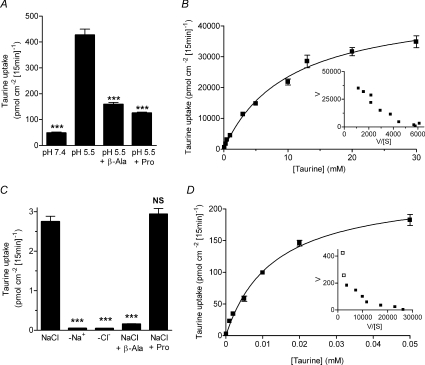
We have identified clear functional differences for β amino acid transport via the cloned transporters PAT1 and TauT (Figs 1–4). Firstly, the affinity for taurine differs by a 1000-fold (Fig. 2) so that even at concentrations below the Km for PAT1, taurine transport via TauT will be saturated. Secondly, PAT1 functions as a H+-coupled, pH-dependent, Na+- and Cl−-independent transporter whereas TauT is a Na+- and Cl−-coupled transport system (Fig. 3). Thirdly, TauT is highly selective for β amino acids whereas PAT1 accepts β amino acids and other amino acids (e.g. proline and MeAIB) with similar affinity (Fig. 4). The question arises as to which of these transporters mediates taurine uptake across the luminal surface of the human small intestine. Therefore, the next stage in this investigation was to exploit the distinctive characteristics of these two transporters to identify whether or not PAT1 and TauT are functionally coexpressed at the brush-border membrane of intact monolayers of the human intestinal epithelial cell line Caco-2 and to identify the relative contribution of these two transport systems to taurine uptake. The results in Fig. 5 demonstrate that both PAT1 and TauT can mediate taurine uptake across the apical membrane of human intestinal Caco-2 cell monolayers if measured under conditions that favour transport via each individual transport system. [3H]Taurine uptake was first measured under conditions that will favour transport via the low-affinity H+-coupled, Na+-independent, PAT1 (pH 5.5, Na+-free solutions, high taurine concentration of 100 μm) (Fig. 5A and B). Under these conditions, where Na+-dependent TauT will not function, [3H]taurine uptake has all the characteristics of PAT1 being increased as luminal pH is reduced from pH 7.4 to 5.5, and being inhibited by β-alanine and proline (both 10 mm, Fig. 5A). This pH-dependent, Na+-independent, taurine uptake across the apical membrane of Caco-2 cell monolayers is saturable with similar affinity (Km 10.1 ± 1.2 mm, Vmax 47 200 ± 2300 pmol cm−2 (15 min)−1, Fig. 5B) to that observed using PAT1-expressing oocytes (Fig. 2A). In contrast, when [3H]taurine uptake is measured under conditions that will favour transport via the high-affinity TauT (pH 7.4, Na+- and Cl−-containing solutions, low taurine concentration of 0.1 μm), a separate transport function can be identified (Fig. 5C). Under these conditions (0.1 μm taurine, apical pH 7.4), where PAT1 function will be minimal, [3H]taurine uptake has all the characteristics of TauT, transport being abolished in the absence of Na+ and Cl−, and being selectively inhibited by β-alanine (500 μm, P < 0.001) but not proline (500 μm, P > 0.05, Fig. 5C). It is possible to fit Michaelis–Menten kinetics to a single saturable component over the taurine concentration range 0.1–50 μm with an affinity similar to that identified for TauT (Km 12.9 ± 1.3 μm, Vmax 232 ± 9 pmol cm−2 (15 min)−1, Fig. 5D). However, if the taurine concentration range used is extended to 0.1–200 μm, a second low-affinity component is revealed (the non-linear Eadie-Hofstee plot, Fig. 5D inset), suggesting that both TauT and PAT1 contribute to taurine uptake even under the TauT-favouring conditions.
Figure 5. Functional characteristics of taurine uptake across the brush-border membrane of human intestinal (Caco-2) cell monolayers demonstrate the involvement of both PAT1 and TauT.
A, to demonstrate H+-coupled, pH-dependent, Na+-independent, PAT1-mediated transport, [3H]taurine uptake across the apical membrane of Caco-2 cell monolayers was measured at an extracellular taurine concentration of 100 μm under Na+-free conditions at apical pH 5.5 and 7.4 (basolateral pH 7.4). At pH 5.5, [3H]taurine uptake was measured in the presence and absence of the PAT1 substrates β-alanine and proline (both 10 mm). Data are mean ±s.e.m. (n= 10). ***, P < 0.001; all versus pH 5.5. B, concentration-dependent PAT1-mediated [3H]taurine uptake (0.1–30 mm taurine) was measured at apical pH 5.5 in the absence of extracellular Na+ (TauT will be inactive under these conditions). Michaelis–Menten kinetics are fitted to the data which are mean ±s.e.m. (n= 6). Inset, Eadie–Hofstee plot: V, taurine uptake; S, taurine concentration. C, to demonstrate Na+- and Cl−-dependent TauT-mediated transport, [3H]taurine uptake across the apical membrane of Caco-2 cell monolayers was measured at an extracellular concentration of 0.1 μm taurine at apical pH 7.4 in NaCl-containing solutions and in the absence of extracellular Na+ (–Na+) or Cl− (–Cl−) (basolateral pH 7.4). In the presence of both Na+ and Cl−, [3H]taurine uptake was measured in the presence and absence of the TauT substrate β-alanine (500 μm) or proline (500 μm) which is excluded from TauT at this concentration. Data are mean ±s.e.m. (n= 10). NS, P > 0.05; ***, P < 0.001; all versus NaCl. D, concentration-dependent TauT-mediated [3H]taurine uptake (0.0001–0.05 mm taurine) was measured at apical pH 7.4 in NaCl-containing solutions. Michaelis–Menten kinetics are fitted to the data which are mean ±s.e.m. (n= 6). Inset, Eadie–Hofstee plot (filled symbols represent data in main panel D over taurine concentration range 0.0001–0.05 mm; open symbols represent data for uptake at 0.1 and 0.2 mm): V, taurine uptake; S, taurine concentration.
Thus, although it is apparent that either PAT1 or TauT can mediate taurine uptake across the brush-border membrane of these human enterocytes under certain conditions, it is unclear what the relative contributions of the two transport mechanisms are under physiological conditions. Measurements made using pH microelectrodes have identified an area of low pH at the luminal surface of the mammalian small intestine where the surface pH in the jejunum is in the range pH 6.2–6.7 (McEwan et al. 1988; Daniel et al. 1989). To determine the relative contribution of the two transporters to taurine influx, [3H]taurine uptake was therefore measured under pseudo-physiological conditions (apical pH 6.5, in the presence of extracellular Na+) over a range of luminal taurine concentrations (0.1 μm to 1 mm) in the presence and absence of extracellular Cl− (Fig. 6). Removal of extracellular Cl− selectively inhibits TauT-mediated uptake (Fig. 3B) but does not affect transport via PAT1 (Fig. 3A). It should be noted that amino acid transport via PAT1 and TauT in intact epithelia cannot be distinguished by removal of extracellular Na+. PAT1 activity in intact epithelia is dependent upon NHE3 function resulting in partial Na+ dependence (Anderson et al. 2004; Anderson & Thwaites, 2005).
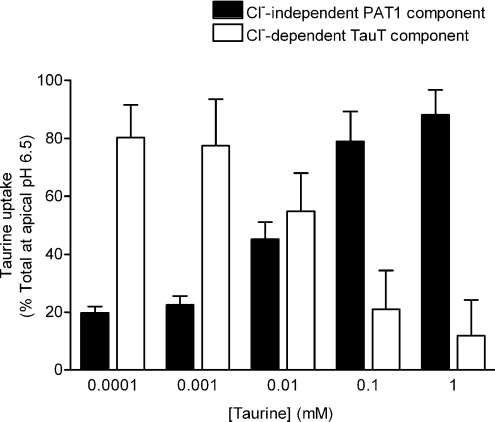
Figure 6. The relative contributions of PAT1 and TauT to total taurine uptake across the brush-border membrane of human intestinal (Caco-2) cell monolayers varies with the luminal taurine concentration.
Taurine (0.0001–1 mm) uptake across the apical membrane of Caco-2 cell monolayers was measured at the physiological apical pH of 6.5 (basolateral pH 7.4) in the presence and absence of extracellular Cl−. Removal of extracellular Cl− will selectively inhibit TauT whereas PAT1 will remain active. At each extracellular taurine concentration, taurine uptake is expressed as a percentage of the total uptake in the presence of Cl−. The PAT1-mediated component is taken as the uptake in the absence of Cl− (filled columns) and the TauT-mediated component is the difference between uptake in the presence and absence of Cl− (open columns). Data are mean ±s.e.m. (n= 10).
At 0.1 μm taurine, 80% of taurine uptake was inhibited by removal of Cl−, uptake being 2.44 ± 0.27 and 0.48 ± 0.06 pmol cm−2 (15 min)−1 (P < 0.001) in the presence and absence of extracellular Cl−, respectively. This Cl− dependence suggests that the majority of taurine uptake (at 0.1 μm taurine) is via the high-affinity Cl−-dependent TauT (Fig. 6). However, as the extracellular taurine concentration increases, the degree of Cl− dependence decreases suggesting that, at higher substrate concentrations, taurine uptake is mediated primarily via the low-affinity Cl−-independent PAT1, such that at 100 μm and 1 mm taurine there is no significant difference (P > 0.05) between uptake in the presence (total uptake at apical pH 6.5) and absence of Cl−. PAT1-mediated (Cl−-independent) taurine uptake represents 79% and 88% of total uptake at extracellular taurine concentrations of 100 μm and 1 mm, respectively (Fig. 6). At 1 mm taurine, uptake was 4251 ± 379 and 3746 ± 366 pmol cm−2 (15 min)−1 in the presence and absence of extracellular Cl−, respectively (Fig. 6). The residual Cl−-dependent component (appproximately 500 pmol cm−2 (15 min)−1) is broadly consistent with the estimate of the maximal capacity of TauT in Fig. 5D. Therefore, the relative contribution of PAT1 will continue to increase at increasing extracellular taurine concentrations whereas the magnitude of TauT-mediated transport will remain constant (above saturating levels) but will decrease as a proportion of total taurine uptake (Fig. 6).
The third intestinal transporter of β amino acids is ATB0,+. However, ATB0,+ will play no role in taurine absorption as, when expressed in Xenopus oocytes, ATB0,+ transports β-alanine but not taurine (Fig. 1). ATB0,+ has a distinct substrate selectivity, compared to either TauT or PAT1 (Fig. 4), as [3H]β-alanine uptake into ATB0,+-expressing oocytes is inhibited by 2 mm lysine and leucine (both P < 0.001) but not taurine or MeAIB (both P > 0.05, Fig. 7A). To identify if ATB0,+ can contribute to β-alanine uptake across the brush-border membrane of human intestinal Caco-2 cell monolayers, [3H]β-alanine uptake was measured under conditions favouring the function of both ATB0,+ and TauT (NaCl-containing solutions, apical pH 7.4, 0.1 μmβ-alanine) (Fig. 7B). [3H]β-Alanine uptake is both Na+- and Cl−-dependent (Fig. 7B) consistent with uptake via either TauT or ATB0,+. In addition, [3H]β-alanine uptake was measured in the presence and absence of amino acids at concentrations that selectively inhibit ATB0,+, TauT or PAT1. Neither the ATB0,+ substrates leucine or lysine (2.5 mm), nor the PAT1 substrate MeAIB (30 mm), had any effect on [3H]β-alanine uptake (all P > 0.05) whereas taurine (500 μm) caused significant inhibition (P < 0.001, Fig. 7B). These data are consistent with β-alanine uptake under these conditions (where PAT1 function will be minimal) being via TauT and not ATB0,+. The lack of ATB0,+ function at the luminal surface of these human intestinal cells is also suggested by the inability of an excess of the ATB0,+ substrate lysine (2.5 mm) to inhibit the uptake of the ATB0,+ substrate [3H]carnitine (10 μm) (Nakanishi et al. 2001), uptake being 14.88 ± 1.29 and 16.56 ± 0.37 pmol cm−2 (15 min)−1 in the absence and presence of lysine, respectively (n= 6) (P > 0.05).
Figure 7. ATB0,+ has a distinct substrate selectivity, compared to PAT1 and TauT, and does not contribute to β-alanine uptake across the brush-border membrane of human intestinal (Caco-2) cell monolayers.
A, [3H]β-alanine (100 μm) uptake into ATB0,+-expressing oocytes was measured in NaCl-containing, pH 7.4 solutions in the presence and absence of various amino acids and the amino acid analogue MeAIB (all 2 mm). Uptake in water-injected oocytes was subtracted from that in cRNA-injected oocytes to give ATB0,+-specific uptake. Data are mean ±s.e.m. (n= 16–20). NS, P > 0.05; ***, P < 0.001; all versus Control. B: [3H]β-alanine uptake across the brush-border membrane of Caco-2 cell monolayers was measured under conditions favourable for both TauT and ATB0,+ function (0.1 μmβ-alanine, pH 7.4 NaCl-containing apical solution). [3H]β-Alanine uptake was measured in the presence (NaCl) or absence of either Na+ (–Na+) or Cl− (–Cl−). [3H]β-Alanine uptake was also measured at pH 7.4 in the presence of NaCl and in the presence and absence of various amino acids at concentrations that discriminate between function of ATB0,+, TauT and PAT1: 2.5 mm leucine (to inhibit ATB0,+); 2.5 mm lysine (to inhibit ATB0,+); 500 μm taurine (to inhibit TauT); 30 mm MeAIB (to inhibit PAT1). Data are mean ±s.e.m. (n= 12). NS, P > 0.05; ***, P < 0.001; all versus NaCl. C, pH-dependent PAT1-mediated β-alanine uptake across the apical membrane of Caco-2 cell monolayers is observed when [3H]β-alanine uptake is measured under conditions favourable for PAT1 function (100 μmβ-alanine, apical pH 5.5, Na+-free solutions). Inhibition of [3H]β-alanine uptake was observed in the presence of the PAT1 substrate MeAIB (30 mm). Data are mean ±s.e.m. (n= 11–12). ***, P < 0.001; versus–Na+ pH 5.5.
As observed for taurine uptake (Fig. 5C), the lack of effect of MeAIB demonstrates that PAT1 does not contribute significantly to [3H]β-alanine uptake across the brush-border membrane of Caco-2 cell monolayers under the TauT-favouring conditions (NaCl-containing solutions, apical pH 7.4, 0.1 μmβ-alanine). However, if [3H]β-alanine uptake across the brush-border membrane of Caco-2 cell monolayers is measured under conditions favouring PAT1 function (pH 5.5, Na+-free solutions, substrate concentration of 100 μm), [3H]β-alanine uptake displays PAT1 characterictics being increased as pH is reduced from pH 7.4 to 5.5 and being inhibited by 30 mm MeAIB (Fig. 7C). Thus, both TauT and PAT1 (but not ATB0,+) contribute to β-alanine uptake across the brush-border membrane of Caco-2 cell monolayers and, as observed for taurine (Fig. 6), the relative contribution of each is probably dependent upon substrate concentration and the ionic composition of the local microenvironment bathing the luminal surface of the small intestinal epithelium.
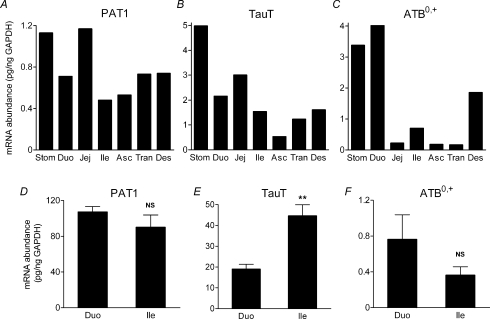
Relative quantitative real-time RT-PCR was used to assess the distribution of PAT1, TauT and ATB0,+ along the proximal–distal axis of the human gastrointestinal tract. Consistent with the functional measurements described in Figs 5–7, real-time PCR using Caco-2 mRNA detected transcripts for both PAT1 (420 ± 160 pg ng−1 GAPDH) and TauT (26 ± 8 pg ng−1 GAPDH) whereas ATB0,+ was essentially undetectable. Samples from a commercial human gastrointestinal tract cDNA panel, in which each sample comprises a pool of cDNAs from multiple donors, were assayed in duplicate (Fig. 8A–C). Results are expressed relative to GAPDH mRNA. All transcripts were found in the stomach and throughout the length of the intestine, from duodenum to descending colon (Fig. 8). PAT1 was most abundant in the jejunum, where expression was approximately 1.5-fold higher than elsewhere in the intestine (Fig. 8A). These observations are similar to a previous study when mRNA levels were detected by Northern blot (Chen et al. 2003). TauT appears to be similarly distributed, although expression in ascending colon was notably less than in other regions (Fig. 8B). In contrast, there were significant regional differences in ATB0,+ expression. It was most abundant in stomach, duodenum and descending colon (duodenum 2.2-fold higher than descending colon), and was lower in all other samples (Fig. 8C). This higher (relative to other regions of the gastrointestinal tract) ATB0,+ mRNA expression in stomach has been observed previously in human tissues (Sloan & Mager, 1999). In the small intestine the relatively high expression levels of ATB0,+ in duodenum, compared to jejunum and ileum, contrast with observations of mRNA distribution in mouse where greater transcipt levels are found in ileum (Nakanishi et al. 2001; Hatanaka et al. 2002). mRNA abundance of each transcript was also measured in a set of human duodenal (n= 4–5) and ileal (n= 8) biopsies (Fig. 8D–F). In contrast to the commercial tissue panel samples where RNA was extracted from all layers of the intestinal wall, endoscopic biopsies comprise mucosa including villus and crypt down to the submucosa layer but exclude muscle layers. All transcripts were readily detected in all samples. Comparison of the two groups showed that, in agreement with the results from the panel samples, PAT1 expression did not differ (P > 0.05) between duodenum and ileum (Fig. 8D). Biopsy data for TauT and ATB0,+ contradicted that obtained from the commercial panel with TauT transcript levels being significantly greater (P < 0.01) in ileum than duodenum (Fig. 8E) whereas ATB0,+ expression was similar in duodenum and ileum (Fig. 8F). In general, ATB0,+ expression in the biopsy samples was comparable to those in the panel whereas both PAT1 and TauT mRNAs were more concentrated in the biopsies. The differences between panel samples and biopsies probably reflect the difference in tissue source (whole wall versus mucosa) of the two sets of samples.
Figure 8. Real-time PCR detection of PAT1, TauT and ATB0,+ transcripts in a human gastrointestinal tract cDNA panel and human mucosal biopsy samples.
Expression of PAT1, TauT and ATB0,+ transcripts in a human gastrointestinal tract cDNA panel (A–C) and human mucosal biopsy samples (D–F). The tissues investigated were stomach (Stom), duodenum (Duo), jejunum (Jej), ileum (Ile), ascending colon (Asc), transverse colon (Tran) and descending colon (Des). The cDNA panel samples each comprised a pool of cDNAs from multiple donors and were assayed in duplicate whereas human duodenal mucosal biopsy samples were n= 4–5 (individual patients) and ileal samples n= 8 (individual patients). Data are expressed as mRNA abundance relative to GAPDH (pg/ng GAPDH). NS, P > 0.05; **, P < 0.01; all versus duodenum.
Discussion
Distinct functional characteristics of β amino acid transport via the cloned transporters PAT1, TauT and ATB0,+ are demonstrated following expression in Xenopus laevis oocytes (Figs 1, 2, 3, 4 and 7). The functional characteristics of the three transporters vary in ion dependency, substrate specificity and substrate affinity, such that each can be identified as follows: PAT1 is a H+-coupled, pH-dependent, Na+- and Cl−-independent, low-affinity, high-capacity transporter for both taurine and β-alanine; TauT is a Na+- and Cl−-dependent, high-affinity, low-capacity transporter of both taurine and β-alanine (Liu et al. 1992; Smith et al. 1992; Uchida et al. 1992; Ramamoorthy et al. 1994); ATB0,+ is a Na+- and Cl−-dependent, high-affinity, low-capacity transporter of neutral (e.g. leucine) and cationic (e.g. lysine) amino acids which accepts β-alanine with low affinity but excludes taurine (Anderson et al. 2008b).
As stated above, the focus of attention in intestinal taurine transport studies over recent years has been the high-affinity, low-capacity Na+- and Cl−-dependent transporter TauT (for examples see Barnard et al. 1988; Miyamoto et al. 1989; Brandsch et al. 1995; Satsu et al. 1997; Roig-Pérez et al. 2005). The potential contribution of a low-affinity, high-capacity transport system for β amino acids has not been addressed which is perhaps a little surprising considering the high concentration of taurine found in many carnivorous/omnivorous diets. However, there is evidence for such a transporter in both rat small intestine and human intestinal Caco-2 cell monolayers (Thwaites et al. 1993, 1995, 1999; Munck et al. 1994; Thwaites & Stevens, 1999). Following the molecular identification of PAT1 as a low-affinity transporter of imino and amino acids in rat and human (Sagnéet al. 2001; Chen et al. 2003), and immunolocalization of PAT1 to the luminal surface of human intestinal Caco-2 cell monolayers and human and rat small intestine (Anderson et al. 2004), we proposed that a potential physiological function of PAT1 was to allow efficient utilization of taurine in species with an abundance of taurine in the diet (Anderson et al. 2004).
This study provides the first demonstration of taurine uptake via a PAT1 transporter expressed in isolation (Figs 1 and 2). In addition, we provide the first demonstration that both PAT1 and TauT are functionally coexpressed at the brush-border membrane of intact monolayers of the human intestinal epithelial cell line Caco-2. It is also evident that the relative contribution of each transport system to total intestinal taurine transport will vary depending upon local conditions including taurine concentration and the ionic composition (Na+, Cl− and H+) of the solution bathing the mucosal surface of the enterocyte. Under physiological conditions (apical pH 6.5, NaCl-containing solutions), both transporters will mediate taurine uptake across the brush-border membrane with TauT being the dominant transporter at low extracellular taurine concentrations whereas PAT1 will mediate the bulk of taurine uptake at concentrations above 10 μm (which is around the Km of TauT for taurine) (Fig. 6). The relative importance of PAT1 will thus increase as luminal taurine concentrations increase suggesting that PAT1 may mediate the bulk of taurine absorption from taurine-rich meals (e.g. meat and fish; Laidlaw et al. 1990), taurine-containing energy drinks (which contain approximately 30 mm taurine), and the large doses used in clinical trials (Darling et al. 1985; Franconi et al. 1995; Militante & Lombardini, 2002).
The presence of two taurine transporters in the human small intestine is similar to observations made in rat small intestine (Munck & Munck, 1994; Munck et al. 1994). Both Cl−-dependent (via the high-affinity, low-capacity TauT) and Cl−-independent (via the imino acid carrier/PAT1) β amino acid transporters were detected in rat small intestine with the Cl−-independent, PAT1-mediated component being the dominant mechanism even at taurine and β-alanine concentrations as low as 5 μm (Munck & Munck, 1994). In contrast to the observations in rat small intestine, there is no evidence for a PAT1 component of taurine uptake in rabbit or guinea pig small intestine where taurine uptake appears to be mediated solely by TauT (Munck & Munck, 1992, 1994). Thus, there are clear species differences in the complement of amino acid transport systems expressed at the luminal surface of the small intestinal epithelium. This species variability is also evident for ATB0,+. We were unable to detect ATB0,+ function in human intestinal Caco-2 cell monolayers (Fig. 7). A previous report (Hu & Borchardt, 1992) has suggested that ATB0,+ may be responsible for Na+-dependent phenylalanine uptake in Caco-2 cells but the low affinity (Km 2.7 mm) and poor inhibition by 10 mm lysine (a concentration 100-fold greater than the Km for lysine; Sloan & Mager, 1999) reported suggest that ATB0,+ is not involved. In contrast to the lack of ATB0,+ in Caco-2 cells, ATB0,+ is the predominant transport system for β-alanine in some other intestinal tissues, for instance, rabbit ileum (whereas it plays only a minor role in the rabbit jejunum; Munck & Munck, 1992). Although ATB0,+ is generally considered to be primarily located in the distal small intestine and colon in many species (Munck & Munck, 1992; Hatanaka et al. 2002) we found a higher expression of ATB0,+ (albeit at the mRNA level) in the duodenum than ileum in both the human tissue panel and human biopsy samples (Fig. 8). Therefore, although ATB0,+ will not be involved in taurine absorption, it may play a role in transport of both nutrients and orally active drugs in proximal and distal regions of the human small intestine (Nakanishi et al. 2001; Hatanaka et al. 2002).
The variation in the expression of the taurine transporters, particularly PAT1, in the small intestine of different species most likely represents adaptation to dietary availability of taurine. The relative requirement for dietary taurine versus endogenous synthesis, in any particular animal or species, will vary with age, environment and physiological state but will generally reflect the relative magnitudes of synthetic capacity (cysteinesulfinate decarboxylase) and demand (due to catabolism, biliary secretion and renal excretion). In man, one of the major sinks for whole body taurine content is in the conjugation of bile acids in the liver. Interestingly, strong parallels can be made between the relative dependence of certain species on dietary taurine and their ability to utilize taurine in bile acid conjugation. A progressive dependence upon dietary taurine has been observed in the series: guinea pig, rat, monkey, human and cat (Huxtable, 1992). Most vertebrates conjugate bile acids exclusively to taurine although some placental animals have adapted to use glycine wholly or partially (Vessey, 1978; Huxtable, 1992). Cats conjugate taurine to bile acids but have minimal capacity for taurine synthesis making them reliant on diet (Hayes, 1988). This may represent an adaptation to maximize use of the abundant taurine in carnivorous diets which negates the requirement for energy expenditure on taurine synthesis. Humans conjugate bile acids to either taurine or glycine with the ratio varying during development and in response to changes in dietary taurine but not dietary glycine (Garbutt et al. 1971; Sturman, 1993). Interestingly, PAT1 can also transport glycine (Thwaites et al. 1995; Chen et al. 2003). However, compared to many other species, humans have a low capacity for taurine synthesis and are still relatively dependent upon dietary sources (Laidlaw & Kopple, 1987; Huxtable, 1992). Rats conjugate bile acids exclusively to taurine but, unlike cats, have significant synthetic capacity (Worden & Stipanuk, 1985) to generate endogenous taurine. In contrast, herbivores such as the guinea pig and rabbit, whose diet is essentially devoid of taurine, conjugate bile acids exclusively to glycine, presumably reflecting an adaptive response to the relative taurine deficiency in the liver (Vessey, 1978). Thus, it appears that the relative dependence upon dietary (as opposed to endogenous) taurine may reflect, at least in part, the use of taurine as the conjugate in bile acid synthesis. As a result, it seems likely that the relative capacities of the small intestine of different species will have adapted to, firstly, maximize taurine absorption from diet and, secondly, ensure the efficient reabsorption of taurine following deconjugation of bile salts (e.g. taurocholate) from the intestinal lumen. In human and rat intestine, where a requirement for luminal taurine uptake either from dietary or biliary sources might be expected, there are two taurine transport systems at the small intestinal luminal surface: PAT1, a low-affinity, high-capacity transporter; and TauT, a high-affinity, very low-capacity transporter. In contrast, in certain herbivorous mammals (guinea pig and rabbit) with a diet essentially devoid of taurine and which do not conjugate bile acids to taurine, the low-affinity (PAT1-like) transporter for taurine seems to have been lost and uptake across the small intestinal brush-border membrane can be accounted for by a TauT-like transporter (Miyamoto et al. 1989; Munck & Munck, 1992, 1994).
In conclusion, we have identified the key functional characterictics of taurine transport via PAT1 and TauT that will allow discrimination between these two carriers in intestine and other tissues. Interpretation of data using commercial multiple tissue panels is limited. However, the relative contributions of PAT1 and TauT to taurine uptake across the Caco-2 cell monolayers, plus relative estimates of PAT1 and TauT transcript expression by real-time PCR in human duodenal and ileal mucosal samples (Fig. 8), as well as Caco-2 cells, support a role for both transporters in taurine absorption. The high-capacity PAT1 transporter may be responsible for the bulk of taurine uptake during a high-taurine meal whereas the low-capacity TauT transporter may be more important for either supplying taurine to the intestinal epithelium (e.g. as an osmolyte) or for capturing taurine released between meals (e.g. from sloughed cells) (Thwaites & Anderson, 2007). The expression of both transporters in the distal intestine indicates that both could play a role in reabsorption of taurine deconjugated from bile acids by bacteria in distal parts of the lumen. How the relative expression levels of the two transporters vary spatially (e.g. along the crypt–villus axis), developmentally and in response to changes in diet, hormonal levels or pharmacological regimes, requires further investigation.
Acknowledgments
We thank Mrs Lisa Burdis for excellent technical assistance. This work was supported by the Wellcome Trust grant 078640/Z/05/Z.
References
- Anderson CMH, Ganapathy V, Thwaites DT. High- and low-capacity taurine transport across the luminal membrane of the small intestinal epithelium. FASEB J. 2008a;22:1202.3. [Google Scholar]
- Anderson CMH, Ganapathy V, Thwaites DT. Human solute carrier SLC6A14 is the β-alanine carrier. J Physiol. 2008b;586:4061–4067. doi: 10.1113/jphysiol.2008.154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, Munck BG, Daniel H, Ganapathy V, Thwaites DT. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. doi: 10.1053/j.gastro.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J Cell Physiol. 2005;204:604–613. doi: 10.1002/jcp.20337. [DOI] [PubMed] [Google Scholar]
- Balesaria S, Pell RJ, Abbott LJ, Tasleem A, Chavele KM, Barley NF, Khair U, Simon A, Moriarty KJ, Brydon WG, Walters JRF. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur J Gastroenterol Hepatol. 2008;20:413–422. doi: 10.1097/MEG.0b013e3282f41b82. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Thaxter S, Kikuchi K, Ghishan FK. Taurine transport by rat intestine. Am J Physiol Gastrointest Liver Physiol. 1988;254:G334–G338. doi: 10.1152/ajpgi.1988.254.3.G334. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem. 2002;277:22966–22973. doi: 10.1074/jbc.M200374200. [DOI] [PubMed] [Google Scholar]
- Brandsch M, Ramamoorthy S, Marczin N, Catravas JD, Leibach JW, Ganapathy V, Leibach FH. Regulation of taurine transport by Escherichia coli heat-stable enterotoxin and guanylin in human intestinal cell lines. J Clin Invest. 1995;96:361–369. doi: 10.1172/JCI118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fei YJ, Anderson CMH, Wake KA, Miyauchi S, Huang W, Thwaites DT, Ganapathy V. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol. 2003;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Fett C, Kratz A. Demonstration and modification of intervillous pH profiles in rat small intestine in vitro. Am J Physiol Gastrointest Liver Physiol. 1989;257:G489–G495. doi: 10.1152/ajpgi.1989.257.4.G489. [DOI] [PubMed] [Google Scholar]
- Darling PB, Lepage G, Leroy C, Masson P, Roy CC. Effect of taurine supplements on fat absorption in cystic fibrosis. Pediatr Res. 1985;19:578–582. doi: 10.1203/00006450-198506000-00015. [DOI] [PubMed] [Google Scholar]
- De la Noüe J, Newey H, Smyth DH. Transfer of alanine isomers by rat small intestine. J Physiol. 1971;214:105–114. doi: 10.1113/jphysiol.1971.sp009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, Gironi A, Anichini R, Seghieri G. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr. 1995;61:1115–1119. doi: 10.1093/ajcn/61.4.1115. [DOI] [PubMed] [Google Scholar]
- Garbutt JT, Lack L, Tyor MP. Physiological basis of alterations in the relative conjugation of bile acids with glycine and taurine. Am J Clin Nutr. 1971;24:218–228. doi: 10.1093/ajcn/24.2.218. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Nakanishi T, Bridges CC, Smith SB, Prasad PD, Ganapathy ME, Ganapathy V. Transport of d-serine via the amino acid transporter ATB0,+ expressed in the colon. Biochem Biophys Res Commun. 2002;291:291–295. doi: 10.1006/bbrc.2002.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KC. Taurine nutrition. Nutr Res Rev. 1988;1:99–113. doi: 10.1079/NRR19880009. [DOI] [PubMed] [Google Scholar]
- Hu M, Borchardt RT. Transport of a large neutral amino acid in a human intestinal epithelial cell line (Caco-2): uptake and efflux of phenylalanine. Biochim Biophys Acta. 1992;1135:233–244. doi: 10.1016/0167-4889(92)90226-2. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. JPEN Parenter Enteral Nutr. 1990;14:183–188. doi: 10.1177/0148607190014002183. [DOI] [PubMed] [Google Scholar]
- Laidlaw SA, Kopple JD. Newer concepts of the indispensable amino acids. Am J Clin Nutr. 1987;46:593–605. doi: 10.1093/ajcn/46.4.593. [DOI] [PubMed] [Google Scholar]
- Liu QR, López-Corcuera B, Nelson H, Mandiyan S, Nelson N. Cloning and expression of a cDNA encoding the transporter of taurine and β-alanine in mouse brain. Proc Natl Acad Sci U S A. 1992;89:12145–12148. doi: 10.1073/pnas.89.24.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan GTA, Daniel H, Fett C, Burgess MN, Lucas ML. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B Biol Sci. 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Tiruppathi C, Ganapathy V, Leibach FH. Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 1989;257:G65–G72. doi: 10.1152/ajpgi.1989.257.1.G65. [DOI] [PubMed] [Google Scholar]
- Munck BG. Intestinal transport of amino acids. In: Kramer M, Lauterbach F, editors. Intestinal Permeation. Vol. 4. Amsterdam: Excerpta Medica; 1977. pp. 123–135. [Google Scholar]
- Munck BG. Comparative aspects of amino acid transport in guinea pig, rabbit and rat small intestine. In: Gilles-Baillien M, Gilles R, editors. Intestinal Transport. Berlin, Heidelberg: Springer-Verlag; 1983. pp. 260–283. [Google Scholar]
- Munck BG. Transport of imino acids and non-α-amino acids across the brush-border membrane of the rabbit ileum. J Membr Biol. 1985;83:15–24. doi: 10.1007/BF01868734. [DOI] [PubMed] [Google Scholar]
- Munck LK, Munck BG. Distinction between chloride-dependent transport systems for taurine and β-alanine in rabbit ileum. Am J Physiol Gastrointest Liver Physiol. 1992;262:G609–G615. doi: 10.1152/ajpgi.1992.262.4.G609. [DOI] [PubMed] [Google Scholar]
- Munck LK, Munck BG. Chloride-dependent intestinal transport of imino and β-amino acids in the guinea pig and rat. Am J Physiol Regul Integr Comp Physiol. 1994;266:R997–R1007. doi: 10.1152/ajpregu.1994.266.3.R997. [DOI] [PubMed] [Google Scholar]
- Munck BG, Munck LK, Rasmussen SN, Polache A. Specificity of the imino acid carrier in rat small intestine. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1154–R1161. doi: 10.1152/ajpregu.1994.266.4.R1154. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Hatanaka T, Huang W, Prasad PD, Leibach FH, Ganapathy ME, Ganapathy V. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB0,+ from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532:297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paauw JD, Davis AT. Taurine concentrations in serum of critically injured patients and age- and sex-matched healthy control subjects. Am J Clin Nutr. 1990;52:657–660. doi: 10.1093/ajcn/52.4.657. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall HG, Evered DF. Amino acid uptake by living cell. I. Amino group requirement by small intestine of the rat in vitro. Biochim Biophys Acta. 1964;93:98–105. doi: 10.1016/0304-4165(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Roig-Pérez S, Moretó M, Ferrer R. Transepithelial taurine transport in Caco-2 cell monolayers. J Membr Biol. 2005;204:85–92. doi: 10.1007/s00232-005-0750-y. [DOI] [PubMed] [Google Scholar]
- Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, Gasnier B, Giros B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci U S A. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsu H, Watanabe H, Arai S, Shimizu M. Characterization and regulation of taurine transport in Caco-2 human intestinal cells. J Biochem. 1997;121:1082–1087. doi: 10.1093/oxfordjournals.jbchem.a021698. [DOI] [PubMed] [Google Scholar]
- Schofield FA, Lewis HB. A comparative study of the metabolism of α-alanine, β-alanine, serine and isoserine. I. Absorption from the gastrointestinal tract. J Biol Chem. 1947;168:439–445. [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na+ and Cl−-dependent neutral and cationic amino acid transporter B0+ J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Wang CHD, Hartig PR, Branchek TA, Weinshank RL. Cloning and expression of a high affinity taurine transporter from rat brain. Mol Pharmacol. 1992;42:563–569. [PubMed] [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Thompson E, Levin RJ, Jackson MJ. The stimulating effect of low pH on the amino acid transferring systems of the small intestine. Biochim Biophys Acta. 1970;196:120–122. doi: 10.1016/0005-2736(70)90175-6. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. Deciphering the mechanisms of intestinal imino (and amino) acid transport: the redemption of SLC36A1. Biochim Biophys Acta. 2007;1768:179–197. doi: 10.1016/j.bbamem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest. 1999;104:629–635. doi: 10.1172/JCI7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. Na+-independent, H+-coupled transepithelial β-alanine absorption by human intestinal Caco-2 cell monolayers. J Biol Chem. 1993;268:18438–18441. [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Simmons NL. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol. 1995;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Stevens BC. H+/zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (Caco-2) cells. Exp Physiol. 1999;84:275–284. [PubMed] [Google Scholar]
- Uchida S, Kwon HM, Yamauchi A, Preston AS, Marumo F, Handler JS. Molecular cloning of the cDNA for an MDCK cell Na+- and Cl−-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci U S A. 1992;89:8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey DA. The biochemical basis for conjugation of bile acids with either glycine or taurine. Biochem J. 1978;174:621–626. doi: 10.1042/bj1740621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JRF, Balesaria S, Chavele KM, Taylor V, Berry JL, Khair U, Barley NF, van Heel DA, Field J, Hayat JO, Bhattacharjee A, Jeffery R, Poulsom R. Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women. J Bone Miner Res. 2006;21:1770–1777. doi: 10.1359/jbmr.060721. [DOI] [PubMed] [Google Scholar]
- Worden JA, Stipanuk MH. A comparison by species, age and sex of cysteinesulfinate decarboxylase activity and taurine concentration in liver and brain of animals. Comp Biochem Physiol B. 1985;82:233–239. doi: 10.1016/0305-0491(85)90232-9. [DOI] [PubMed] [Google Scholar]