Abstract
We aimed to determine whether there is a differential stimulation of the contractile myofibrillar and the cellular sarcoplasmic proteins after ingestion of protein and how this is affected by resistance exercise. Fasted (FAST) muscle protein synthesis was measured in seven healthy young men with a primed constant infusion of l-[ring-13C6]phenylalanine. Participants then performed an intense bout of unilateral resistance exercise followed by the consumption of 25 g of whey protein to maximally stimulate protein synthesis. In the rested (FED) leg myofibrillar (MYO) protein synthesis was elevated (P < 0.01) above FAST at 3 h (∼163%) but not at 1 and 5 h (P > 0.05). In contrast, MYO protein synthesis in the exercised (FED-EX) leg was stimulated above FAST at 1, 3 and 5 h (∼100, 216, and 229%, respectively; P < 0.01) with the increase at 5 h being greater than FED (P < 0.01). Thus, the synthesis of muscle contractile proteins is stimulated by both feeding and resistance exercise early (1 h) but has a greater duration and amplitude after resistance exercise. Sarcoplasmic (SARC) protein synthesis was similarly elevated (P < 0.01) above FAST by ∼104% at 3 h in both FED and FED-EX suggesting SARC protein synthesis is stimulated by feeding but that this response is not augmented by resistance exercise. In conclusion, myofibrillar and sarcoplasmic protein synthesis are similarly, but transiently, stimulated with protein feeding. In contrast, resistance exercise rapidly stimulates and sustains the synthesis of only the myofibrillar protein fraction after protein ingestion. These data highlight the importance of measuring the synthetic response of specific muscle protein fractions when examining the effects of exercise and nutrition.
The ability of amino acids to increase the synthesis of mixed muscle proteins during the transition from a fasted to fed state has been well characterized (Rennie et al. 1982; Bohe et al. 2001,2003; Paddon-Jones et al. 2004; Fujita et al. 2007). However, less is known about the stimulatory effect of dietary amino acids on the synthesis of specific muscle protein fractions. For example, myofibrillar protein synthesis responds to dietary amino acids in a dose-related manner but becomes refractory to persistent hyperaminoacidaemia (Bohe et al. 2001,2003; Cuthbertson et al. 2005). Saroplasmic protein synthesis is also responsive to dietary amino acids but may be less sensitive to nutrient availability than myofibrillar proteins (Mittendorfer et al. 2005; Cuthbertson et al. 2005). Overall, we still do not know the amplitude or duration of the enhanced synthetic response after a discrete meal (i.e. protein bolus).
Resistance exercise has been shown to increase the synthesis of mixed muscle proteins (that must include myofibrillar proteins) and feeding potentiates this response (Borsheim et al. 2002; Tipton et al. 2003; Wilkinson et al. 2007; Dreyer et al. 2008; Moore et al. 2009); presumably, this is the basis for an expansion of the muscle protein pool that, over time, leads to hypertrophy (Tipton et al. 2003; Hartman et al. 2007; Wilkinson et al. 2007). Most acute studies have reported the anabolic effect of nutrients after exercise as a stimulation of mixed muscle protein fraction (Tipton et al. 2003; Wilkinson et al. 2007; Dreyer et al. 2008; Drummond et al. 2008). We, and others, have reported that resistance exercise stimulates myofibrillar protein synthesis in the fasted state (Kim et al. 2005) and in the presence of a constant supply of amino acids (Louis et al. 2003; Moore et al. 2005; Cuthbertson et al. 2006; Wilkinson et al. 2008). What we remain uncertain about are the latency, amplitude, and duration of acute changes in the synthesis of myofibrillar proteins in response to bolus protein ingestion after resistance exercise.
The purpose of this study was to examine the acute changes in muscle protein synthesis at rest after ingestion of a bolus of dietary protein. Additionally, the synthetic response of both the myofibrillar and non-myofibrillar muscle protein fraction was measured to determine the relative stimulation after protein ingestion both at rest and after resistance exercise. We hypothesized that resistance exercise would potentiate the feeding-induced stimulation of protein synthesis primarily in the contractile myofibrillar protein fraction.
Methods
Subjects
Seven healthy recreationally active males (26 ± 3 years; 84.8 ± 4.5 kg; 1.76 ± 0.05 m; means ±s.e.m.) volunteered to participate in the study. Participants reported engaging in recreational lower body resistance exercise no more than once per week for the prior 3 months. Participants were informed about the experimental procedure to be used as well as the purpose of the study and all potential risks prior to obtaining written consent. All participants were deemed healthy based on their response to a routine medical screening questionnaire. The study carried approval by the local Research Ethics Board of McMaster University and Hamilton Health Sciences and conformed to all standards for the use of human subjects in research as outlined in the 1963 Declaration of Helsinki on the use of human subjects in research.
General design
To define the changes in myofibrillar and non-myofibrillar (sarcoplasmic) protein synthesis a primed constant infusion of l-[ring-13C6]phenylalanine was used to measure basal muscle protein synthesis prior to performing an acute bout of unilateral leg resistance exercise. By using a within-subject design, whereby each individual served as his own rested control, we are better able to attribute the acute changes in protein synthesis to the respective feeding and/or exercise stimuli rather than to interindividual response variation. After exercise, participants consumed a drink containing 25 g of whey protein to maximally stimulate muscle protein synthesis in both the rested and exercised legs (Cuthbertson et al. 2005; Moore et al. 2009) and changes in protein synthesis were measured over the subsequent 5 h of recovery.
Infusion protocol
Participants reported to the laboratory at least 1 week prior to the infusion trial to undergo a muscle biopsy procedure for the measurement of baseline protein enrichment. A single biopsy was taken from the vastus lateralis of a randomly selected thigh using a Bergström needle modified for manual suction under local anaesthesia (2% xylocaine). All muscle biopsy samples taken throughout the study were blotted and freed of any visible fat and connective tissue, frozen (within ∼20 s of excision) in liquid nitrogen, and stored at –80°C until further analysis. On the morning of the trial, participants reported to the laboratory at 07.00 h after an overnight fast and having refrained from any strenuous physical activity for the previous 3 days. A catheter was inserted in the antecubital vein of one arm for blood sampling. After a baseline blood sample was drawn, a second catheter was inserted in the contralateral arm for the application of a primed constant infusion of l-[ring-13C6]phenylalanine (prime: 2 μmol kg−1; 0.05 μmol kg−1 min−1). The participants rested comfortably on a bed throughout the infusion. Blood samples were drawn every 0.5–1 h (Fig. 1) and processed as previously described (Moore et al. 2009). At 3 h after the start of the infusion, a single muscle biopsy was taken from the contralateral leg from the baseline biopsy to measure fasted (FAST) rates of protein synthesis. Participants then performed an intense bout of unilateral leg resistance exercise consisting of five sets each of leg press and knee extension using a predetermined weight that elicited voluntary failure in 8–10 repetitions. The exercise bout was completed in 24 ± 1 min. Upon completion of the exercise, a blood sample was drawn and then participants consumed a drink containing 25 g of whey protein to maximize muscle protein synthesis in the rested (FED) and exercised (FED-EX) legs. To minimize disturbances in isotopic equilibrium, drinks were enriched to 6% with tracer according to a measured phenylalanine content of ∼3.5% in whey protein. Bilateral muscle biopsies were obtained from separate incisions (∼5 cm apart) at 1, 3 and 5 h after protein ingestion to characterize the acute changes in protein synthesis in FED and FED-EX. To minimize the number of biopsies, muscle samples were not taken from each leg immediately upon completion of the exercise bout but rather only 1 h after exercise cessation and the subsequent protein ingestion. Therefore, since an additional ∼24 min of tracer incorporation occurred during the exercise bout at a fasted (FED) rate that would be further suppressed with exercise (FED-EX), the reported protein synthetic rates measured at 1 h (i.e. ∼84 min of incorporation) represent slight underestimations of the true rates of protein synthesis in FED (∼10%) and FED-EX (∼25%). These average estimates were calculated by comparing our measured rate to an estimated rate of protein synthesis, as determined by extrapolating the basal rate of protein synthesis during exercise in FED and a 30% reduction during exercise in FED-EX (Dreyer et al. 2006). However, we have presented the uncorrected data as we did not directly measure the additional tracer incorporation occurring during the brief exercise time. In addition, we speculate that the relatively brief incorporation time during exercise is unlikely to significantly affect the rates of protein synthesis when expressed over 3 and 5 h.
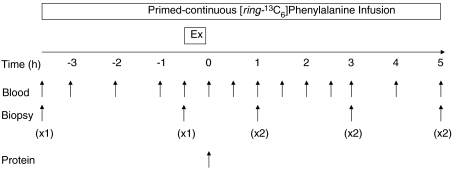
Figure 1. Schematic diagram of the experimental protocol.
Single muscle biopsies (1×) were taken from alternate thighs to characterize fasted protein synthesis after which a unilateral bout of resistance exercise (EX) was performed. After a drink containing 25 g of whey protein (Protein) was ingested at 0 h, bilateral muscle biopsies (2×) were taken from the rested (FED) and exercised (FED-EX) legs.
Analyses
Blood amino acid concentrations were analysed by HPLC as previously described (Moore et al. 2005). Blood glucose concentration was measured using a commercially available spectrophotmetric kit (Standbio Laboratory, Boerne, TX, USA) and plasma insulin concentration was determined using a standard radioimmuno assay kit (Diagnostics Products Corporation, Los Angeles, CA, USA).
Intracellular proteins were isolated from a piece of wet muscle (∼30 mg) by hand-homogenizing on ice using a Teflon pestle in a standard 20 mm Tris (pH 7.2) Western blotting buffer (10 μl mg−1) (Glover et al. 2008). The sample was spun for 10 min at 1500 g at 4°C to separate the myofibrillar and collagen proteins. The resultant homogenate was removed and the sarcoplasmic proteins were precipated by addition of 1 ml of 1 m perchloric acid (PCA). The myofibrillar and collagen pellet was washed once with buffer and the myofibrillar proteins were solubilized by adding 1.5 ml of 0.3 m NaOH and heating to 37°C for 30 min with vortex mixing every 10 min. Samples were centrifuged for 10 min at 4000 g at 4°C and the supernatant containing the myofibrillar fraction was collected. Myofibrillar protein was precipitated by adding 1 ml of 1 m PCA and spinning for 10 min at 4000 g at 4°C. The sarcoplasmic and myofibrillar proteins were washed twice with 70% EtOH before lyophilization. Amino acids were liberated from myofibrillar and sarcoplasmic proteins by adding 1.5 ml of 6 m HCl and heating to 110°C for 24 h. Free amino acids were purified using cation exchange chromatography (Dowex 50WX8-200 resin; Sigma-Aldrich Ltd) and converted to their N-acetyl-n-propyl ester derivatives for analysis by gas chromatography combustion-isotope ratio mass spectrometry (GC-C-IRMS: Hewlett Packard 6890; IRMS model Delta Plus XP, Thermo Finnigan, Waltham, MA, USA). Derivitized amino acids were separated on a 30 m DB-1701 column (temperature programme: 110°C for 2 min; 20°C min−1 ramp to 210°C; 5°C min−1 ramp to 280°C; hold for 5 min) prior to combustion.
Intracellular amino acids were isolated from a separate piece of muscle (∼20 mg) by homogenizing on ice with 500 μl of acetonitrile. Samples were centrifuged at 10000 g at 20°C for 10 min and the supernatant containing the intracellular amino acids was collected and dried under nitrogen at 70°C. Amino acids were converted to their heptafluorobutyric (HFB) derivatives before analysis by GC-MS (models 6890 GC and 5973 MS; Hewlett-Packard, Palo Alto, CA, USA). Intracellular phenylalanine enrichment was determined using electron-impact ionization by monitoring ions 316 and 322 (m+ 0 and m+ 6, respectively).
Calculations
The fractional synthetic rates (FSR) of myofibrillar and sarcoplasmic protein were calculated using the standard precursor–product method:
where ΔEp is the change in bound protein enrichment between two biopsy samples, Em is the average enrichment of intracellular phenylalanine across the two biopsy samples, and t is the time between biopsies. Specifically, fasted FSR was calculated from the change in bound protein enrichment between the baseline biopsy and the biopsy taken at 3 h after the start of the infusion using the intracellular phenylalanine enrichment at 3 h. FSR at 1 h was calculated from the change in bound protein enrichment from the fasted biopsy to the 1 h biopsy after feeding in both the FED or FED-EX conditions, respectively. The change in bound protein enrichment between subsequent biopsies within each condition was used to calculate FSR in FED and FED-EX at 1–3 and 3–5 h (i.e. FSR expressed over 2 h intervals). Additionally, FSR in FED and FED-EX was also calculated over 3 and 5 h intervals using the change in bound protein enrichment between the fasted biopsy and biopsies at 3 and 5 h, respectively, to allow for better comparisons between the present results and those of other studies that typically measure protein synthesis over these longer time frames.
Statistics
Because we used a within-subject study design, myofibrillar and sarcoplasmic protein synthesis were first analysed using a one-factor (1 × 7; FSR) repeated measures ANOVA with a Holm–Sidak post hoc test for comparison to control (Fast) to determine whether changes in protein synthesis were elevated above fasted. Myofibrillar and sarcoplasmic protein synthesis were then analysed using a two-factor (2 × 3; condition, time) repeated measures ANOVA to determine significant differences between conditions and across time (i.e. over 2 h intervals). Additionally, myofibrillar and sarcoplasmic protein synthesis expressed over 3 and 5 h were analysed using a one-factor (condition) ANOVA. Differences in the relative stimulation of myofibrillar and sarcoplasmic protein synthesis between conditions were determined by Student's paired t test. Blood glucose, plasma insulin, and blood amino acid concentrations were analysed using a one-factor (time) repeated measures ANOVA. Unless otherwise stated, Tukey's post hoc test was performed to determine differences between means for all significant main effects and interactions. For all analyses, differences were considered significant at P < 0.05. Values are expressed as means ±s.e.m.
Results
Blood glucose and plasma insulin
Fasted blood glucose was 4.2 ± 0.3 mm and remained stable for the duration of the infusion trial. Fasted plasma insulin concentration was 4.5 ± 0.4 μIU ml−1 and increased by ∼190% 1 h after protein ingestion but returned to basal values by 2 h.
Blood amino acid concentration
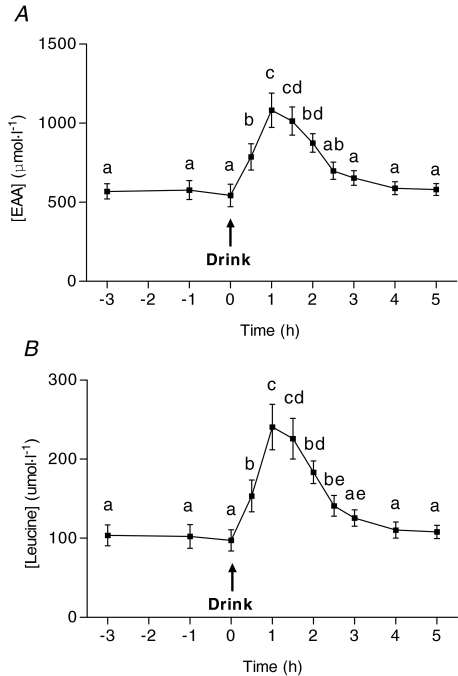
Blood EAA and leucine concentrations were stable during the fasted condition. After protein ingestion, blood EAA concentration increased immediately at 0.5 h and reached a peak at 1 h after which it declined to basal levels by 3 h (Fig. 2). Blood leucine concentration showed a similar pattern to that of EAA (Fig. 2).
Figure 2. Blood essential amino acid (EAA; top) and leucine (bottom) concentration after ingestion of 25 g of protein (Drink).
Values are means ±s.e.m. (n= 7). EAA are sum of His, Ile, Leu, Lys, Met, Phe, Thr, Val (note: Cys not measured). Means with different letters are significantly different from each other, P < 0.05.
Intracellular precursor labelling
Fasted intracellular phenylalanine enrichment was 3.9 ± 0.3 APE. Intracellular phenylalanine enrichment was stable across time for both FED (4.8 ± 0.3, 5.4 ± 0.3 and 4.8 ± 0.3 APE at 1, 3, and 5 h, respectively; P > 0.05) and FED-EX (5.8 ± 0.1, 5.7 ± 0.3 and 5.1 ± 0.4 APE at 1, 3 and 5 h, respectively; P > 0.05). More importantly, the slope of intracellular phenylalanine enrichment by time curve for both FED and FED-EX was not different from zero (P= 0.9 and 0.3, respectively) further confirming all measurements were made at isotopic plateau, the requisite condition for application of the steady-state precursor product equation.
Muscle protein synthesis
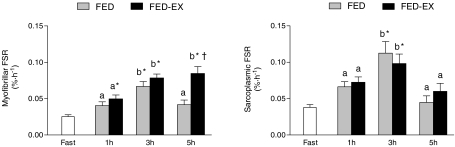
In FED, myofibrillar protein synthesis was elevated ∼2.6-fold above the fasted state (FAST) only at 1–3 h (P < 0.01; Fig. 3). In contrast, FED-EX increased myofibrillar protein synthesis above FAST at 1 h (P < 0.05) and was further elevated at 1–3 and 3–5 h (P < 0.01) with the increase at 3–5 h being greater than FED (P < 0.01). In contrast, sarcoplasmic protein synthesis was similarly elevated (P < 0.01) above FAST at 1–3 h in both FED and FED-EX (∼2.2 and 1.9-fold, respectively) with no difference between the conditions (P > 0.05; Fig. 3). In FAST, the rate of incorporation of labelled phenylalanine into sarcoplasmic proteins was greater (P < 0.01) than myofibrillar (Table 1). There was an expected graded response of feeding and resistance exercise (FAST < FED < FED-EX; all P < 0.01) when the rate of myofibrillar protein synthesis was expressed over 3 and 5 h (Table 1); however, the difference between FED and FED-EX was greater over 5 h compared to 3 h (0.020 ± 0.003 versus 0.012 ± 0.004% h−1, respectively; P < 0.05, paired t test). In addition, although both were greater than FAST (P < 0.01), there was no difference (both P > 0.05) between FED and FED-EX when sarcoplasmic protein synthesis was expressed over 3 or 5 h (Table 1). Additionally, there was no difference in the increase above fasted of myofibrillar and sarcoplasmic protein synthesis after feeding at rest (FED) with respect to the increase in peak rate (118 ± 19 and 121 ± 33%, respectively; P= 0.8; paired t test) or when the rates were expressed over 3 h (P= 0.31; paired t test) or 5 h (P= 0.15; paired t test) (Table 1).
Figure 3. Myofibrillar (top) and sarcoplasmic (bottom) protein synthesis (FSR) in the fasted state (Fast) and after protein ingestion at rest (FED) and after resistance exercise (FED-EX).
Values are means ±s.e.m. (n= 7). *Significantly different from Fast, P < 0.01. †Significantly different from FED at same time point, P < 0.01. Times with different letters are significantly different from each other within each condition, P < 0.01.
Table 1.
Myofibrillar and sarcoplasmic protein synthesis after feeding at rest (FED) and resistance exercise (FED-EX) expressed over 3 and 5 h
| 3 h |
5 h |
||||
|---|---|---|---|---|---|
| Fasted | FED | FED-EX | FED | FED-EX | |
| Myofibrillar | |||||
| Rate (% h−1) | 0.025 ± 0.002* | 0.051 ± 0.003*† | 0.066 ± 0.003†‡ | 0.049 ± 0.003*† | 0.070 ± 0.003†‡ |
| Change (%) | — | 112 ± 19 | 179 ± 36‡§ | 103 ± 22 | 204 ± 48‡§ |
| Sarcoplasmic | |||||
| Rate (% h−1) | 0.052 ± 0.004 | 0.086 ± 0.009† | 0.084 ± 0.006† | 0.074 ± 0.004† | 0.077 ± 0.005† |
| Change (%) | — | 71 ± 20 | 66 ± 15 | 44 ± 8 | 51 ± 9 |
Values are means ±s.e.m. (n= 7) for myofibrillar and sarcoplasmic protein synthesis expressed over 3 and 5 h in absolute rate (Rate; % h−1) and relative increase above fasted (Change;%). *Different from sarcoplasmic at same time, P < 0.01. †Different from fasted, P < 0.01. ‡Different from FED at same time, P < 0.01. §Different from sarcoplasmic at same time, P < 0.01.
Discussion
Here we report for the first time changes in myofibrillar and non-myofibrillar (sarcoplasmic) protein synthesis after ingestion of a bolus dose of protein. The novel findings of the study were that myofibrillar and sarcoplasmic protein synthesis are similarly, but transiently, enhanced with protein feeding with the stimulation peaking at 3 h and returning to basal levels by 5 h. Additionally, resistance exercise stimulates a rapid (i.e. within 1 h) increase in myofibrillar protein synthesis and sustains the synthetic response of only the myofibrillar protein fraction after discrete protein ingestion.
In response to feeding alone the amplitude and duration of myofibrillar and sarcoplasmic protein synthesis was similar. The acute time course for the stimulation of muscle protein synthesis in the present study is consistent with observed changes in whole body protein metabolism after ingestion of a discrete meal that would contribute to the repletion fasted state losses in muscle protein balance (Boirie et al. 1997; Dangin et al. 2001). Our data are in contrast to previous studies reporting that sarcoplasmic protein synthesis is less sensitive to nutrient availability than myofibrillar protein (Mittendorfer et al. 2005; Cuthbertson et al. 2005). Previous studies measuring changes in both myofibrillar and sarcoplasmic protein synthesis with nutrients provided amino acids either orally, which only induced a fold change in blood EAA concentration, or via a sustained intravenous infusion (Cuthbertson et al. 2005; Mittendorfer et al. 2005). Accepting that muscle protein synthesis is regulated by acute changes in extracellular amino acid concentration (Bohe et al. 2003), it is possible that the 2-fold increase in EAA concentration after ingestion of a bolus of 25 g of protein in the present study enhanced the sensitivity of the sarcoplasmic protein fraction to nutrients as a result of a greater physiological increase in EAA concentration compared to previous studies. Nonetheless, we observed that myofibrillar and sarcoplasmic protein fractions respond in a qualitatively similar fashion with respect to the timing of peak rates and duration of the response suggesting the measurement of mixed muscle protein synthesis is an appropriate surrogate for determining the anabolic effect of a protein meal at rest.
Resistance exercise induced a rapid (i.e. within 1 h) stimulation of myofibrillar protein synthesis that persisted for up to 5 h, which is consistent with the temporal changes measured in mixed muscle protein synthesis and the anabolic nature of the exercise stimulus (Dreyer et al. 2006; Drummond et al. 2008). The observation that the stimulation of myofibrillar protein synthesis demonstrates a lag period of ∼1 h in the fasted state could suggest that the immediate ingestion of dietary amino acids may have contributed to the rapid onset of myofibrillar protein synthesis after resistance exercise in the present study (Kumar et al. 2008). Interestingly, there was little additional stimulation in the amplitude of myofibrillar protein synthesis with feeding after exercise compared to feeding at rest suggesting that muscle contractile proteins are primarily responsive to physical stress/stretch after resistance exercise. These data would seem at odds with studies reporting a synergistic effect of exercise and nutrition on muscle protein synthesis (Biolo et al. 1997; Koopman et al. 2005; Tang et al. 2007; Dreyer et al. 2008; Moore et al. 2009). It is possible that the synergistic effect of exercise and nutrition previously reported in mixed muscle may be related to the amino acid-induced increase in sarcoplasmic protein synthesis, which in our hands is responsive only to nutrients after exercise. Notwithstanding, in light of recent work in the fasted state (Kumar et al. 2008), our data demonstrate it is the duration and not the amplitude for which the synergism of exercise and nutrition is observed in the myofibrillar protein fraction. Our decision to measure changes in protein synthesis over 2 h intervals likely afforded us the temporal resolution necessary to accurately measure changes in the peak rates of protein synthesis. For instance, when the present data are expressed over 3 and, especially, 5 h the expected increase in myofibrillar protein synthesis to ingestion of dietary amino acids after resistance exercise was observed (Table 1); these data suggest the timing of muscle biopsies, as has been highlighted by Bohe et al. (2001) at rest, should be considered in studies investigating the combined effects of exercise and nutrition. Nonetheless, the sustained synthesis of the myofibrillar protein fraction with protein feeding and resistance exercise is likely to represent an adaptive response to exercise within the contractile proteins that, over time, would ultimately translate into training-induced myofiber hypertrophy.
We found that resistance exercise had no additional stimulatory effect on the synthesis of the sarcoplasmic protein fraction after physiological feeding. This is in contrast to earlier work reporting an elevation in the synthesis of non-myofibrillar proteins after exercise (Louis et al. 2003; Miller et al. 2005; Cuthbertson et al. 2006; Wilkinson et al. 2008). Relative to the present bolus protein ingestion, it is possible that the previous studies may have provided an optimal, albeit non-physiological, anabolic environment for the synthesis of sarcoplasmic and mitochondrial proteins after exercise as a result of a feeding regime that induced a sustained hyperaminoacidaemia (Louis et al. 2003; Miller et al. 2005; Cuthbertson et al. 2006; Wilkinson et al. 2008). However, we cannot discount the possibility that a greater exercise volume (20 versus 10 sets to failure) (Louis et al. 2003) or a more aerobic-based exercise stimulus (Miller et al. 2005; Cuthbertson et al. 2006) in previous studies may have induced adaptations typically associated with endurance exercise such as, for example, mitochondrial biogenesis, angiogenesis, and/or the synthesis of metabolic enzymes and metabolite transporters (Dubouchaud et al. 2000; Prior et al. 2004; Wilkinson et al. 2008), which could have been included in the non-myofbrillar protein faction and therefore contributed to the acute postexercise increase in sarcoplasmic protein synthesis.
In summary, our data demonstrate that the synthesis of myofibrillar and sarcoplasmic protein fractions are similarly elevated after ingestion of a physiologically discrete protein bolus. Resistance exercise induces a rapid and sustained increase in the synthesis of myofibrillar proteins but fails to augment the synthetic response of the sarcoplasmic protein fraction after feeding. These data highlight the importance of measuring the response of specific protein fractions when investigating the combined effects of exercise and nutrition.
Acknowledgments
We would like to thank Dr Yifan Yang and Andrea Josse for their help in data collection and the participants for their time and effort. D.R.M. was supported by a Canada Graduate Scholarship award from the Canadian Institutes for Health Research (CIHR). J.E.T. was supported by a doctoral graduate scholarship from CIHR. S.M.P. was the recipient of a CIHR New Investigator Career Award and gratefully acknowledges that source of funding. This research was supported by a research grant from Natural Sciences and Engineering Research Council of Canada to S.M.P.
References
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose–response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2Bɛ phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008;295:R604–R610. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568:283–290. doi: 10.1113/jphysiol.2005.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in dose–response of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2008;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003;285:E1089–E1094. doi: 10.1152/ajpendo.00195.2003. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose–response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2008.26401. in press. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–1138. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003;284:E76–E89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Macdonald MJ, MacDonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]