Abstract
Rod bipolar cells transmit visual signals from their dendrites, where they receive input from rod photoreceptors, to their axon terminals, where they synapse onto amacrine cells. Little is known, however, about the transmission and possible transformation of these signals. We have combined axon terminal recording in retinal slices, quantitative, light-microscopic morphological reconstruction and computer modelling to obtain detailed compartmental models of rat rod bipolar cells. Passive cable properties were estimated by directly fitting the current responses of the models evoked by voltage pulses to the physiologically recorded responses. At a holding potential of −60 mV, the average best-fit parameters were 1.1 μF cm−2 for specific membrane capacitance (Cm), 130 Ω cm for cytoplasmic resistivity (Ri), and 24 kΩ cm2 for specific membrane resistance (Rm). The passive integration of excitatory and inhibitory synaptic inputs was examined by computer modelling with physiologically realistic synaptic conductance waveforms. For both transient and steady-state synaptic inhibition, the inhibitory effect was relatively insensitive to the location of the inhibition. For transient synaptic inhibition, the time window of effective inhibition depended critically on the relative timing of inhibition and excitation. The passive signal transmission between soma and axon terminal was examined by the electrotonic transform and quantified as the frequency-dependent voltage attenuation of sinusoidal voltage waveforms. For the range of parameters explored (axon diameter and length, Ri), the lowest cutoff frequency observed was ∼300 Hz, suggesting that realistic scotopic visual signals will be faithfully transmitted from soma to axon terminal, with minimal passive attenuation along the axon.
Rod bipolar cells receive input from rod photoreceptors at their dendrites in the outer plexiform layer (Dowling & Boycott, 1966) and are presynaptic to AII and A17 amacrine cells at their axon terminals in the innermost stratum of the inner plexiform layer (Famiglietti & Kolb, 1975; Nelson & Kolb, 1985). The transmission of signals from the soma-dendritic region to the axon terminal region is thought to be mediated by passive, or electrotonic, spread of changes in membrane potential, with no evidence for expression of voltage-gated Na+ channels (Karschin & Wässle, 1990). Thus, this transmission is determined by the morphological properties of the rod bipolar cells and their passive membrane parameters, specifically membrane resistance (Rm), membrane capacitance (Cm) and cytoplasmic resistivity (Ri). In addition, the visual signals can be shaped by the interaction between excitatory and inhibitory synaptic inputs and by voltage-gated currents found in rod bipolar cells, such as IK (Karschin & Wässle, 1990; Klumpp et al. 1995; Hu & Pan, 2002; Ma et al. 2003), ICa (de la Villa et al. 1998; Protti & Llano, 1998; Hartveit, 1999; Pan, 2000, 2001) and the hyperpolarization-activated Ih (Karschin & Wässle, 1990; Ma et al. 2003; Ivanova & Müller, 2006). While the exact role of inhibitory input from GABAergic horizontal cells to rod bipolar cells in the soma-dendritic region is unresolved (see, e.g. Duebel et al. 2006), there is strong evidence for inhibitory input from GABAergic and glycinergic amacrine cells along the axon shaft and at the axon terminal (Freed et al. 1987; Strettoi et al. 1990; Chun et al. 1993; Kim et al. 1998). The GABAergic input (Hartveit, 1999; Singer & Diamond, 2003; Chávez et al. 2006) is mediated both as reciprocal inhibition where the inhibitory amacrine cell process receives excitation from the same rod bipolar cell axon terminal (Dowling & Boycott, 1966) and as non-reciprocal inhibition. The glycinergic input (Cui et al. 2003; Eggers & Lukasiewicz, 2006; Ivanova et al. 2006) is mediated as non-reciprocal inhibition.
To understand signal transmission and transformation in rod bipolar cells, it is necessary to understand, at a quantitative level, the interaction between morphology, passive membrane properties, and voltage- and ligand-gated conductances, including the spatial and temporal patterns of activation of the synaptic inputs. Here, we have used a combination of electrophysiological recording and morphological reconstruction to estimate the passive membrane properties of rod bipolar cells by fitting the responses of morphologically accurate compartmental models evoked by voltage pulses to the current responses evoked in the physiological recordings. The use of axon terminal recordings was an advantage with respect to obtaining an accurate measurement of Ri. Such recordings also allowed us to obtain morphological reconstructions without damage to the cell body, a particular advantage for bipolar cells where the cell body constitutes a large fraction of the total membrane area. The compartmental models were used to study the electrotonic signal processing of rod bipolar cells and the passive integration of synaptic inputs. These compartmental models go beyond the previously developed analytical two-compartment model (Mennerick et al. 1997; Oltedal et al. 2007) and will allow the incorporation of quantitative models of voltage- and ligand-gated currents to address their potential role in signal transmission and transformation in rod bipolar cells.
Methods
Retinal slice preparation
General aspects of the methods have previously been described in detail (Oltedal et al. 2007). Albino rats (4–7 weeks postnatal) were deeply anaesthetized with isoflurane in oxygen and killed by cervical dislocation (procedure approved under the surveillance of the Norwegian Animal Research Authority). Retinal slices were cut by hand with a curved scalpel blade at a thickness of ∼50 to ∼150 μm. The slices were visualized (Axioskop 2 FS, Zeiss) with a ×60 water immersion objective (0.9 NA; Olympus) and infrared differential interference contrast (IR-DIC) videomicroscopy. Recordings were carried out at room temperature (22–25°C).
Solutions and drugs
The extracellular perfusing solution was continuously bubbled with 95% O2–5% CO2 and had the following composition (in mm): 125 NaCl, 25 NaHCO3, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose, pH 7.4. The recording pipettes were filled either with intracellular solution A (in mm): 125 potassium gluconate, 10 KCl, 8 NaOH, 1 CaCl2, 5 EGTA, 10 Hepes, 4 MgATP, 0.3 NaGTP (pH adjusted to 7.3 with KOH); or with intracellular solution B (in mm): 125 caesium methanesulphonate (CsCH3SO3), 10 tetraethylammonium chloride (TEA-Cl), 8 NaOH, 1 CaCl2, 5 EGTA, 10 Hepes, 4 MgATP, 0.3 NaGTP (pH adjusted to 7.3 with CsOH). Each intracellular solution also contained Lucifer yellow (1 mg ml−1) and biocytin (3 mg ml−1). The osmolarity was ∼285 mosmol l−1 and ∼300 mosmol l−1 for intracellular solution A and B, respectively. Theoretical liquid junction potentials (the potential of the extracellular solution relative to that of the intracellular solution) were calculated to be +14.6 mV and +9.2 mV for intracellular solution A and B, respectively (JPCalcW; Molecular Devices, Sunnyvale, CA, USA). Membrane holding potentials were automatically corrected for the liquid junction potential on-line.
Drugs were added directly to the extracellular solution used to perfuse the slices. The concentrations of drugs were as follows (μm; supplier Tocris Bioscience, Bristol, UK, unless otherwise noted): 10 bicuculline methchloride, 10 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288), 1 strychnine (Research Biochemicals Inc., Natick, MA, USA), 50 (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA), and 50 dl-threo-β-benzyloxyaspartic acid (TBOA).
Electrophysiological recording and data acquisition
Patch pipettes were pulled from thick-walled borosilicate glass (outer diameter, 1.5 mm; inner diameter, 0.86 mm). Electrodes were coated with dental wax (Kerr's sticky wax) or Parafilm (American National Can; Greenwich, CT, USA) to reduce their capacitance and were heat-polished before use. In addition, the fluid level both in the recording chamber and in the pipette was kept low to minimize the electrode capacitance. The open-tip resistance of the pipettes ranged from ∼6 to ∼8 MΩ when filled with either intracellular solution. Whole-cell voltage-clamp recordings from rod bipolar cell axon terminals (Oltedal et al. 2007) were performed with an EPC9-dual amplifier controlled by PatchMaster software (HEKA Elektronik, Lambrecht/Pfalz, Germany). After establishing gigaohm seals (initial seal resistances 2–25 GΩ), currents caused by the recording electrode capacitance (Cfast) were automatically measured and neutralized by the amplifier. The Cfast time constant ranged from 690 to 940 ns (n= 19). After breaking into the cell, currents caused by the cell membrane capacitance (Cslow) were partially neutralized by the amplifier. For measurements of capacitative current transients, the Cslow capacitance neutralization circuitry was transiently disabled and the time constant of the internal stimulus filter was set to 2 μs. Series resistance (Rs) was not compensated, but instead was included as a free parameter in the off-line modelling (see below). The sampling interval was set to 10 μs and before sampling, signals were low-pass filtered (analog 3-pole Bessel filter) with a corner frequency (−3 dB) of 10 kHz. Current transients were evoked by 15 or 25 ms long voltage pulses of alternating amplitudes of ±5, ±10 or ±20 mV from the holding potential. When we sampled other current responses, the Cslow capacitance neutralization circuitry was re-enabled and the time constant of the internal stimulus filter was set to 20 μs. Signals were low-pass filtered (analog 3- and 4-pole Bessel filters in series) with a corner frequency (−3 dB) between 1/5 and 1/3 of the inverse of the sampling interval (10–50 μs, depending on protocol).
Histological procedures
All cells were filled with Lucifer yellow and after the recording, fluorescence microscopy allowed visualization of the cell's full morphology. Each cell was sketched by hand, identifying characteristic morphological features. For some cells, fluorescence images at a series of focal planes were acquired with a digital CCD camera (CoolSnap ES; Photometrics/Roper Scientific, Tucson, AZ, USA) controlled by RSImage (Photometrics/Roper Scientific) running under Windows XP. During image acquisition, exposure to UV-light was controlled by an electronic shutter (Uniblitz VCM-D1, Vincent Associates, NY, USA), thereby minimizing the total exposure time. Subsequently, the images were assembled in a montage to reveal the cell's morphology (ImageJ version 1.37v, NIH, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/ or Adobe Photoshop 7.0, Adobe Systems) and used to verify the morphology obtained during quantitative morphological reconstruction (see below).
The rod bipolar cells were filled with biocytin (3 mg ml−1) during recording and this was used for staining the cells and subsequent off-line morphological reconstruction. To obtain adequate morphology, it was necessary to minimize damage to the axon terminal during withdrawal of the recording pipette. The most successful procedure involved slow withdrawal of the pipette. This resulted in formation of an outside-out patch (Veruki et al. 2006; Oltedal et al. 2007), suggesting that the gigaohm seal remained intact during recording. Shortly after removing the recording pipette, the slice was fixed for 2 min in 2% paraformaldehyde (PFA; 4% PFA in 0.1 m phosphate buffer was added to an equal volume of Hepes-buffered extracellular solution). Thereafter, the slice was fixed in 4% PFA in phosphate buffer at room temperature for 30 min and at 4°C overnight. To minimize problems with tissue shrinkage, we did not dehydrate or resection the slices.
After fixation, slices were rinsed in phosphate buffered saline (PBS) four times (10 min each). Following this, endogenous peroxidase activity was quenched by incubation in PBS containing 0.3% hydrogen peroxide and 10% methanol for 15 min. Slices were then rinsed three times (10 min each) in PBS and permeabilized for 1 h in PBS containing 0.5% Triton X-100 (Sigma). The slices were then incubated with avidin-biotinylated horseradish peroxidase complex (VECTASTAIN ABC-elite kit; Vector Laboratories, CA, USA) in PBS containing 0.5% Triton X-100 at 4°C overnight with gentle agitation. After rinsing three times in PBS (10 min each), slices were preincubated for 30 min in diaminobenzidine (0.7 mg ml−1), followed by addition of hydrogen peroxide (SigmaFast tablet kit; Sigma). The ensuing reaction was monitored under a microscope and stopped after 3–8 min by rinsing in PBS. After three additional rinses in PBS (10 min each), slices were mounted in Mowiol (Hoechst AG, Frankfurt/M, Germany) in a mixture with glycerol and 0.2 m Tris-HCl (pH 8.5). To minimize compression of the slices by the coverslip, each slice was positioned in the middle of a circle drawn on the microscope slide by a PAP pen (Daido Sangyo Co. Ltd, Japan). Illustrations of Lucifer yellow-filled or biocytin-filled cells were prepared by assembling images acquired at a series of focal planes in a montage, including digital enhancement for improving visualization of the cells' morphology (Adobe Photoshop 7.0).
Three-dimensional (3D) reconstructions and quantitative morphological measurements
Quantitative morphological reconstruction of the labelled cells was done with a motorized microscope (Olympus BX 51) equipped with a ×100 oil immersion objective (NA 1.25; Olympus), a digital CCD camera (MicroFire S99808, Optronics) and Neurolucida software (v7; MicroBrightField Bioscience, Williston, VT, USA). The soma of each cell was traced as a single contour. In addition, a 3D-reconstruction of the soma was made by tracing it with multiple contours at a series of different focal planes. For each cell, the 3D-tracing of the soma was repeated 3–5 times and the average surface area was used for further analysis. The surface area of the 3D-reconstructed cells was calculated with the help of the software programs Neurolucida Explorer (MicroBrightField Bioscience) or NEURON (see below). Neurolucida Explorer was also used for general quantitative morphological analysis. For morphological analysis, the cells were regarded as composites of three main parts: dendrite, soma and axon + terminal. Here, the phrase axon + terminal will encompass both the axon and the terminal swellings at the end of the axon. No attempt was made to correct for errors in morphological measurements due to shrinkage and consequent distortion, but in other studies the errors for similar morphological measurements have been estimated to be < 5% (Roth & Häusser, 2001; Perreault & Raastad, 2006). To reduce observer bias, each cell was reconstructed independently by two different operators (LO and MLV). If marked discrepancies were found between the two operators, the tracings were re-evaluated until the difference between the estimates of the surface area of each compartment (soma, dendrites, axon + terminal) was < 20%. The average difference between the operators' estimates of the surface area was 5 ± 4% for the soma compartment, 12 ± 7% for the dendritic compartment and 3 ± 3% for the axon + terminal compartment (n= 10 cells). For population averages, the value reported for each cell is the average of the two operators' measurements.
The Neurolucida data files containing the quantitative morphological representation of each reconstructed neuron were imported to NEURON (see below) using the Import3D tool (Hines & Carnevale, 2005). For the soma, the major axis of the soma single contour was used to slice it into a series of disks from edge to edge (along the minor axis). The disks would then slide on the plane normal to the major axis to remove all the curvature of the centroid and the resultant cylindrically symmetric shape was then used for the quantitative simulations. Functions in NEURON were used to calculate the surface area of each compartment class (dendrite, soma, axon + terminal) of the complete morphology. The results for dendrites and axons (with axon terminals) were found to be in excellent agreement with those reported by Neurolucida Explorer.
Compared to many types of neurons elsewhere in the central nervous system, the soma of rod bipolar cells makes up a relatively large part of the total surface area. Because of this, we estimated the soma surface area in two different ways. The first method (implemented in Neurolucida Explorer) calculated the surface area from the 3D-reconstruction based on tracing with multiple contours in a series of focal planes and is based on the unbiased stereological Cavalieri technique (Gundersen & Jensen, 1987). It will be referred to here as the ‘multiple contour 3D-reconstruction method’. The second method used the Import3D tool in NEURON, such that the soma surface area was taken as the sum of the areas of the disk edges into which the soma single contour was sliced. This method will be referred to as the ‘single contour method’ of the Import 3D tool in Neuron.
Computer modelling and simulation
Computer simulations of passive cable models were performed with NEURON (version 6.0.3) running under Mac OS X (10.4) (Carnevale & Hines, 2006). Unless otherwise noted, simulations were run with an integration time step of 1 μs. For analysis, data were decimated to give a sampling interval of 5 μs. Spatial discretization (compartmentalization) was implemented by applying the d_lambda rule (Carnevale & Hines, 2006). Briefly, the alternating current (AC) length constant at 100 Hz (λ100) was calculated for each section and the number of segments (nseg) in each section was adjusted such that the length of each segment was smaller than a fraction d_lambda of λ100. The fraction was set by the adjustable parameter d_lambda= 0.01 for all simulations. Corresponding to this, the average number of segments for each cell was 70 ± 19 (s.d.; n= 19 cells with full morphological reconstruction). Decreasing d_lambda to 0.001 had virtually no impact on the simulation results (tested for 5 cells). In the simulations, an idealized single-electrode voltage-clamp (SEClamp; taken from the standard repertoire of NEURON point processes) was connected to the terminal compartment at which the recording pipette was located during electrophysiological recording. Before each simulation run, the model was initialized to steady-state (Carnevale & Hines, 2006). For quantitative analysis of signal transmission between soma and axon terminal, we used a single, morphologically reconstructed rod bipolar cell with morphology and passive membrane parameters that were close to and representative for the population averages. The functional consequences of variability of morphological parameters were analysed by varying the corresponding parameters of this cell.
Passive membrane parameters were obtained using NEURON's Multiple Run Fitter (MRF) to directly fit the current responses of a given morphological model evoked by voltage pulses to the physiological data obtained for the same cell. The MRF tool uses the principal axis (PRAXIS) algorithm (Brent, 1973) to minimize the sum of squared errors (χ2) between the model current response to voltage pulses and the experimental data. Four free parameters were included in the fitting: series resistance (Rs; specified by the parameter SEClamp_rs of the SEClamp point process in NEURON), cytoplasmic (internal) resistivity (Ri), specific membrane capacitance (Cm) and specific membrane resistance (Rm). Ri, Cm and Rm were each assumed to be uniform throughout the neuron. Because the initial seal resistance was > 2 GΩ and withdrawal of the recording pipette resulted in the formation of an outside-out patch, it is likely that the seal remained intact during recording. Accordingly, a shunt at the recording electrode was not included in the model. For fitting, we used the following starting values: SEClamp_rs= 30–50 MΩ, Ri= 100 Ω cm, Cm= 1.0 μF cm−2 and Rm= 14 kΩ cm2 (corresponding to a specific membrane conductance, Gm, of 7.14 × 10−5 S cm−2; Mennerick et al. 1997; Oltedal et al. 2007). For some model simulations, the randomization factor of the optimizer was increased in order to reduce the possibility that the results of the fitting should correspond to a local minimum. The reversal potential (Erev) of the leak current (e_pas), was set to the holding potential used during acquisition of the experimental traces. Only current responses evoked by the negative voltage pulses (15 or 25 ms duration; −10 mV from Vhold=−60 mV; −20 mV from Vhold=−90 mV) were used for the direct fitting. Fitting was started from the data point 55 μs after onset of the voltage pulse. Experimental traces were zero-subtracted before the direct fitting procedure. The passive membrane parameters for each cell were calculated as the average of the parameters determined from both morphological reconstructions (LO and MLV).
Statistical errors in the best-fit parameters (Ri, Cm and Rm) were estimated by bootstrap analysis (Efron & Tibshirani, 1993) of the physiological responses (Roth & Häusser, 2001; Schmidt-Hieber et al. 2007). Balanced resampling was done by generating 100 random lists of N event numbers by random permutation (with N equal to the number of current responses in the original data set for a given cell). Each number corresponded to an event in the original data set and each list of numbers was subsequently used to generate a synthetic data set with N events. For a given original data set, the 100 synthetic data sets were used for model fitting in NEURON to obtain 100 best-fit values for Ri, Cm and Rm and the coefficient of variation (CV) for each parameter (morphological data from only one operator used).
In some computer simulations, we included a more physically realistic model of the tip of the recording electrode (Roth & Häusser, 2001; Schmidt-Hieber et al. 2007). A pipette tip model was adapted from the model of Roth & Häusser (2001; computer code kindly provided by A. Roth). The tip was modelled as a conical arrangement of eight cylindrical sections with an initial diameter of 1.27 μm, a final diameter of 162 μm, and a total length of 2.55 mm. Each section consisted of 10 compartments. The value of Ri in the pipette tip was adjusted such that it matched a series resistance of 60 MΩ, a value slightly above the average found during model fitting (see Results). The conductivity of the pipette wall was assumed to be zero. The capacitance per unit length of each section was assumed to be constant and the total capacitance of the pipette tip was set to either 0, 1 or 2.55 pF for different simulations. The narrow end of the pipette tip was connected to the axon terminal compartment of the model of the prototypical rod bipolar cell and a SEClamp point process with Rs= 1 mΩ was connected to the other end of the pipette tip model.
Synaptic conductance waveforms injected into the theoretical computer models were calculated from current responses obtained during whole-cell voltage-clamp recording according to the following equation,
| (1) |
where I(t) is the recorded current, Vm is the voltage-clamp holding potential and Erev is the assumed or measured reversal potential. To obtain an inhibitory conductance waveform, we first idealized a glycinergic spontaneous inhibitory postsynaptic current recorded at a rod bipolar axon terminal (S. H. Mørkve & E. Hartveit, unpublished observations) by fitting it with the following function,
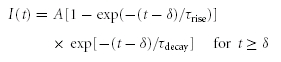 |
(2) |
where δ is the delay to onset of the response, A describes the peak amplitude and τrise and τdecay are the time constants of the rise and decay phases, respectively. The idealized waveform was scaled to a peak amplitude of 20 pA, close to the population average of ∼17 pA (S. H. Mørkve & E. Hartveit, unpublished observations). An excitatory conductance waveform (‘single-photon response’) was adapted from Field & Rieke (2002; their Fig. 3B) without further modification.
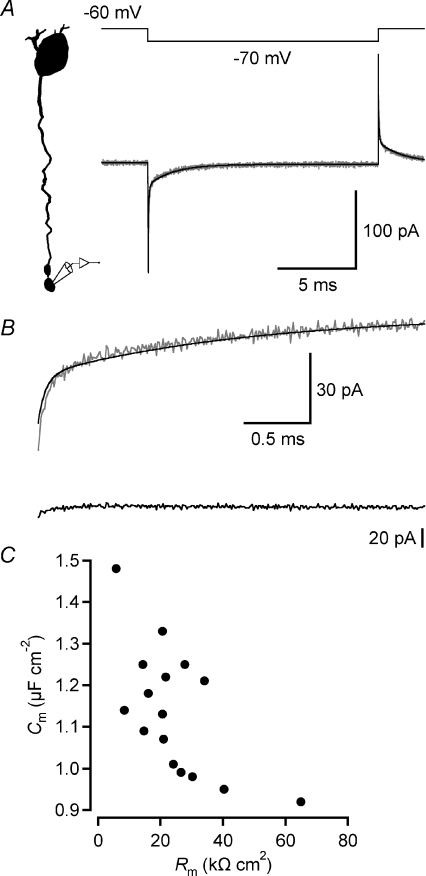
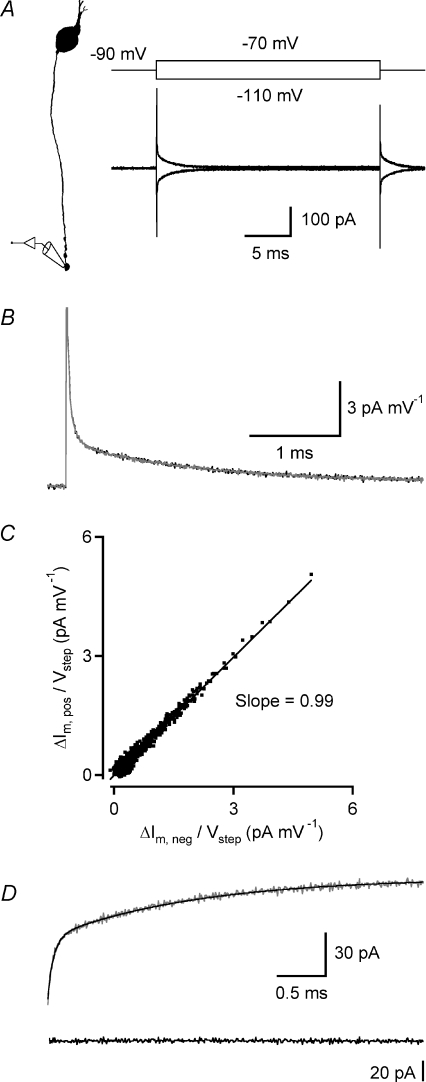
Figure 3. Estimating passive membrane properties after electrophysiological recording and morphological reconstruction of rod bipolar cells.
A, current response of rod bipolar cell (bottom; grey trace; average of 120 trials) and passive compartment model (bottom; black trace) to 10 mV hyperpolarizing voltage pulse (15 ms; top) applied in axon terminal whole-cell recording. Diagram (left) indicates recording configuration and shape plot of the morphologically reconstructed cell. The compartment model was obtained by directly fitting the current responses of the model evoked by voltage pulses to the experimental data in NEURON with four free parameters (Ri, Cm, Rm and Rs). B, top, same as in A, time scale expanded to display decay of current response of rod bipolar cell (grey trace) and passive compartmental model (black trace). B, bottom, curve fit residual (difference between experimental and model current response). C, scatter plot displaying relationship between specific membrane capacitance (Cm) and specific membrane resistance (Rm) obtained from direct fitting of passive membrane parameters for 16 morphologically reconstructed rod bipolar cells (as in A and B). Notice negative correlation between Cm and Rm.
Statistical analysis and data presentation
In addition to NEURON, data were analysed with Neurolucida Explorer, FitMaster (HEKA Elektronik) and IGOR Pro (WaveMetrics, Lake Oswego, OR, USA). Data are presented as means ±s.d. (n= number of cells). Statistical analyses with comparisons between or within groups were performed using Student's two-tailed t test (unpaired or paired as stated). Differences were considered statistically significant at the P < 0.05 level. The number of individual traces included in the averaged current traces in the figures are stated for each case.
Results
Electrophysiological recording and morphological reconstruction of rod bipolar cells
Axon terminals of rod bipolar cells were targeted for recording as previously described (Oltedal et al. 2007). After recording, fluorescence microscopy of the Lucifer yellow-filled cells identified them as rod bipolar cells (Fig. 1A and B). The slices were then fixed and a visible reaction product was developed based on the intracellular filling with biocytin (Fig. 1C). The cells were morphologically reconstructed with the Neurolucida system (Fig. 1D) and the digitized morphological data were imported to NEURON. Computer simulations in NEURON were used to obtain passive membrane parameters (see Methods). This involved optimizing the model parameters by adjusting the values of four free parameters (Ri, Cm, Rm and Rs) such that the responses generated by the model matched the experimental responses. In total, 19 rod bipolar cells were morphologically reconstructed. Of these cells, 10 cells were recorded with intracellular solution A (based on potassium gluconate) and nine cells were recorded with intracellular solution B (based on caesium methanesulphonate). Because cells recorded with solution A yielded qualitatively better morphologies than cells recorded with solution B, only the former were used for detailed analysis of morphological parameters. Two of the morphologically reconstructed cells were not used for model optimizations because of insufficient sampling of electrophysiological data.
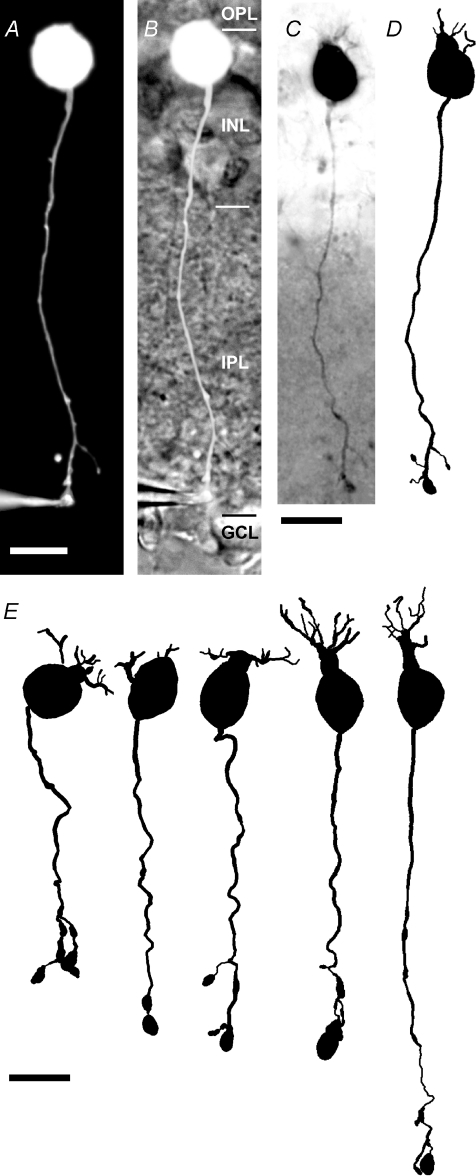
Figure 1. Axon terminal whole-cell recording and morphological reconstruction of rod bipolar cells in a retinal slice preparation.
A, composite fluorescence digital micrograph of a rod bipolar cell (same cell in A–D) filled with Lucifer yellow from a recording patch pipette at the axon terminal (A and B). The micrograph was generated by assembling a series of images taken with epifluorescent illumination at different focal planes. Scale bar (A and B): 10 μm. B, micrograph in A overlaid on a digital micrograph showing the position of the cell in the retinal slice. The retinal layers are indicated by abbreviations (OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer) and the borders between them are indicated by horizontal lines. C, composite digital photomicrograph of rod bipolar cell filled with biocytin during recording. The micrograph was generated by assembling a series of images taken at different focal planes. Scale bar (C and D): 10 μm. D, shape plot of the morphologically reconstructed cell. E, shape plots of reconstructed cells illustrate range of morphological variability among rod bipolar cells. Scale bar: 10 μm.
Quantitative morphological characteristics of rod bipolar cells
The general morphological characteristics of rod bipolar cells have previously been characterized in a variety of different species at both the light microscopic (Cajal, 1893; Boycott & Dowling, 1969; Dacheux & Raviola, 1986; Greferath et al. 1990) and electron microscopic level (Dowling & Boycott, 1966; Dacheux & Raviola, 1986; Greferath et al. 1990; Strettoi et al. 1990; Chun et al. 1993). Projections of the reconstructed morphology of five rod bipolar cells are illustrated in Fig. 1E and exemplify the range of morphological variability within the population of rod bipolar cells obtained in this study. When the soma surface area was estimated with the multiple contour 3D-reconstruction method implemented in Neurolucida Explorer (see Methods), the total surface area of the rod bipolar cells was 525 ± 61 μm2 (n= 10). Of this area, the soma represented 254 ± 35 μm2, the dendrites represented 100 ± 39 μm2 and the axon + terminals represented 170 ± 18 μm2. Thus, the soma contributed 49 ± 6% of the total surface area, underscoring the importance of an accurate estimate of the soma size for these cells. When the soma surface area was estimated by the single contour method of the Import3D tool in NEURON (see Methods), the results were similar to those obtained with the multiple contour 3D-reconstruction method (240 ± 30 μm2versus 254 ± 35 μm2; n= 10; P= 0.43; paired t test).
Rod bipolar cells, including rat rod bipolar cells, have a number of thin dendrites, originating from the soma, that branch in the outer plexiform layer and enter into triadic synaptic relationships with rod spherules and processes from horizontal cells (Dowling & Boycott, 1966; Greferath et al. 1990; Chun et al. 1993). For our reconstructed rod bipolar cells (n= 10), the average number of main dendritic trunks (directly originating from the soma) was 2.4 ± 1.1. The average number of dendritic branchlets (terminals) was 9.6 ± 3.7 (range 5–15). Because of the resolution limit of light microscopy, we might have missed some of the finest dendritic branchlets. The Abbe resolution limit of light microscopy (d) is given by λ/2NA (e.g. Lanni & Keller, 2005), where λ is the wavelength of the light and NA is the numerical aperture of the microscope objective. With λ= 550 nm for green light and NA= 1.25 for the objective used, d becomes 0.22 μm, and thus, we were unable to adequately resolve the diameter of processes thinner than this. In rabbit retina, the number of dendritic branchlets in Golgi-impregnated rod bipolar cells examined with light microscopy was considerably higher, ranging between 80 and 120 (Dacheux & Raviola, 1986), but in cat retina, Kolb & Nelson (1983) estimated approximately 15 dendritic branchlets from a Golgi-impregnated rod bipolar cell located in the central area. In our material the total dendritic length was 46 ± 18 μm and the dendritic processes contributed 19 ± 6% of the total surface area of the rod bipolar cells.
Rod bipolar cells also have a single, long axon that emanates from the proximal end of the soma and continues to stratum 5 (S5) of the inner plexiform layer where it expands into a small number of knob-shaped axon terminal swellings (Fig. 1D and E). The total length of the axon (including branches) and terminals was 77 ± 10 μm (range 67–96 μm). The average diameter of the axon (length-weighted mean; excluding axon terminals) was 0.66 ± 0.07 μm. The average number of axon endings was 2.4 ± 0.8. The axon + terminal compartment contributed 33 ± 3% of the total surface area.
Linearity of current responses to small voltage steps
To develop a passive cable model of a cell studied in voltage-clamp, it is necessary that the cell's current response scales linearly with the applied voltage, without activation or deactivation of voltage-gated currents. It is well established that rod bipolar cells express both L- and T-type voltage-gated Ca2+ currents (de la Villa et al. 1998; Protti & Llano, 1998; Satoh et al. 1998; Hartveit, 1999; Pan, 2000, 2001; Pan et al. 2001). The L-type current is predominantly located in the axon terminal region and displays an activation threshold between −50 and −40 mV (de la Villa et al. 1998; Protti & Llano, 1998; Satoh et al. 1998; Hartveit, 1999; Pan, 2000, 2001). The T-type current is located both in the soma region and in the axon terminal region and displays an activation threshold between −70 and −60 mV (de la Villa et al. 1998; Satoh et al. 1998; Hartveit, 1999; Pan, 2000, 2001; Pan et al. 2001). However, T-type currents also display strong steady-state inactivation, with half-maximum inactivation typically between −60 and −80 mV (reviewed by Perez-Reyes, 2003). Accordingly, we considered that with a membrane holding potential of −60 mV, close to the resting membrane potential of rod bipolar cells (Veruki et al. 2006), the T-type current should be predominantly inactivated and it should be possible to apply low-amplitude voltage pulses, without significant activation of L-type current.
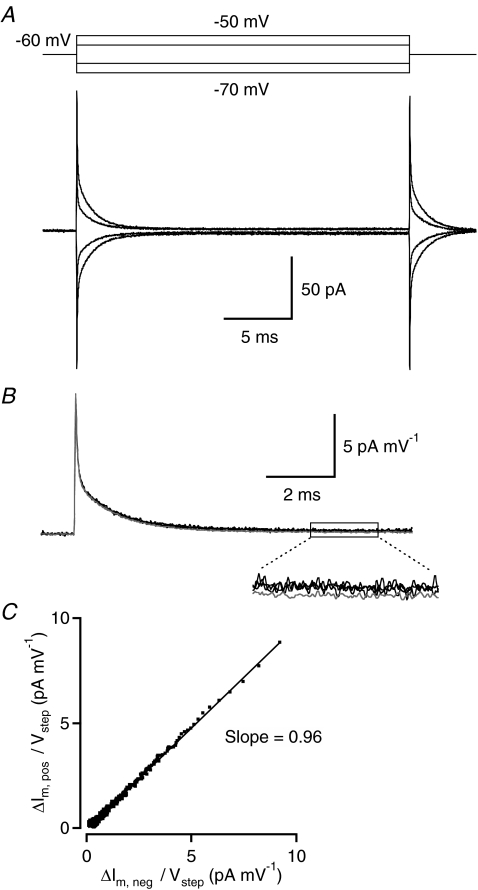
We examined the linearity of the membrane current response to small voltage pulses by applying short (25 ms) hyperpolarizing (to −65 and −70 mV) and depolarizing (to −55 and −50 mV) voltage pulses from a holding potential of −60 mV (Fig. 2A). This evoked transient current responses with steady-state components ranging from approximately ±1 pA to approximately ± 4 pA (calculated as the average of the last 5 ms of the voltage pulse; Fig. 2A). Only very few sweeps needed to be excluded from analysis because they contained spontaneous PSCs. We evaluated the linearity of the membrane response by inverting and scaling the average response evoked by the hyperpolarizing voltage pulse and superimposing it on the average response evoked by the depolarizing voltage pulse (Fig. 2B). The −5 and −10 mV hyperpolarizing voltage pulses and the +5 mV depolarizing voltage pulse, but not the +10 mV depolarizing voltage pulse, evoked predominantly linear, passive responses (Fig. 2A). For the +10 mV voltage pulse, the evoked current did not superimpose exactly with the currents evoked by the other voltage pulses (Fig. 2B) and suggested weak activation of an inward, voltage-gated current, most likely the L-type Ca2+ current. To quantify the linearity of evoked membrane currents, we plotted the average responses evoked by depolarizing and hyperpolarizing voltage pulses against each other for corresponding points in time (Fig. 2C). When we fitted the relationship with a straight line, the slope was 0.97 ± 0.02 for the ±5 mV steps and 0.99 ± 0.02 for the +5/−10 mV steps (after appropriate scaling; n= 6 cells). Taken together, we conclude that the responses to hyperpolarizing voltage pulses from −60 mV to −65 or −70 mV and to depolarizing voltage pulses from −60 mV to −55 mV appear to be passive without measurable activation or deactivation of voltage-gated membrane currents.
Figure 2. Linear membrane properties of rod bipolar cells at Vm=−60 mV.
A, current responses (bottom; average of 100 trials) of a rod bipolar cell to 25 ms voltage pulses with different amplitudes (top; −10, −5, +5, and +10 mV from holding potential) applied in axon terminal whole-cell recording. B, current responses in A were scaled with the applied voltage pulse amplitude and superimposed to examine linearity. Superposition of current responses to −10, −5 and +5 mV voltage pulses indicate that they scale linearly with voltage (black traces). Current response to +10 mV voltage pulse revealed activation of an inward current and did not superimpose exactly with the other current responses (grey trace; inset displays selected epoch at higher magnification). Time scale expanded (relative to A) to display onset and initial decay of current transients with higher temporal resolution. C, the current responses to the ±5 mV voltage pulse stimuli were plotted against each other for corresponding points in time during a 25 ms time interval, starting 50 μs after onset of the stimulus. The straight line indicates a linear fit to the data points and has a slope of 0.96.
Detailed cable models and passive membrane parameters of rod bipolar cells
To investigate the passive membrane properties of rod bipolar cells, we used NEURON to fit the current responses of the morphological model evoked by voltage pulses (to −70 mV from a holding potential of −60 mV) to the experimental current responses (two morphological reconstructions for each recorded cell, see Methods). An example of the experimentally recorded and fitted current responses for a morphologically reconstructed rod bipolar cell is shown in Fig. 3A and B. For this cell, the model fitting resulted in Cm= 1.22 μF cm−2, Ri= 187 Ω cm, Rm= 22 kΩ cm2 and Rs= 54 MΩ. For cells recorded with intracellular solution A (potassium gluconate), the average best-fit parameters were: Cm= 1.19 ± 0.17 μF cm−2, Ri= 135 ± 45 Ω cm, Rm= 25 ± 19 kΩ cm2 and Rs= 51 ± 20 MΩ (n= 7). We next estimated the statistical errors by analysis of 100 bootstrap re-sampled synthetic data sets from each of the original data sets (Roth & Häusser, 2001; Schmidt-Hieber et al. 2007), with a data set corresponding to a morphologically reconstructed cell (one operator only) and the ensemble of current responses obtained from the cell in response to a square wave pulse. As indicated in Table 1, the coefficient of variation (CV) was close to 0.01 for the estimates of Cm and Ri. For Rm, the CV was ∼0.04 and for Rs, it was ∼0.02. This suggests that the best-fit parameters of the reconstructed cells are well constrained by the experimental data (cf. Schmidt-Hieber et al. 2007). The average membrane time constant calculated from the model estimates (τm=Rm×Cm) was 28 ± 17 ms (n= 7).
Table 1.
Best-fit parameters obtained for rod bipolar cells
| Cm (μF cm−2) | CV (%) | Ri (Ω cm) | CV (%) | Rm (kΩ cm2) | CV (%) | Rs (MΩ) | CV (%) | |
|---|---|---|---|---|---|---|---|---|
| Cell 1 | 1.23 | 0.8 | 166 | 1.1 | 21.6 | 4.8 | 52 | 0.7 |
| Cell 2 | 1.08 | 0.7 | 190 | 0.8 | 20.9 | 3.7 | 47 | 0.6 |
| Cell 3 | 0.91 | 0.4 | 96 | 0.9 | 65.7 | 6.0 | 53 | 7.8 |
| Cell 4 | 1.19 | 0.7 | 128 | 0.8 | 16.0 | 2.6 | 43 | 0.6 |
| Cell 5 | 1.22 | 0.8 | 83 | 1.2 | 34.3 | 3.4 | 27 | 1.3 |
| Cell 6 | 1.46 | 0.4 | 100 | 0.6 | 5.8 | 1.2 | 29 | 1.4 |
| Cell 7 | 1.21 | 1.3 | 159 | 1.2 | 14.9 | 3.3 | 111 | 1.9 |
| Mean ± s.e.m. | 1.18 ± 0.06 | 0.73 | 132 ± 15 | 0.93 | 25.6 ± 7.4 | 3.6 | 51 ± 11 | 2.0 |
The electrophysiological data were obtained with whole-cell recordings at the axon terminal. Passive membrane parameters (specific membrane capacitance, Cm; cytoplasmic resistivity, Ri; specific membrane resistance, Rm) and series resistance (Rs) obtained using NEURON's multiple run fitter to directly fit the response of each cell's morphological model to the physiological data obtained for the same cell. Statistical errors in the best-fit parameters were estimated by bootstrap analysis of the physiological responses. For a given original data set, 100 synthetic data sets were used for model fitting in NEURON to obtain 100 best-fit values for each parameter and the variability of best-fit parameter values is given by the coefficient of variation (CV).
When we instead recorded cells with an intracellular solution where potassium was replaced with caesium (caesium methanesulphonate; solution B), the average best-fit parameters were: Cm= 1.10 ± 0.13 μF cm−2, Ri= 130 ± 51 Ω cm, Rm= 24 ± 9 kΩ cm2 and Rs= 48 ± 16 MΩ (n= 9). The average membrane time constant calculated from the model estimates (τm=Rm×Cm) was 26 ± 9 ms (n= 9). For each parameter, there was no statistically significant difference between the two groups of cells (P= 0.23–0.85). The similarity of Rm estimates obtained with potassium- and caesium-based internal solutions is, perhaps, surprising, but consistent with similar observations for goldfish bipolar cells (Mennerick et al. 1997). The ionic basis of the resting leak conductance in rod bipolar cells remains to be determined. If we lumped together the results for all rod bipolar cells examined with voltage pulses at Vh=−60 mV, irrespective of the intracellular solution used, the average best-fit parameters were: Cm= 1.14 ± 0.15 μF cm−2, Ri= 132 ± 47 Ω cm, Rm= 24 ± 14 kΩ cm2 and Rs= 49 ± 17 MΩ (n= 16). The average membrane time constant calculated from the model estimates (τm=Rm×Cm) was 27 ± 13 ms (n= 16).
For two rod bipolar cells, currents related to spontaneous synaptic input, including tonic activation of presynaptic glutamate transporters (Veruki et al. 2006) and GABAC receptors (Hull et al. 2006), were blocked pharmacologically (Methods). Even though the passive membrane parameters estimated for these cells (Ri, Cm and Rm) were within the range of values obtained for cells recorded in control solution without pharmacological blockers, it is possible that the estimates for Rm would have been even higher if all cells had been recorded with pharmacological blockers.
When we plotted the specific membrane capacitance versus the specific membrane resistance obtained for each cell, there was a negative correlation between these two parameters (Fig. 3C). This suggested that the larger estimates of Cm might be caused by erroneous underestimation of the surface area (cf. Perreault & Raastad, 2006), potentially related to the inherent difficulty in tracing small dendrites at the resolution limit of light microscopy.
Activation of Ih and effects on membrane parameters of rod bipolar cells
In addition to voltage-gated Ca2+ currents, there is evidence that rod bipolar cells also express Ih, a slow, hyperpolarization-activated, non-selective cation current with an activation threshold reported to range from −50 to −70 mV (Karschin & Wässle, 1990; Ma et al. 2003; Ivanova & Müller, 2006). In different types of neurons, Ih typically has an Erev between −40 and −25 mV (reviewed by Robinson & Siegelbaum, 2003). The degree of activation of Ih might be important for the integrative properties of rod bipolar cells. Because Ih might be tonically active at membrane potentials within the physiological operating range of rod bipolar cells, we wanted to investigate its contribution to the membrane currents under steady-state conditions.
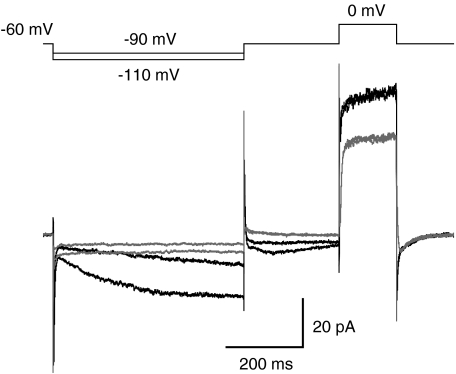
We examined activation of Ih by applying hyperpolarizing voltage pulses from a holding potential of −60 mV. When the membrane potential was stepped to either −90 mV or to −110 mV for 500 ms, a slowly activating inward current was observed (Fig. 4). After the hyperpolarizing voltage step, the membrane potential was returned to −60 mV. Following this, we applied a 150 ms depolarization to 0 mV to fully deactivate Ih to avoid sequential effects from one sweep to the next (cf. Ivanova & Müller, 2006). The inward, hyperpolarization-activated current was strongly suppressed by adding 50 μm ZD7288, a relatively specific blocker of Ih (BoSmith et al. 1993), to the extracellular solution (Fig. 4). At a membrane potential of −60 mV, there was no difference between the holding current in the control condition (−15 ± 5 pA) and in the presence of 50 μm ZD7288 (−16 ± 5 pA; n= 12; P= 0.22; paired t test), suggesting that there was no or minimal activation of Ih at this membrane potential. At a membrane holding potential of −90 mV, application of 50 μm ZD7288 reduced the holding current from −26 ± 6 pA to −21 ± 6 pA (P= 0.0048; paired t test; n= 12), suggesting a contribution of Ih to the membrane current at this potential.
Figure 4. Presence of hyperpolarization-activated current (Ih) in rod bipolar cells.
Currents (bottom) evoked during whole-cell recording at the axon terminal of a rod bipolar cell by hyperpolarizing voltage pulses (top; 500 ms duration) to −90 mV and −110 mV from a holding potential of −60 mV, both in the control condition (black traces) and in the presence of ZD7288 to block the hyperpolarization-activated Ih (grey traces). Each current trace is the average of five trials (no correction for leak or capacitive current). After the hyperpolarizing voltage pulses, the cell was depolarized to 0 mV (right; 150 ms duration) to fully deactivate Ih before the next stimulus.
When cells were held at −90 mV, application of short (25 ms) hyperpolarizing (to −110 mV) and depolarizing (to −70 mV) voltage pulses evoked predominantly linear, passive responses (Fig. 5A). The linearity of the membrane response was evaluated by inverting the average response evoked by the hyperpolarizing voltage pulse and superimposing it on the average response evoked by the depolarizing voltage pulse (Fig. 5B). In addition, we plotted the average responses evoked by depolarizing and hyperpolarizing voltage pulses against each other for corresponding points in time (Fig. 5C) and fitted the relationship with a straight line, yielding a slope of 1.00 ± 0.01 (n= 13). These results suggested that at −90 mV, activation of Ih contributes a steady inward current, but with brief hyperpolarizing and depolarizing pulses, there is no change in the level of activation of Ih. Confirming this, when the duration of the hyperpolarizing (to −110 mV) or depolarizing (to −70 mV) voltage pulses was increased to 500 ms, we observed additional activation or deactivation of Ih (data not shown).
Figure 5. Influence of steady-state activation of Ih on membrane properties of rod bipolar cells.
A, current responses (bottom; average of 45 trials) of a rod bipolar cell to 25 ms voltage pulses to −110 and −70 mV from a holding potential of −90 mV (top) applied in axon terminal whole-cell recording. Diagram (left) indicates recording configuration and shape plot of the morphologically reconstructed cell. Same cell in A–D. B, current responses in A were scaled with the applied voltage pulse amplitude and superimposed to examine linearity. Superimposition of current response to −110 mV (black trace) and current response to −70 mV (grey trace) indicate that they scale linearly with voltage. Time scale expanded to display onset and initial decay of current transients with higher temporal resolution. C, the current responses to the ±20 mV voltage pulse stimuli were plotted against each other for corresponding points in time during a 25 ms time interval, starting 50 μs after onset of the stimulus. The straight line indicates a linear fit to the data points and has a slope of 0.99. D, top, current response of rod bipolar cell (grey trace; average of 45 trials) and passive compartment model (black trace) to 20 mV hyperpolarizing voltage pulse (as in A). The compartment model was obtained by directly fitting the current responses of the model evoked by voltage pulses to the experimental data in NEURON with four free parameters (Ri, Cm, Rm and Rs). Time scale expanded to display decay of current responses at higher temporal resolution. D, bottom, curve fit residual (difference between experimental and model current response).
We investigated the membrane properties at −90 mV by fitting the passive membrane parameters of each cell to the reconstructed morphology and the current responses evoked by the hyperpolarizing voltage pulses (to −110 mV). For the cell illustrated in Fig. 5D, the model fitting resulted in Cm= 0.91 μF cm−2, Ri= 93 Ω cm, Rm= 23 kΩ cm2 and Rs= 67 MΩ. If we lumped together the results for all rod bipolar cells examined at Vh=−90 mV (irrespective of the intracellular solution used), the average best-fit parameters were: Cm= 1.14 ± 0.17 μF cm−2, Ri= 125 ± 47 Ω cm, Rm= 16 ± 8 kΩ cm2 and Rs= 54 ± 24 MΩ (n= 12). Rm estimated at −90 mV was significantly smaller than Rm estimated at −60 mV (24 ± 14 kΩ cm2; P= 0.009; paired t test; n= 12). Corresponding to this, the average calculated membrane time constant at −90 mV (18 ± 6.4 ms; n= 12) was significantly smaller than the one at −60 mV (P= 0.04). There was a small, but significant difference between Cm estimated at −90 mV and at −60 mV (1.12 ± 0.17 μF cm−2; P= 0.04; paired t test). For Ri and Rs, there was no significant difference between the estimates at −90 and −60 mV. For cells examined in the presence of ZD7288 to block Ih (Vm=−90 mV), the average best-fit parameters were: Cm= 1.24 ± 0.12 μF cm−2, Ri= 167 ± 64 Ω cm, Rm= 27 ± 20 kΩ cm2 and Rs= 105 ± 67 MΩ (n= 6). There was no significant difference between Rm estimated at −90 mV in the presence of ZD7288 and Rm estimated at −60 mV in the absence of ZD7288 (P= 0.19; paired t test; n= 5).
Influence of uncompensated pipette capacitance on best-fit passive membrane parameters
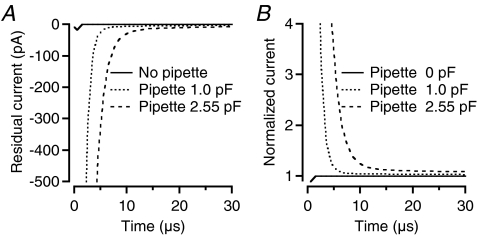
We used optimization tools in NEURON to obtain a cell's passive membrane parameters by fitting the current responses of the model evoked by voltage pulses to the experimental data. The free parameters in the model were Ri, Cm, Rm and Rs. This approach represents a simplification. First, modelling Rs as a point process (SEClamp in NEURON), neglects the fact that it is distributed in a pipette (albeit concentrated at the tip). Second, the pipette capacitance typically cannot be completely neutralized by the fast transient cancellation of the amplifier (Cfast). This is partly because the pipette capacitance is distributed along the length of the pipette such that each element of capacitance has a different amount of resistance in series with it. This means that a single time constant is insufficient to provide perfect cancellation. In addition, the time course of the capacitive transients also reflects dielectric relaxations (in the pipette glass and pipette holder) that do not follow simple exponential functions. Coating the pipettes (see Methods) will reduce, but not eliminate, the relaxations. To investigate the consequences of uncompensated pipette capacitance for our recordings and model fitting, we explicitly included different models of the recording pipette and simulated current relaxations (models kindly supplied by Arnd Roth; see Roth & Häusser, 2001). A total of four different pipette models were simulated: a voltage-clamp electrode without pipette (this corresponded to the standard SEClamp point process), a 2.55 mm long pipette with 0 pF capacitance, a 2.55 mm long pipette with 1.0 pF capacitance, and a 2.55 mm long pipette with 2.55 pF capacitance. In each case, the pipette was attached to the axon terminal of a rod bipolar cell (Fig. 1E, middle cell) and the series resistance was set to 60 MΩ. We then sampled the current responses evoked by 10 mV hyperpolarizing voltage steps. The response from the pipette without capacitance (the second model as indicated above) was used as a reference response. For each of the other model simulations, we calculated a residual response by subtracting the reference response from the response evoked by the model. From the residual responses illustrated in Fig. 6A, it can be seen that the residual currents related to uncompensated pipette capacitance decayed very rapidly. When the passive membrane parameters of each cell were obtained using NEURON's MRF tool, the fitting started 55 μs after onset of the voltage pulse. At this point in time, the uncompensated pipette capacitances contributed very little current (Fig. 6A), indicating that the corresponding errors introduced in the best-fit passive membrane parameters should be small.
Figure 6. Influence of uncompensated pipette capacitance and representation of series resistance (Rs) on current transient time course.
A, computer simulations of current transients evoked in a morphologically reconstructed rod bipolar cell by 10 mV hyperpolarizing voltage pulses applied in axon terminal whole-cell recording. Time scale expanded to display initial decay of current transient (peak truncated; time 0 corresponding to start of stimulus). Each line corresponds to simulation of a specific pipette model (Rs= 60 MΩ). ‘No pipette’ corresponds to a voltage-clamp electrode without capacitance and with Rs represented as a point process (NEURON SEClamp; standard model). ‘Pipette 1.0 pF’ and ‘Pipette 2.55 pF’ correspond to a pipette with either 1.0 pF or 2.55 pF uncompensated capacitance and with Rs distributed along the length of the pipette tip (2.55 mm). In each case, the response evoked by a pipette with 0 pF capacitance was used as a reference and subtracted from the response evoked for each model. Note that the residual currents (related to uncompensated pipette capacitance) decay rapidly with no significant contribution at the point in time when curve fitting was started (55 μs). The current responses have been plotted at a resolution corresponding to the simulation time step (1 μs; A and B). B, decay of current transients of three simulated pipette models with Rs distributed along the length of the pipette tip, normalized by the decay of the current transient of the pipette model with Rs represented as a point process (the standard model). Each line corresponds to simulation of a specific pipette model with either 0, 1.0 or 2.55 pF capacitance. Note the similarity of the current evoked by the model with distributed Rs (and 0 capacitance) and by the model with Rs represented as a point process (standard model).
To analyse the consequence of modelling Rs as a point process rather than distributed in the pipette, we normalized the current responses evoked in the three models where the pipette was explicitly simulated (with or without capacitance neutralization) to the current decay evoked in the standard model (with Rs represented as a point process). As can be seen in Fig. 6B, the model with Rs distributed in the pipette, but with no capacitance, did not change the relaxation current notably, indicating that the assumption of simulating Rs as a point process does not cause significant artefacts.
The influence of axon morphology on the passive signal attenuation between soma and axon terminal
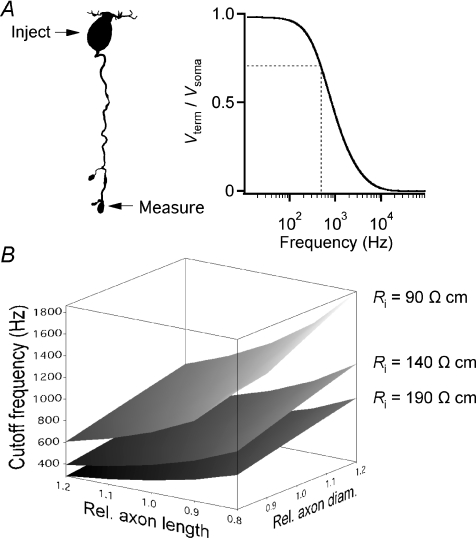
The axon of rod bipolar cells will mediate an attenuation of the signal transfer between the soma-dendritic and the axon terminal compartments. To investigate the magnitude and frequency dependence of this attenuation, we used morphologically reconstructed cells with their best-fit passive membrane parameters as models in computer simulations with NEURON. The rod bipolar cell selected for the simulations in Fig. 7A represented a cell with morphological parameters close to the population averages. The best-fit passive membrane parameters for this cell were: Cm= 1.07 μF cm−2, Ri= 190 Ω cm and Rm= 21 kΩ cm2. The axon length was 79 μm and the average axon diameter (length-weighted) was 0.71 ± 0.17 μm (calculated from 189 segments). To quantify the signal transmission characteristics, we used the frequency tool in NEURON. This tool, which is based on the electrotonic transform developed by Carnevale et al. (1995), calculates the attenuation between the voltage at the site of current injection (Vinject) and the voltage at a specific site of interest (Vmeasure). In the case of the rod bipolar cell studied here, we injected sinusoidal current stimuli in the soma and measured the voltage responses both at the soma (Vinject) and in the distal axon terminal (Vmeasure). The stimulus frequency ranged from 1 Hz to 100 kHz and for each frequency, the passive signal transmission was calculated as an attenuation factor (Vmeasure/Vinject) (e.g. Spruston et al. 1994). There was very little attenuation under steady-state conditions, but the degree of attenuation increased with increasing stimulus frequency and displayed a cutoff frequency (−3 dB) of approximately 490 Hz (Fig. 7A). To explore the effect of variations in axon diameter and length, we repeated the simulations for a series of combinations of each parameter, effectively scaling them by the factors 0.8, 0.9, 1, 1.1 and 1.2. For axon length, this corresponded to varying the absolute length by a maximum of ±16 μm and for the axon diameter, this corresponded to varying the absolute diameter by a maximum of ±0.14 μm. For each combination of axon diameter and length, we repeated the simulations for three different values of Ri: 90, 140 and 190 Ω cm (140 ± 50 Ω cm). For each simulation, the cutoff frequency was extracted from the attenuation versus frequency plot (as in Fig. 7A). The relationship between the two axon parameters, a given value of Ri and the resulting cutoff frequency could be visualized as a surface in a three-dimensional plot (Fig. 7B). For the total range of parameters explored (axon diameter and length, Ri), the cutoff frequency varied between ∼300 and ∼1800 Hz (Fig. 7B).
Figure 7. Influence of axon morphology and cytoplasmic resistivity (Ri) on the signal attenuation between soma and axon terminal.
A, left, shape plot of the morphologically reconstructed rod bipolar cell used for computer simulation of signal attenuation. Arrows indicate voltage measurements at site of sinusoidal current injection (Inject; Vsoma) and at site of measurement in the axon terminal (Measure; Vterm). A, right, voltage attenuation (Vterm/Vsoma) in axon terminal as a function of the frequency of the sinusoidal current stimulus at the soma. Vterm/Vsoma= 1 corresponds to no attenuation and Vterm/Vsoma= 0 corresponds to complete attenuation. The dotted lines indicate the frequency (cutoff frequency) where the response at the axon terminal is attenuated to 1/√2 (∼0.707) of the steady-state response. B, voltage attenuation (expressed as cutoff frequency) in axon terminal as a function of relative axon length and relative axon diameter (see text for details) for three different values of Ri (90, 140, and 190 Ω cm). Same cell as in A.
Position dependence of a shunting conductance at the axon of rod bipolar cells
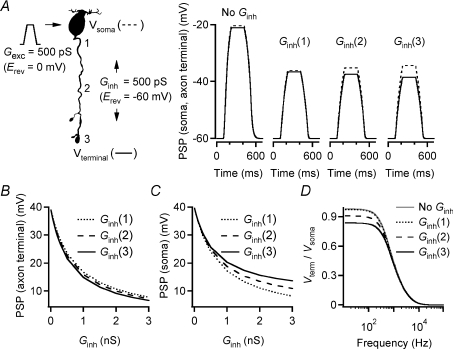
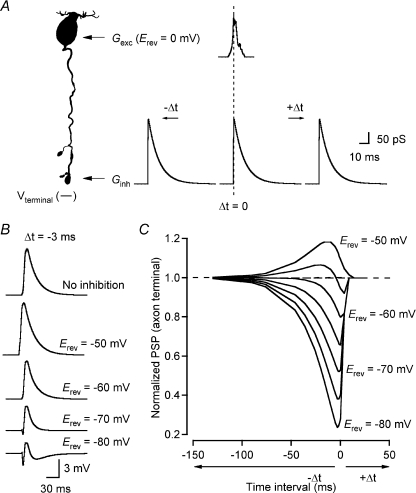
There is strong physiological evidence for inhibitory synaptic input from both GABAergic (Hartveit, 1999; Singer & Diamond, 2003) and glycinergic (Cui et al. 2003; Eggers & Lukasiewicz, 2006; Ivanova et al. 2006) amacrine cells to rod bipolar cells. Morphological investigations suggest that synaptic input from amacrine cells may be localized both at the axon terminals and along the axonal shafts of the cells (Freed et al. 1987; Strettoi et al. 1990; Chun et al. 1993; Kim et al. 1998). The inhibitory input along the axonal shaft is typically non-reciprocal, i.e. there is no synapse from the rod bipolar cell to the amacrine cell that provides the inhibitory input. Synaptic input mediated by GABA and glycine receptors activates chloride conductances in rod bipolar cells (Karschin & Wässle, 1990; Suzuki et al. 1990) and because both the resting membrane potential (Veruki et al. 2006) and the chloride equilibrium potential (ECl) are close to −60 mV (Yamashita & Wässle, 1991; Billups & Attwell, 2002; Duebel et al. 2006), the inhibition is likely to have the characteristics of shunting inhibition. In electrophysiological whole-cell recordings at the axon terminal of rod bipolar cells, we have previously used conductance injection (dynamic clamp) to demonstrate that steady-state shunting conductances (Erev=−60 mV) of a few hundred pS can be sufficient to reduce the peak amplitude of EPSPs evoked by a transient excitatory stimulus (Erev= 0 mV; 400 pS peak amplitude) (Veruki et al. 2006). Corresponding experiments with conductance injection at different positions along the axon will be extremely difficult to perform. As an alternative, we used computer simulations in NEURON with a morphologically reconstructed rod bipolar cell and its best-fit passive membrane parameters (Fig. 8A; same cell as in Figs 6 and 7). At the soma, we injected an excitatory conductance waveform (500 pS peak amplitude; Erev= 0 mV) designed to mimic a light-evoked, excitatory conductance measured in rod bipolar cells (Euler & Masland, 2000). With no inhibition, the excitatory input evoked a PSP with virtually identical amplitude in the soma (39.6 mV) and in the axon terminal (39.0 mV) (Fig. 8A). We then repeated the simulations while injecting a constant shunting conductance (500 pS; Erev=−60 mV) at either the proximal end of the axon (location 1), the middle of the axon (location 2) or the distal end of the axon (axon terminal; location 3; Fig. 8A). In the presence of a shunting conductance, the amplitude of the PSP recorded at the soma was reduced in amplitude. The reduction was largest when the conductance was located near the soma and diminished slightly as the location of the conductance moved towards the axon terminal (Fig. 8A). When instead the PSP was recorded at the axon terminal, its amplitude was also reduced in the presence of a shunting conductance, but the reduction was largest when the conductance was located near the axon terminal and diminished slightly as the location of the conductance moved towards the soma (Fig. 8B). We explored these relationships systematically by repeating the simulations for a series of values for the shunting conductance (Ginh; 0–3000 pS) while keeping the excitatory conductance stimulus constant at 500 pS. Irrespective of whether the PSP was recorded at the axon terminal or at the soma, the relationship between the magnitude of Ginh and the resulting PSP was non-linear, with the largest slope of the PSP versus Ginh relationship occurring for the lowest magnitudes of Ginh (Fig. 8B and C). At the axon terminal, the difference in PSP amplitude between the conditions with the shunting conductance located at either end of the axon (proximal versus distal) was relatively insensitive to the magnitude of the shunt conductance and was ∼2 mV for conductance values larger than ∼250 pS (Fig. 8B). This was in marked contrast to the results obtained when we measured the PSP at the soma, where there was a much stronger effect of the location of the inhibitory conductance on the reduction of the PSP amplitude (Fig. 8C).
Figure 8. Position dependence of shunting inhibition located along the axon of rod bipolar cells.
A, left, shape plot of the morphologically reconstructed rod bipolar cell used for computer simulation of interaction between transient excitatory conductance (Gexc; peak conductance 500 pS; 10–90% rise and decay time 90 ms; Erev= 0 mV) injected at soma (arrow) and steady-state inhibitory conductance (Ginh; 500 pS; Erev=−60 mV) injected at one of three different positions between the soma and axon terminal (1, 2 and 3). Voltage recorded at soma (Vsoma; dotted line) and axon terminal (Vterminal; continuous line). A, right, postsynaptic potentials (PSPs) recorded at soma and axon terminal (as in A left) in response to Gexc with either no Ginh or Ginh located at either position 1, 2 or 3 (as in A left). B and C, amplitude of PSP recorded at axon terminal (B) or soma (C) in response to transient Gexc (as in A) injected at soma during steady-state injection of Ginh at either position 1 (dotted line), 2 (dashed line), or 3 (continuous line) (as in A left). Amplitude of Ginh varied between 0 and 3 nS. D, voltage attenuation (Vterm/Vsoma) as a function of the frequency of the sinusoidal current stimulus at the soma, either in the control condition with no inhibitory conductance or with a steady-state inhibitory conductance (Ginh= 500 pS) injected at one of three locations (1–3; as in A left).
We next explored the effect of the location of the shunting conductance on the filtering properties of the axon by using the frequency tool of NEURON (see above). For a fixed shunt conductance of 500 pS, changing the location of the inhibitory input from the proximal to the distal part of the axon increased the signal attenuation for the steady-state condition and frequencies up to 500–600 Hz (Fig. 8D). Corresponding to this, the cutoff frequency (−3 dB) increased from ∼490 Hz to ∼570 Hz (Fig. 8D).
Interaction between transient excitatory and inhibitory synaptic inputs
When excitatory input occurs on a background of an invariant inhibitory conductance, as in the situations analysed above, the relative timing between excitatory and inhibitory input is irrelevant. When transient excitatory input is combined with transient inhibitory input, however, the relative timing between the two can become important for the integration. It is also of consequence whether the inhibition acts as a shunt or not. To study this systematically, we used NEURON simulations and the prototypical rod bipolar cell (same as in Figs 6–8). We injected two different synaptic conductance waveforms, one excitatory (Erev= 0 mV) at the soma-dendritic region and one inhibitory at the axon terminal region (Fig. 9A). The excitatory conductance was adapted from Field & Rieke (2002; their Fig. 3B; peak conductance ∼200 pS) and the inhibitory conductance corresponded to an idealized version of the average waveform of spontaneous glycinergic IPSCs recorded at the axon terminal of a rod bipolar cell and displayed a peak conductance ∼320 pS (S. H. Mørkve & E. Hartveit; unpublished observations). The idealized waveform of the glycineric IPSC had a 10–90% rise time of 360 μs and a single-exponential decay (τ= 11 ms). In the simulations, Erev of the inhibitory input was varied between −50 and −80 mV (steps of 5 mV). In addition, we varied the relative timing between the peak of the excitatory and inhibitory conductance from Δt=−130 ms (peak of inhibitory waveform occurring before peak of excitatory waveform) to Δt=+24 ms (peak of inhibitory waveform occurring after peak of excitatory waveform; Fig. 9A). The response was measured as the peak amplitude of the integrated PSP and for each combination of Δt and Erev the peak was normalized to the peak of the PSP evoked by the excitatory stimulus in the absence of inhibition. The strength of inhibition increased markedly with increasing negative values of Erev. A series of integrated PSPs obtained at a constant value of Δt (−3 ms), with Erev ranging from −50 to −80 mV is illustrated in Fig. 9B. For each value of Erev, the effectiveness of inhibition depended strongly on the relative timing of inhibition. When the peak of the inhibitory waveform occurred after the peak of the excitatory waveform (Δt > 0), the time window of effective inhibition was very narrow and no reduction of the integrated PSP could be observed when Δt > 9 ms, with little variation for different values of Erev (Fig. 9C). When the peak of the inhibitory waveform occurred before the peak of the excitatory waveform (Δt < 0), the time window of effective inhibition was much broader and a reduction of the integrated PSP by ∼10% could be observed for intervals (Δt) up to ∼70 ms (Fig. 9C). With Erev=−60 mV, the width at half-maximum of the PSP amplitude versusΔt curve was ∼16 ms and with Erev=−80 mV, the width increased to ∼28 ms (Fig. 9C).
Figure 9. Interaction between transient excitatory and inhibitory synaptic conductances in rod bipolar cells, effect of temporal summation and Erev of inhibitory conductance.
A, left, shape plot of the morphologically reconstructed rod bipolar cell used for computer simulation of interaction between a transient excitatory conductance (Gexc; Erev= 0 mV; peak ∼210 pS; taken from Field & Rieke (2002)) injected at soma (arrow) and a transient inhibitory conductance (Ginh; Erev as indicated; peak ∼330 pS; glycinergic postsynaptic conductance, see text for details) injected at axon terminal (arrow). Integrated PSP recorded at axon terminal (Vterminal). A, right, Gexc and Ginh waveforms injected at different time intervals (Δt) relative to each other, with Δt= 0 corresponding to coincidence of the peaks of Gexc and Ginh, Δt < 0 corresponding to peak of Ginh preceding peak of Gexc and Δt > 0 corresponding to peak of Gexc preceding peak of Ginh. B, integrated PSPs recorded at axon terminal after injection of Gexc alone (no inhibition) or coincident with Ginh (as in A; Δt=−3 ms) for different values of Erev for Ginh (as indicated). C, peak amplitude of axon terminal PSP as a function of time interval between Gexc and Ginh (Δt) for different values of Erev for Ginh (−50 to −80 mV; 5 mV intervals). For each value of Erev, PSP amplitudes were normalized to the amplitude evoked by Gexc without coincident Ginh (indicated by horizontal dashed line).
There was almost no influence of the location of Ginh relative to Gexc on the axon terminal PSP amplitude. We examined this by keeping the excitatory stimulus fixed at the soma-dendritic region, while moving the inhibitory stimulus between the axon terminal region and the soma-dendritic region. For Δt=−3 ms and Erev=−60 mV, the PSP amplitudes corresponding to different locations of Ginh differed by less than 0.2 mV and the PSP durations differed by less than 0.5 ms (not shown).
Discussion
In this study we have characterized the structure of rod bipolar cells in rat retina by quantitative morphological reconstruction of cells filled with biocytin during whole-cell, axon terminal recordings from an in vitro thin-slice preparation. We developed compartmental models from the morphological reconstructions and used these models to determine the passive membrane properties and to study electrotonic signal processing in rod bipolar cells. From the modelling studies, the main conclusion is that the voltage response evoked by a realistic transient excitatory synaptic conductance in the soma-dendritic compartment is conveyed to the axon terminal with almost no attenuation or distortion. This conclusion is likely to pertain to signal transmission at physiological temperatures as well because passive membrane properties do not change much with temperature (Trevelyan & Jack, 2002; see also Schmidt-Hieber et al. 2007). We investigated the integrative properties of rod bipolar cells by examining the passive interaction between an excitatory synaptic conductance change in the soma-dendritic compartment and an inhibitory synaptic conductance change located along the axon or at the axon terminal. We found that the relative timing between excitation and inhibition was critical for the interaction between transient inputs, but there was little influence of the exact location of the inhibitory synaptic input on the amplitude of the axon terminal PSP.
Accuracy of compartmental models
In general, the accuracy of the estimates for the passive membrane parameters will depend critically on three conditions. First, the membrane responses obtained should be passive, i.e. obtained from a linearly behaving membrane without activation of voltage-gated currents. Second, the computational model should be based on a morphologically accurate reconstruction. Third, the computer simulations should be numerically accurate and make reasonable assumptions concerning the representation of the model. In addition, the choice of recording mode, voltage-clamp or current-clamp, may also be of consequence for the results obtained, and arguments have been presented in favour of either mode (e.g. Major, 2001; Jackson, 2006). An important point concerns the problems introduced by including an incorrect value for Rs in the subsequent computer modelling. In the ideal case, computer modelling would require either full compensation or independent knowledge of Rs, as even relatively small errors in Rs can lead to large errors in the estimates for Ri (Perreault & Raastad, 2006). In a previous study we found that accurate estimation of Rs in whole-cell recordings at the axon terminal is non-trivial (Oltedal et al. 2007). An alternative strategy is to include Rs as a free parameter in the direct fitting of the current responses of the morphological models. In their comparison of voltage-clamp and current-clamp recording, Perreault & Raastad (2006) concluded that when Rs was included as a free parameter, voltage-clamp recording resulted in a more accurate parameter estimation than current-clamp recording. In our study, we included Rs as a free parameter in the direct fitting and subsequent bootstrap analysis suggested that all four fitted parameters (Ri, Cm, Rm and Rs) were well constrained (cf. Roth & Häusser, 2001; Schmidt-Hieber et al. 2007).
Linearity of membrane responses and passive membrane parameters
Ri, Cm and Rm are important parameters that influence the integrative properties and summation of synaptic inputs in single neurons. In our study we obtained estimates for the passive membrane properties under the most physiological conditions possible, without pharmacological block of voltage-gated conductances. This is similar to procedures used in several investigations of single neurons in other systems (e.g. Spruston & Johnston, 1992; Briska et al. 2003; Perreault & Raastad, 2006). In general, there are two ways in which unblocked, voltage-gated conductances could influence the estimates. First, the applied voltage pulses may change the activation level of a voltage-gated conductance. In this case, the membrane will not be passive and the current responses will not be linear. Second, a conductance may be activated in the steady-state condition corresponding to the membrane holding potential. It is important to note that even when a voltage-gated conductance contributes to the total conductance at the membrane holding potential, its activation and/or deactivation kinetics may be too slow for the conductance to change its contribution in response to relatively brief voltage pulses and the current responses might appear linear.
In the case of rod bipolar cells, we obtained linear membrane responses with application of brief voltage pulses from membrane holding potentials of both −60 and −90 mV. This did not necessarily demonstrate that voltage-gated conductances were not active at the membrane potentials used, only that the brief voltage pulses applied did not change the degree of activation. With respect to steady-state activation of voltage-gated conductances, we have focused our attention on two conductances, the hyperpolarization-activated, non-selective cation current (Ih) and the depolarization-activated T-type Ca2+ current (ICa(T)).
In the case of Ih, further examination with the pharmacological blocker ZD7288 suggested that there was minimal or no steady-state activation at −60 mV, but significant activation at −90 mV. Corresponding to this, Rm was found to be significantly lower at −90 mV than at −60 mV, while Cm and Ri were similar at the two holding potentials. Corresponding to this, τm was significantly shorter at −90 mV than at −60 mV. This is consistent with results from other systems where blocking Ih results in a marked increase in τm (e.g. Spruston & Johnston, 1992). At either holding potential, the voltage pulses used to evoke charging transients were too brief to cause a measurable change in the activation level of Ih, consistent with the slow time constants of activation and deactivation reported (Karschin & Wässle, 1990; Ma et al. 2003; Ivanova & Müller, 2006).
In the case of ICa(T), a ‘window-current’ could potentially be present at a membrane potential of −60 mV if there is a sufficient degree of overlap between the steady-state activation and inactivation curves. In recordings from isolated rat rod bipolar cells, Pan (2000) reported that ICa(T) displays half-maximal steady-state inactivation at ∼−60 mV and full activation is only reached at ∼0 mV. If there is a steady-state contribution of ICa(T) to the total membrane conductance at −60 mV, this could lead to an understimation of Rm. However, it is likely that half-maximal inactivation of ICa(T) in rod bipolar cells is reached at a more negative membrane potential than the estimate of −60 mV suggests. First, in other studies of neuronal ICa(T), half-maximal inactivation is typically ∼−80 mV (reviewed by Huguenard, 1996; Perez-Reyes, 2003). Second, it is known that increasing the extracellular Ca2+ concentration leads to a positive shift of the half-maximal inactivation voltage. For example, increasing the Ca2+ concentration from ∼2 to 10 mm, as in the study of isolated rod bipolar cells (Pan, 2000), can shift the voltage for half-maximal steady-state inactivation by +10 mV (Fukushima & Hagiwara, 1985), most likely through a surface charge screening effect (Hille, 2001). Third, decreasing the duration of the conditioning voltage pulses used to evoke inactivation leads to a positive shift of the half-maximal inactivation voltage. For example, decreasing the duration from 10 to 1 s can result in a shift of +10 mV (Bossu & Feltz, 1986; Takahashi et al. 1991). Pan (2000) used conditioning pulses of 3 s duration. Taken together, this suggests that the true voltage for half-maximal inactivation for ICa(T) in rod bipolar cells is more negative than −60 mV, consistent with studies that did not find evidence for a transient ICa upon depolarization from a holding potential of −60 mV (Hartveit, 1999; Singer & Diamond, 2003).
Axonal morphology and Ri
With excitatory synaptic input to rod bipolar cells at the soma-dendritic region and synaptic output at the axon terminal region, the axonal resistance becomes an important parameter for the signal transmission between these two regions. In experiments with simultaneous somatic and dendritic whole-cell recordings from layer 5 neurons in cerebral cortex (Stuart & Spruston, 1998), CA1 pyramidal neurons (Golding et al. 2005) and cerebellar Purkinje neurons (Roth & Häusser, 2001), computer modelling has indicated values for Ri between 70 and 200 Ω cm. The values obtained from these experiments have been considered to represent the most reliable estimates of Ri because the filtering of transient voltage changes in dendrites is very sensitive to the value of Ri (Spruston et al. 2008). Consistent with this, we found previously that the decay of capacitative charging transients obtained in axon terminal recordings, as opposed to somatic recordings, is strongly influenced by changes of Ri (Oltedal et al. 2007). Here, our average estimate for Ri was ∼130 Ω cm, very similar to the values obtained in the simultaneous, dual-recording experiments.
Whereas axonal length can be measured reliably, influenced only by the degree of shrinking in fixed tissue, the resolution limit of light microscopy might compromise the accuracy of measurements of axonal diameter. Axial resistance (ri) is given by the following equation: ri=Ri× (L/A), where L is the length of the axon and Ai is the cross-sectional area of the axon. For a given axial resistance, this means that if the true diameter was, e.g. 0.7 μm, but was measured as 0.8 μm, the resulting Ri would be overestimated by a factor of 1.3 (see also Major, 2001). Though the resulting compartmental model might still be electrically valid, in the sense that it can reproduce the experimental observations, the morphological error would trade off for accuracy in the estimates for Ri, Cm and Rm. We attempted to reduce the influence of observer bias by performing the morphological reconstructions twice for each cell by independent observers. Our average value for the axonal diameter (0.66 μm) compares favourably with other data in the literature. Chun et al. (1993) examined rat rod bipolar cells by electron microscopy. From their Fig. 6 that illustrates five different axons, we calculate an average diameter of 0.64 μm.
In our material, we found an average of 2.4 axonal terminal branches. This compares well with data from electron microscopic studies where one to three branches were found for rat rod bipolar cells (Chun et al. 1993) and two to four branches were found for rabbit rod bipolar cells (Strettoi et al. 1990). We found an axonal length, including axon terminal branches, ranging from 67 to 96 μm. This compares reasonably well to rough measurements made on published micrographs of PKC-stained rat rod bipolar cells (∼60 μm; Greferath et al. 1990; their Fig. 3A; Chun et al. 1993, their Fig. 1A).
Membrane surface area and Cm
Although Cm is generally regarded as a biological constant, with estimates typically being close to 1 μF cm−2 (Gentet et al. 2000; Major, 2001), it is important to obtain an accurate estimate of Cm when modelling specific types of neurons. For rod bipolar cells, the soma contributes ∼50% of the total surface area, thus an accurate estimate of Cm for these cells requires that the morphological reconstruction and surface area measurement are performed as carefully as possible. In this study, we took advantage of the recently developed technique of axon terminal recordings in situ to avoid damage to the soma at the end of recording when the recording pipette was pulled away. In general, the somata of the recorded cells were located > 10–20 μm below the surface of the slice, suggesting that structural damage of the dendritic processes should have been reduced to a minimum. For our reconstructed rod bipolar cells we obtained average estimates for Cm at two different membrane potentials, 1.12 μF cm−2 at −60 mV and 1.14 μm cm−2 at −90 mV. These values are very similar to values obtained from other neuronal cells and suggest that our surface area estimates were reasonably accurate.
Integrative mechanisms in rod bipolar cells
Because the passive signal attenuation between the soma and axon terminal will be influenced by parameters such as axon length, axon diameter and Ri, we explored the consequences of varying these parameters within realistic ranges around the average values obtained from model fitting. Depending on the specific combination of parameters, the strongest attenuation observed corresponded to a cutoff frequency of ∼300 Hz. This is quite remarkable and suggests that even in the absence of potential amplification mechanisms, there is very little attenuation of ordinary visual signals. Because rod bipolar cells can convey and transmit single-photon signals, we used computer modelling to inject conductance waveforms corresponding to voltage-clamp recorded single-photon responses (Field & Rieke, 2002) at the soma. There was only a negligible difference between the voltage responses at the soma and axon terminal. With repetitive visual signals, the critical fusion (flicker) frequency of the rod response measured in psychophysical experiments has been estimated at ∼20 Hz (Hecht et al. 1948; see also Sharpe & Stockman, 1999), suggesting that it is very unlikely that passive signal attenuation along the rod bipolar cell axon will limit transmission of signals from soma to axon terminal. However, the attenutation of responses mediated by voltage-gated conductances is unknown.
In general, there are two potential mechanisms for shaping the signals conveyed by rod bipolar cells: integration of excitatory and inhibitory synaptic inputs and activation of voltage-dependent conductances. EPSPs evoked in the soma-dendritic region can interact with inhibitory synaptic input, in principle located anywhere between the soma and axon terminal. This inhibitory input can be either feedforward (GABAergic or glycinergic) or feedback (GABAergic) input. With respect to a stimulus-evoked PSP recorded at the axon terminal, the amplitude reduction evoked by steady-state (tonic) shunting inhibition was relatively insensitive to the location of the inhibition. For the interaction of physiologically realistic transient excitatory and inhibitory input waveforms, the time window of effective inhibition depended critically on the relative timing of the inputs and was considerably longer when the inhibitory input occurred before the excitatory input. Surprisingly, when we measured the effect of the spatial location of the inhibitory input on the PSP amplitude measured at the axon terminal, the passive integration of excitatory and inhibitory inputs was practically insensitive to the spatial location of the inhibitory input. One interpretation is that the morphological clustering of inhibitory inputs at the axon terminal is a consequence of the requirement for tightly coupled feedback inhibition, with the inhibitory feedback being driven by excitation from the axon terminal itself.
An alternative, but not mutually exclusive interpretation is that the functional importance of targeting inhibitory input to the axon terminal is related to interaction with voltage-gated conductances concentrated in the same compartment, e.g. the L-type ICa (see above). Specifically, the output characteristics at the axon terminal will differ, depending on whether a regenerative or non-regenerative depolarization occurs at the axon terminal. In goldfish bipolar cell terminals, voltage-gated Ca2+ channels can initiate a regenerative process, eventually triggering a Ca2+ spike (Zenisek & Matthews, 1998). If a similar mechanism can operate in rod bipolar cells in situ, the balance between regenerative and non-regenerative depolarization could be controlled by inhibition targeted to the axon terminal (cf. Hull et al. 2006). Another current with the highest density at the axon terminal is Ih (Ivanova & Müller, 2006). The functional importance of Ih in rod bipolar cells is unclear, but there is evidence that it facilitates membrane potential rebound after hyperpolarization (Ma et al. 2003) and it has recently been suggested that it might be a mechanism for high pass filtering of input signals (Cangiano et al. 2008). An important goal for future work will be to obtain quantitative models of voltage-gated conductances at physiological temperatures, incorporate them in the compartmental models developed in the present study and examine their role in shaping the responses of rod bipolar cells evoked by inputs mimicking physiologically realistic stimuli.
Acknowledgments
We thank Dr Arnd Roth for kindly providing computer code for implementing a recording pipette tip in the NEURON modelling environment. Financial support from the Norwegian Research Council (NFR 161217, 165328, 178105) is gratefully acknowledged.
References
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu JL, Feltz A. Inactivation of the low-threshold transient calcium current in rat sensory neurones: evidence for a dual process. J Physiol. 1986;376:341–357. doi: 10.1113/jphysiol.1986.sp016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philos Trans R Soc Lond B Biol Sci. 1969;255:109–194. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Brent RP. Algorithms for Minimization Without Derivatives. Englewood Cliffs, NJ, USA: Prentice Hall; 1973. A new algorithm for minimizing a function of several variables without calculating derivatives; pp. 116–167. [Google Scholar]
- Briska AM, Uhlrich DJ, Lytton WW. Computer models of passive signal integration based on whole-cell in vitro studies of rat lateral geniculate nucleus. Eur J Neurosci. 2003;17:1531–1541. doi: 10.1046/j.1460-9568.2003.02579.x. [DOI] [PubMed] [Google Scholar]
- Cajal SR. La rétine des vertébrés. La Cellule. 1893;9:119–255. [Google Scholar]
- Cangiano L, Gargini C, Santina LD, Demontis GC, Cervetto L. High-pass filtering of input signals by the Ih current in a non-spiking neuron, the retinal rod bipolar cell. PLoS ONE. 2008;2:e1327. doi: 10.1371/journal.pone.0001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale NT, Hines ML. The NEURON Book. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Carnevale NT, Tsai KY, Claiborne BJ, Brown TH. The electrotonic transformation: a tool for relating neuronal form to function. In: Tesauro G, Touretzky DS, Leen TK, editors. Advances in Neural Information Processing Systems. Vol. 7. Cambridge, MA, USA: The MIT Press; 1995. pp. 69–76. [Google Scholar]
- Chávez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Cui J, Ma Y-P, Lipton SA, Pan Z-H. Glycine receptors and glycinergic synaptic input at the axon terminals of mammalian retinal rod bipolar cells. J Physiol. 2003;553:895–909. doi: 10.1113/jphysiol.2003.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur J Neurosci. 1998;10:317–323. doi: 10.1046/j.1460-9568.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL, USA: Chapman & Hall/CRC; 1993. [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci. 2006;26:9413–9425. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Rod bipolar array in the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol. 1987;266:445–455. doi: 10.1002/cne.902660310. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Mickus TJ, Katz Y, Kath WL, Spruston N. Factors mediating powerful attenuation along CA1 pyramidal neuron dendrites. J Physiol. 2005;568:69–82. doi: 10.1113/jphysiol.2005.086793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hecht S, Shlaer S, Smith EL, Haig C, Peskin JC. The visual functions of the complete colorblind. J Gen Physiol. 1948;31:459–472. doi: 10.1085/jgp.31.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA, USA: Sinauer; 2001. [Google Scholar]
- Hines ML, Carnevale NT. Recent developments in NEURON. Brains, Minds and Media. 2005;1 bmm221 (urn:nbn:de:0009–3-2210) [Google Scholar]
- Hu H-J, Pan Z-H. Differential expression of K+ currents in mammalian retinal bipolar cells. Vis Neurosci. 2002;19:163–173. doi: 10.1017/s0952523802191140. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Hull C, Li G-L, von Gersdorff H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci. 2006;26:6979–6984. doi: 10.1523/JNEUROSCI.1386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Müller F. Retinal bipolar cell types differ in their inventory of ion channels. Vis Neurosci. 2006;23:143–154. doi: 10.1017/S0952523806232048. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Müller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Molecular and Cellular Biophysics. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Karschin A, Wässle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- Kim IB, Lee MY, Oh SJ, Kim KY, Chun MH. Double-labeling techniques demonstrate that rod bipolar cells are under GABAergic control in the inner plexiform layer of the rat retina. Cell Tissue Res. 1998;292:17–25. doi: 10.1007/s004410051030. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, Ito S, Sheng MH, Jan LY, Pinto LH. The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current. J Neurosci. 1995;15:5004–5013. doi: 10.1523/JNEUROSCI.15-07-05004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Nelson R. Rod pathways in the retina of the cat. Vision Res. 1983;23:301–312. doi: 10.1016/0042-6989(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Lanni F, Keller HE. Microscopy and microscope optical systems. In: Yuste R, Konnerth A, editors. Imaging in Neuroscience and Development. A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 2005. pp. 711–765. [Google Scholar]
- Ma Y-P, Cui J, Hu H-J, Pan Z-H. Mammalian retinal bipolar cells express inwardly rectifying K+ currents (IKir) with a different distribution than that of Ih. J Neurophysiol. 2003;90:3479–3489. doi: 10.1152/jn.00426.2003. [DOI] [PubMed] [Google Scholar]
- Major G. Passive cable modeling – a practical introduction. In: De Schutter E, editor. Computational Neuroscience. Realistic Modeling for Experimentalists. Boca Raton, FL, USA: CRC Press; 2001. pp. 209–232. [Google Scholar]
- Mennerick S, Zenisek D, Matthews G. Static and dynamic membrane properties of large-terminal bipolar cells from goldfish retina: experimental test of a compartment model. J Neurophysiol. 1997;78:51–62. doi: 10.1152/jn.1997.78.1.51. [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Oltedal L, Mørkve SH, Veruki ML, Hartveit E. Patch-clamp investigations and compartmental modeling of rod bipolar axon terminals in an in vitro thin-slice preparation of the mammalian retina. J Neurophysiol. 2007;97:1171–1187. doi: 10.1152/jn.01010.2006. [DOI] [PubMed] [Google Scholar]
- Pan Z-H. Differential expression of high- and two types of low-voltage-activated calcium currents in rod and cone bipolar cells of the rat retina. J Neurophysiol. 2000;83:513–527. doi: 10.1152/jn.2000.83.1.513. [DOI] [PubMed] [Google Scholar]
- Pan Z-H. Voltage-activated Ca2+ channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Vis Neurosci. 2001;18:279–288. doi: 10.1017/s095252380118212x. [DOI] [PubMed] [Google Scholar]
- Pan Z-H, Hu H-J, Perring P, Andrade R. T-type Ca2+ channels mediate transmitter release in retinal bipolar cells. Neuron. 2001;32:89–98. doi: 10.1016/s0896-6273(01)00454-8. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Raastad M. Contribution of morphology and membrane resistance to integration of fast synaptic signals in two thalamic cell types. J Physiol. 2006;577:205–220. doi: 10.1113/jphysiol.2006.113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Llano I. Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J Neurosci. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Roth A, Häusser M. Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J Physiol. 2001;535:445–472. doi: 10.1111/j.1469-7793.2001.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Aoki K, Watanabe S-I, Kaneko A. L-type calcium channels in the axon terminal of mouse bipolar cells. Neuroreport. 1998;9:2161–2165. doi: 10.1097/00001756-199807130-00002. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Subthreshold dendritic signal processing and coincidence detection in dentate gyrus granule cells. J Neurosci. 2007;27:8430–8441. doi: 10.1523/JNEUROSCI.1787-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 1999;22:497–504. doi: 10.1016/s0166-2236(99)01458-7. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994;17:161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Spruston N, Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992;67:508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Spruston N, Stuart G, Häusser M. Dendritic integration. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. 2nd edn. Oxford, UK: Oxford University Press; 2008. pp. 351–399. [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295:449–466. doi: 10.1002/cne.902950309. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tachibana M, Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol. 1990;421:645–662. doi: 10.1113/jphysiol.1990.sp017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ueno S, Akaike N. Kinetic properties of T-type Ca2+ currents in isolated rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1991;65:148–155. doi: 10.1152/jn.1991.65.1.148. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Jack J. Detailed passive cable models of layer 2/3 pyramidal cells in rat visual cortex at different temperatures. J Physiol. 2002;539:623–636. doi: 10.1113/jphysiol.2001.013291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9:1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Wässle H. Reversal potential of GABA-induced currents in rod bipolar cells of the rat retina. Vis Neurosci. 1991;6:399–401. doi: 10.1017/s0952523800006635. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. Calcium action potentials in retinal bipolar neurons. Vis Neurosci. 1998;15:69–75. doi: 10.1017/s0952523898151064. [DOI] [PubMed] [Google Scholar]