Abstract
In order to evaluate the neuronal mechanisms underlying functional abnormalities of swallowing in orofacial pain patients, this study investigated the effects of noxious orofacial stimulation on the swallowing reflex, phosphorylated extracellular signal-regulated kinase (pERK) and γ-aminobutyric acid (GABA) immunohistochemical features in brainstem neurons, and also analysed the effects of brainstem lesioning and of microinjection of GABA receptor agonist or antagonist into the nucleus tractus solitarii (NTS) on the swallowing reflex in anaesthetized rats. The swallowing reflex elicited by topical administration of distilled water to the pharyngolaryngeal region was inhibited after capsaicin injection into the facial (whisker pad) skin or lingual muscle. The capsaicin-induced inhibitory effect on the swallowing reflex was itself depressed after the intrathecal administration of MAPK kinase (MEK) inhibitor. No change in the capsaicin-induced inhibitory effect was observed after trigeminal spinal subnucleus caudalis lesioning, but the inhibitory effect was diminished by paratrigeminal nucleus (Pa5) lesioning. Many pERK-like immunoreactive neurons in the NTS showed GABA immunoreactivity. The local microinjection of the GABAA receptor agonist muscimol into the NTS produced a significant reduction in swallowing reflex, and the capsaicin-induced depression of the swallowing reflex was abolished by microinjection of the GABAA receptor antagonist bicuculline into the NTS. The present findings suggest that facial skin–NTS, lingual muscle–NTS and lingual muscle–Pa5–NTS pathways are involved in the modulation of swallowing reflex by facial and lingual pain, respectively, and that the activation of GABAergic NTS neurons is involved in the inhibition of the swallowing reflex following noxious stimulation of facial and intraoral structures.
Swallowing abnormalities often are a consequence of stroke in a variety of brain regions or of head and neck cancer surgery (Robbins & Levine, 1993; Kronenberger & Meyers, 1994; McConnel & O'Connor, 1994; Paterson, 1996). Several neuronal deficits in the sensory and motor systems are thought to be involved in oropharyngeal dysphagia (Buchholz, 1994). It is known that interactions between sensory and motor functions have an important role in the control of jaw and other orofacial movements. Indeed, it has been shown that high-intensity orofacial stimulation or electrical stimulation of branches of the trigeminal nerve causes strong inhibition of jaw-closing motor neurons, and this inhibitory reflex is a well-documented nocifensive reflex in the orofacial region (Cruccu et al. 1986; Cadden, 2007). In addition, electrical stimulation of the lingual nerve may produce inhibition of swallowing reflex (Sumi, 1970; Zoungrana et al. 2000). These findings suggest that the trigeminal nociceptive inputs may be involved in the modulation of orofacial reflex function, including swallowing.
Some previous reports have described that inflammatory myopathy or severe oral pain after dental extraction is involved in swallowing abnormalities (Ertekin et al. 2004; Vaiman et al. 2006), and so it is important to evaluate trigeminal nociceptive effects on the swallowing reflex, in order to develop appropriate methods to treat dysphagic patients. The nucleus tractus solitarii (NTS) is a major brainstem site involved in the modulation of swallowing reflex (Kessler & Jean, 1985; Jean, 2001). However, the input–output mechanisms in the NTS involved in the swallowing reflex are not well known. It has been reported that the extracellular signal-regulated kinase (ERK) is phosphorylated not only in many neurons in the trigeminal spinal subnucleus caudalis (Vc) and upper cervical spinal cord (C1–C2) following noxious orofacial stimuli (Noma et al. 2008), but also in NTS neurons following noxious visceral stimulation (Xu et al. 2002). Therefore, phosphorylated ERK (pERK) is thought to be a reliable marker of neurons activated by a variety of noxious stimuli. Furthermore, γ-aminobutyric acid (GABA) is known to have a major inhibitory function in the NTS since the swallowing reflex can be inhibited by microinjection of GABA agonist and enhanced by GABA antagonist microinjection into the NTS (Wang & Bieger, 1991).
In the present study, an animal model of swallowing naturally induced by distilled water (DW) administration was developed to investigate the effects of noxious orofacial stimulation on the swallowing reflex, pERK and GABA immunohistochemical features in brainstem neurons, and also analysed the effects of brainstem lesioning and of microinjection of GABAA receptor agonist or antagonist into the NTS on the swallowing reflex in anaesthetized rats.
Methods
This study was approved by the Animal Experimentation Committee of Nihon University School of Dentistry, and procedures in animals were performed according to the guidelines of the International Association for the Study of Pain (Zimmermann, 1983). Experiments were performed on a total of 177 male Sprague–Dawley rats weighing between 250 and 350 g. For the measurement of swallows we used urethane to obtain a stable light anaesthetic level for a long time period (2–3 h), whereas pentobarbital anaesthesia was used for the pERK-LI neuron analysis to obtain a deep stable anaesthetic level for a short period and to allow for comparison of our data with those of previous data in many CNS regions, most of which have used animals under pentobarbital anaesthesia for pERK-LI neuron analysis (Wang & Bieger, 1991; Ji et al. 1999; Kajii et al. 2002; Kitagawa et al. 2002; Fukui et al. 2007; Noma et al. 2008).
Analysis of swallows and capsaicin injection
Rats were anaesthetized with urethane (1.3 g kg−1i.p.) and a longitudinal midline incision was made in the ventral surface of the neck. The trachea was cannulated so that respiration could be maintained and the rat was then carefully placed on a warm mat in a supine position in the stereotaxic frame, and the EMG activity was recorded during and after DW administration. Bipolar enamel-coated stainless steel wire electrodes (interpolar distance: 5 mm) were inserted into the left mylohyoid muscle to record electromyographic (EMG) activity. Swallowing was identified by laryngeal movement and by the mylohyoid EMG activity, which is recognized as an ‘obligate muscle’ involved in swallowing (Dubner et al. 1978; Kitagawa et al. 2002). Mylohyoid EMG activities were stored in the hard disk of a microcomputer system and analysed off line. Spike2 software (Cambridge Electronic Design, Cambridge, UK) was used to analyse the EMG activity and the number of swallows was calculated from the EMG burst discharges.
DW was infused through an infusion tube placed into the pharyngeal region to elicit the swallowing reflex. Another tube was placed into the lower oesophagus to drain off the DW. During the EMG recording experiment, the guide tube (2.2 mm in diameter) was fixed at the midline of the hard palate. An infusion tube (0.9 mm in diameter) used to administer DW was passed through the guide tube. The distal tip of the infusion tube was placed near the end of the soft palate. According to a previous report (Kajii et al. 2002), this procedure can produce localized stimulation of the pharyngolaryngeal region. To study capsaicin-induced modulation of the swallowing reflex, capsaicin (Wako Co. LTD, Japan) was dissolved in 100% ethanol and 7% Tween 80 in saline (1, 10 or 30 mm), and 20 μl of capsaicin or vehicle solution was injected into the left whisker pad skin subcutaneously, or into the left lingual muscle in each animal. After these procedures, DW was applied at a flow rate of 5.0 μl s−1 for 20 s using an infusion pump. The number of swallows was calculated at 6 min after capsaicin injection. The effect of 1, 10, or 30 mm capsaicin or vehicle solution injection on the number of swallows was compared in different groups of rats (with a different concentration of capsaicin or vehicle injection into whisker pad skin and lingual muscle in each group, n= 5 in each group).
pERK, neuronal nuclei (NeuN) and GABA immunohistochemistries
In order to define the peak time point for the expression of pERK-like immunoreactive (pERK-LI) neurons in the NTS after capsaicin injection, pERK immunohistochemistry was first carried out in rats (anaesthetized with sodium pentobarbital, 50 mg kg−1, i.p.) at different time periods following capsaicin or vehicle injection (20 μl) into the whisker pad skin (n= 5 in each group). Since these initial experiments indicated that the number of pERK-LI neurons peaked at 6 min after capsaicin injection into the whisker pad skin, other rats were subsequently perfused at 6 min after capsaicin injection into the whisker pad skin or lingual muscle. Rats were perfused through the aorta with 0.9% saline (50 ml) followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.4, 500 ml). Twenty microlitres of vehicle was subcutaneously injected into the whisker pad skin (n= 5) to test for vehicle effects on pERK expression and rats were perfused 6 min later, as above. The medulla and upper cervical spinal cord were removed and postfixed in 4% paraformaldehyde for 3 days at 4°C. The tissues were then transferred to 20% sucrose (w/v) in phosphate-buffered saline (PBS) for several days for cryoprotection. Thirty-micrometre-thick sections were cut with a freezing microtome and every fourth section was collected in PBS. Free-floating tissue sections were rinsed in PBS, 10% normal goat serum in PBS for 1 h, and then incubated in rabbit anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) antibody (1 : 1000, Cell Signaling Technology, Inc., Beverly, MA, USA) for 72 h at 4°C. The sections were incubated in biotinylated goat anti-rabbit IgG (1 : 600; Vector Laboratories, Inc., Burlingame, CA, USA) for 2 h at room temperature. After washing in PBS, the sections were incubated in peroxidase-conjugated avidin–biotin complex (1 : 100; ABC, Vector Laboratories) for 2 h at room temperature. They were then washed in 0.05 m Tris buffer (TB) (pH 7.4), and next incubated in 0.035% 3,3′-diaminobenzidine-tetra HCl (DAB; Sigma Co. Ltd, Tokyo), 0.2% nickel ammonium sulphate, and 0.05% peroxide in 0.05 m TB. The sections were then washed in PBS, serially mounted on gelatin-coated slides, dehydrated in a series of alcohols (from 50 to 100%) and coverslipped.
The pERK-LI neurons were drawn under a light microscope with an attached camera lucida drawing tube (Neurolucida 2000, MicroBrightField, Inc., Williston, VT, USA). The number of pERK-LI neurons was counted from every sixth section. The total number of pERK-LI neurons in three sections was calculated and the mean number of pERK-LI neurons (3 sections per rat) was obtained from each animal.
Double immunofluorescence histochemistry was also conducted in pentobarbital-anaesthetized rats receiving 10 mm capsaicin injection into the whisker or lingual muscle. Seven rats were perfused through the aorta with 0.9% saline followed by 4% paraformaldehyde in 0.1 m PB. Thirty-micrometre-thick sections were cut and processed for double-labelling immunohistochemistry for pERK and NeuN or GABA. Free-floating tissue sections were rinsed in PBS, 10% normal goat serum in PBS for 1 h, and then incubated in rabbit anti-phospho-p44/42 MAPK antibody (1 : 300) 3 days and mouse anti-NeuN antibody (1 : 1000, Chemicon, USA) or guinea pig anti-GABA antibody (1 : 1000, Chemicon (Millipore), Temecula, CA, USA) overnight at 4°C and secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 568 goat anti-mouse IgG, 1 : 400 or Alexa Fluor 568 goat anti-guinea pig IgG, 1 : 400; Invitrogen, USA) conjugated for 2 h at room temperature in a dark room. Then the sections were washed in PBS 3 times for 10 min and were mounted on slides and coverslipped in PermaFluor (Sigma, USA). Immunofluorescent and immunohistochemical images were taken with a confocal laser-scanning microscope (LSM 510-V2.8; Carl Zeiss Co., Germany) and a light microscope (Olympus, Japan) with a digital camera controlled by DP Controller (Olympus, Japan), respectively.
PD98059 administration
We tested whether intrathecal (i.t.) administration of PD98059, which has been used as a MAPK kinase (MEK) inhibitor in previous studies (Kawasaki et al. 2004; Zhuang et al. 2005), would suppress ERK phosphorylation and modulate swallowing reflex in the rats receiving capsaicin administration to the whisker pad skin or lingual muscle. PD98059 was dissolved in 10% DMSO and saline solution (0.1 μg μl−1) and was administered to the capsaicin- or vehicle-treated rats (n= 20 in each group), which were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.). A microsilicon tube (0.8 mm in diameter) was connected to an Alzet mini-osmotic-pump (model 2001, Durect Co., USA; total volume of the pump: 200 μl, drug infusion: 1 μl h−1 for 7 days); the pump was placed subcutaneously into the back of the rats. After exposing the L1–L4 vertebrae, a L2–L3 laminectomy was performed and the dura mater removed. Then the microsilicon tube was inserted into the subdural space and its tip was placed dorsally in the C2–C3 region of the spinal cord so as to prevent mechanical damage of the medulla.
NTS, Vc or paratrigeminal nucleus (Pa5) lesioning
Each of the three target regions (NTS, Vc and Pa5) was defined according to the stereotaxic criteria by Paxinos & Watson (1998) and Barraco et al. (1992). Rats (n= 35) were anaesthetized with urethane (1.3 g kg−1i.p.). Then the rats were mounted in the prone position in a stereotaxic frame, the brainstem was exposed, and enamel-coated tungsten microelectrodes were inserted into the NTS at three different points (at the obex level and 0.3 mm lateral from the midline bilaterally, at 0.25 mm depth from the brainstem surface in each penetration) and 100 μA negative direct current was applied for 1 min at each site. For Vc and C1–C2 lesioning (at the left side 2.0 mm from the midline and 2 mm rostro-caudally from the obex) a sharp 26-gauge needle was used to remove these structures. For Pa5 lesioning, a microelectrode was inserted into the Pa5 bilaterally (at the 2.5 mm lateral from the midline and 0.8 and 0.3 mm rostral from the obex, at 0.2 mm depth from the brainstem surface in each penetration) and 50 μA negative direct current was applied for 30 s at each site. Sham operations without lesioning (different group of rats, n= 5) were carried out without microelectrodes insertion. For the NTS lesioning or sham groups (n= 5 in each group), EMG activity was recorded. In another NTS lesioning group (n= 5), EMG activity was recorded as above with capsaicin administration into whisker pad skin after NTS lesioning. For the Vc and Pa5 lesioning or sham groups (n= 5 in each group), EMG activity was recorded before Vc, Pa5 lesioning or sham operation. After lesioning or sham operation, EMG activity was also recorded 6 min following the capsaicin injection into the whisker pad skin or lingual muscle.
Microinjection of muscimol or bicuculline into the NTS
Muscimol (Sigma, USA) or bicuculline (Fluka, Switzerland) was dissolved in a freshly sterile saline (muscimol: 10 mm, bicuculline: 5 mm). Rats were anaesthetized with urethane (1.3 g kg−1i.p.) and placed in a stereotaxic frame. The brainstem was exposed by neck skin and muscle incision. A dialysis tube (1 mm length cellulose membrane, 0.22 mm o.d., Mr 50 000 ‘cut-off’, Eicom A-I-4–01 type, Japan) was inserted into the middle of the NTS (0.2 mm caudal to the obex and 0.5 mm in depth with 45 deg angle). The dialysis tube was then fixed to the skull with dental acrylic and the neck muscles and skin were sutured. The probe was perfused at a flow rate of 2 μl min−1 using a syringe pump. The number of swallows was counted after DW administration to the pharyngolaryngeal region during continuous microinjection of muscimol, bicuculline or vehicle into the NTS for 15 min. The number of swallows was similarly counted in those rats receiving 10 mm capsaicin injection into the lingual muscle during continuous microinjection of bicuculline or vehicle into the NTS for 15 min.
Statistical analyses
Statistical analyses were performed using SigmaStat3.5 (Systat Software Inc., San Jose, CA, USA). Results are presented as means ±s.e.m. The effect of different dose of capsaicin on the number of swallows and the time course change in the number of pERK LI-neurons were analysed using a non-parametric Kruskal–Wallis test followed by Dunn's test (post hoc). A non-parametric Mann–Whitney U test was used to analyse differences between two groups. Differences were considered significant at P < 0.05.
Results
Modulation of swallowing reflex following capsaicin injection into whisker pad skin or lingual muscle
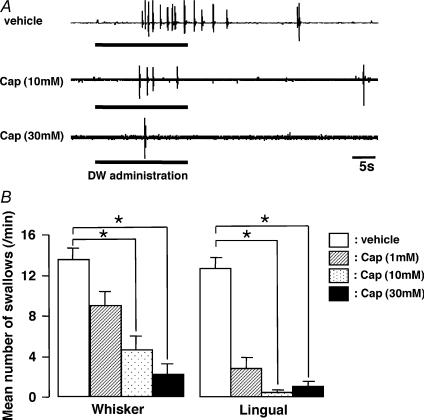
EMG burst discharges could be recorded from the mylohyoid muscle during and after DW application to the pharyngolaryngeal region, as illustrated in Fig. 1A. Each EMG burst indicated the occurrence of a swallow, as previously described (Dubner et al. 1978; Kitagawa et al. 2002). The number of EMG bursts was counted for 60 s and the number of swallows was calculated during and after the administration of DW. The number of swallows was significantly reduced after injection of capsaicin compared to vehicle into the whisker pad skin or lingual muscle, as illustrated in Fig. 1B (Whisker: P < 0.05, Dunn's test; Lingual: P < 0.05, Dunn's test, n= 5 in each group). The number of swallows was decreased after capsaicin injection into the whisker pad skin. The reduction in the number of swallows was greater following capsaicin injection into the lingual muscle than into the whisker pad skin, as illustrated in Fig. 1B.
Figure 1. EMG recordings from mylohyoid muscle during and after DW administration into the pharyngolaryngeal region in the rats with capsaicin or vehicle injection to the whisker pad skin or lingual muscle.
The frequent EMG burst discharges were recorded from mylohyoid muscle during and after DW administration and each burst indicates the occurrence of a swallow. The number of EMG bursts was counted for 60 s and the number of swallows was calculated during and after the administration of DW. A, typical burst EMG discharges following capsaicin or vehicle injection to the whisker pad skin in the rat with DW administration. B, effect of different concentrations of capsaicin to whisker pad skin or lingual muscle on DW-induced swallowing reflex. Whisker: whisker pad skin, Lingual: lingual muscle, Cap: capsaicin, DW: distilled water. *P < 0.05.
Effect of PD98059 on pERK-LI neurons in NTS, Vc and Pa5 and swallowing reflex
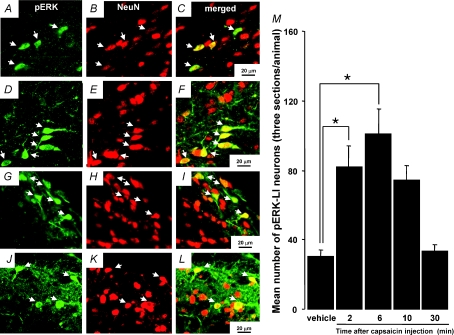
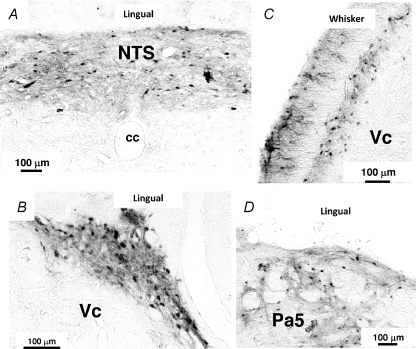
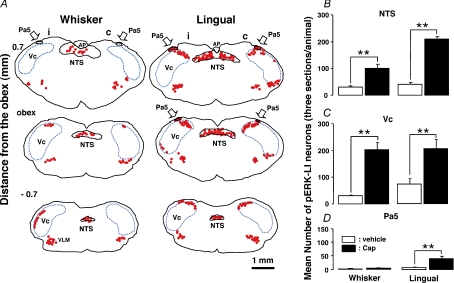
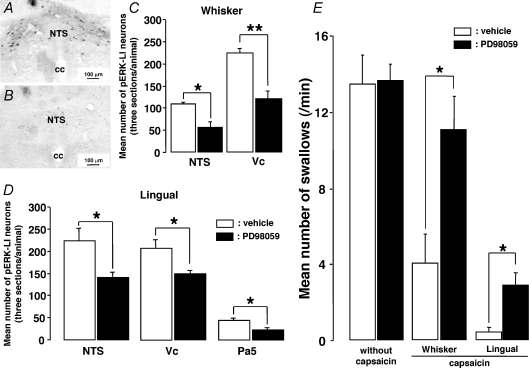
Many pERK-LI neurons were expressed in the NTS (whisker pad skin and lingual muscle), Vc (whisker pad skin) or Pa5 (lingual muscle) following capsaicin injection into the whisker pad skin (Fig. 2A, C, G and I) or lingual muscle (Fig. 2D, F, J and L). All pERK-LI neurons also showed NeuN immunoreactivity (Fig. 2A–L). The pERK-LI neurons in the NTS were expressed within 2 min and peaked at 6 min after capsaicin injection into the whisker pad skin (Fig. 2M, P < 0.05, Dunn's test). Figure 3 illustrates pERK-LI neurons in the NTS, Vc and Pa5. The pERK-LI neurons were observed as small dots with fibres. After capsaicin injection into the lingual muscle or whisker pad skin, many pERK-LI neurons were observed in the NTS, Vc and the Pa5 (Fig. 3). Since pERK-LI neurons were observed in the VLM in vehicle-injected rats as well as capsaicin-treated rats, we analysed the pERK-LI neurons in NTS, Vc and Pa5. In Vc, many pERK-LI neurons were distributed in the superficial laminae on the side ipsilateral to the capsaicin injection into the whisker pad skin and in the superficial laminae of Vc bilaterally following lingual muscle injection (Fig. 4A). We observed only a few pERK-LI neurons in the deep laminae of Vc and upper cervical spinal cord after capsaicin stimulation. On the other hand, bilateral expression occurred in the NTS after capsaicin injection into whisker pad skin or lingual muscle (Fig. 4A). A dense packing of pERK-LI neurons was also observed in the Pa5 bilaterally after capsaicin injection into the lingual muscle but only a small number of pERK-LI neurons was expressed in the Pa5 after capsaicin injection into the whisker pad skin (Fig. 4A and D). The pERK-LI neurons were mainly distributed rostro-caudally in the lateral portion of the Vc at 0.7 mm to 2.2 mm caudal from the obex after capsaicin injection into the whisker pad skin. The number of pERK-LI neurons expressed following 10 mm capsaicin injection into the whisker pad skin or lingual muscle was significantly increased in the NTS and Vc compared to vehicle administration (Fig. 4B and C: P < 0.01, Mann–Whitney U test, n= 5 in each group). After capsaicin injection into the lingual muscle, the number of pERK-LI neurons was significantly increased in the Pa5 compared to vehicle administration (Fig. 4D, P < 0.01, Mann–Whitney U test, n= 5 in each group). The number of pERK-LI neurons was larger in NTS and Pa5 after capsaicin injection into the lingual muscle compared to those after whisker pad skin injection (Fig. 4B and D).
Figure 2. pERK-LI neurons and NeuN-LI neurons in the NTS, Vc and Pa5.
pERK-LI neurons and NeuN-LI neurons in the NTS (A, B and C: 10 mm capsaicin injection to the whisker pad skin; D, E and F: 10 mm capsaicin injection to the lingual muscle), Vc (G, H and I: 10 mm capsaicin injection to the whisker pad skin) and Pa5 (J, K and L: 10 mm capsaicin injection to the lingual muscle). M, time course change in the number of pERK-LI neurons in the NTS following capsaicin injection to the whisker pad skin. *P < 0.05.
Figure 3. Photomicrographs of NTS, Vc and Pa5 in rats with 10 mm capsaicin injection into the whisker pad skin or lingual muscle.
A, NTS following capsaicin injection into the lingual muscle. B, Vc following capsaicin injection into the lingual muscle. C, Vc following capsaicin injection into the whisker pad skin. D, Pa5 following capsaicin injection into the lingual muscle. NTS: nucleus tractus soritarii; Vc: trigeminal spinal subnucleus caudalis; Pa5: paratrigeminal nucleus – in this and following figures.
Figure 4.
A, camera lucida drawings of pERK-LI neurons and mean number of pERK-LI neurons in the NTS, Vc and Pa5 following 10 mm capsaicin injection to the whisker pad skin or lingual muscle. Arrows indicate dense labellings of pERK-LI neurons could be detected in NTS, Vc and Pa5. B, C and D, mean number of pERK-LI neurons in the NTS (B), Vc (C) and Pa5 (D) after 10 mm capsaicin or vehicle injection to the whisker pad skin or lingual muscle. Filled column: capsaicin-treated rats, open column: vehicle-treated rats, AP: area postrema, VLM: ventrolateral medulla. **P < 0.01.
The number of pERK-LI neurons expressed following 10 mm capsaicin injection into the whisker pad skin or lingual muscle was significantly depressed in the NTS, Vc and Pa5 following i.t. administration of PD98059 compared to vehicle administration, as illustrated in Fig. 5A–D (P < 0.05 or 0.01, Mann–Whitney U test, n= 5 in each group). The swallowing reflex was not affected by i.t. injection of PD98059 (Fig. 5E, P= 0.732, Mann–Whitney U test, n= 10 in each group). On the other hand, the swallowing reflex depression induced by 10 mm capsaicin injection into the whisker pad skin or lingual muscle (see above) was significantly reduced after i.t. injection of PD98059 (Fig. 5E, P < 0.05, Mann–Whitney U test, n= 5 in each group). We did not observe any differences in the decrement ratio of the number of pERK-LI neurons in NTS or Vc following PD98059 i.t. administration between lingual muscle and whisker pad skin capsaicin-injected rats (lingual + PD/vehicle versus whisker + PD/vehicle, NTS, P= 0.151, Mann–Whitney U test, n= 5 in each group, lingual: 62.8 ± 4.9%, whisker: 51.5 ± 11.1%; Vc, P= 0.151, Mann–Whitney U test, n= 5 in each group, lingual: 66.8 ± 2.9%, whisker: 53.7 ± 7.7%). On the other hand, the increment ratio of the number of swallows following PD98059 i.t. administration was significantly higher in lingual muscle capsaicin-injected rats compared to whisker pad skin capsaicin-injected rats (P < 0.05, Mann–Whitney U test, n= 5 in each group, lingual: 700 ± 165.8%, whisker: 275 ± 44.7%).
Figure 5. Effect of i.t. administration of PD98059 on pERK-LI neurons expression in the NTS, Vc, Pa5 and swallowing reflex induced by capsaicin injection to the whisker pad skin or lingual muscle.
A and B, photomicrographs of the NTS after vehicle (A) or PD98059 (B) i.t. administration in the rat with whisker pad skin capsaicin stimulation, respectively. C and D, mean number of pERK-LI neurons in the NTS, Vc and Pa5 in the rats with capsaicin injection to the whisker pad skin (C) or lingual muscle (D) following PD98059 i.t. administration compared to that after vehicle administration. E, effect of the i.t. administration of PD98059 on the number of swallows in the rats with capsaicin injection to the whisker pad skin or lingual muscle compared to that following vehicle administration. cc: central canal. *P < 0.05, **P < 0.01.
NTS, Vc or Pa5 lesioning on swallowing reflex
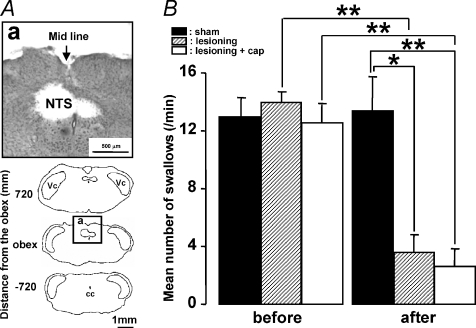
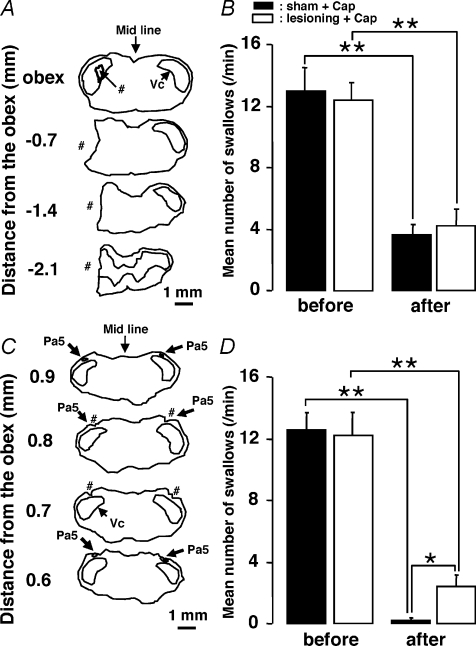
Since a large number of pERK-LI neurons were expressed in the NTS, Vc and Pa5 following capsaicin injection into the whisker pad skin or lingual muscle (see Fig. 4B–D), NTS, Vc or Pa5 lesioning was carried out to test if the swallowing reflex depression induced by capsaicin injection into the whisker pad skin or lingual muscle could be influenced by disruption of these structures. Based on the technical limitations of our lesioning procedure, it is likely that the lesioning disrupted the afferent pathways to the NTS as well as damaged the NTS, Vc and Pa5 themselves. We first tested the effects of NTS lesioning on swallowing to confirm that NTS is involved in the swallowing reflex (Dubner et al. 1978; Jean, 2001). We observed a number of pERK-LI neurons in the NTS at the obex level. It has been reported that the rostral and caudal NTS to the obex level is involved in swallowing (Amirali et al. 2001). Thus, NTS lesioning was made at the obex level in this study; it is possible that the dorsal motor nucleus of the vagus (DMV) could also have been partly lesioned. A lesion was made at the NTS level as illustrated in Fig. 6A. The number of swallows induced by DW administration was significantly reduced after the lesioning of the NTS (Fig. 6B, P < 0.05, Mann–Whitney U test, n= 5 in each group). The number of swallows between NTS-lesioned rats and NTS-lesioned rats with capsaicin injection was, however, not significantly different (Fig. 6B, P= 0.548, Mann–Whitney U test, in each group). The number of swallows was also significantly reduced after the capsaicin injection into the whisker pad skin in Vc- and C1–C2-lesioned rats as well as sham-operated rats, and there was no significant difference between both groups (Fig. 7A and B, P < 0.01, before versus after: Mann–Whitney U test, P= 0.690, sham versus lesioning: Mann–Whitney U test, n= 5 in each group). We also studied the effect of bilateral Pa5 lesionings on the depression of swallowing reflex by capsaicin injection into the lingual muscle but not in rats receiving whisker pad skin capsaicin, because a large number of pERK-LI neurons in Pa5 were only observed after lingual muscle capsaicin injection (Figs 4D and 7C). The number of swallows following capsaicin injection was significantly larger in Pa5-lesioned rats compared to sham-operated rats, as illustrated in Fig. 7D (P < 0.01, before versus after: Mann–Whitney U test, P < 0.05, sham versus lesioning: Mann–Whitney U test, n= 5 in each group).
Figure 6. Effect of the NTS lesioning on the number of swallows.
A, photomicrograph and camera lucida drawings of the sections from the brainstem with NTS lesioning. B, mean number of swallows before and after the NTS lesioning with or without capsaicin injection, or the sham operation. Before: before lesioning or sham operation; after: after lesioning or sham operation. *P < 0.05, **P < 0.01.
Figure 7. Effect of the Vc and C1–C2 or Pa5 lesioning on the number of swallows.
A, camera lucida drawings of sections from the brainstem with Vc and C1–C2 lesioning. B, mean number of swallows before and after the Vc and C1–C2 lesioning or the sham operation. Before: before capsaicin injection to the whisker pad skin in the rats without Vc lesioning; after: after capsaicin injection to the whisker pad skin in the rats with Vc lesioning or sham operation. C, camera lucida drawings of sections from the brainstem with bilateral Pa5 lesionings. D, mean number of swallows before and after Pa5 lesioning or sham operation. Before: before capsaicin injection to the lingual muscle in the rats without Pa5 lesionings; after: after capsaicin injection to the lingual muscle in the rats with bilateral Pa5 lesionings or sham operation. #The lesioning areas. *P < 0.05, **P < 0.01.
GABA-LI and pERK-LI neurons in the NTS and the effect of microinjection of muscimol and bicuculline into the NTS on swallowing reflex
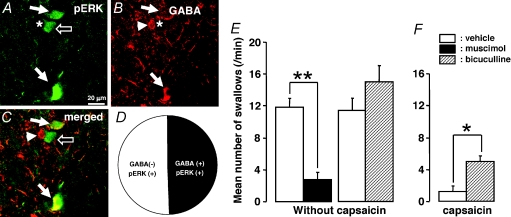
A number of pERK-LI and GABA-LI neurons were widely distributed in the NTS following 10 mm capsaicin injection into the lingual muscle, as illustrated in Fig. 8A–C. Surprisingly, about 49.5% of the pERK-LI neurons in NTS also showed GABA immunoreactivity (pERK-LI +GABA-LI neurons/pERK-LI neurons: 50/101), as illustrated in Fig. 8D. The effect of microinjection of muscimol and bicuculline into the NTS on the swallowing reflex was shown in Fig. 8E and F. After the microinjection of muscimol into the NTS, the number of swallows was significantly depressed compared to vehicle-treated rats (Fig. 8E, P < 0.01, vehicle versus muscimol: Mann–Whitney U test, n= 5 in each group). On the other hand, the number of swallows was slightly larger following microinjection of bicuculline into the NTS compared to vehicle microinjection (Fig. 8E, P= 0.222, vehicle versus bicuculline: Mann–Whitney U test, n= 5 in each group). Furthermore, the number of swallows was significantly larger in capsaicin-treated rats following microinjection of bicuculline into the NTS compared to capsaicin-treated rats with microinjection of vehicle into the NTS (Fig. 8F, P < 0.05, vehicle versus muscimol: Mann–Whitney U test, n= 5 in each group).
Figure 8. GABA-LI neurons or pERK-LI neurons in the NTS of the rats with 10 mm capsaicin injection to the lingual muscle and the effect of muscimol or bicuculline microinjection into the NTS on swallowing reflex.
A, pERK-LI neurons in the NTS; B, GABA-LI neurons in the NTS; C, pERK-LI neurons with GABA immunoreactivity; D, pie chart showing the distribution of the GABA-LI and GABA-negative pERK-LI neurons in the NTS. White arrows in A indicate pERK-LI neurons, those in B indicate GABA and those in C indicate GABA and pERK-LI neurons. The open arrows in A and C indicate pERK-LI neuron without GABA immunoreactivity. The arrowhead in B indicates pERK negative/GABA positive. The star in A corresponds to the arrowhead in B and C, and that in B corresponds open arrows in A and C. E and F, mean number of swallows in vehicle-treated rats and that following microinjection of muscimol or bicuculline to the NTS, and that following microinjection of vehicle or bicuculline to the NTS in capsaicin-treated rats. *P < 0.05, **P < 0.01.
Discussion
Modulation of swallowing reflex following capsaicin injection
It has been reported that a variety of noxious stimuli in the orofacial region can cause modulation of jaw and orofacial motor reflexes (McGrath et al. 1981; Cruccu & Romaniello, 1998; Cadden, 2007). For example, mustard oil injection into the TMJ facilitates jaw-closing and jaw-opening muscles EMG activities (Cairns et al. 1998, 2001; Lam et al. 2005). Mustard oil stimulation causes a barrage of action potentials in small-diameter nociceptive afferent fibres (Handwerker et al. 1991; Schmidt et al. 1995), and the Vc, C1–C2, Pa5, NTS and reticular formation are target nuclei in the brainstem for small-fibre terminations from the orofacial region (Li et al. 1993; Takemura et al. 1996; Takemura et al. 1998). These nuclei are known to have strong connections with neurons involved in motor output such as motoneurons in the trigeminal motor nucleus, facial (VII) nucleus, hypoglossal motor nucleus and nucleus ambiguous and DMV (Caous et al. 2001; Zec & Kinney, 2003; Luo et al. 2006). Thus, it is highly likely that trigeminal small-fibre nociceptive afferents are involved in modulating the activity of motoneurons related to swallowing, but if and how they do this has been unclear. We have provided evidence that orofacial noxious stimulation can modulate swallowing by using as the noxious stimulus capsaicin, which is an irritant that activates C-fibres and small-diameter Aδ-fibres (Szolcsanyi et al. 1988; Takeda et al. 2005). We have documented that a significant reduction in the number of swallows occurs following capsaicin injection into the whisker pad skin or lingual muscle. These findings suggest that orofacial nociceptive inputs are strongly involved in modulating the swallowing reflex. Furthermore, the effect of lingual capsaicin injection on the swallowing reflex was much stronger compared to that of whisker pad skin injection, suggesting that deep nociceptive afferents have stronger effects in modulating of the swallowing reflex compared to superficial nociceptive afferents. As outlined below, we have also provided some insights into the pathways and processes by which this modulation occurs.
Involvement of NTS, Vc and Pa5 neurons in swallowing reflex
It is well known that the Vc, NTS and Pa5 neurons are strongly activated by a variety of noxious stimuli in the orofacial region (Imbe et al. 1999; Tsai et al. 1999; Chattipakorn et al. 2005). We observed a significant increase in the number of pERK-LI neurons in these nuclei after capsaicin injection into the whisker pad skin and lingual muscle, and also observed that the expression pattern of pERK-LI neurons in each nucleus differed depending upon the capsaicin injection site. A large number of pERK-LI neurons was expressed in Vc and NTS after capsaicin injection into the whisker pad skin. On the other hand, many pERK-LI neurons were observed in Pa5 as well as Vc and NTS after capsaicin injection into the lingual muscle. The different expression patterns of pERK-LI neurons in each nucleus may be related to the different roles played by each nucleus in sensory and motor functions in the orofacial region. The NTS is known as a major relay nucleus sending sensory and autonomic information to the trigeminal motor nucleus, hypoglossal nucleus, VII nucleus, DMV and nucleus ambiguus, resulting in swallowing (Jean, 2001). We found that NTS lesioning causes a significant reduction in the number of swallows (see Fig. 6), which is consistent with earlier findings that the NTS is the key nucleus relaying sensory inputs to cranial nerve motoneurons related to swallowing (Doty et al. 1967; Jean, 2001). However, the NTS is involved in a variety of functions such as gustation, visceral sensation and respiration, as well as swallowing (Travers & Smith, 1979; Schwartzbaum & DiLorenzo, 1982; Katz & Karten, 1983; Scott et al. 1986; Feldman & Felder, 1989; Jean, 2001; Boscan et al. 2002). Furthermore, nociceptive neurons have been reported in the NTS and are thought to be involved in modulating autonomic functions and respiration (Boscan et al. 2002). These findings as well as the present data showing that pERK-LI neurons in the NTS are involved in an inhibition of the swallowing reflex suggest that NTS nociceptive neurons may have a very important role in regulating motor functions including swallowing.
Previous anatomical studies have reported that the NTS receives prominent afferent inputs from the Pa5 as well as the trigeminal ganglion (Caous et al. 2001; de Sousa Buck et al. 2001). Furthermore, many Pa5 neurons can be activated by noxious stimulation of the TMJ, masseter muscle or tooth pulp (Imbe & Ren, 2000; Chattipakorn et al. 2005; Shimizu et al. 2006). We also observed a strong activation of Pa5 neurons following capsaicin injection into the lingual muscle, although not after capsaicin injection into the whisker pad skin, and Pa5 lesioning caused significant reduction of the capsaicin-induced depression of swallowing. Thus, it is highly likely that nociceptive inputs from some deep structures in the orofacial region could be directed to the NTS via the Pa5 nucleus, resulting in a modulation of the swallowing reflex.
It is also well documented that many Vc neurons receive nociceptive afferent inputs from the orofacial region (Dubner, 1978; Imbe et al. 1999; Chattipakorn et al. 2005). The Vc is also known as an important relay nucleus for orofacial nociceptive inputs to higher CNS regions (Meng et al. 2000). Therefore, we tested the effects of Vc lesioning on the swallowing reflex inhibition induced by noxious stimulation of the whisker pad skin. Since the inhibitory effect of capsaicin stimulation was not affected by Vc and C1–C2 lesionings (see Fig. 7A and B), it is likely that nociceptive afferent inputs that relay through the Vc have little effect on the swallowing reflex.
Effect of PD98059 administration
PD98059 is known as a specific inhibitor for ERK phosphorylation in spinal dorsal horn neurons (Ji et al. 1999). Some previous studies have also reported that PD98059 administration causes a strong inhibition of nociceptive reflexes, suggesting that ERK phosphorylation in the dorsal horn neurons is involved in an enhancement of the nocifensive behaviour (Ji et al. 1999; Cruz et al. 2005a; Cruz et al. 2005b). We observed a significant reduction of the number of pERK-LI neurons in the NTS and a significant increase in the number of swallows following i.t. infusion of PD98059 in capsaicin-treated rats. Together with the previous observations, the present results suggest that ERK phosphorylation in NTS neurons is involved in the inhibition of the swallowing reflex induced by the capsaicin stimulation of the orofacial region.
Involvement of GABAergic NTS neurons in swallowing reflex
It is well known that GABAergic neurons are strongly involved in the inhibition of neuronal excitability in a variety of CNS nuclei (Jorgensen, 2005; Da Settimo et al. 2007). In the present study, about half of the pERK-LI NTS neurons activated by capsaicin injection into the lingual muscle also showed GABA immunoreactivity (see Fig. 8D), suggesting that many NTS neurons activated by noxious stimulation of the lingual muscle may be involved in inhibitory actions on NTS neurons. Microinjection of GABA agonist into the NTS is known to produce an inhibition of swallowing behaviour in rats, cats and dogs (Wang & Bieger, 1991; Hockman et al. 1996; Lehmann et al. 2002), and we also observed significant reduction of the number of swallows following the microinjection of the GABAA receptor agonist muscimol into the NTS, whereas the number of swallows was slightly larger following microinjection of the GABAA receptor antagonist bicuculline into the NTS compared to vehicle microinjection. Furthermore, the capsaicin-induced depression of the swallowing reflex was reversed by microinjection of bicuculline into the NTS. These earlier findings and the present data raise the possibility that the activation of GABAergic NTS neurons by orofacial noxious stimulation may be involved in the inhibition of NTS output neurons, resulting in an inhibition of swallowing reflex.
Urethane was used during the behavioural analysis of swallowing reflex and pentobarbital was used during the immunohistochemical analysis (pERK expression). Although we could not discount the theoretical possibility that the use of different anaesthetics may affect the outcome, the present study investigated three possible pathways involved in the modulation of the swallowing reflex. These pathways may have parallel roles in this modulatory process, as follows: the facial skin–NTS pathway may be involved in the modulation of swallowing that can occur as a result of superficial (i.e. facial skin) pain, whereas the lingual muscle–NTS and lingual muscle–Pa5–NTS pathways may be involved in modulation of the swallowing reflex by deep (i.e. lingual muscle) pain. The present data also suggest that the activation of GABAergic NTS neurons is involved in the modulation of swallowing reflex following a variety of noxious stimuli to the facial skin and intraoral structures.
Acknowledgments
This study was supported in part by Research grants from the Sato and Uemura Funds from Nihon University School of Dentistry, and a Grant from the Dental Research Center, Nihon University School of Dentistry; a Nihon University multidisciplinary research grant and Individual Research Grant; a grant from the Ministry of Education, Culture, Sports, Science, Technology to promote multidisciplinary research projects ‘Translational Research Network on Orofacial Neurological Disorders’ at Nihon University school of Dentistry; Canadian Institutes of Health grant MT-4918; and the Japan-Canada Joint Health Research Program.
References
- Amirali A, Tsai G, Schrader N, Weisz D, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol. 2001;110:502–513. doi: 10.1177/000348940111000603. [DOI] [PubMed] [Google Scholar]
- Barraco R, el-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002;87:259–266. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- Buchholz DW. Neurogenic dysphagia: what is the cause when the cause is not obvious? Dysphagia. 1994;9:245–255. doi: 10.1007/BF00301918. [DOI] [PubMed] [Google Scholar]
- Cadden SW. Modulation of human jaw reflexes: heterotopic stimuli and stress. Arch Oral Biol. 2007;52:370–373. doi: 10.1016/j.archoralbio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci. 1998;18:8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Temporomandibular- evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. Neuroreport. 2001;12:1875–1878. doi: 10.1097/00001756-200107030-00022. [DOI] [PubMed] [Google Scholar]
- Caous CA, de Sousa Buck H, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus. A topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Auton Neurosci. 2001;94:14–24. doi: 10.1016/s1566-0702(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Chattipakorn S, Chattipakorn N, Light AR, Narhi M, Maixner W. Comparison of Fos expression within the ferret's spinal trigeminal nuclear complex evoked by electrical or noxious-thermal pulpal stimulation. J Pain. 2005;6:569–580. doi: 10.1016/j.jpain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Agostino R, Lahuerta J, Manfredi M. Inhibition of jaw-closing muscles by electrical stimulation of the ophthalmic division in man. Brain Res. 1986;371:298–304. doi: 10.1016/0006-8993(86)90366-5. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Romaniello A. Jaw-opening reflex after CO2 laser stimulation of the perioral region in man. Exp Brain Res. 1998;118:564–568. doi: 10.1007/s002210050312. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci. 2005a;21:773–781. doi: 10.1111/j.1460-9568.2005.03893.x. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Neto FL, Castro-Lopes J, McMahon SB, Cruz F. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005b;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Da Settimo F, Taliani S, Trincavelli ML, Montali M, Martini C. GABAA/Bz receptor subtypes as targets for selective drugs. Curr Med Chem. 2007;14:2680–2701. doi: 10.2174/092986707782023190. [DOI] [PubMed] [Google Scholar]
- de Sousa Buck H, Caous CA, Lindsey CJ. Projections of the paratrigeminal nucleus to the ambiguus, rostroventrolateral and lateral reticular nuclei, and the solitary tract. Auton Neurosci. 2001;87:187–200. doi: 10.1016/S1566-0702(00)00259-9. [DOI] [PubMed] [Google Scholar]
- Doty RW, Richmond WH, Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol. 1967;17:91–106. doi: 10.1016/0014-4886(67)90125-2. [DOI] [PubMed] [Google Scholar]
- Dubner R. Neurophysiology of pain. Dent Clin North Am. 1978;22:11–30. [PubMed] [Google Scholar]
- Dubner R, Sessle BJ, Storey AT. The Neural Basis of Oral and Facial Function. New York: Plenum Press; 1978. [Google Scholar]
- Ertekin C, Secil Y, Yuceyar N, Aydogdu I. Oropharyngeal dysphagia in polymyositis/dermatomyositis. Clin Neurol Neurosurg. 2004;107:32–37. doi: 10.1016/j.clineuro.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Felder RB. α-Adrenergic influences on neuronal responses to visceral afferent input in the nucleus tractus solitarius. Neuropharmacology. 1989;28:1081–1087. doi: 10.1016/0028-3908(89)90121-4. [DOI] [PubMed] [Google Scholar]
- Fukui T, Dai Y, Iwata K, Kamo H, Yamanaka H, Obata K, Kobayashi K, Wang S, Cui X, Yoshiya S, Noguchi K. Frequency-dependent ERK phosphorylation in spinal neurons by electric stimulation of the sciatic nerve and the role in electrophysiological activity. Mol Pain. 2007;3:18. doi: 10.1186/1744-8069-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- Hockman CH, Weerasuriya A, Bieger D. GABA receptor-mediated inhibition of reflex deglutition in the cat. Dysphagia. 1996;11:209–215. doi: 10.1007/BF00366388. [DOI] [PubMed] [Google Scholar]
- Imbe H, Dubner R, Ren K. Masseteric inflammation- induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–175. doi: 10.1016/s0006-8993(99)01913-7. [DOI] [PubMed] [Google Scholar]
- Imbe H, Ren K. The up-regulation of preprodynorphin mRNA in trigeminoparabrachial neurons after inflammation. Neuroreport. 2000;11:845–847. doi: 10.1097/00001756-200003200-00037. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive- specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM. GABA. WormBook. 2005:1–13. doi: 10.1895/wormbook.1.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajii Y, Shingai T, Kitagawa J, Takahashi Y, Taguchi Y, Noda T, Yamada Y. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol Behav. 2002;77:321–325. doi: 10.1016/s0031-9384(02)00854-5. [DOI] [PubMed] [Google Scholar]
- Katz DM, Karten HJ. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol. 1983;218:42–73. doi: 10.1002/cne.902180104. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res. 1985;57:256–263. doi: 10.1007/BF00236530. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1342–R1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- Kronenberger MB, Meyers AD. Dysphagia following head and neck cancer surgery. Dysphagia. 1994;9:236–244. doi: 10.1007/BF00301917. [DOI] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Peripheral NMDA receptor modulation of jaw muscle electromyographic activity induced by capsaicin injection into the temporomandibular joint of rats. Brain Res. 2005;1046:68–76. doi: 10.1016/j.brainres.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Bremner-Danielsen M, Branden L, Karrberg L. Inhibitory effects of GABAB receptor agonists on swallowing in the dog. Eur J Pharmacol. 2002;448:67–70. doi: 10.1016/s0014-2999(02)01907-6. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Mizuno N. Identification of premotor interneurons which project bilaterally to the trigeminal motor, facial or hypoglossal nuclei: a fluorescent retrograde double-labeling study in the rat. Brain Res. 1993;611:160–164. doi: 10.1016/0006-8993(93)91789-u. [DOI] [PubMed] [Google Scholar]
- Luo P, Zhang J, Yang R, Pendlebury W. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jaw-tongue coordination via hypoglossal premotor neurons. Eur J Neurosci. 2006;23:3269–3283. doi: 10.1111/j.1460-9568.2006.04858.x. [DOI] [PubMed] [Google Scholar]
- McConnel FM, O'Connor A. Dysphagia secondary to head and neck cancer surgery. Acta Otorhinolaryngol Belg. 1994;48:165–170. [PubMed] [Google Scholar]
- McGrath PA, Sharav Y, Dubner R, Gracely RH. Masseter inhibitory periods and sensations evoked by electrical tooth pulp stimulation. Pain. 1981;10:1–17. doi: 10.1016/0304-3959(81)90041-5. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Parabrachial area and nucleus raphe magnus inhibition of corneal units in rostral and caudal portions of trigeminal subnucleus caudalis in the rat. Pain. 2000;87:241–251. doi: 10.1016/S0304-3959(00)00289-X. [DOI] [PubMed] [Google Scholar]
- Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, Saito K, Iwata K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol. 2008;507:1428–1440. doi: 10.1002/cne.21620. [DOI] [PubMed] [Google Scholar]
- Paterson WG. Dysphagia in the elderly. Can Fam Physician. 1996;42:925–932. [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego: Academic Press; 1998. [Google Scholar]
- Robbins J, Levine R. Swallowing after lateral medullary syndrome plus. Clin Commun Disord. 1993;3:45–55. [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum JS, DiLorenzo PM. Gustatory functions of the nucleus tractus solitarius in the rabbit. Brain Res Bull. 1982;8:285–292. doi: 10.1016/0361-9230(82)90061-2. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET. Gustatory responses in the nucleus tractus solitarius of the alert cynomolgus monkey. J Neurophysiol. 1986;55:182–200. doi: 10.1152/jn.1986.55.1.182. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Asano M, Kitagawa J, Ogiso B, Ren K, Oki H, Matsumoto M, Iwata K. Phosphorylation of extracellular signal-regulated kinase in medullary and upper cervical cord neurons following noxious tooth pulp stimulation. Brain Res. 2006;1072:99–109. doi: 10.1016/j.brainres.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Sumi T. Changes of hypoglossal nerve activity during inhibition of chewing and swallowing by lingual nerve stimulation. Pflugers Arch. 1970;317:303–309. doi: 10.1007/BF00586579. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ito M, Nasu M, Matsumoto S. Role of capsaicin-sensitive primary afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp Brain Res. 2005;160:107–117. doi: 10.1007/s00221-004-1990-2. [DOI] [PubMed] [Google Scholar]
- Takemura M, Tsujio A, Iwase K, Shimada T, Shigenaga Y. Central terminals of orofacial primary afferents and NADPH-diaphorase activity in the trigemino-solitary complex of rats. Brain Res. 1998;781:77–89. doi: 10.1016/s0006-8993(97)01210-9. [DOI] [PubMed] [Google Scholar]
- Takemura M, Wakisaka S, Iwase K, Yabuta NH, Nakagawa S, Chen K, Bae YC, Yoshida A, Shigenaga Y. NADPH-diaphorase in the developing rat: lower brainstem and cervical spinal cord, with special reference to the trigemino-solitary complex. J Comp Neurol. 1996;365:511–525. doi: 10.1002/(SICI)1096-9861(19960219)365:4<511::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Travers JB, Smith DV. Gustatory sensitivities in neurons of the hamster nucleus tractus solitarius. Sens Processes. 1979;3:1–26. [PubMed] [Google Scholar]
- Tsai CM, Chiang CY, Yu XM, Sessle BJ. Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 1999;81:115–128. doi: 10.1016/s0304-3959(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Vaiman M, Nahlieli O, Eliav E. Oynophagia in patients after dental extraction: surface electromyography study. Head Face Med. 2006;2:34. doi: 10.1186/1746-160X-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Bieger D. Role of solitarial GABAergic mechanisms in control of swallowing. Am J Physiol Regul Integr Comp Physiol. 1991;261:R639–R646. doi: 10.1152/ajpregu.1991.261.3.R639. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang BR, Ding YQ, Kuang F, Wang X, Duan XL, Jiao XY, Ju G. Extracellular signal-regulated kinases1/2 in neurons of the dorsal motor nucleus of the vagus nerve and nucleus of the solitary tract are activated by noxious visceral stimulus in mice. Neurosci Lett. 2002;334:103–106. doi: 10.1016/s0304-3940(02)01113-8. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus of the solitary tract in the medulla oblongata: a DiI labeling study. Auton Neurosci. 2003;105:131–144. doi: 10.1016/S1566-0702(03)00027-4. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zoungrana OR, Lamkadem M, Amri M, Car A, Roman C. Effects of lingual nerve afferents on swallowing in sheep. Exp Brain Res. 2000;132:500–509. doi: 10.1007/s002210000361. [DOI] [PubMed] [Google Scholar]