Abstract
Significant alterations in maternal nutrition may induce long-term metabolic consequences in offspring, in particular obesity and leptin and insulin resistance. Although maternal nutrient deprivation has been well characterized in this context, there is a relative paucity of data on how high fat (HF) nutrition impacts on the subsequent generation. The present study investigated the effects of maternal HF nutrition either throughout the mother's life up to and including pregnancy and lactation or HF nutrition restricted to pregnancy and lactation, on growth and metabolic parameters in male and female offspring. Virgin Wistar rats were assigned to one of three experimental groups: (1) controls (Cont): dams fed a standard chow diet throughout their life and throughout pregnancy and lactation; (2) maternal high fat (MHF) group: dams fed a HF diet from weaning up to and throughout pregnancy and lactation; and (3) pregnancy and lactation high fat (PLHF): dams fed a chow diet through their life until conception and then fed a HF diet throughout pregnancy and lactation. At weaning, all offspring were fed either a chow or HF diet for the remainder of the study (160 days). Litter size and sex ratios were not significantly different between the groups. MHF and PLHF offspring had significantly lower body weights and were hypoleptinaemic and hypoinsulinaemic at birth compared to Cont offspring. As adults however, chow-fed MHF and PLHF offspring were significantly more obese than Cont offspring (DEXA scanning at day 150, P < 0.001 for maternal HF diet). As expected a postweaning HF diet resulted in increased adiposity in all groups; MHF and PLHF offspring, however, always remained significantly more obese than Cont offspring. Increased adiposity in MHF and PLHF offspring was paralleled by hyperinsulinaemia and hyperleptinaemia (P < 0.001; MHF and PLHF versus Cont). It is of interest that a lifetime of HF nutrition produced a similar offspring phenotype to HF nutrition restricted to pregnancy and lactation alone, thus suggesting that the postnatal sequelae of maternal HF nutrition occurs independent of preconceptional diet. These data further reinforce the importance of maternal nutrition during these critical windows of development and show that maternal HF feeding can induce a markedly obese phenotype in male and female offspring completely independent of postnatal nutrition.
Obesity and its sequelae may prove to be the greatest threat to human lifestyle and health in the developed world this century (Armitage et al. 2008). The obesity epidemic has seen the incidence of obesity and overweight almost double in Western societies and the trend is mirrored in developing nations that are transitioning to first-world economies. Obesity is strongly associated with the morbidities of type 2 diabetes, hypertension and ischaemic heart disease and represents an enormous burden to the health care system. Of even more concern is the rise of over 40% over the last 20 years in the prevalence of childhood obesity – with concomitant increases in childhood type 2 diabetes.
Metabolic disease results from a complex interaction of many factors, including genetic, physiological, behavioural, and environmental influences. The recent rate at which these diseases have increased suggests that environmental and behavioural influences, rather than genetic causes, are fuelling the present epidemic. In this context, it is of particular relevance that epidemiological and experimental studies have highlighted a relationship between the periconceptual, fetal and early infant phases of life and the subsequent development of obesity and type 2 diabetes. This relationship, referred to as the ‘developmental origins of health and disease’ (DOHaD) model, speculates that the fetus makes predictive adaptations in response to intrauterine cues, resulting in permanent adjustments in homeostatic systems to aid immediate survival and improve success in an adverse postnatal environment. However, inappropriate interpretations of prenatal cues or changes to that immediate environment may result in a mismatch between prenatal predictions and postnatal reality. As a result, these adaptations, known as predictive adaptive responses (PARs) (Gluckman et al. 2005; Gluckman et al. 2007), may ultimately be disadvantageous in postnatal life, leading to an increased risk of chronic non-communicable disease in adulthood and/or the inheritance of risk factors and a cycle of disease transmission across generations.
Initial epidemiological studies have suggested that fetal growth restriction is correlated with later disease, implying that fetal nutritional deprivation may be a strong programming stimulus. This prompted the development of experimental animal models using controlled maternal calorie, protein or macronutrient deficiency during key periods of development. However, in many societies, maternal and postnatal nutrition are either sufficient or excessive. As a result, excessive weight gain and/or obesity is one of the more common nutritional problems complicating pregnancy in developed countries.
In view of the rising prevalence of obesity in pregnancy and the association with gestational diabetes, there is now increasing interest in the detrimental influence of maternal obesity and excess maternal nutrition on the risk of disease in childhood and beyond (Catalano, 2003; Ehrenberg et al. 2004; Oken et al. 2007; Gillman et al. 2008). However, to date, relatively few studies have investigated long-term consequences of maternal nutrient excess during pregnancy or lactation on development of obesity in the offspring (Armitage et al. 2004, 2005; Vickers et al. 2007).
Within human populations, problems with maternal obesity are well documented – the incidence of gestational diabetes rises, as does pre-eclampsia and rates of caesarean section. Moreover, these effects may be self-perpetuating, as offspring of overweight mothers are themselves prone to obesity in adulthood thus giving rise to transgenerational effects. Although maternal obesity in the human is often paralleled by an increased risk for fetal macrosomia, there is also a growing body of evidence suggesting that obese mothers are also characterized by an increasing prevalence of IUGR infants compared to non-obese mothers. The present study was therefore designed to investigate the effects of a moderate maternal HF diet during the preconceptional period and/or throughout pregnancy and lactation on birth phenotype and risk of obesity and related metabolic sequelae in male and female offspring in adult life when fed either a chow or postnatal HF diet.
Methods
Animal model
Male and female Wistar rats were acquired at a weaning age (22 days) and housed two per cage under standard conditions with a 12 : 12 light–dark cycle and free access to water. Females were weight-matched and assigned to receive either standard rat chow (n= 16, Diet 2018, Harlan Teklad, Blackthorn, Bicester, UK) or a HF diet (n= 8, 45% kcals as fat, D12451, Research Diets, New Brunswick, NJ, USA) to be fed ad libitum for the duration of the trial. Males were fed chow ad libitum for the duration of the premating period. Body weights were recorded every 3 days until postnatal day 120.
At postnatal day 110, body composition in females was quantified using dual energy X-ray absorptiometry (DEXA, Lunar Prodigy, GE Medical Systems, Madison, WI, USA) as previously described. At postnatal day 120, females were time-mated using an oestrus cycle monitor (Fine Science Tools, Foster City, CA, USA). Qualitative measures of the effect of the HF diet on oestrus cycling were made by observation. Upon confirmation of mating, three maternal dietary groups were established: (1) controls (Cont): females fed a standard chow diet throughout their life and maintained on a standard chow diet throughout pregnancy and lactation; (2) maternal high fat diet (MHF): females fed a HF diet throughout their life and maintained on the HF diet throughout pregnancy and lactation; and (3) pregnancy + lactation HF diet (PLHF): females fed a standard chow diet until conception and then a HF diet throughout pregnancy and lactation. All pregnant dams were weighed and had food intakes measured daily throughout pregnancy. Following birth, pups were weighed and had lengths recorded, and litter size was randomly adjusted to 8 pups (4 males and 4 females) to ensure standardized nutrition until weaning. Non-assigned pups were killed by decapitation at postnatal day (P)2 and plasma samples pooled for later analysis. Lactating dams had body weights and food intakes measured throughout the lactation period and pups were weighed every 3 days until weaning.
After weaning, dams were fasted overnight and killed by decapitation following anaesthesia with sodium pentobarbitone (60 mg kg−1, i.p.) and plasma samples collected for insulin and leptin analyses. At weaning (d22), male and female offspring were housed two per cage (2 per litter/sex/maternal background) and randomly assigned to receive either the standard chow or the HF diet ad libitum until the end of the trial. At postnatal day 150, animals (n= 10–12 per group) had body composition quantified by DEXA scanning while under light isoflurane (2%) anaesthesia. At postnatal day 160, animals were fasted overnight and killed by decapitation following anaesthesia with sodium pentobarbitone (60 mg kg−1, i.p.). Blood was collected into heparinized vacutainers and centrifuged, and plasma supernatant stored for future analysis. All animal experiments were approved under guidelines of the Animal Ethics Committee at the University of Auckland (R402).
Plasma analyses
Leptin and insulin concentrations were analysed on plasma from male and female pups at postnatal day 2, lactating dams at postnatal day 22 (weaning) and postnatal male and female offspring using commercial rat-specific ELISAs (CrystalChem 90040 and 90060, respectively, Uppsala, Sweden). Fasting plasma glucose concentrations were measured using a glucose meter at the time of cull (Roche AccuChek).
Statistical analysis
Data for pups (P2) were analysed by two-way factorial ANOVA with maternal background and sex as factors. Data for postnatal animals (P160) were analysed by three-way factorial ANOVA with maternal background, postnatal diet and sex as factors. Data on lactating dams were analysed using one-way ANOVA with maternal dietary background as a factor. Data from adult age-matched control female offspring (non-lactating females) were used to investigate effects of lactation. Analysis was performed using StatView statistical software (SAS Institute Inc., Cary, NC, USA). All data are presented as means ±s.e.m. unless otherwise stated.
Results
Maternal body weights
Dams fed a HF diet throughout their life including pregnancy and lactation (MHF) had significantly increased body weights (Cont 265 ± 3.5 g versus MHF 295 ± 1.2 g, P < 0.05), body fat mass and fat to lean ratios compared to females raised on chow (Cont and PLHF) at the time of mating (Fig. 1). Of note, the HF diet resulted in reduced cycling (as monitored over 3 cycle periods via the daily oestrus probe method) in MHF dams compared to chow fed animals (Cont 95% cycling, MHF 45% cycling). Total caloric intake during gestation (intake or kcals consumed per g body weight) was not different between Cont and MHF dams. Caloric intake was significantly increased in the PLHF dams during the early period of gestation compared to Cont and MHF dams and normalized to that of Cont dams by the second week of gestation (Table 1 and Fig. 2). There were no significant differences in maternal weight gain across the treatment groups during gestation (Cont 136 ± 5.3 g, MHF 132 ± 3.4 g, PLF 142 ± 6.1 g, Fig. 3) although the MHF animals remained significantly heavier than the other groups (Day 21 gestation weights, Cont 401 ± 11 g, MHF 451 ± 10, PLHF 412 ± 19, P < 0.0001 for MHF versus Cont and PLHF). Gestation length (monitored every 3 h during the daylight period) was increased by approximately 24 h in MHF and PLHF dams compared to controls, particularly noticeable in PLHF dams (P < 0.005, Table 2).
Figure 1. Body fat mass and fat : lean ratios in female dams prior to mating as quantified by DEXA scanning.
Data are means ±s.e.m.; n= 8–17 per group. *P < 0.0001.
Table 1.
Food intake (total kcals) consumed per dam during gestation and lactation in Cont, MHF and PLHF dams
| Gestation day (kcals total) |
Lactation day (kcals total) |
|||||
|---|---|---|---|---|---|---|
| Group | G1–7 | G8–14 | G15–21 | L22–28 | L29–35 | L36–42 |
| Cont | 481 ± 11 | 535 ± 12 | 596 ± 17 | 769 ± 34 | 1267 ± 21 | 1624 ± 17 |
| MHF | 524 ± 10 | 503 ± 14 | 605 ± 16 | 629 ± 45 | 1404 ± 12 | 2049 ± 17 |
| PLHF | 659 ± 15 | 592 ± 12 | 609 ± 15 | 609 ± 31 | 1236 ± 13 | 1877 ± 16 |
| Cont versus MHF | NS | NS | NS | NS | NS | P < 0.05 |
| Cont versus PLHF | P < 0.0001 | P < 0.05 | NS | NS | NS | P < 0.05 |
| MHF versus PLHF | P < 0.0001 | P < 0.05 | NS | NS | NS | NS |
G – gestation, L – lactation. Data are means ±s.e.m., n= 8–12 per group.
Figure 2. Daily caloric intake (expressed as kcals consumed per gram body weight) in dams during the period of pregnancy and lactation.
*P < 0.0001 for effect of PLHF versus Cont and MHF; **P < 0.0001 for effect of MHF and PLHF versus Cont. Data are means ±s.e.m.; n= 8–9 per group.
Figure 3. Body weight gain in dams during pregnancy and lactation.
P < 0.001 for effect of MHF versus Cont and PLHF. Data are means ±s.e.m., n= 8–9 per group.
Table 2.
Gestation length, birth weights and weaning weights in dams and offspring following Cont, MHF or PLHF nutrition
| Birth Weights (g) |
Weaning Weights (g) |
||||
|---|---|---|---|---|---|
| Group | Gestation (days) | Male | Female* | Male | Female* |
| Cont | 21.6 ± 0.14 | 6.3 ± 0.07 | 6.1 ± 0.06 | 61.6 ± 0.7 | 59.1 ± 0.7 |
| MHF | 22.5 ± 0.18 | 5.9 ± 0.05 | 5.7 ± 0.05 | 70.5 ± 0.9 | 66.3 ± 0.9 |
| PLHF | 22.8 ± 0.14 | 5.5 ± 0.04 | 5.3 ± 0.03 | 64.2 ± 1.2 | 63.8 ± 0.8 |
| Cont versus MHF | P < 0.05 | P < 0.0005 | P < 0.0005 | P < 0.0001 | P < 0.0001 |
| Cont versus PLHF | P < 0.05 | P < 0.0001 | P < 0.0001 | P < 0.05 | P < 0.05 |
| MHF versus PLHF | NS | P < 0.05 | P < 0.005 | P < 0.05 | P < 0.05 |
Data are means ±s.e.m., n= 8–12 per group (dams) and 24–36 per group per sex (offspring).
Females were lighter than males at both time points (P < 0.0001).
Birth and weaning weights
Maternal HF feeding led to a small but significant reduction in birth weight in males and females compared to controls (Table 2). There was a further significant reduction in birth weight in offspring in the PLHF group compared to MHF and Cont offspring. Caloric intake of dams was similar among all groups from birth until neonatal day 10 when MHF and PLHF dams significantly increased caloric intake compared to controls, an increase which persisted until weaning (Fig. 2). Weaning weights were slightly but significantly increased in MHF and PLHF male and female offspring compared to controls (Table 2).
Postnatal growth and caloric intake
A postnatal HF diet significantly increased body weight gain in all groups. Postnatal body growth was significantly increased in male and female offspring of MHF and PLHF dams, independent of postnatal diet (Fig. 4A–D). By postnatal day 150, male and female offspring of MHF and PLHF fed dams were significantly heavier than Cont animals on both chow and HF diets. Total caloric intake of male and female offspring was measured at postnatal days 30, 60, 100 and 150. In males, PLHF offspring had increased caloric intake compared to Cont and MHF animals until postnatal day 60 from which point on caloric intakes were not significantly different between any of the treatment groups. In females, there were no significant differences in caloric intake across the treatment groups at the ages measured (data not shown).
Figure 4. Postnatal growth curves from weaning until day 150.
A, female offspring of Cont or MHF mothers fed either the C or HF diet postnatally; B, male offspring of Cont or MHF mothers fed either the C or HF diet postnatally; C, female offspring of Cont or PLHF mothers fed either the C or HF diet postnatally; D, male offspring of Cont or PLHF mothers fed either the C or HF diet postnatally. P < 0.001 for effect of maternal HF diet and postnatal HF nutrition. MHF versus PLHF not significant. No interactions. Data are means ±s.e.m., n= 12–18 per group.
Body composition
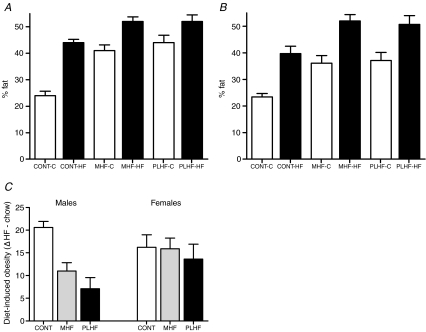
Total body fat mass and fat : lean ratios were significantly increased in male and female offspring of HF-fed dams even when fed a chow diet postnatally (Fig. 5A and B). Total body fat was further increased in all animals fed a HF diet postnatally. There were no significant differences in total body fat or fat : lean ratios between MHF and PLHF offspring. There was a highly significant maternal diet–postnatal diet interaction in males (P < 0.05) but not in females (P > 0.6). Female offspring fed the HF diet displayed the same relative weight gain as chow fed animals independent of maternal dietary group (Fig. 5C). However, Cont males showed a marked increase in HF diet-induced obesity compared to offspring of HF-fed mothers where the response to HF feeding was reduced relative to that of chow fed animals (Fig. 5C).
Figure 5.
A and B, total body fat (%) as quantified by DEXA scanning in male (A) and female (B) offspring at postnatal day 150. P < 0.05 for effect of maternal HF nutrition and postnatal HF nutrition. MHF versus PLHF not significant, no statistical interactions. C, effect of diet-induced obesity (mean % body fat HF-fed minus mean % body fat chow fed for each maternal group). Males P < 0.05 for Cont versus MHF and PLHF, females NS; male–female interaction P < 0.05. Data are means ±s.e.m., n= 8–10 per group.
Bone mineral content (BMC) and bone mineral density (BMD) were significantly increased in all postnatally HF-fed animals (Table 3) and BMC correlated highly with body weight across all groups (r2= 0.94, P < 0.0001). BMD and BMC were significantly increased in all male MHF and PLHF offspring compared to Cont animals on either postnatal diet (Table 3). BMC and BMD were not different between male MHF and PLHF offspring. BMC in males was highly correlated with total fat mass (r2= 0.76, P < 0.0001). In females, BMC and BMD were significantly lower compared to males. BMC was significantly increased in female MHF and PLHF offspring on both postnatal diets compared to Cont females and was not different between MHF and PLHF animals. BMC in females was highly correlated with total fat mass (r2= 0.81, P < 0.0001). In contrast to males, there were no significant differences in BMD among any of the treatment groups.
Table 3.
Bone mineral content and bone mineral density at postnatal day 150 in male and female offspring of Cont, MHF and PLHF fed dams as quantified by DEXA scanning
| Bone mineral content (BMC, g) |
Bone mineral density (BMD, g/cm2) |
|||
|---|---|---|---|---|
| Group | Male | Female* | Male | Female* |
| Cont-chow | 13.13 ± 0.28 | 7.90 ± 0.16 | 0.173 ± 0.002 | 0.159 ± 0.001 |
| Cont-HF | 16.94 ± 0.40 | 10.19 ± 0.34 | 0.184 ± 0.002 | 0.170 ± 0.002 |
| MHF-chow | 15.89 ± 0.64 | 9.75 ± 0.4 | 0.178 ± 0.003 | 0.165 ± 0.002 |
| MHF-HF | 18.98 ± 0.61 | 12.15 ± 0.34 | 0.191 ± 0.003 | 0.168 ± 0.001 |
| PLHF-chow | 16.59 ± 0.43 | 9.54 ± 0.39 | 0.179 ± 0.003 | 0.164 ± 0.001 |
| PLHF-HF | 18.59 ± 0.50 | 11.50 ± 0.40 | 0.187 ± 0.002 | 0.169 ± 0.003 |
| Maternal effect | P < 0.0001 | P < 0.0001 | P < 0.05 | NS |
| Postnatal diet effect | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.005 |
| Interactions | NS | NS | NS | NS |
Data are means ±s.e.m., n= 12–18 per group.
Effect of sex P < 0.0001.
Plasma insulin and leptin concentrations
Maternal
Fasting leptin concentrations in lactating dams at postnatal day 22 (at the time of weaning) were significantly lower when compared to control female age-matched HF-fed offspring, representing an effect of lactation on leptin concentrations. Leptin levels in lactating dams, however, were not affected by a high fat diet (Table 4). Maternal fasting plasma insulin concentrations, however, were significantly increased in MHF and PLHF dams compared to Cont dams (P < 0.05, Table 4) and were further increased in PLHF dams compared to MHF dams. Fasting plasma insulin concentrations were higher in lactating PLHF dams compared to non-lactating age-matched chow- and HF-fed females (Table 4) but were not different among the other groups.
Table 4.
Plasma leptin and insulin in lactating dams and in pups at postnatal day 2
| Offspring, postnatal Day 2 (ng ml−1) |
Dam, day 22 lactation |
|||
|---|---|---|---|---|
| Group | Leptin | Insulin | Leptin | Insulin |
| Cont | 5.50 ± 1.60a | 2.9 ± 0.62a | 1.71 ± 0.19a | 1.91 ± 0.4a,d |
| MHF | 1.54 ± 0.49b | 1.9 ± 0.25b | 1.86 ± 0.38a | 3.18 ± 0.6b |
| PLHF | 1.58 ± 0.33b | 1.6 ± 0.23b | 2.55 ± 0.77a | 4.53 ± 0.7c |
| NLF-chow | — | — | 3.47 ± 0.31a | 1.14 ± 0.1a |
| NLF-HF | — | — | 7.52 ± 1.07b | 2.42 ± 0.2d |
Pup plasma was pooled from male and females within litter. Means with different letters are significantly different from each other. Data are means ±s.e.m., n= 8–12 per group (dams) and 6–8 litters per offspring analysis.
Offspring leptin
Plasma leptin concentrations in pups at postnatal day 2 were significantly reduced in offspring of MHF and PLHF dams compared to Cont dams (P < 0.001, Table 4). There were no significant differences in fasting leptin concentrations between MHF and PLHF offspring at postnatal day 2. In contrast, in adulthood, fasting plasma leptin concentrations were significantly increased in MHF and PLHF male and female chow-fed offspring compared to Cont offspring and were increased even further if offspring were fed a postnatal HF diet (Fig. 6A and B). Fasting leptin concentrations positively correlated with fat mass across all treatment groups (r2= 0.72, P < 0.0001).
Figure 6.
A and B, fasting plasma leptin concentrations in male and female offspring at postnatal day 175. P < 0.05 for effect of maternal HF nutrition and postnatal HF diet; MHF versus PLHF not significant. C and D, fasting plasma insulin concentrations in male and female offspring at postnatal day 175. P < 0.05 for effect of maternal HF nutrition and postnatal HF diet; MHF versus PLHF not significant. No statistical interactions. Data are means ±s.e.m., n= 12–18 per group.
Insulin and glucose
As with leptin, plasma insulin concentrations in pups at postnatal day 2 were significantly reduced in MHF and PLHF offspring compared to Cont offspring and were not different between MHF and PLHF offspring (P < 0.001, Table 4). At postnatal day 160, fasting plasma insulin concentrations were significantly higher in MHF and PLHF male and female chow-fed offspring compared to Cont offspring and were increased even further following a postnatal HF diet (Fig. 6C and D). There were no significant differences in fasting insulin concentrations between MHF and PLHF offspring. Plasma glucose concentrations were not different in any of the maternal dietary groups but were slightly but significantly elevated in HF fed males (P < 0.005) (treatment groups: Males: Cont-C 5.6 ± 0.2 mmol l−1, Cont-HF 6.0 ± 0.2, MHF-C 5.7 ± 0.3, MHF-HF 6.4 ± 0.2, PLHF-C 5.7 ± 0.2, PLHF-HF 6.1 ± 0.1; Females: Cont-C 5.6 ± 0.1, Cont-HF 5.6 ± 0.1, MHF-C 5.4 ± 0.1, MHF-HF 5.6 ± 0.1, PLHF-C 5.5 ± 0.1, PLHF-HF 5.7 ± 0.2).
Discussion
In the current study we have demonstrated that mothers fed a high fat diet throughout their lifetime up to and including pregnancy and lactation give birth to smaller offspring that are predisposed to developing obesity in a manner that is independent of postnatal diet. Although other models of maternal high fat nutrition have been established in the context of developmental programming (Taylor et al. 2003; Khan et al. 2004, 2005; Armitage et al. 2005; Morris & Chen, 2008; Samuelsson et al. 2008; Chen et al. 2008), the present trial using a moderate high fat diet is the first to compare specific windows of maternal dietary exposure coupled with postweaning dietary challenges in both sexes. These observations underscore the importance of considering the early life environment as a contributer to later life metabolic health. Further, a lifetime consumption of a HF diet appears to have not dissimilar influences on offspring obesogenic phenotypes compared to HF consumption restricted to pregnancy and lactation alone. Importantly, male and female offspring appear equally at risk for developing obesity and related endocrine disturbances in adult life.
A relationship has been established between early life nutritional adversity and a greater risk of obesity and metabolic disease in later life, presumably due to significant environmental changes during a phase of developmental plasticity (Gluckman & Hanson, 2007; Gluckman et al. 2007). Prenatal nutrition-induced obesity risk has been described experimentally in several animal models including the rat, sheep and guinea pig. Importantly, the level of increased susceptibility to programmed obesity risk is dependent upon several factors including timing and severity of the manipulation, postnatal dietary composition, species and sex. To date, however, a primary focus has been on models utilizing relative prenatal undernutrition such as global or protein nutrient restriction (Vickers et al. 2000; Ozanne, 2001; Lillycrop et al. 2005; Vickers et al. 2007) or uterine artery ligation (Simmons et al. 2001) and relatively few studies have elaborated on exposure to excessive nutrition. In this regard we have presented novel data demonstrating long-term effects of exposure to high fat nutrition early in life on growth and metabolism.
Maternal adaptations to HF nutrition
Although undernutrition and protein deficiency remain an issue in many countries, a major problem now relates to current obesogenic environments with caloric over-consumption (Shankar et al. 2008). In developed countries, 15–20% of reproductive aged women are obese (Seidell, 2000) and in a recent reports, > 40% of women gained more weight during pregnancy than is considered ideal (May, 2007; Olson, 2008). Inappropriate pregnancy weight gain and maternal obesity have long-term effects on the developing offspring (Taylor & Poston, 2007), who then go on to develop early puberty and have a greater risk for obesity – leading to a cycle of overweight mothers preparing their children for the same destiny. Animal models of neonatal overnutrition have demonstrated a link between excessive weight gain early in life and later metabolic complications (Plagemann et al. 1992). Oken et al. (2007) have shown that high gestational weight gain significantly increases the risk of childhood obesity. Intriguingly, we demonstrate in the present study that the mere consumption of HF both prior to and including pregnancy did not increase maternal weight gain over and above control pregnancies. Although dams fed HF diet throughout their lifetime began pregnancy heavier with increased body fat, their gestational weight gain profile followed identical growth trajectories to those of the control dams. These data may suggest that it is the composition of the diet rather than maternal weight gain per se that has an effect on offspring phenotype.
We demonstrated that during lactation, dams were hypoleptinaemic and hyperinsulinaemic. Lactation-associated hypoleptinaemia in the rat has previously been reported by others (Seeber et al. 2002). These observations were associated with marked increases in food consumption during the late lactation period and may suggest that these endocrine changes are physiologically appropriate, providing increased energy reserves to help meet the high metabolic demands of lactation (Grattan et al. 2007).
We have also demonstrated a significantly longer gestation length in pregnant mothers fed a HF diet. These data are not dissimilar to those reported in human populations known to consume high levels of n–3 fatty acids (Szajewska et al. 2006), where n–3 fatty acids are thought to interfere with the production of prostaglandins necessary for the activation of parturition. The diet used in our study derives its fat from animal lard, which is primarily composed of oleic acid, an n–9 fatty acid. The impact of these fatty acids on gestation length is intriguing although the mechanisms are unclear and warrant further investigation.
Postnatal consequences of maternal HF nutrition
Birth weight
In rodent models maternal HF feeding has been reported to have variable effects on birth weight with some studies reporting no effects (Khan et al. 2003, 2005; Gorski et al. 2006; Caluwaerts et al. 2007; Ferezou-Viala et al. 2007; Shankar et al. 2008) while others report either decreased (Hausman et al. 1991; Rasmussen, 1998; Taylor et al. 2003; Cerf et al. 2005) or increased birth weights (Samuelsson et al. 2008) although the latter study used a high fat : high sugar diet. These discrepancies are likely to be due to differing fatty acid composition of the fat enriched diets across studies with variable levels in maternal saturated fat intakes (Armitage et al. 2008; Samuelsson et al. 2008). It has also been shown recently that maternal obesity preconceptionally results in no differences in birth weights in offspring (Shankar et al. 2008). High birth weight, or macrosomia, in human populations of obese mothers normally relates to the development of gestational diabetes (GDM) (Khan, 2007; Plagemann et al. 2008). Although we did not measure maternal glucose and insulin levels throughout pregnancy in the present study, the fact that we demonstrated birth weight reduction rather than augmentation would suggest that it is unlikely that the results we observe are due to the development of GDM. It is possible that the growth restriction may relate to different maternal adaptations to HF feeding between species.
Body fat mass
We have shown that a lifetime of HF nutrition up to and including pregnancy and lactation resulted in an obese phenotype in offspring irrespective of postnatal nutrition. This effect was present in both males and females and was paralleled by fasting plasma hyperinsulinaemia and hyperleptinaemia. These observations are underpinned by a highly significant interaction between maternal diet and postnatal HF diet strengthening the hypothesis that effects of a nutritionally high fat diet during childhood and adulthood are predicted by maternal nutritional history. When control males were fed a postnatal HF diet, they demonstrated a greater increase in diet-induced body fat accumulation compared to offspring of HF-fed mothers, suggesting that these latter offspring are better able to metabolically handle a postnatal HF diet. It is possible that offspring of HF-fed mothers are adaptively more suited to a postnatal HF diet and would support a predictive adaptive response in these offspring (Gluckman et al. 2005). Alternatively, male HF exposed offspring may have reached a maximal threshold of body fat accumulation. In contrast, the degree of body fat accumulation in female offspring fed a postnatal high fat diet increased to the same degree in all groups, suggesting that maternal nutritional history did not influence offspring obesogenic responses to postnatal diet in females.
We have demonstrated that mothers fed a lifetime of HF produced offspring with a similar phenotype as mothers fed HF during pregnancy and lactation alone. These data suggest that at least in rats, maternal body composition at the time of conception has no additive effect on offspring phenotypic outcome. However, the level of diet-induced maternal obesity at the time of conception, although highly significant, may have not been great enough for synergistic effects to have been observed. Excessive degrees of preconceptional obesity in rodents can present a twofold problem. Firstly, marked obesity results in lack of normal oestrus cycling (as observed in the present study) and reduced reproductive success and secondly, is also known to result in lactational failure and high mortality in offspring (Shaw et al. 1997; Taylor et al. 2003; Samuelsson et al. 2008). Nonetheless, the observation that a HF diet from weaning to conception did not confer phenotypic differences in offspring distinguishable from those who were fed the HF diet through pregnancy and lactation alone is intriguing and underscores the importance of nutrition throughout the lifetime of a reproductively active individual.
Bone mineral density
We have demonstrated in the present study a significant relationship between body weight and bone mass, and fat mass and bone mass that is in agreement with data reported by others (Goulding & Taylor, 1998). As expected BMC and BMD values were significantly higher in males than in females. Obesity during childhood and adolescence is known to be associated with increased vertebral bone density and increased whole-body bone mass (Leonard et al. 2004).
Circulating leptin and insulin concentrations in adulthood
We have shown in the present study that maternal HF consumption resulted in a suppression of neonatal plasma insulin and leptin concentrations at postnatal day 2. As adults however, these offspring are hyperinsulinaemic and hyperleptinaemic and circulating levels were increased even further following a postnatal HF diet. A maternal HF diet has been shown to result in hypothalamic leptin resistance in offspring leading to increased weight gain in adulthood (Ferezou-Viala et al. 2007). Work by Khan et al. (2003, 2004, 2005) using diets high in lard have shown that rats fed a high saturated fat diet for 10 days prior to conception through to weaning leads to obesity and hyperinsulinaemia in male and female offspring. In this case females but not males also became hypertensive (Taylor et al. 2005). A neonatal leptin surge occurs in rodents at approximately 10 days of life and has been hypothesized to be associated with the establishment of neuronal projections in the arcuate nucleus of the hypothalamus rather than fat accumulation and body weight gain (Ahima et al. 1998; Bouret et al. 2004). Although we did not measure leptin repeatedly throughout the first 10 days of life, it is possible that reduction in leptin production or sensitivity in early neonatal life (postnatal day 2) may significantly alter the establishment of a neuronal appetite sensing pathway that has long-term repercussions on adult metabolic capabilities (Vickers et al. 2005; Ikenasio-Thorpe et al. 2007). Further investigation into hypothalamic regulation in these offspring is currently underway.
In summary, the present study has demonstrated that a lifetime of HF nutrition and HF consumption during pregnancy and lactation results in a significantly increased risk of obesity in offspring independent of postweaning diet with associated hyperinsulinaemia and hyperleptinaemia in both male and female offspring in adulthood. This postnatal phenotype bears a striking similarity to offspring born of mothers undernourished during pregnancy (Vickers et al. 2000), thus underscoring the hypothesis that a ‘U’ shaped relationship exists between maternal dietary intakes and offspring phenotypic adaptations. Further work is now required to examine whether common mechanisms underlie the phenotypic development induced by suboptimal levels of maternal nutrition at either end of the intake spectrum.
References
- Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism. 2007;56:1431–1438. doi: 10.1016/j.metabol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Catalano PM. Obesity and pregnancy – the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–3506. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- Cerf ME, Williams K, Nkomo XI, Muller CJ, Du Toit DF, Louw J, Wolfe-Coote SA. Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1122–R1128. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1056–R1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16:1651–1656. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental plasticity and human disease: research directions. J Intern Med. 2007;261:461–471. doi: 10.1111/j.1365-2796.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci U S A. 2007;104:12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63:456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Ladyman SR, Augustine RA. Hormonal induction of leptin resistance during pregnancy. Physiol Behav. 2007;91:366–374. doi: 10.1016/j.physbeh.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Hausman DB, McCloskey HM, Martin RJ. Maternal dietary fat type influences the growth and fatty acid composition of newborn and weanling rats. J Nutr. 1991;121:1917–1923. doi: 10.1093/jn/121.12.1917. [DOI] [PubMed] [Google Scholar]
- Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol. 2007;193:31–37. doi: 10.1677/joe.1.07017. [DOI] [PubMed] [Google Scholar]
- Khan NA. Role of lipids and fatty acids in macrosomic offspring of diabetic pregnancy. Cell Biochem Biophys. 2007;48:79–88. doi: 10.1007/s12013-007-0019-4. [DOI] [PubMed] [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation. 2004;110:1097–1102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- May R. Prepregnancy weight, inappropriate gestational weight gain, and smoking: Relationships to birth weight. American Journal of Human Biology. 2007;19:305–310. doi: 10.1002/ajhb.20572. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. International J Obes (Lond) 2008 doi: 10.1038/ijo.2008.213. (in press) [DOI] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322e1–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutrition. 2008;28:411–423. doi: 10.1146/annurev.nutr.28.061807.155322. [DOI] [PubMed] [Google Scholar]
- Ozanne SE. Metabolic programming in animals. Br Med Bull. 2001;60:143–152. doi: 10.1093/bmb/60.1.143. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Dudenhausen JW. The diabetic pregnancy, macrosomia, and perinatal nutritional programming. Nestle Nutr Workshop Series Pediatr Program. 2008;61:91–102. doi: 10.1159/000113179. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- Rasmussen KM. Effects of under- and overnutrition on lactation in laboratory rats. J Nutr. 1998;128:390S–393S. doi: 10.1093/jn/128.2.390S. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Seeber RM, Smith JT, Waddell BJ. Plasma leptin-binding activity and hypothalamic leptin receptor expression during pregnancy and lactation in the rat. Biol Reprod. 2002;66:1762–1767. doi: 10.1095/biolreprod66.6.1762. [DOI] [PubMed] [Google Scholar]
- Seidell JC. Obesity, insulin resistance and diabetes – a worldwide epidemic. Br J Nutrition. 2000;83(Suppl. 1):S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R528–R538. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J Nutr. 1997;127:64–69. doi: 10.1093/jn/127.1.64. [DOI] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutrition. 2006;83:1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Khan IY, Lakasing L, Dekou V, O'Brien-Coker I, Mallet AI, Hanson MA, Poston L. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol. 2003;88:389–398. doi: 10.1113/eph8802495. [DOI] [PubMed] [Google Scholar]
- Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Krechowec SO, Breier BH. Is later obesity programmed in utero? Curr Drug Targets. 2007;8:923–934. doi: 10.2174/138945007781386857. [DOI] [PubMed] [Google Scholar]