Abstract
Fragile X syndrome is one of the most common forms of mental retardation, yet little is known about the physiological mechanisms causing the disease. In this study, we probed the ionotropic glutamate receptor content in synapses of hippocampal CA1 pyramidal neurons in a mouse model for fragile X (Fmr1 KO2). We found that Fmr1 KO2 mice display a significantly lower AMPA to NMDA ratio than wild-type mice at 2 weeks of postnatal development but not at 6–7 weeks of age. This ratio difference at 2 weeks postnatally is caused by down-regulation of the AMPA and up-regulation of the NMDA receptor components. In correlation with these changes, the induction of NMDA receptor-dependent long-term potentiation following a low-frequency pairing protocol is increased in Fmr1 KO2 mice at this developmental stage but not later in maturation. We propose that ionotropic glutamate receptors, as well as potentiation, are altered at a critical time point for hippocampal network development, causing long-term changes. Associated learning and memory deficits would contribute to the fragile X mental retardation phenotype.
Fragile X mental retardation syndrome (FRAX) is one of the most common forms of mental retardation, and causes mild to severe mental retardation, hyperactivity and autism. It is typically caused by hypermethylation of CGG repeats in the promoter and 5′ untranslated region (5′ UTR) of the fragile X mental retardation 1 gene, FMR1 (de Vries et al. 1997), which effectively silences the gene. Mutations in FMR1 leading to mutant fragile X mental retardation protein (FMRP) expression, as well as mutations in Rho family GTPase associated genes, may also cause FRAX or FRAX-like mental retardation (De Boulle et al. 1993; Castellvi-Bel & Mila, 2001; Endris et al. 2002; Ramakers, 2002). A prominent neuronal phenotype detected in Fmr1 knockout (KO) mice, as well as in FRAX patients, is supernumerary, thin tortuous spines (Comery et al. 1997). Interestingly, a family of Rac (a Rho family protein) effectors, the WAVE complex, which has been shown to interact with FMRP, was also shown to be localized to dendritic spines and to have effects on spine numbers (Pilpel & Segal, 2005). The persistence of the spine phenotype into adulthood is controversial. Spine differences in different cortical regions have been reported to be maintained throughout adulthood (Irwin et al. 2001), though other studies show that these differences are very small by 3–4 weeks after birth in the mouse (Nimchinsky et al. 2001).
The physiological effects of the Fmr1 deletion require better characterization. Recent studies have shown a lack of spike-timing dependent potentiation (STDP) in the cortex of Fmr1 KO mice (Desai et al. 2006; Meredith et al. 2007). In the hippocampus, a protein-translation, metabotropic glutamate receptor (mGluR1/5)-dependent form of long-term depression (LTD) is significantly increased (Huber et al. 2002; Nosyreva & Huber, 2006). Changes in long-term potentiation (LTP), however, have not been convincingly shown (Godfraind et al. 1996; Li et al. 2002), though a small but significant effect of Fmr1 deletion on LTP induced by a theta-burst protocol in hippocampus has recently been reported (Lauterborn et al. 2007).
A study performed in primary hippocampal neuron cultures demonstrated a delayed synapse maturation, but no differences in miniature AMPA-receptor (AMPAR)-mediated currents (Braun & Segal, 2000). Another study in organotypic hippocampal slice cultures reported slight but detectable reduction in AMPA miniature currents in Fmr1 KO cells, only detectable when pairs of cells, Fmr1 KO and wild-type (WT) controls, were patched within the same slice (Pfeiffer & Huber, 2007). In acute neocortex slices, no differences in AMPA mEPSCs or in NMDA to AMPA ratios were found (Desai et al. 2006). It is still an open question whether the physiological properties of hippocampal synapses are altered during postnatal development in Fmr1 KO mice.
To investigate synaptic changes in the hippocampus, we used a new generation of knockout mice in which the remaining levels of mRNA are effectively null, unlike the previous model (Bakker et al. 1994). These mice will henceforth be referred to as Fmr1 KO2 mice (Mientjes et al. 2006). We prepared acute slices at two developmental stages, equivalent to prepubertal and young adult, from Fmr1 KO2 mice and WT littermates. Herein, we describe the developmental effects of Fmr1 deletion on hippocampal synapses. Our data reveal a surprising transient phase of unbalanced AMPA and NMDA content in the juvenile Fmr1 KO2 mouse, and a large increase in an NMDA-dependent form of LTP. These changes disappeared later in development.
Methods
Animals
All experimental procedures were approved by the Animal Research Committee of the Max Planck Society and complied with the guidelines laid out in the EU directive regarding the protection of animals used for experimental and scientific purposes, 86/609/EEC. Mice were kept on a 12 h–12 h light–dark cycle and had ad libitum access to food and water at all times. A total of 122 mice were used in this study.
Mice lacking Fmr1 (Fmr1 KO2) were generated by deletion of both the promoter and exon 1 of the Fmr1 gene (Mientjes et al. 2006). These mice are distinct from the original Fmr1 KO mouse line (Bakker et al. 1994), because they are deficient for both Fmr1 RNA and FMRP protein (Mientjes et al. 2006). Mice were backcrossed four generations into a C57BL/6 (Charles River, Sulzfeld, Germany or The Jackson Laboratory, Bar Harbour, ME, USA) background and maintained in this mixed (129/Sv/C57BL/6/FVB) background for most experiments described herein. In cases that mice backcrossed five or six times were used, the same genetic background was then used constantly throughout the analysis. Male WT and KO littermates were used and analysed in a blind manner. Mice were genotyped by tail PCR as described by Mientjes et al. (2006).
Preparation of slices and solutions
Transverse hippocampal slices (250 μm thick) were prepared from postnatal day 14 (P14 ± 2) and postnatal day 40–50 (6–7 weeks) Fmr1 KO2 mice and WT littermate control animals. Briefly, animals were deeply anaesthetized by inhalation of isoflurane released as fumes from liquid stock (Baxter Deutschland GmbH, Unterschleissheim, Germany), and killed by decapitation. The brains were quickly removed and placed in a slice chamber containing an ice-cold solution comprising (mm): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 3 myo-inositol, 2 sodium pyruvate, 0.4 vitamin C, 1 CaCl2, 6 MgCl2 and 25 glucose; or, alternatively (mm): 2.5 KCl, 1.25 NaH2PO4, 28 NaHCO3, 1 CaCl2, 7 MgCl2, 7 glucose and 240 sucrose. Slices were allowed to recover in the slicing solution for 1 h at 37°C, followed by slow cooling to room temperature (23–24°C). For some experiments (theta-burst pairing-induced LTP), the connection between the CA3 and CA1 was cut, and the slices kept in standard Ringer solution (mm: 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 25 glucose and 2 CaCl2), supplemented with 1 mm vitamin C and 3 mm sodium pyruvate and kept at 37°C. For low-frequency stimulation-induced depression (LFS-depression) slices were kept in standard Ringer solution at 37°C. All solutions (both slicing chamber and incubation chamber) were constantly perfused with a 95%–5% O2–CO2 gas mixture (carbogen).
Recording and stimulation
Generally, an effort was made to adhere to standard conditions used in our laboratory or described in the literature for the different electrophysiological protocols. Exact conditions are stated hereforth.
For recording, slices were transferred to a recording chamber placed in a Zeiss Axioscope upright microscope, fitted with a ×63 water immersion objective, and differential interference contrast (DIC) optics. Ringer solution was saturated with carbogen, and allowed to flow through the recording chamber at a rate of 1–2 ml min−1 (depending on the type of experiment), kept constant using a peristaltic pump. For low-frequency pairing LTP experiments, a solution was used containing (mm): 124 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 10 glucose, 4 CaCl2 and 4 MgCl2. Whole-cell recording pipettes (3–7 MΩ) were pulled from borosilicate glass and backfilled with intracellular solution comprising (mm; for AMPA/NMDA current ratio, miniature current and paired-pulse analysis): 125 caesium gluconate, 20 CsCl2, 10 NaCl, 10 Hepes, 0.2 EGTA, 4 MgATP and 0.3 NaGTP; or (mm; for low-frequency pairing LTP): 120 caesium gluconate, 10 CsCl2, 10 Hepes, 10 phosphocreatine, 8 NaCl, 4 MgATP, 0.3 NaGTP and 0.2 EGTA; or (mm; for theta-burst LTP): 120 potassium gluconate, 20 KCl, 10 Hepes, 0.2 EGTA, 2 MgCl2, 4 NaATP, 0.3 Tris–GTP and 14 phosphocreatinine. All intracellular solutions were at a pH of 7.2–7.3, adjusted with KOH or CsOH. Liquid junction potential was adjusted to 11 mV for caesium gluconate-based internal solutions and to 4 mV for potassium gluconate-based internal solutions. Whole-cell patch-clamp recordings were made from the somata of CA1 pyramidal neurons. For field recordings, a 0.5–2 MΩ pipette filled with standard Ringer solution was placed in the dendritic field of stratum radiatum (CA1 apical dendrites). Signals were acquired at 5000 Hz and filtered at 2900 Hz using a HEKA EPC9 amplifier (HEKA Instruments Inc., Bellmore, NY, USA). Stimulation of the Schaffer collaterals was carried out with a monopolar stimulation electrode via a pipette filled with NaCl (1 m) in the stratum radiatum of the hippocampal CA1 region. The stimulation electrode was placed 150–200 μm from the soma along the dendritic axis and 150–200 μm lateral from the recorded cell dendrites. In some experiments, a second stimulation electrode was used to stimulate a control pathway in the stratum oriens. There was no compensation for series resistance in whole-cell recordings.
For the experiments measuring AMPA/NMDA current and paired-pulse ratios, and for those using the low-frequency stimulation protocol, bicuculline methiodide (Sigma-Aldrich Chemie Gmbh, Munich, Germany, 10 μm) and CGP55845 (Tocris Bioscience, Bristol, UK, 0.1–0.5 μm) were added to the solution to block GABAA and GABAB receptors, and glycine (10 μm) was added to serve as a coagonist for the NMDA receptor. For miniature NMDA current analysis, tetrodotoxin citrate (Tocris Bioscience, 1 μm), NBQX (Tocris Bioscience, 5 μm) and bicuculline (10 μm) were added to the solution, and QX-314 (Alomone Labs Ltd, Jerusalem, Israel, 1–2 mm) was added in the patch pipette to block voltage-dependent sodium channels and to prevent spontaneous firing. Cells were held at −40 mV, a holding potential at which the voltage-dependent magnesium block of NMDA receptors is greatly attenuated. For rectification analysis, QX-314 and 100 μm spermine were added in the patch pipette to inhibit firing at depolarized potentials and to replace endogenous polyamines and allow rectification of the calcium-permeable AMPA receptors. For theta-burst pairing-induced LTP, 10 μm bicuculline, 10 μm picrotoxin and 10 μm glycine were added to enhance the induction of NMDA receptor-dependent LTP. The recording temperatures were different between voltage clamp (22–23°C) and current clamp or field experiments (30–32°C). Series resistance was monitored throughout voltage clamp experiments, and was between 15 and 25 MΩ. Input resistance was monitored throughout current clamp experiments (see Table 1 for average input resistance). Cells in which the monitored resistance changed over 20% during the recording were excluded from the analysis. In order to reliably quantify current flow in all analyses in which synaptic response was measured, stimulation intensities were set to obtain responses of 75–300 pA (average 150 pA) at a holding potential of −70 to −90 mV. Most traces shown are scaled in order to afford an easier comparison of AMPA to NMDA ratios and rectification.
Table 1.
Intrinsic excitability of hippocampal CA1 pyramidal cells at P14
| Measurement | WT | Fmr1 KO2 | P valuec |
|---|---|---|---|
| Resting membrane potential (mV) | −65.6 ± 3.2 | 65.8 ± 2.9 | 0.82 |
| Input resistance (MΩ) | 151 ± 63 | 155 ± 32 | 0.84 |
| mAHP* amplitude (mV) | 6.3 ± 3.5 | 6.1 ± 3.2 | 0.93 |
| Spike threshold (mV) | −49.0 ± 3.2 | −50.8 ± 3.1 | 0.13 |
| Rheobase (Rh; pA)a | 70 ± 28 | 52 ± 28 | 0.1 |
| Number of spikes at (Rh + 20 mV)b | 7.5 ± 4.5 | 7.5 ± 3.1 | 1 |
Measurements were made by whole-cell recordings in WT (n= 12) and Fmr1 KO cells (n= 18)
Injected current required to evoke one spike (600 ms);
600 ms current injection;
Student's unpaired t test; *mAHP, membrane afterhyperpolarization.
Paired-pulse ratios were measured as the ratio between the peak current response to two consecutive stimulations delivered at interpulse intervals ranging from 15 to 120 ms. The paired-pulse ratio for each interpulse interval was taken as the ratio between the peak EPSC response of the second pulse to the first.
Ratios of AMPA to NMDA were measured as described by Myme et al. (2003). At a highly depolarized holding current (–90 mV), the AMPA receptor index is measured at the peak of the current trace, and at a polarized holding current (+40 mV), the NMDA index is measured as the remaining level of current flow 50 ms after the stimulation artifact. At this point, the AMPA receptor-mediated current is virtually gone (see Fig. 2A).
Figure 2. AMPA/NMDA ratios are altered at P14, but not at 6–7 weeks.
A and B, scaled sample traces of AMPA/NMDA ratio measurements at P14 and 6–7 weeks, respectively. Black traces are from WT control cells and grey traces are from Fmr1 KO2 littermates. In all cases, in order to afford easy comparison of the ratio between the AMPA and the NMDA responses, the current at a holding potential of −90 mV was scaled to the level of peak current flow at a +40 mV holding potential in the same recording. Numbers on the X-axis denote time after stimulation. Note that AMPA currents (negative current traces) are virtually gone 50 ms after stimulation (as seen by the stimulus artifact). In A, a vertical line has been drawn to denote where NMDA current values are measured (NMDA). The AMPA current values are measured at the peak at a −90 mV holding current (AMPA). C and D, mean values of the AMPA/NMDA ratios in the developing and young adult stage, respectively. E, histogram showing the distribution of the AMPA/NMDA ratios in young (P14) mice. The distribution is skewed towards significantly lower AMPA/NMDA ratios in the Fmr1 KO2 mice.
Rectification was measured by substituting intracellular polyamines with spermine, which allows rectification of calcium-permeable AMPA receptors (Verdoorn et al. 1991). In the presence of the NMDA channel blocker d-aminophosphonovalerate (d-APV), we measured the peak current in response to stimulation at increasing holding potentials (from −40 to +40 mV), and calculated a rectification index as the ratio of the response at +40 to that at −40 mV. To reduce offset errors, liquid junction potential was set at 11 mV, and current flow at 0 mV holding potential (a negligible amount) was subtracted (see Fig. 3C).
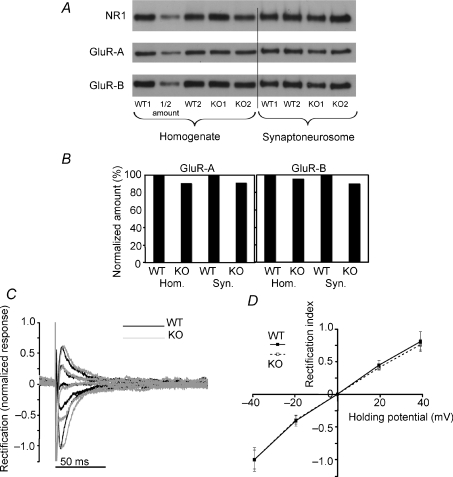
Figure 3. AMPA/NMDA protein levels are reduced at P14 in Fmr1 KO2 mice.
A, Western blot showing NR1, GluR-A and GluR-B levels at P14 in the homogenate and synaptoneurosome fractions. Shown are 2 pooled fractions from 2 mice each, for each genotype. For quantification of synaptoneurosomal GluR-A/NR1 protein levels, another 2 mice of each genotype were added to the analysis (not shown). Ten micrograms were loaded for each homogenate fraction (lane 2 is an internal loading control in which ½ amount was loaded). In order to obtain similar signal intensities as for the homogenate fraction for reliable quantification, only 15 μl of 500 μl of the synaptoneurosomal fraction was loaded. B, quantification of GluR-A/NR1 and GluR-B/NR1 protein amounts relative to the WT fraction (taken as 100%). C and D show AMPA receptor rectification. C, sample scaled traces showing AMPA-receptor-mediated currents in response to synaptic stimulation in the presence of an NMDA receptor blocker (d-APV, 30 μm) in the recording medium and spermine in the patch pipette (black traces, WT; grey traces, Fmr1 KO2). Traces were obtained at holding currents of −40 to +40 mV, in 20 mV steps (Y-axis is current normalized to the value at −40 mV). D, summary data for 9 WT and 18 Fmr1 KO2 cells shows overlapping rectification curves of the AMPA-receptor-mediated synaptic responses.
Induction protocols for LTP
Two stimulation protocols were used to induce LTP. The first was a low-frequency pairing (LFP) protocol, in which the cells were recorded in the voltage clamp configuration and held at a depolarized membrane potential of 0 mV for 3 min, while a long train of 120 pulses at 0.67 Hz of synaptic stimulation was delivered. To assess potentiation significance, the values from the control pathway were subtracted from the values of the potentiated pathway (Mack et al. 2001). Both pathways are shown in all figures.
The second protocol was a theta-burst pairing protocol (Frick et al. 2004) to induce LTP by pairing bursts of five synaptic inputs with five postsynaptic action potentials (induced by a 2 ms injection of 1000 pA current) at 100 Hz, with the presynaptic stimulation leading the postsynaptic stimulation by 5 ms. A train consisting of 10 such bursts at theta-frequency (5 Hz, 200 ms intervals) was applied three times at 0.1 Hz.
Induction of short- and long-term depression (STD and LTD)
For field recordings of low-frequency stimulation (LFS)-depression we used conditions similar to Huber et al. (2002) for NMDA-dependent LTD. Briefly, a 10 min stable baseline was recorded, followed by 900 stimulations at 1 Hz for the induction of depression. Field potentials were recorded for up to 60 min postinduction. Where indicated, metabotropic glutamate receptor activity was blocked by adding the mGluR1 antagonist LY367385 (15 μm, IC50 of 8.8 μm) and the mGluR5 antagonist 2-Methyl-6-(phenylethynyl)pyridine (MPEP) (0.5–1 μm, IC50 of 36 nm; blockers from Tocris Bioscience, Ellisville, MI, USA).
In a second set of experiments, depression was induced using the patch-clamp configuration as described by Terashima et al. (2008). In this protocol, a 5 min stable baseline was recorded at a holding potential of −70 mV, followed by 300 stimulations at 1 Hz at a holding potential of −40 mV for the induction of depression, and 30 min of recording at a holding potential of −70 mV. For details of solutions, see Terashima et al. (2008). Liquid junction potential was not adjusted in these experiments.
In a third set of experiments, LTD was induced in whole-cell, voltage clamp mode. This was done by recording a 5 min stable baseline at a holding potential of −70 mV, followed by 900 stimulations at 3 Hz at a holding potential of −50 mV for the induction of LTD, and 30 min of recording at a holding potential of −70 mV. Standard Ringer solution was used for slicing and recording (bicuculline was used to inhibit GABA receptors), and a caesium gluconate-based solution (see ‘Recording and stimulation’), with QX-100 in the recording pipette. Liquid junction potential was not adjusted in these experiments.
Analysis
HEKA Pulse and Pulsefit software (Heka Elektronik, Lambrecht/Pfalz, Germany) were used to acquire and analyse data. Statistical analysis was performed with GB-STAT (Dynamic Microsystems Inc., Silver Spring, MD, USA) or with Sigmastat (Systat Software GmbH, Erkrath, Germany).
Mini-Analysis program (Synaptosoft Inc., Fort Lee, NJ, USA) was used to analyse miniature currents further. Traces were also digitally low-pass filtered to reduce noise (600 and 150 Hz for AMPA and for the slower NMDA mEPSC analysis, respectively). For cumulative distribution analysis of mEPSC amplitudes, a similar number of events were randomly selected by the analysis software for each cell (or all events where the total number of events was less than 10 events for NMDA mEPSCs or 20 events for AMPA mEPSCs). All recordings and analyses were performed blind to the genotype.
All values are presented as means ±s.e.m.
Synaptoneurosome preparation
Synaptoneurosomal fractions were prepared from P14 mice as described by Carlin et al. (1980). Briefly, whole hippocampi were dissected from brains in ice-cold phosphate-buffered saline and homogenized in solution A (0.32 m sucrose, 1 mm NaHC03, 1 mm MgCl2 and 0.5 mm CaCl2; complete-EDTA-free protease inhibitor cocktail, Roche) using 12 strokes in a Potter homogenizer. Pooled supernatants of the homogenates I (1400g, 10 min) and homogenates II (710g, 10 min) were centrifuged at 13800g for 10 min. Pellets were resuspended with six strokes in solution B (0.32 m sucrose and 1 mm NaHCO3) and fractionated on a discontinuous 1.2–1.0–0.85 m sucrose gradient at 82500g for 2 h. The fraction at the top of the 1.2 m sucrose solution was collected and solubilized in SDS sample buffer. Fifteen microlitres of a total volume of 500 μl of each sample was fractionated by 8% SDS-PAGE and transblotted onto Polyvinylidene Fluoride membrane (PVDF). Membranes were probed with primary antibodies diluted in 1% milk in Tris Buffered Saline with Tween for 2 h or overnight. After incubation with secondary antibodies coupled to horseradish peroxidase (Amersham) immunoreactions were visualized with ECL Plus (Amersham) on autoradiography films (Amersham Hyper ECL). After stripping, membranes were subsequently probed with anti-glutamate receptor A, glutamate receptor B, and NMDA receptor 1 subunits antibodies.
Results
Presynaptic paired-pulse potentiation is unchanged in Fmr1 KO2 mice
The postsynaptic compartment in neurons, the dendritic spine, is altered in FRAX patients and Fmr1 KO mice (Comery et al. 1997). This, however, does not exclude a participation of the presynaptic compartment, or, indeed, a cause-and-effect relationship of the presynaptic onto the postsynaptic compartment. Miniature current analysis in the hippocampus has shown no significant differences in miniature current frequency, a parameter for presynaptic release probability, between Fmr1 KO and WT animals (Braun & Segal, 2000; Pfeiffer & Huber, 2007). To probe changes in presynaptic release properties further, we used the paired-pulse stimulation protocol. In the hippocampal CA1 neurons, paired-pulse stimulation of the Schaffer collaterals at short intervals (< 500–1000 ms) leads to potentiation of the second response (paired-pulse ratio > 1). This has been interpreted as an enhancement of the presynaptic transmitter release probability (e.g. Thomson, 2000). We therefore tested this protocol in P14 mice by pairing two consecutive stimulations at different interstimulus intervals (15, 30, 60 and 120 ms) and measuring the potentiation of the second pulse (see Methods). No significant differences were seen between KO animals and WT littermate controls for any interval (Fig. 1A and B). This provides further evidence that the release probability is largely unaffected in the hippocampus of Fmr1 KO2 animals.
Figure 1. Paired-pulse facilitation and intrinsic excitability are unaltered in Fmr1 KO2 mice.
A, electrophysiological trace showing an overlay of four paired-pulse protocols scaled to the first stimulus, at 15–120 ms intervals. Scale bar: 1 fold is equivalent to no change. Values above stimulus artifacts denote initiation of second pulse at said interval (in ms) from first pulse. B, quantification of paired-pulse facilitation (PPF) at different interstimulus intervals. C displays sample traces showing similar intrinsic excitability in a WT control cell (upper panel) and an Fmr1 KO2 cell (lower panel).
Intrinsic excitability of Fmr1 KO2 CA1 pyramidal neurons is unaltered
Basic changes in the postsynaptic excitability can also lead to changes in the responses of Fmr1 KO2 cells. We therefore measured intrinsic excitability by current clamping the neurons and injecting hyperpolarizing and depolarizing current steps of different amplitudes. No change was observed in the passive and active membrane properties (e.g. resting membrane potential, input resistance and rheobase) between Fmr1 KO2 and WT littermate control cells (Table 1 and Fig. 1C). We conclude that intrinsic postsynaptic excitability is unaltered in Fmr1 KO2 mice.
The ratio of postsynaptic AMPA and NMDA glutamate receptors is altered during early postnatal development, but not later in maturation in Fmr1 KO2 mice
To investigate changes in glutamatergic neurotransmission in the hippocampal synapses of Fmr1 KO2 animals during postnatal development, we compared the AMPA to NMDA current ratios in acute hippocampal slices at two different developmental stages, P14 and 6–7 weeks. The AMPA receptor-mediated responses were isolated from NMDA receptor-mediated responses by strong hyperpolarization (–90 mV) in the presence of 1 mm Mg2+ (Nowak et al. 1984). At a depolarized membrane voltage (+40 mV), owing to the relief of the Mg2+ block, we measured strong NMDA currents with slow kinetics lasting hundreds of milliseconds (Fig. 2A and B). The current flow value measured at 50 ms after stimulation at a positive holding current of 40 mV was therefore taken as the NMDA index. At P14, CA1 neurons from Fmr1 KO2 animals displayed a large reduction in the AMPA/NMDA ratio (0.90 ± 0.06, compared with 1.38 ± 0.14 seen in the WT littermate control animals; P < 0.002, Student's unpaired t test, n= 21 and 29 cells, respectively). At 6–7 weeks of age, these differences were abolished (2.56 ± 0.30 in Fmr1 KO2 mice versus 2.25 ± 0.11 in WT littermate control animals; no significant differences, P > 0.27, Student's unpaired t test; Fig. 2), indicating that the glutamatergic neurotransmission at synapses of hippocampal CA1 pyramidal neurons is altered during the early period of postnatal development but not in adulthood.
The AMPA/NMDA protein ratio is down-regulated in synaptoneurosomes from Fmr1 KO2 mice at P14
The measured reduction in the AMPA/NMDA current ratio at P14 of about 35% might be detected biochemically if it is dependent on protein levels in the synapse (in addition to post-translational changes such as internalization or different phosphorylation states of the receptors). We therefore purified the synaptoneurosomal fraction from the hippocampi of four Fmr1 KO2 and four WT littermate mice (pooled into 2 extracts from 2 mice each, for each genotype, and an additional 2 mice from each genotype were assayed for synaptoneurosomal GluR-A levels) and performed Western blots against the two main subunits of the AMPA receptors found in the hippocampus (GluR-A and -B; Pandis et al. 2006), as well as the obligatory subunit of the NMDA receptor, NR1. Protein amounts in the synaptoneurosomal fraction are too low to be reliably quantified. We therefore measured the levels of GluR-A and -B relative to the levels of the NR1 protein. In good agreement with our electrophysiological data, we found a reduction of 8.9% in relative GluR-A and 10% in relative GluR-B levels in synaptoneurosomes of Fmr1 KO mice (Fig. 3A and B). For GluR-A, the relative reduction in the total homogenate was about the same level as it was in the synaptoneurosomal fraction, and for GluR-B, the reduction was slightly lower in the total homogenate (9.4% GluR-A protein level reduction and 4.5% GluR-B reduction in in the total homogenate fraction of Fmr1 KO2 mice). This indicates that at least some of the changes in AMPA/NMDA ratio observed here are due to a change in the amounts of the AMPA and NMDA proteins in the synapse. Remarkably, the reduction in protein levels was also transient, and in older animals (6–8 weeks), there was virtually no change in the GluR-A or -B levels in the synaptoneurosomal fraction. The differences were only 0.7% higher and 0.4% lower relative GluR-A and -B levels, respectively, in the Fmr1 KO2 mice (n= 3 WT and 2 KO animals). The total homogenate protein levels followed a similar trend, yielding only 3.8% lower and 1.3% higher relative levels of GluR-A and -B in Fmr1 KO2 animals at this age, respectively (data not shown).
While this study was in progress, a detailed study using the previous mouse model (the Fmr1 KO mouse; Bakker et al. 1994) had detailed some phosphorylation differences in GluR-A (reduced phosphorylation of residue 831 in some conditions), but had not found differences in GluR-A protein levels (Hu et al. 2008). Hu et al. (2008), however, also found no differences in basal transmission in the previous, Fmr1 KO, mouse model (no differences in AMPA/NMDA ratios were observed in their study, or in GluR-A-mediated rectification of synaptic currents). This prompted us to test whether GluR-A might be differentially localized to the synapse. Discrimination between different AMPA subtypes is also possible electrophysiologically, and is based on differential divalent ion permeability of the receptors. The AMPA receptors lacking the GluR-B subunit are inwardly rectifying in the presence of polyamines and do not allow any outward current flow at positive holding potentials, whereas those receptors that also contain the GluR-B subunit do not rectify (Verdoorn et al. 1991). It was our prediction that if relative GluR-A to GluR-B levels in the synapse were lower in the Fmr1 KO2 mouse, we would expect a more linear rectification curve. We calculated a rectification index as the average current flow at positive (+40 mV) over negative (–40 mV) holding potentials. Lack of rectification would result in a rectification index of 1. As shown in Fig. 3C and D, there was no change in the rectification index between WT and KO cells (rectification index was 0.84 ± 0.12 for WT and 0.84 ± 0.08 for Fmr1 KO2 cells; P > 0.99, Student's unpaired t test, n= 9 and 18 cells, respectively), indicating no change in the proportion of AMPA receptor subtypes in the hippocampal CA1 neurons of Fmr1 KO2 mice.
Differences in the synaptic NMDA and AMPA receptor complement cause the AMPA/NMDA ratio shift
Next, we tested whether the synaptic AMPA and NMDA currents were altered, which may lead to the observed change in the AMPA/NMDA ratio. Recordings AMPA mEPSCs in Fmr1 KO mice have yielded conflicting results depending on the preparation used, e.g. no differences were found between WT and KO mice in primary hippocampal cultures (Braun & Segal, 2000), whereas a slight decrease in AMPA mEPSCs was measured in organotypic slice cultures at Days In Vitro (DIV)16 prepared from heterozygous females (Pfeiffer & Huber, 2007). N-Methyl-d-aspartate mEPSCs have not been recorded from Fmr1 KO mice. To quantify synaptic AMPA and NMDA receptor currents in the Fmr1 KO2 mice, we recorded mEPSCs in the presence of tetrodotoxin plus either NMDA or AMPA receptor antagonists. The following parameters were analysed: rise time constant and half-width (a more sensitive measure of the decay constant), frequency of miniature currents and amplitude cumulative distribution (used instead of average amplitude since small-amplitude miniature currents are not detected because a detection threshold must be applied, and this has strong effects on the average amplitude).
We first measured AMPA mEPSCs in the presence of 10 μm d-APV and 1 mm Mg2+ in the recording medium at a holding potential of −70 mV; a total of 41 WT and 33 Fmr1 KO2 CA1 cells were analysed in acute slices from P14 mice. Indeed, the cumulative histogram of AMPA mEPSC amplitudes varied significantly at P14 between WT and KO cells (Mann–Whitney U test, P < 0.001, n= 778 and 612 randomly selected events, respectively. The 50% or median values were 10.38 and 9.59 pA for WT and KO cells, respectively, Fig. 4A and B). This is a decrease of 7.5% in the Fmr1 KO2 mice. Rise and decay parameters were not significantly different (rise time, 3.1 ± 0.07 and 3.4 ± 0.06 ms, P > 0.18; half-width, 6.2 ± 0.2 and 6.8 ± 0.2 ms, P > 0.45, Student's unpaired t test, in WT and KO cells, respectively; Fig. 4E and F). Frequency changes may stem from a change in presynaptic release efficiency or in synapse number. The AMPA miniature current frequency was apparently slightly lower in the Fmr1 KO2 mice (though not significantly so; frequency was 0.25 ± 0.05 and 0.21 ± 0.03 Hz, in WT and KO cells, respectively, P > 0.34; Fig. 4G). This trend is, however, a consequence of the detection of more events in those cells in which the average mEPSC amplitudes are higher, owing to the setting of a detection threshold (7.5 pA). In agreement with this explanation, a non-significant increase in frequency was seen for NMDA miniature currents (see next paragraph). In conclusion, AMPA mEPSCs are reduced in amplitude but not in frequency in the Fmr1 KO2 mice.
Figure 4. Miniature currents are altered in Fmr1 KO2 mice.
A, cumulative histogram showing the distribution of miniature AMPA currents in WT and Fmr1 KO2 mice. A significant shift is seen in the distribution. B, sample traces for A. A 3 s recording epoch is shown. C and D, same as for A and B, only for miniature NMDA currents. Here, too, a significant shift is seen in the cumulative histogram. Average rise (E), decay (half-width; F) and frequency (G) of AMPA miniature currents. There are no significant differences between genotypes. H, I and J, same as E–G, only for NMDA miniature events. Here, too, there are no significant differences between genotypes.
Spontaneous NMDA mEPSCs were recorded at a holding potential of −40 mV in the presence of the AMPA antagonist NBQX (5 μm). Here, too, we found significant differences (Mann–Whitney U test, P < 0.002, n= 102 and 342 events from 18 WT and 45 KO cells, respectively; the median value was 7.6 and 8.7 pA for WT and Fmr1 KO2 cells, respectively; Fig. 4C and D). This is an increase of about 15% of the NMDA component in the Fmr1 KO2 mice. There was a non-significant trend towards a higher frequency of miniature NMDA currents in the Fmr1 KO2 mice compared with the WT mice (1.53 ± 0.17 versus 1.17 ± 0.2 events min−1 in Fmr1 KO2 and WT cells, respectively, P < 0.172; Fig. 4J), once again attributable to detection of more events past the detection threshold (3.5 pA). Rise time and half-width constants of the NMDA miniature currents were not significantly different between WT and Fmr1 KO2 mice, and were in any event much longer than typical values obtained for AMPA mEPSCs (e.g. Pilpel & Segal, 2004). Average rise and decay times for WT and Fmr1 KO2 mice were: rise, 26.8 ± 2.2 versus 25.6 ± 1.2 ms; and half-width, 50.2 ± 3.9 versus 49.9 ± 2.8 ms (P > 0.63 and 0.95, respectively, Student's unpaired t test, Fig. 4H and I).
Long-term potentiation is increased in the hippocampus of Fmr1 KO2 mice during early postnatal development
N-Methyl-d-aspartate receptors are key molecules in synaptic plasticity induced by a variety of activity patterns (Malenka & Bear, 2004). A possible outcome of an increase in the NMDA component in the synapse could be a concurrent increase in NMDA-dependent long-term potentiation. Two well-characterized stimulation protocols that require NMDA receptor activation were used to induce LTP, namely a low-frequency pairing protocol (LFP) and a theta-burst pairing protocol (see Methods for details of stimulation). At P14, application of the LFP protocol led to a significantly larger increase in LTP for Fmr1 KO2 mice compared with WT littermates (Fig. 5A). This difference was already present at 2.5–3.0 min postpotentiation and remained significant throughout the experiment (up to 35 min after potentiation, P < 0.005 at 3 min postpotentiation; potentiation reached 125 versus 189% in the test pathways, while control pathways were at 97 and 98% in the WT and KO cells, respectively; and P < 0.05 at 30 min postpotentiation; 116 versus 159% in the test pathways, while control pathways were at 107 and 84% in the WT and KO cells, respectively, Student's unpaired t test, n= 24 Fmr1 KO2 and 13 WT littermate cells). In general, the lower potentiation measured in our mice compared with mice having a pure C57BL/6 background (Mack et al. 2001) could be attributed to a measure of inbreeding and a different genetic background in our mice (see Methods).
Figure 5. Long-term potentiation is increased in juvenile Fmr1 KO2 mice.
A, induction of LTP at the hippocampal CA3–CA1 synapses using the LFS-LTP protocol in P14 mice. Filled squares represent WT and open squares Fmr1 KO2 test pathway values; filled and open circles represent control pathway. The duration of the induction protocol is omitted for clarity. Five minutes of baseline are shown, and potentiation following application of LFP started at time 0. Values are fold increase, after control-pathway subtraction (0 equals baseline). Insets show overlayed sample traces during baseline and 25 min after potentiation for WT and KO cells. B, as A, in 6- to 7-week-old mice. C, LTP induction using the theta-burst pairing (TBP) protocol. Input resistance changed < 10% for the duration of the recording. Five minutes of baseline are shown, and TBP was applied at time 0. Values are fold increase (1 equals baseline because there is no control-pathway subtraction). Insets 1 and 2 are sample traces illustrating the synaptic responses before and after LTP induction, and during potentiation. Inset 1, overlayed sample traces before and 30 min after potentiation showing the stimulus artifact, response of the cell to stimulation, and then response of the cell membrane voltage to a long depolarizing pulse for measuring input resistance. Inset 2, sample trace during the application of the TBP protocol. One train of a total of 30 is shown; stimulus artifacts can be seen 5 ms before a current injection evokes a postsynaptic spike. Five such pairings were repeated in each train, 10 times at theta frequency (5 Hz), and this was repeated for 3 times at 10 s intervals (Frick et al. 2004). D, LFS-LTP in C57BL/6 WT mice with and without the presence of an mGluR5 inhibitor, MPEP. Squares are test pathway, circles control pathway. Filled symbols are without, open symbols with MPEP.
We also performed the same experiment at 6–7 weeks of postnatal age, a developmental period at which the difference in the AMPA/NMDA ratio was no longer measurable. At this age, there was no sign of a stronger potentiation in the Fmr1 KO2 mice compared with their WT littermates (Fig. 5B; n= 7 control and 9 Fmr1 KO2 cells; test pathways were potentiated to 173 and 194%, and control pathways were 93 and 130% in WT and KO cells, respectively; P < 0.862, 25 min postpotentiation, Student's unpaired t test).
Since STDP was previously shown to be reduced in the neocortex of Fmr1 KO animals (Desai et al. 2006; Meredith et al. 2007), we tested a hippocampal variation of STDP, the theta-burst pairing protocol (TBP; Frick et al. 2004; Fig. 5C). Theta-burst activity is associated with exploratory behaviour in rodents (Buzsaki et al. 1990). Using the TBP in P14 animals, we observed a strong potentiation of the synaptic input in both WT and Fmr1 KO2 mice (259 ± 27 versus 260 ± 60% of baseline EPSC at 20 min postpotentiation; Figs 5C; n= 11 WT and 13 Fmr1 KO2 cells, respectively; potentiation was not significantly different at any time point, Student's unpaired t test). These results indicate that the capacity for increased LTP is protocol specific.
The increased activity of mGluR5 in FRAX has been implicated in this disease (Yan et al. 2005; Dolen et al. 2007), culminating in the proposed mGluR hypothesis (Bear et al. 2004). A recent study (Neyman & Manahan-Vaughan, 2008) has shown strong effects of mGluR5 inhibition on tetanus-induced LTP in the CA1. We therefore decided to test whether our LFS-LTP protocol, which had yielded large differences between genotypes, might also be susceptible to mGluR5 inhibition by the highly specific inhibitor MPEP (in C57BL/6 P14 WT mice). We did not see any significant differences between control and MPEP-treated slices (not significant at any time point, Student's unpaired t test, n= 9 control and 16 MPEP-treated cells; Fig. 5D). The inhibitor MPEP did not have any apparent effect on basal synaptic transmission, as can be seen from the lack of change in the control pathway with MPEP (Fig. 5D).
No difference in NMDA-dependent short- and long-term depression
In a previous study, Huber et al. (2002) found no differences in hippocampal NMDA receptor-dependent LTD in 2- to 3-month-old mice, using a low-frequency stimulation protocol (LFS-depression protocol). We used the same protocol in order to see whether differences in NMDA-dependent LTD might be present earlier in development, at P14. We did not detect significant differences between Fmr1 KO2 and WT littermate mice (n= 8 WT and 13 KO slices; Fig. 6A and C). Remarkably, in our genetic background, the same protocol yielded only a transient depression (short-term depression, STD). In contrast to Huber et al. (2002), we found an increase in field responses within 20 min of induction. We had reasoned that the different age and genetic background of the mice, and the inhibition of metabotropic glutamate receptors, might have contributed to this effect. In order to understand the cause for this difference better, we analysed the response of the control pathway to stimulation. Following the LFS-depression protocol, we saw that the control pathway displays an increase in the response for the entire duration of the recording (between 10 and 20%). There was no significant difference between genotypes. In other LTP protocols in which pathway-independent effects on response to stimulation may occur, control-pathway subtracted values are generally used as the measure of potentiation (e.g. Mack et al. 2001). Control-subtracted values were virtually identical between genotypes and showed a return to baseline within 30–35 min of induction (Fig. 6B and C). This indicates that the increase in response following this protocol is the result of a global effect on CA1 neurons, and that FMRP deletion has no effect on it. Metabotropic glutamate receptors have recently been shown to play a significant role in LTP and LTD in the CA1, although without affecting basal synaptic transmission (Neyman & Manahan-Vaughan, 2008). To see whether metabotropic receptor inhibition may have had an effect on this protocol, we repeated it in a C57BL/6 background in P14 mice (n= 15 slices) without mGluR inhibition. Here, too, we saw a transient depression, and when comparing test and control pathways, both reached similar normalized values 30 min following induction (Fig. 6D). To prove the dependence of this protocol on NMDA receptors, we repeated it in the presence of 50 μm d-APV without mGluR inhibitors (n= 10 slices). The addition of d-APV prevented any depression (barring a transient small decrease, probably the result of a small number of unblocked NMDA receptors at this d-APV concentration), but did not prevent the post-LFS increase in the field response (Fig. 6D). These findings indicate that net depression is not dependent on metabotropic glutamate receptors or on genetic background and is not associated with lack of FMRP.
Figure 6. Short- and long-term depression protocols in field and whole-cell recordings.
Test (A) and control pathway response (B) to the LFS-depression protocol performed in field recordings according to Huber et al. (2002). Shown is a 10 min baseline, followed by a 15 min induction period (time points 0–15, indicated by black bar). The responses of both pathways to stimulation were monitored for another hour. C, control-subtracted values show identical response to LFS-depression protocol for both genotypes, and a return to baseline values about 30–35 min after induction (indicated by black bar), without overshooting. D, LFS-depression protocol in C57BL/6 mice without mGluR inhibitors (▪), with control pathway shown (□), and in the presence of d-APV (•), showing similar transient depression (lasting about 30 min post-LFS-depression protocol), and complete blockage by d-APV. E, sample trace showing the field EPSP (fEPSP) response of the test pathway followed by the response of the control pathway. The control pathway was recorded in stratum oriens and the detected fEPSP is therefore positive. For both pathways, the stimulus artifact can be seen, followed closely by the presynaptic fibre volley (easier to see for the test pathway owing to the direction of the stimulus artifact), and then the field response. F, depression protocol in patch-clamp whole-cell mode according to Terashima et al. (2008), showing no differences between WT (▪) and Fmr1 KO2 cells (□). Only STD was detected. G, depression protocol in patch-clamp whole-cell mode according to Zhang et al. (2005), showing no differences between genotypes. H, input–output curve and sample traces (I; from a WT slice). Fibre volley is easier to distinguish in I because of extended time scale.
Several protocols exist for inducing LTD. We also tested an NMDA-dependent protocol in patch-clamp mode, recently reported by Terashima et al. (2008). Here, too, we found no significant differences between WT and Fmr1 KO2 neurons (n= 21 and 11 cells, respectively; Fig. 6F). In this protocol, too, in our genetic background, values returned to baseline within 30 min, allowing us to evaluate only STD.
Differences in LTD (though not NMDA dependent) have been reported between WT and Fmr1 KO mice (e.g. Huber et al. 2002). It was therefore important to address the question of NMDA-dependent LTD in our mice, especially given the recorded differences in LTP. To this end, we finally employed a third protocol, based on the study of Zhang et al. (2005). Using this protocol, we successfully induced robust NMDA-dependent LTD (efficiently blocked by d-APV; not shown) in Fmr1 KO2 mice and WT littermates. At 30 min postinduction, there was no significant difference between genotypes (WT neurons depressed to 73.5 ± 0.06%, KO neurons to 75.9 ± 0.08% of pre-induction value; n= 9 and 24 neurons, respectively). This indicates that NMDA-dependent short- and long-term depression are normal in Fmr1 KO2 mice (Fig. 6G).
No differences in evoked EPSPs between Fmr1 KO2 CA1 cells and WT cells
Differences in the AMPA to NMDA ratio and the difference seen in miniature currents may indicate a global synaptic reduction in the AMPA content. We therefore also tested the input–output response of the CA1 cell layer using field recordings. There was no significant difference between Fmr1 KO2 and WT animals in this parameter, indicating that the average postsynaptic response to stimulation is normal in Fmr1 KO2 mice (not significant at any point, P= 0.43–0.81, Student's unpaired t test, n= 10 WT and 20 Fmr1 KO2 slices; Fig. 6H and I).
Discussion
In this study, we examined the ionotropic glutamate receptor content in synapses of hippocampal CA1 pyramidal neurons of Fmr1 KO2 mice. We found that Fmr1 KO2 mice displayed a significantly lower AMPA to NMDA ratio compared with WT mice at P14, but not at 6–7 weeks. This difference in ratio at P14 was caused by an up-regulation of the NMDA receptor component concurrent with a down-regulation of the AMPA receptor component. In line with an up-regulation of the NMDA component, the induction of NMDA receptor-dependent long-term potentiation following a low-frequency pairing protocol was increased in Fmr1 KO2 mice at this developmental stage but not later in maturation. To our knowledge, this is the first study to demonstrate a difference in the synaptic NMDA component in the hippocampus of Fmr1 KO2 mice.
Although we see no direct contradiction between our and existing data acquired with the previous generation of Fmr1 KO mice, it should be stated that our data were obtained from Fmr1 KO2 mice in which Fmr1 mRNA is undetectable, which is not the case for the former mouse model (Mientjes et al. 2006). Thus it is possible this may have facilitated the detection of small but pertinent differences that were previously difficult to detect (e.g. differences in miniature synaptic currents). While this manuscript was in preparation, a study was published which partly contradicts our results, using the commercially available Fmr1 KO mouse from Jackson Laboratories. In this study, a reduction in pairing LTP in the KO mice and no difference in the AMPA/NMDA ratio or protein levels were detected (Hu et al. 2008). However, LTP differences, as we have seen, depend heavily on the protocol used. The AMPA/NMDA ratios tend to show a large variability, and it is also possible that AMPA/NMDA ratio differences would not be detected in the previous Fmr1 KO mouse, where small levels of the FMR protein and mRNA are still present. The differences in protein levels that we detect are small and may also not manifest in the previous mouse model. It is hard to determine which is the better mouse model for the human disease, since the number of triplet repeats in the FMR1 gene, and therefore the reduction in the level of the protein, as well as the phenotype, all differ in humans (de Vries et al. 1997). A future comparison of behavioural, biochemical and physiological phenotypes between the different mouse lines is certainly in order.
The dendritic spine phenotype in Fmr1 KO mice has been compared to that originating from sensory deprivation (reviewed by Koukoui & Chaudhuri, 2007). In some model systems (e.g. Drosophila melanogaster), it has been suggested that FMRP is involved in the formation of the presynaptic compartment as well (Gatto & Broadie, 2008). However, this does not seem to be the case in the hippocampus, since we observed normal paired-pulse facilitation, which is a measure of presynaptic activity. In addition, miniature synaptic currents have not been shown to differ in frequency (Braun & Segal, 2000), and our data show that any detected change is probably due to the increase in the amplitude of near-threshold events to the point where they might be detected. Indeed, a trend towards an increased frequency was detected in WT mice for AMPA miniature currents, which were also larger in amplitude in the WT mice, and for KO2 mice for NMDA miniature currents, which were larger in amplitude in the KO2 mice, supporting this explanation. It is therefore unlikely that the knockout mice suffer from insufficient presynaptic stimulation. All our data support the idea that the main defect is on the postsynaptic side.
Our findings stand in contrast to recent studies in the cortex, which suggest that the neurons are impaired in their ability to potentiate (at least following a STDP stimulation protocol; Desai et al. 2006; Meredith et al. 2007) and that there are no differences in AMPA to NMDA ratios in the cortex (Desai et al. 2006). In the case of the LTP, one explanation is the nature of the stimulation protocol used. Indeed, using two different protocols (LFP and TBP), we see an increase in LTP in Fmr1 KO2 mice only in one protocol (LFP).
A reduction in L-type calcium channels (LTCC) in somatosensory cortex is cited as the explanation for the defect in potentiation. This does not necessarily occur in the hippocampus. In fact, a reduction in the α1D subunit of LTCC has only been shown in the cortex, since the hippocampus was not examined (Chen et al. 2003). It is also very likely that different brain regions are differentially affected in their ability to potentiate in the absence of FMRP (like the differences in mGluR-dependent depression which have been shown for the hippocampus but not the cortex). Such comparisons are, of course, extremely difficult, because cortex and hippocampus are intrinsically different in both cell type and connectivity.
Changes in AMPA to NMDA ratio are a consequence of two factors. The first is that functional AMPA receptors are down-regulated or mature more slowly, which might lead to synaptic dysfunction (e.g. Braun & Segal, 2000; Pfeiffer & Huber, 2007). This could occur through various means, including a change in translation/stability of receptors or their mRNA, alterations in trafficking, changes in receptor internalization or other functional modifications. All of the above have been demonstrated in cultured neurons (Li et al. 2002; Muddashetty et al. 2007; Nakamoto et al. 2007; Korkotian & Segal, 2007). Our biochemical data indicate that some of the differences are due to a reduction in AMPA receptor protein levels in the synapse. Another very likely mechanism discussed in these reports is that increased internalization of the AMPA receptor and failure to up-regulate translation from its dendritically localized mRNA following the induction of mGluR1/5 receptors would bring about a reduced synaptic AMPA component.
A second reason we have seen for the change in AMPA to NMDA ratios is that NMDA receptors are enriched in the synapse of Fmr1 KO2 mice. In good correlation with this fact, when challenged with a robust NMDA-dependent potentiation protocol (LFP), Fmr1 KO2 hippocampal neurons showed much greater potentiation than WT neurons.
Fragile X mental retardation protein is most likely to play a critical role in the formation of synapses. The levels of FMRP decline in the brains of mice as a function of age (Singh & Prasad, 2008). Indeed, Gatto & Broadie (2008) have recently shown that in D. melanogaster, the early reintroduction of FMRP was beneficial, whereas a late introduction was not. Most physiological data, however, have been acquired in animals equivalent at least to a young adult, after sexual maturity (though not all, and in the cortex of young mice both structural and physiological findings have been reported; Nimchinsky et al. 2001; Desai et al. 2006; Bureau et al. 2008). The hippocampus plays a major role in the translation of events to memories (Bachevalier, 1990; Neves et al. 2008) and is therefore extremely important in the development of the CNS. We therefore suggest that some of the later life cognitive defects are the consequence of early life impairments in hippocampal function, together with parallel developmental irregularities in the cortex.
An important point is whether or not plasticity differences in Fmr1 KO2 mice are bi-directional. This is a very difficult issue to address, since potentiation and depression can both depend on calcium levels, but on different molecular agents (Teyler et al. 1994). We found increased LTP using our NMDA-dependent LFP protocol, whereas earlier studies (e.g. Huber et al. 2002) found increased LTD, albeit non-NMDA dependent (mGluR5 receptor dependent). In our genetic background, two NMDA-dependent depression protocols (Huber et al. 2002; Terashima et al. 2008) yielded only transient depression, not different between genotypes. A third protocol produced normal short- and long-term depression. This is in good agreement with Huber et al. (2002), who also did not detect NMDA-dependent LTD differences. In short, enhanced LTP can be achieved in Fmr1 KO2 mice, very likely due to increased NMDA receptor levels, whereas enhanced LTD may be achieved via the mGluR5 receptor (Huber et al. 2002; Nosyreva & Huber, 2006).
How may the effects of the two proteins, and an overall apparently widened plasticity spectrum, be reconciled with the mental retardation phenotype? The answer may lie in the complex nature of LTD and LTP. Although increased plasticity is correlated with improved performance on memory trials, such as in the case for the NMDA Receptor Subunit 2B (NR2B) overexpressing mice (Tang et al. 1999), it is obviously also correlated with impaired intelligence in human mental retardation. It should also be noted that, although both LTP and LTD differences have now been demonstrated in the hippocampus of fragile X mice, the differences in spatial learning in these mice are either very mild, or dependent on genetic background (Peier et al. 2000; Kooy, 2003; Yan et al. 2004; Errijgers & Kooy, 2004). Indeed, the TBP protocol we used, which is designed to mimic the activity in hippocampal neurons during exploratory behaviour in rodents, did not show any differences. On the whole, these findings are more indicative of incorrect ‘wiring’, i.e. an aberrant connectivity, of the hippocampus to higher brain structures, e.g. the cortex, than a dysfunction in the function of the hippocampus per se.
Several studies performed in sensory cortex also describe a similar time line for the appearance of physiological phenotypes. Nimchinsky et al. (2001) describe dendritic spine phenotypes at 2 but not 4 weeks of age in somatosensory barrel cortex. Bureau et al. (2008) recently described the selective weakening of the Layer 4 to Layer 3 projection in the same region (due to a decreased connection probability), also detectable only at 2 weeks but not later. These data correlate nicely with our own, and would indicate that the lack of FMRP during a critical period of synaptic development is a global event, with consequences in different regions of the CNS. Bureau et al. (2008) also show that experience-induced plasticity in the barrel cortex (the weakening of L4–L3 connections as a result of whisker trimming) is lacking in the Fmr1 KO mice. We show an increase in the capacity for LTP. These different results would align well if the assumption that Fmr1-lacking neurons are in a virtual state of understimulation is correct. In this case, Fmr1 KO neurons in L3 in somatosensory barrel cortex are effectively in a state which is equivalent to sensory deprivation. Another possibility, which does not contradict this explanation and would also fit well with the data presented by Bureau et al. (2008), is that an altered connectivity would also result in an apparently different response for AMPA and NMDA. Thus, through an altered distribution of synapses, one would obtain an apparent difference in the AMPA/NMDA ratio, which could affect miniature EPSCs without affecting average synaptic response, as we see in our study.
We detected AMPA miniature current and AMPA/NMDA ratio differences, but no differences in evoked responses. An interesting possibility is raised by a recent study (Atasoy et al. 2008). This study showed that two pools of NMDA receptors respond to spontaneous and to evoked glutamate release, with partial overlap. Since both AMPA and NMDA receptors are glutamate receptors, there could potentially be distinct AMPA receptor pools as well, only one of which is affected in the Fmr1 KO2 mice.
Is the increase in the NMDA receptor component shown here indeed the cause of an increased LFS-LTP? Since several parameters (spine morphology, connectivity in cortex, and synaptic transmission shown here in hippocampus) all show age-dependent differences with the same time scale, it is difficult to prove a cause and effect relationship between the two, since increases in LTP may be a consequence of other factors as well. Neyman & Manahan-Vaughan (2008) recently reported a strong effect of mGluR5 on plasticity in CA1. We did not see an effect of mGluR5 inhibition on LFS-LTP. However, chronically altered mGluR5 signalling is strongly implicated in FRAX (Yan et al. 2005; Dolen et al. 2007). An interesting possibility is that the increase in NMDA-receptor-mediated currents is the downstream consequence of an increased activation of mGluR5 (Neyman & Manahan-Vaughan, 2008, and references within; Jia et al. 1998; Attucci et al. 2001). This would nicely explain the increased NMDA component detected here and a consequent facilitation of NMDA-dependent LTP in the Fmr1 KO mice.
These two molecules, NMDA receptors and mGluR5 receptors, may be the key to pharmacological treatment of FRAX. Dolen et al. (2007) showed that it is possible to genetically reduce the mGluR5 levels in order to rescue many behavioural phenotypes. Indeed, antagonists for mGluR5 were shown to be effective in ameliorating both the propensity for seizures and aberrant open field activity in Fmr1 KO mice (Yan et al. 2005). Approaches towards efficient gene therapy of FRAX must be weighed carefully. The dose of the corrected gene is very important; overexpression of FMRP caused increased anxiety (Peier et al. 2000). Also, the fact that the phenotypes we detected revert to normality at the postpubertal (6–7 week) stage, and also that differences in dendritic spines appear to be reduced at older ages, make any gene therapy technically challenging, since the timing of the intervention would be critical and must start very early in development. Would FRAX be helped by antagonists of NMDA? This depends very much on the nature of the increased LTP detected in the hippocampus of young mice. It is quite possible that the up-regulation of NMDA receptors subserves a compensation mechanism, and antagonists of NMDA would, if anything, prove deleterious in the case of FRAX. This is supported by the fact that an enriched environment improves cognitive functions in Fmr1 KO mice (Restivo et al. 2005), as well as increasing AMPA receptor levels, which we find to be reduced in the Fmr1 KO2 hippocampus. Fragile X mental retardation syndrome in humans displays a large phenotypic variability, which would reflect, among other things, the environmental heterogeneity in different individuals. In conclusion, we provide new physiological evidence for a synaptic malfunction at an early age. Our data and those of others comply with the theory that increased mental stimulation to FRAX individuals at an early age may be beneficial. We also emphasize the role of the hippocampal formation in FRAX.
Acknowledgments
Y.P. was supported by a postdoctoral fellowship from the Minerva Foundation. M.G. was supported by the Deutsche Forschungsgemeinschaft, grant SFB 636. We wish to thank Simone Hundemer for help with genotyping.
References
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. Ontogenetic development of habit and memory formation in primates. Ann N Y Acad Sci. 1990;608:457–477. doi: 10.1111/j.1749-6632.1990.tb48906.x. discussion 477–484. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, Vanderhelm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, Deboulle K, Dhooge R, Cras P, Van Velzen D, Nagels G, Martin JJ, Dedeyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Braun K, Segal M. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex. 2000;10:1045–1052. doi: 10.1093/cercor/10.10.1045. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Chen LS, Gage FH. Spatial organization of physiological activity in the hippocampal region: relevance to memory formation. Prog Brain Res. 1990;83:257–268. doi: 10.1016/s0079-6123(08)61255-8. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellvi-Bel S, Mila M. Genes responsible for nonspecific mental retardation. Mol Genet Metab. 2001;72:104–108. doi: 10.1006/mgme.2000.3128. [DOI] [PubMed] [Google Scholar]
- Chen L, Yun SW, Seto J, Liu W, Toth M. The fragile X mental retardation protein binds and regulates a novel class of mRNAs containing U rich target sequences. Neuroscience. 2003;120:1005–1017. doi: 10.1016/s0306-4522(03)00406-8. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- de Vries BB, van den Ouweland AM, Mohkamsing S, Duivenvoorden HJ, Mol E, Gelsema K, van Rijn M, Halley DJ, Sandkuijl LA, Oostra BA, Tibben A, Niermeijer MF. Screening and diagnosis for the fragile X syndrome among the mentally retarded: an epidemiological and psychological survey. Collaborative Fragile X Study Group. Am J Hum Genet. 1997;61:660–667. doi: 10.1086/515496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endris V, Wogatzky B, Leimer U, Bartsch D, Zatyka M, Latif F, Maher ER, Tariverdian G, Kirsch S, Karch D, Rappold GA. The novel Rho-GTPase activating gene MEGAP/srGAP3 has a putative role in severe mental retardation. Proc Natl Acad Sci U S A. 2002;99:11754–11759. doi: 10.1073/pnas.162241099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errijgers V, Kooy RF. Genetic modifiers in mice: the example of the fragile X mouse model. Cytogenet Genome Res. 2004;105:448–454. doi: 10.1159/000078218. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–2648. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Kooy RF. Of mice and the fragile X syndrome. Trends Genet. 2003;19:148–154. doi: 10.1016/s0168-9525(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Morphological constraints on calcium dependent glutamate receptor trafficking into individual dendritic spine. Cell Calcium. 2007;42:41–57. doi: 10.1016/j.ceca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev. 2007;53:27–38. doi: 10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in GluR-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myme CI, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol. 2003;90:771–779. doi: 10.1152/jn.00070.2003. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140:163–175. doi: 10.1016/j.neuroscience.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9:1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci. 2004;19:3151–3164. doi: 10.1111/j.0953-816X.2004.03380.x. [DOI] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Rapid WAVE dynamics in dendritic spines of cultured hippocampal neurons is mediated by actin polymerization. J Neurochem. 2005;95:1401–1410. doi: 10.1111/j.1471-4159.2005.03467.x. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Prasad S. Differential expression of Fmr-1 mRNA and FMRP in female mice brain during aging. Mol Biol Report. 2008;35:677–684. doi: 10.1007/s11033-007-9140-0. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Cavus I, Coussens C, DiScenna P, Grover L, Lee YP, Little Z. Multideterminant role of calcium in hippocampal synaptic plasticity. Hippocampus. 1994;4:623–634. doi: 10.1002/hipo.450040602. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr Fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang Y, Li H, Cao J, Xu L. Amplitude/frequency of spontaneous mEPSC correlates to the degree of long-term depression in the CA1 region of the hippocampal slice. Brain Res. 2005;1050:110–117. doi: 10.1016/j.brainres.2005.05.032. [DOI] [PubMed] [Google Scholar]