Abstract
Secreted Frizzled-related protein-1 (sFRP1) associates with Wnt proteins and its loss can lead to activation of Wnt/β-catenin signalling. It is frequently downregulated in cancer, including prostate cancer, but its function in prostate cancer is unclear because it can increase proliferation of prostate epithelial cells. We investigated the function of sFRP1 in androgen-dependent prostate cancer and found that sFRP1 inhibited androgen receptor (AR) transcriptional activity. In addition, sFRP1 inhibited the proliferation of androgen-dependent LNCaP cells but not of an androgen-independent subline LNCaP-r, suggesting a role in androgen-dependent growth. The inhibition of AR by sFRP1 was unaffected by co-expression of Wnt3a, stabilised β-catenin or β-catenin shRNA, suggesting it does not involve Wnt/β-catenin signalling. Wnt5a also inhibited AR and expression of Wnt5a and sFRP1 together did not further inhibit AR, suggesting that Wnt5a and sFRP1 activate the same signal(s) to inhibit AR. However, sFRP1 inhibition of AR was unaffected by inhibitors of kinases involved in Wnt/Ca2+ and Wnt/planar cell polarity non-canonical Wnt signalling. Interestingly, the cysteine-rich domain of sFRP1 interacted with Frizzled receptors expressed in prostate cancer cells, suggesting that sFRP1/Frizzled complexes activate a signal that leads to repression of AR. Taken together, these observations highlight the function of β-catenin-independent Wnt signalling in the control of AR activity and provide one explanation for sFRP1 downregulation in prostate cancer.
Keywords: sFRP1, prostate cancer, Wnt signal, Frizzled, Wnt5a
Wnt signalling is important in a wide variety of biological processes and is frequently misregulated in cancer. Wnt ligands bind to Frizzled and low-density lipoprotein receptor-related protein 5/6 receptors, thereby activating the Wnt/β-catenin pathway, also known as the canonical pathway, and the non-canonical planar-cell polarity (PCP) (Boutros et al, 1998) and protein kinase C (PKC)/Ca2+ pathways (Slusarski et al, 1997). In the Wnt/β-catenin pathway, stimulation by Wnt ligand leads to β-catenin accumulation in the cytoplasm and translocation to the nucleus, where it associates with T-cell receptor/lymphoid enhancer factor-1 (TCF/LEF-1) family transcription factors (Behrens et al, 1996; Molenaar et al, 1996) and activates target genes such as c-Myc (He et al, 1998).
Cytoplasmic and/or nuclear β-catenin, which is often used as an indicator of activation of the Wnt/β-catenin pathway, is observed in up to 71% of advanced prostate tumour specimens (Chesire et al, 2002; de la Taille et al, 2003; Yardy et al, 2008). However, unlike colon cancer, where either inactivating mutations in APC or activating mutations in β-catenin are observed in most cases, mutations in intracellular components of the Wnt signalling pathway in prostate cancer are rare. For instance, 5% of prostate tumours harboured activating mutations in β-catenin (Voeller et al, 1998; Chesire et al, 2000), whereas changes in the coding region of Axin1 were recently identified in 6% of advanced prostate cancer (Yardy et al, 2008) and inactivating mutations in APC have not been found in prostate cancer patients (Suzuki et al, 1994; Watanabe et al, 1996).
Wnt signalling is also regulated by Wnt antagonists such as members of the secreted Frizzled-related protein (sFRP) family. Secreted Frizzled-related proteins are glycoproteins that possess two characteristic domains, the cysteine-rich domain (CRD) in the N terminus and the Netrin-like (NTR) domain in the C terminus (Hoang et al, 1996; Finch et al, 1997; Leyns et al, 1997). The CRDs of sFRPs share homology with Frizzled CRDs, and it is thought that the sFRP1 CRD is essential in antagonising Wnt signals by directly binding to Wnts, thereby preventing Wnt interaction with Frizzleds (Lin et al, 1997; Bafico et al, 1999; Dann et al, 2001; Bhat et al, 2007). Although the NTR domain of sFRP1 does not associate with Wnts, it is required for maximal Wnt inhibitory activity (Bhat et al, 2007). Secreted Frizzled-related proteins are involved in wide-ranging biological phenomena. The sFRP1 gene is inactivated in many human cancers either as a result of chromosomal deletions (Stoehr et al, 2004; Huang et al, 2007) or promoter hypermethylation (Suzuki et al, 2002; Takada et al, 2004; Lodygin et al, 2005; Lo et al, 2006; Shih et al, 2006; Veeck et al, 2006; Dahl et al, 2007; Huang et al, 2007; Nojima et al, 2007), and loss of sFRP1 expression contributes to a poor prognosis (Klopocki et al, 2004; Veeck et al, 2006).
The androgen receptor (AR) is a member of the nuclear receptor superfamily, and is a key regulator of prostate cancer cell proliferation and survival (Dehm and Tindall, 2007). The transcriptional activity of AR is regulated by interaction with various cofactors (reviewed in Chesire and Isaacs, 2003 and Cronauer et al, 2003), which include β-catenin (Truica et al, 2000; Chesire et al, 2002; Mulholland et al, 2002; Yang et al, 2002). Because both AR transcriptional activity and the cytoplasmic and/or nuclear levels of β-catenin (Chesire et al, 2002; de la Taille et al, 2003) are elevated in prostate cancer, crosstalk between AR and Wnt/β-catenin signalling pathways may contribute to prostate cancer progression.
We have investigated how the expression level of sFRP family members in prostate cancer cells affects AR signalling. We hypothesised that loss of sFRP leads to activation of canonical Wnt signalling and, as a result, increased AR transcriptional activity. Our results indicate that sFRP1 represses AR, but that the mechanism of repression is independent of Wnt/β-catenin signalling.
Materials and methods
Cells and reagents
Cell lines were from the American Type Culture Collection (Rockville, MD, USA), except for LNCaP-r cells that were obtained from El-Nasir Lalani (Imperial College London). Cells were grown as previously described (Mazor et al, 2004; Zhu et al, 2004; Kawano et al, 2006). In some experiments normal growth media were replaced with phenol red-free media (Invitrogen, Paisley, UK) containing charcoal-stripped serum (First Link UK, Birmingham, UK) and DHT (Sigma, St Louis, MO, USA). Anti-Myc monoclonal antibody (9E10) was purchased from Sigma. Recombinant sFRP1 was purchased from R&D Systems (Abingdon, UK).
Plasmids
Expression plasmids for Myc-tagged full-length human sFRP1, sFRP1-ΔCRD and sFRP1-Δ1 were generous gifts from Jeffrey S Rubin (NCI, Bethesda, MD, USA). Expression plasmids for HA-tagged wild-type and S37A mutant form of β-catenin were from Stephen Byers (Georgetown University, Washington, DC, USA). 16xSuperTOPFLASH was from Randall Moon (University of Washington, Seattle, WA, USA). For expression plasmids encoding extracellular domains of human Frizzled fused to human IgG1 heavy chain (pSMT2-Fz1-IgG, pSMT2-Fz3-IgG, pSMT2-Fz4-IgG and pSMT2-Fz6-IgG), cDNA was obtained by PCR using human Frizzled cDNAs (Origene Technologies, Rockville, MD, USA). Detailed methods including sequences of primers are available upon request. Other plasmids and reporters used have been described previously (Mazor et al, 2004; Zhu et al, 2004; Kawano et al, 2006).
Colony formation assays
LNCaP cells (3 × 105 cells per well) or LNCaP-r cells (2 × 105 cells per well) were plated in six-well plates and transfected with 2 μg of expression plasmids encoding sFRP1 derivatives or pcDNA3.1 as a negative control using FuGENE HD (Roche Diagnostics, Burgess Hill, UK). After 2 days (LNCaP), or on the following day (LNCaP-r), all transfected cells (LNCaP) cells or one-third of transfected cells (LNCaP-r) were re-plated in 100 mm tissue culture plates with 500 μg ml−1 G418 (Merck Chemicals, Nottingham, UK). After 2–3 weeks, colonies were visualised by crystal violet staining. Colonies more than 2 mm in diameter were counted and the results were plotted on graphs.
Transcription assays
All cells were transfected in triplicate in 24-well plates unless otherwise stated, and each well of the 24-well plate was transfected with 40 ng pDM-βGal as an internal control, 200 ng firefly luciferase gene driven by various promoter sequences and the expression plasmids as indicated. The total amount of DNA was brought to 400 ng using empty pcDNA3.1 (Invitrogen). To measure AR transcriptional activity, cells were incubated in hormone-depleted medium before transfection, DHT or vehicle was added 24 h after transfection and cells were grown for a further 24 h. Recombinant sFRP1 or kinase inhibitors were added to cells 24 h after transfection of reporters. Transfected cells were incubated with sFRP1 or kinase inhibitors for 25 h in hormone-depleted medium and for an additional 24 h in the presence of DHT. Measurement, normalisation and calculation of luciferase activity were carried out as previously described (Mazor et al, 2004).
Generation of LNCaP/TR-βi cells
LNCaP cells expressing the Tet repressor (LNCaP/TR2) (Kawano et al, 2006) were transfected with pTER-β-catenin (van de Wetering et al, 2003) and selected for resistance to both 6 μg ml−1 blasticidin and 300 μg ml−1 Zeocin™ (Invitrogen). Positive clones were identified by western blotting for β-catenin.
RT–PCR
RT–PCR was performed essentially as described previously (Zhu et al, 2004), but with modified PCR parameters (2 min at 94°C, 30 s at 94°C, 30 s at 55°C and 30 s at 72°C for 35 cycles). Primers for human Frizzleds were designed as reported by Sala et al (2000) except Frizzled-8 (forward, 5′-AAGACAGGCCAGATCGCTAA-3′; reverse, 5′-GCCATGCCGAAGAAGTAGAC-3′) and GAPDH (forward, 5′-TGTTGCCATCAATGACCCCTT-3′; reverse, 5′-CTCCACGACGTACTCAGCG-3′).
IP and western analyses
293 cells (2 × 105 cells per well) were plated in six-well plates and transfected with 50 ng sFRP1 derivatives and 950 ng Frizzled-IgG using FuGENE HD. Cells were harvested 48 h following transfection and extracted using lysis buffer (0.5% Triton X-100, 10 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA), supplemented with Complete, EDTA-free protease inhibitor cocktail tablets (Roche Diagnostics). Cell extracts were clarified by centrifugation for 15 min at 16 000 g at 4°C and processed for protein A/G precipitation on a rotating wheel in a cold room for 1 h. After five washes in lysis buffer, the beads were re-suspended in SDS sample buffer. For western blotting, extracts and IPs were separated by SDS–PAGE, transferred to nitrocellulose membranes and incubated in 5% Fraction V BSA in TBS-T (20 mM Tris (pH 7.5), 100 mM NaCl, 0.1% Tween 20) for 30 min. After probing with antibodies, antigens were visualised using chemiluminescence (ECL; GE Healthcare, Chalfont St Giles, UK).
Results
Inhibition of AR transcriptional activity by sFRP1
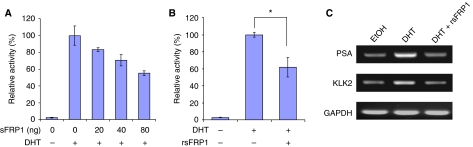
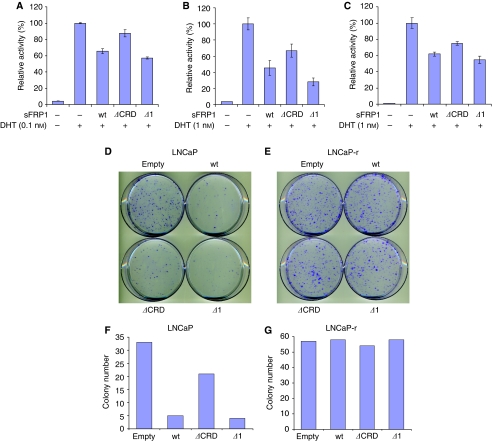
We conducted RT–PCR analysis of sFRP family gene expression using cell lines derived from normal prostate and prostate cancer and confirmed previous reports (Lodygin et al, 2005) that sFRP1 expression is reduced in prostate cancer cells (data not shown). To study whether sFRP1 could affect AR transcriptional activity, we expressed sFRP1 in 22Rv1 cells, an AR-positive prostate cancer cell line that does not express sFRP family members (data not shown), together with the androgen-responsive reporter MMTV-luciferase and a control reporter, and treated cells with the AR ligand dihydrotestosterone (DHT). As expected, DHT increased AR transcriptional activity (Figure 1A). Interestingly, transfection of sFRP1 expression plasmid repressed AR activity in a dose-dependent manner. To confirm this result in a more physiological context, we repeated these experiments using purified recombinant sFRP1 and found that this also repressed AR transcriptional activity (Figure 1B). In addition, RT–PCR analysis indicated that recombinant sFRP1 reduced expression of the endogenous AR target genes PSA and Kallikrein 2 (KLK2) (Figure 1C). These results indicate that sFRP1 has an inhibitory effect on AR transactivation in prostate cancer cells. To investigate which domains of sFRP1 are required for repression of AR, we used sFRP1 mutants lacking either the CRD (ΔCRD) or the C-terminal half containing the NTR domain (Δ1) (Uren et al, 2000). Δ1 and wild-type sFRP1 repressed AR to a similar extent, whereas ΔCRD had a significantly weaker effect (Figure 2A). Similar results were observed using LNCaP, another AR-expressing prostate cancer cell line (Figure 2B). These results indicate that the CRD is important for AR repression by sFRP1.
Figure 1.
sFRP1 inhibits AR signalling. (A) 22Rv1 cells were co-transfected with MMTV-luc, pDM-βGal and increasing amounts of sFRP1 expression plasmid as indicated. At 24 h after transfection, cells were treated with 0.1 nM of the agonist DHT or an equivalent volume of vehicle (ethanol) for 24 h. (B) 22Rv1 cells were co-transfected with MMTV-luc and pDM-βGal. At 24 h after transfection, cells were treated with or without 25 μg ml−1 recombinant sFRP1 for 5 h, and then further treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h. Data are average±standard deviation (s.d.) of a representative experiment carried out in triplicate (*P<0.005; Student's t-test). (C) RT–PCR for AR target genes in 22Rv1 cells treated with recombinant sFRP1 and DHT.
Figure 2.
(A–C) sFRP1 represses AR transcriptional activity and androgen-dependent proliferation of prostate cancer cells principally through the CRD. (A) 22Rv1 cells. (B) LNCaP cells. (C) LNCaP-r cells. All cells were transfected in triplicate in six-well plates, and each well of the six-well plate was transfected with 0.2 μg pDM-βGal as an internal control, 1 μg MMTV-luc and 0.4 μg expression plasmid of sFRP1 derivative. Empty pcDNA3.1 plasmid was used as a negative control. The total amount of DNA was brought to 2 μg using empty pcDNA3.1. At 24 h after transfection, cells were treated with indicated concentration of DHT or an equivalent volume of vehicle (ethanol) for 24 h. Secreted Frizzled-related protein-1 or its derivatives were expressed at comparables level in all cell lines tested (see Supplementary Information 1A–C). (D–G) sFRP1 reduces colony formation of LNCaP cells but not of the androgen-independent subline LNCaP-r. LNCaP cells (D and F) or LNCaP-r cells (E and G) were transfected with sFRP1 or its derivatives, and the number of colonies was determined as described in ‘Experimental procedures’.
sFRP1 reduces proliferation of LNCaP cells but not of the androgen-independent subline LNCaP-r
To determine the importance of the CRD in sFRP1 for androgen-dependent prostate cancer cell proliferation, LNCaP cells and a subline of LNCaP, LNCaP-r, which expresses AR but is hormone resistant (Pousette et al, 1997), were transfected with empty vector, sFRP1 or the sFRP1 deletion mutants and grown in medium containing G418. Compared to empty vector, full-length sFRP1 and Δ1 significantly inhibited colony formation of LNCaP cells (Figure 2D and F). ΔCRD also reduced colony formation in LNCaP cells, but not to the same extent as full-length sFRP1 and Δ1 (Figure 2D and F). The growth inhibitory effects of the mutants correlated with their effects on AR transcriptional activity in LNCaP cells (Figure 2B). Interestingly, none of the sFRP1 constructs inhibited colony formation of LNCaP-r cells (Figure 2E and G), despite the fact that AR transcriptional activity was similarly inhibited by sFRP1 in LNCaP and LNCaP-r cells (Figure 2B and C). These results indicate that sFRP1 specifically represses colony formation of androgen-dependent prostate cancer cells, and that this repression is mediated principally through the sFRP1 CRD.
Repression of AR by sFRP1 does not involve Wnt/β-catenin signalling
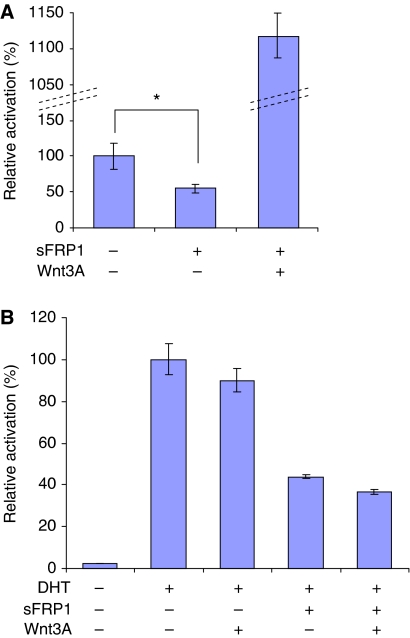
Recently, Wnt3a was shown to enhance AR activity at low concentrations of DHT (Verras et al, 2004). Because the sFRP1 CRD competes with Frizzled receptors for binding to Wnts (Lin et al, 1997; Bafico et al, 1999), we hypothesised that sFRP1 represses AR by antagonising autocrine Wnt signals in prostate cancer cells. If this is the case, then excess Wnt3a should rescue the inhibitory effect of sFRP1 on AR. We tested this possibility by conducting luciferase assays using a Wnt-responsive reporter (Figure 3). 22Rv1 cells were found to have low but measurable β-catenin/Tcf signalling activity (Figure 3A). Moreover, this activity was repressed by sFRP1 and thus most likely resulted from autocrine signals mediated by endogenous Wnts. The inhibitory effects of sFRP1 on β-catenin/Tcf activity were prevented by co-expression of Wnt3a (Figure 3A). We next examined the effect of Wnt3a on sFRP1 repression of AR activity and found that despite preventing sFRP1 repression of β-catenin/Tcf activity, Wnt3a did not affect sFRP1 repression of AR activity (Figure 3B). These results suggest that sFRP1 repression of AR is independent of its ability to bind to endogenous Wnt ligands that activate β-catenin/Tcf-dependent transcription.
Figure 3.
Repression of AR by sFRP1 does not involve Wnt/β-catenin signalling. 22Rv1 cells were transfected with luciferase reporter gene (A, 16XSuperTOPFLASH; B, MMTV-luc), pDM-βGal, 80 ng of sFRP1 plasmid and 80 ng of Wnt3A plasmid as indicated. At 24 h after transfection, cells were treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h (*P<0.00002; Student's t-test). Western blotting of cell lysates shows comparable expression of sFRP1 (Supplementary Information 2A). The levels of Wnt3A were too low to be detected by western blotting in these experiments.
Repression of AR by sFRP1 does not involve β-catenin
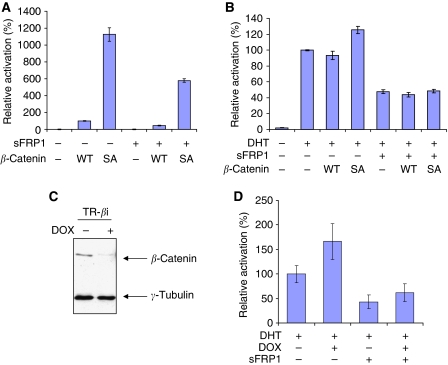
Secreted Frizzled-related protein-1 can also inhibit β-catenin/Tcf activity in colon cancer cells with mutations in APC that stabilise β-catenin (Suzuki et al, 2004). Moreover, exogenous high-level expression of β-catenin increases AR transcriptional activity independently of Tcf/LEF (Truica et al, 2000; Chesire et al, 2002; Yang et al, 2002), suggesting that sFRP1 might repress β-catenin/AR activity in a Wnt-independent manner. To investigate this, we compared the effects of sFRP1 on β-catenin/Tcf and AR activities in the presence of exogenous β-catenin (Figure 4). As expected, expression of wild-type β-catenin increased β-catenin/Tcf activity, and expression of a stable mutant form of β-catenin increased this further (Figure 4A). AR activity was not significantly affected by expression of wild-type β-catenin, but it was increased to a small but significant extent by the stable mutant form of β-catenin (Figure 4B). Interestingly, sFRP1 reduced the effects of wild-type β-catenin and the stable mutant form of β-catenin on β-catenin/Tcf activity to a similar extent (two-fold). It was clear that both wild-type and the stable mutant forms of β-catenin still significantly increased β-catenin/Tcf signalling (26-fold and 330-fold higher than endogenous activity respectively) in sFRP1-transfected cells (Figure 4A). Importantly, sFRP1 inhibited AR activity to a similar extent independently of the expression of wild-type and stabilised β-catenin (Figure 4B), suggesting that sFRP1 does not act through β-catenin to repress AR. To test this further, we established LNCaP cell lines expressing β-catenin shRNA in a doxycycline-inducible manner (LNCaP/TR-βi cells) (Figure 4C). As we previously reported (Mazor et al, 2004), depletion of endogenous β-catenin in 22Rv1 cells increased AR activity (Figure 4D), suggesting that the function of endogenous β-catenin differs from that of exogenously expressed β-catenin. Importantly, depletion of β-catenin did not affect sFRP1 repression of AR (Figure 4D), indicating that sFRP1 repression of AR does not require endogenous β-catenin. To conclude, the inhibitory effects of sFRP1 on AR do not appear to involve canonical Wnt signalling or β-catenin.
Figure 4.
Repression of AR by sFRP1 does not involve β-catenin. (A and B) 22Rv1 cells were transfected with luciferase reporter gene (A, 16xSuperTOPFLASH; B, MMTV-luc), pDM-βGal, 80 ng of sFRP1 plasmid and 80 ng of wild-type (WT) or S37A mutant (SA) β-catenin plasmid as indicated. At 24 h after transfection, cells were treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h. Western blotting of cell lysates shows comparable expression of sFRP1 and β-catenin (Supplementary Information 2B). (C) Establishment of an LNCaP subline that expresses a Dox-inducible β-catenin shRNA. Doxycycline (Dox, 1 μg ml−1) was added to LNCaP/TR-βi cells, and cytosolic extracts were analysed for the level of β-catenin. γ-Tubulin was used as a loading control. (D) Following Dox treatment, LNCaP/TR-βi cells were transfected with MMTV-luc, pDM-βGal and 80 ng of sFRP1 plasmid. At 24 h after transfection, cells were treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h.
Repression of AR by sFRP1 does not involve kinases implicated in non-canonical Wnt signalling
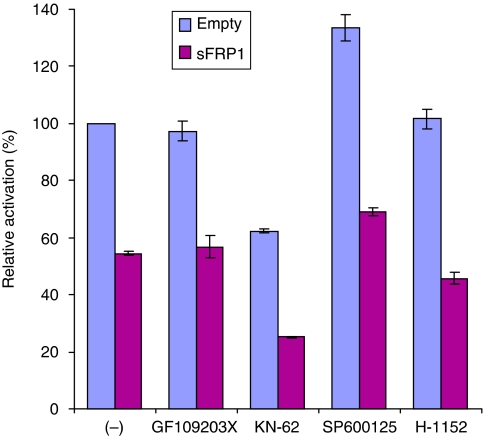
Non-canonical Wnt pathways, such as the PKC/Ca2+ and PCP pathways, involve several key kinases, including PKC, calmodulin kinase II (CaMKII), c-Jun N-terminal kinase (JNK) and ROCK, which all have the potential to regulate AR transcriptional activity (de Ruiter et al, 1995; Sato et al, 1997; Muller et al, 2000, 2002; Jeong et al, 2004; Backs et al, 2006). We reasoned that if sFRP1 repression of AR involves any of these kinases, then inhibitors that target these kinases would not further repress AR in the presence of sFRP1. To test this possibility, 22Rv1 cells were transfected with empty vector or sFRP1 and treated with a panel of kinase inhibitors (Figure 5A). In the absence of sFRP1, the PKC inhibitor GF109203X and the ROCK inhibitor H-1152 did not affect AR transcriptional activity, whereas the CaMKII inhibitor KN-62 repressed AR activity and the JNK inhibitor SP600125 increased AR activity (Figure 5B). Importantly, sFRP1 repressed AR regardless of the presence of any of these kinase inhibitors, with none of the kinase inhibitors significantly reducing the sFRP1 fold repression of AR compared to empty vector. These results suggest that serine/threonine kinases implicated in non-canonical Wnt signalling are not required for sFRP1 repression of AR.
Figure 5.
Repression of AR does not involve kinases implicated in non-canonical Wnt signalling. 22Rv1 cells were co-transfected with 80 ng of sFRP1 plasmid, MMTV-luc and pDM-βGal. At 24 h after transfection, cells were treated with GF109203X (2.5 μM), KN-62 (10 μM), SP600125 (10 μM), H-1152 (1 μM) or an equivalent volume of vehicle for 5 h, and then further treated with 0.1 nM DHT for 24 h.
Interaction of sFRP1 with Frizzled receptors expressed in prostate cancer cells
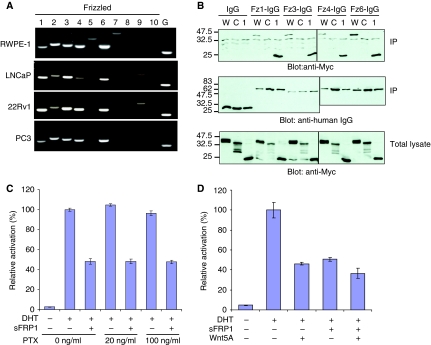
Recent reports indicate that sFRP1 can also directly interact with Frizzled receptors to trigger intracellular signals (Bafico et al, 1999; Rodriguez et al, 2005). Therefore, we hypothesised that sFRP1 represses AR by activating a Frizzled-mediated signal. To identify candidate Frizzled receptors that could be involved, RT–PCR was performed using specific primers targeting each Frizzled family member in a panel of normal prostate and prostate cancer cell lines. Frizzled-1, -2, -3, -4 and -6 were expressed in all prostate cell lines examined (Figure 6A). Because Frizzled-2 expression was relatively weak in the AR-expressing prostate cancer cell lines (22Rv1 and LNCaP), Frizzled-1, -3, -4 and -6 were chosen for the further analysis. To explore the possibility that sFRP1 interacts with these Frizzleds, the extracellular domains of these family members (containing the CRD) were fused to the Fc domain of human IgG1 and expressed in HEK 293 cells together with full-length or deletion mutants of sFRP1 (Figure 6B). Immunoprecipitation (IP) analysis showed that all Frizzled–Fc fusion proteins interacted with full-length sFRP1 and Δ1 but not with ΔCRD (Figure 6B). These results indicate that the sFRP1 CRD is required for interaction with Frizzled-1, -3, -4 and -6. Although further studies will be required, these results raise the interesting possibility that sFRP1 repression of AR is mediated by signals acting directly through Frizzleds. Secreted Frizzled-related protein-1 regulates axon growth of retinal ganglion cells through direct binding to Frizzled and activation of a trimeric G-protein pathway that can be blocked by pertussis toxin (PTX) (Rodriguez et al, 2005). We therefore tested the effect of repressing trimeric G-protein signals on sFRP1 inhibition of AR by treating 22Rv1 cells with PTX. As shown in Figure 6C, sFRP1 repression of AR was not affected by PTX, suggesting that sFRP1 repression of AR does not involve PTX-sensitive G-protein signals.
Figure 6.
sFRP1 associates with Frizzled receptors expressed in prostate cancer cells. (A) RT–PCR analysis of Frizzled expression in normal prostate and prostate cancer cell lines. G, GAPDH. (B) 293 cells were co-transfected with sFRP1 derivatives and Frizzled-IgG, then cell lysates were analysed by immunoprecipitation with protein A/G agarose and western blotting with anti-myc directly (top). To confirm immunoprecipitation of Frizzled-IgG, the immunoprecipitated sample was analysed by western blotting with anti-human IgG antibody (middle). To confirm expression of sFRP1 derivatives in cells, total lysate was analysed by western blotting with anti-myc antibody (bottom). W, sFRP1 wild type; C, sFRP1-ΔCRD; 1, sFRP1-Δ1. (C) 22Rv1 cells were co-transfected with MMTV-luc and pDM-βGal. At 24 h after transfection, cells were treated with the indicated concentration of PTX or an equivalent volume of vehicle for 5 h, and then further treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h. (D) 22Rv1 cells were co-transfected with 80 ng of sFRP1 plasmid, 80 ng of Wnt5a plasmid, MMTV-luc and pDM-βGal. At 24 h after transfection, cells were treated with 0.1 nM DHT or an equivalent volume of vehicle (ethanol) for 24 h. Western blotting of cell extracts shows comparable expression of sFRP1 and Wnt5a (Supplementary Information 2C).
These results suggest that the sFRP1/Frizzled interaction might repress AR through a novel signalling pathway. Although we have ruled out canonical and several of the non-canonical Wnt signals in the repression of AR by sFRP1, Wnt5a is thought to activate additional, as yet uncharacterised, signals (Mikels and Nusse, 2006). Therefore, we examined the effect of Wnt5a on AR activity in 22Rv1 cells. Wnt5a repressed AR, and expression of Wnt5a and sFRP1 together did not lead to further inhibition of AR (Figure 6D), suggesting that sFRP1 and Wnt5a activate the same signalling pathway(s) to repress AR.
Discussion
Secreted Frizzled-related protein-1 expression is downregulated in many cancers including prostate cancer (Ugolini et al, 2001; Suzuki et al, 2002; Caldwell et al, 2004; Klopocki et al, 2004; Stoehr et al, 2004; Takada et al, 2004; Lodygin et al, 2005; Lo et al, 2006; Shih et al, 2006, 2007; Veeck et al, 2006; Dahl et al, 2007; Huang et al, 2007; Nojima et al, 2007). Because sFRP1 is a Wnt antagonist and the Wnt/β-catenin/TCF axis is aberrantly activated in cancer, it is plausible that downregulation of sFRP1 contributes to abnormal activation of the β-catenin/TCF complex. This is indeed the case in some tumours, where restoration of sFRP1 expression inhibits both β-catenin/TCF activity and cancer cell growth (Suzuki et al, 2004; Nojima et al, 2007; Shih et al, 2007). However, although both downregulation of sFRP1 and accumulation of cytoplasmic β-catenin are frequently observed in prostate cancer (Chesire et al, 2002; de la Taille et al, 2003; Lodygin et al, 2005), β-catenin/TCF activity is much lower than in cancers such as colon cancer, in which β-catenin/TCF signalling is essential for tumour cell growth (Lodygin et al, 2005; YK and RMK, unpublished observations). Therefore, it is important to consider the possibility that loss of sFRP1 affects signalling pathways other than those mediated by β-catenin/TCF, and that these drive prostate cancer cell proliferation. In this paper, we have shown that sFRP1 represses AR-dependent transcription both in androgen-dependent LNCaP cells and in the androgen-independent derivative, LNCaP-r. Importantly, sFRP1 inhibited colony formation of LNCaP cells but not of LNCaP-r cells, thus linking the growth inhibitory effects of sFRP1 to androgen-dependent proliferation of prostate cancer cells.
Secreted Frizzled-related protein-1 also reduced the mRNA expression levels of the androgen-regulated genes PSA and KLK2 in 22Rv1 cells. These findings are consistent with a recent report by Joesting et al (2005) demonstrating that sFRP1 negatively regulates expression of androgen-regulated proteins by prostate luminal epithelial cells in vivo. Joesting et al also have shown evidence that proliferation of prostate epithelial cells is reduced in Sfrp1 null mice and increased in sFRP1 transgenic mice. These observations might appear to contradict our results. However, the function of AR in normal prostate epithelial cells in vivo is anti-proliferative (Wu et al, 2007), whereas AR has proliferative function in prostate cancer. Thus, the mouse phenotypes may, at least in part, reflect the function of sFRP1 in the regulation of AR transcriptional activity in the normal prostate.
We have found that the sFRP1 mutant comprising the CRD but not the NTR domain (Δ1) inhibited both AR activity and colony formation to the same extent as wild-type sFRP1, whereas the sFRP1 mutant comprising the NTR domain but not the CRD (ΔCRD) had a weaker effect in both assays (Figure 2). These results indicate that the CRD has an important function in the repression of AR by sFRP1. It is intriguing that ΔCRD retains some inhibitory activity. The NTR domain in this mutant has affinity for heparin, and so it is possible that it sequesters heparan-sulphate proteoglycans and inhibits serum growth factors such as FGF-2, which has been shown to regulate androgen-dependent AR activity and LNCaP cell growth (Kassen et al, 2000). However, in the context of full-length sFRP1, it is clear that the CRD has the predominant function in repressing AR.
Because sFRP1 is best known as a Wnt antagonist, it is plausible that it represses AR by sequestering endogenous Wnt ligands secreted by prostate cancer cells. Indeed, we have previously reported that prostate cancer cell lines express several Wnt family members (Zhu et al, 2004). Therefore, we tested whether Wnt3a, which directly binds to sFRP1 and has been reported to potentiate AR activity in LNCaP cells (Verras et al, 2004), could rescue sFRP1 inhibition of AR. In contrast to what was reported for LNCaP cells, Wnt3a did not significantly increase AR activity in 22Rv1 cells (Figure 3B), consistent with a previous report using this cell line (Cronauer et al, 2005). Moreover, although co-expression of Wnt3a rescued sFRP1 repression of β-catenin/Tcf activity in 22Rv1 cells (Figure 3A), it had no effect on sFRP1 repression of AR activity (Figure 3B). This suggests that repression of AR by sFRP1 does not involve sequestration of endogenous canonical Wnt signals that might be responsible for activating AR.
Similarly, co-expression of β-catenin enhanced β-catenin/Tcf activity in 22Rv1 cells (Figure 4A), but had no effect on sFRP1 repression of AR activity (Figure 4B). Despite having relatively low β-catenin/Tcf activity, prostate cancer cell lines contain significant amounts of cytoplasmic β-catenin. Therefore, it was important to determine whether sFRP1 repression of AR required endogenous β-catenin. Our experiments using shRNA to deplete β-catenin clearly showed that endogenous β-catenin is not required for sFRP1 repression of AR (Figure 4C and D).
Secreted Frizzled-related protein-1 can also interact with Wnt proteins that are involved in so-called Wnt non-canonical signalling pathways, including the Ca2+/PKC (Slusarski et al, 1997) and PCP pathways (Boutros et al, 1998). Activation of these pathways is thought to involve PKC, CaMKII (Slusarski et al, 1997), JNK (Boutros et al, 1998) and ROCK (Marlow et al, 2002). Several of these kinases can affect AR signalling: CaMKII phosphorylates and inactivates HDAC4 (Backs et al, 2006), which represses AR (Jeong et al, 2004), ROCK activates FHL2, a co-activator for AR (Muller et al, 2000, 2002) and JNK activates AP-1, which can repress AR (Sato et al, 1997). Consistent with some of these reports, both KN-62 (a CaMKII inhibitor) and SP600125 (a JNK inhibitor) affected AR activity in 22Rv1 cells (Figure 3B). However, none of the kinase inhibitors tested was able to mimic or rescue sFRP1 inhibition of AR. Activation of CaMKII and JNK was also monitored in 22Rv1 cells expressing sFRP1 or treated with recombinant sFRP1 by western analysis using phospho-specific antibodies. However, neither kinase was activated by sFRP1 in 22Rv1 cells (YK and RMK, unpublished observations), suggesting that sFRP1 repression of AR is not mediated by the kinases implicated in the Ca2+/PKC or PCP pathways.
Recent reports indicate that sFRP proteins can signal independently of Wnts (Bovolenta et al, 2008). Rodriguez et al (2005), for example, showed that sFRP1-induced axonal outgrowth growth is mediated by a direct interaction between sFRP1 and Frizzled-2. The effects of sFRP1 were mediated by the CRD and involved activation of heterotrimeric G proteins (Rodriguez et al, 2005). Although we found that the CRD of sFRP1 is able to interact with each of four frizzled family members that are highly expressed in AR-expressing prostate cancer cell lines, sFRP1 repression of AR was not rescued by PTX treatment, indicating that G proteins are not involved in this phenomenon.
It is intriguing that Wnt5a and sFRP1 inhibited AR to a similar extent. Moreover, co-transfection of sFRP1 and Wnt5a did not produce an additive effect on repression of AR. Wnt5a was recently reported to activate novel signalling pathways (Yamamoto et al, 2007; Fukuda et al, 2008). There are conflicting data on whether sFRP1 and Wnt5a directly interact (Dennis et al, 1999; Wawrzak et al, 2007), but it is plausible that sFRP1 and Wnt5a activate a common downstream pathway(s) that leads to AR inhibition. Future work will address whether signals downstream of Frizzleds affect AR function. One possibility is the involvement of Ror1/2 (Hikasa et al, 2002; Oishi et al, 2003; Fukuda et al, 2008), receptor tyrosine kinases that contain a CRD that binds to Wnt5a (Oishi et al, 2003) and to Frizzleds (Li et al, 2008). The signals downstream of Ror1/2 have not been characterised, but it would be interesting to investigate whether there is a molecular link between Ror1/2 and AR. Alternatively, sFRP1 may inhibit AR through a mechanism that involves the receptor activator of nuclear factor-κB ligand (RANKL). Secreted Frizzled-related protein-1 was reported to inhibit RANKL-dependent osteoclast formation (Hausler et al, 2004) and RANKL is found in prostate cancer cells, where it is thought to mediate the effects of prostate tumour cells on osteoclastogenesis in vivo (Zhang et al, 2001). A more recent report indicates that many cancer cells (including the prostate cancer line LNCaP) express RANK and respond to RANKL (Jones et al, 2006).
To summarise, we have shown that sFRP1 represses AR transcriptional activity and, as a result, inhibits proliferation of androgen-dependent prostate cancer cells and that the CRD is mainly responsible for both of these effects. We have addressed the possible mechanisms of action of sFRP1 and demonstrated that repression of AR by sFRP1 does not involve signals mediated by canonical Wnts, β-catenin or by kinases implicated in Wnt/Ca2+ and Wnt/PCP signalling. Taken together with our demonstration that sFRP1 can associate with Frizzleds expressed in prostate cancer cells, we propose that sFRP1/Frizzled complexes activate a signal that leads to repression of AR and that inactivation of sFRP1 leads to uncontrolled AR activation, which may be a crucial step in prostate cancer progression.
Acknowledgments
We thank Jeffrey S Rubin, Charlotte Bevan, El-Nasir Lalani, Randall Moon and Steven Byers for cells and reagents. We also thank our colleagues in the Prostate Cancer Research Group for daily support. We especially thank Maria Vivanco for critical reading of the paper. This work was supported by grants from the Joron Charitable Trust (YK, JW) and the Prostate Cancer Charity UK (RK). RK was also supported by the Department of Industry, Tourism and Trade of the Government of the Autonomous Community of the Basque Country (Etortek Research Programs 2005/2006), the Innovation Technology Department of Bizkaia County and the Ministry of Education and Science (SAF 2005-06122).
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN (2006) CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA (1999) Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 274: 16180–16187 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Bhat RA, Stauffer B, Komm BS, Bodine PV (2007) Structure–function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J Cell Biochem 102: 1519–1928 [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118 [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121: 737–746 [DOI] [PubMed] [Google Scholar]
- Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P, Chughtai S, Wallis Y, Matthews GM, Morton DG (2004) The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res 64: 883–888 [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Gage WR, Isaacs WB (2002) In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene 21: 2679–2694 [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB (2000) Detection and analysis of beta-catenin mutations in prostate cancer. Prostate 45: 323–334 [DOI] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB (2003) Beta-catenin signaling in prostate cancer: an early perspective. Endocr Relat Cancer 10: 537–560 [DOI] [PubMed] [Google Scholar]
- Cronauer MV, Schulz WA, Ackermann R, Burchardt M (2005) Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. Int J Oncol 26: 1033–1040 [DOI] [PubMed] [Google Scholar]
- Cronauer MV, Schulz WA, Burchardt T, Anastasiadis AG, de la Taille A, Ackermann R, Burchardt M (2003) The androgen receptor in hormone-refractory prostate cancer: relevance of different mechanisms of androgen receptor signaling (Review). Int J Oncol 23: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Dahl E, Wiesmann F, Woenckhaus M, Stoehr R, Wild PJ, Veeck J, Knuchel R, Klopocki E, Sauter G, Simon R, Wieland WF, Walter B, Denzinger S, Hartmann A, Hammerschmied CG (2007) Frequent loss of SFRP1 expression in multiple human solid tumours: association with aberrant promoter methylation in renal cell carcinoma. Oncogene 26: 5680–5691 [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ (2001) Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412: 86–90 [DOI] [PubMed] [Google Scholar]
- de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, Buttyan R, Chopin D (2003) Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res 9: 1801–1807 [PubMed] [Google Scholar]
- de Ruiter PE, Teuwen R, Trapman J, Dijkema R, Brinkmann AO (1995) Synergism between androgens and protein kinase-C on androgen-regulated gene expression. Mol Cell Endocrinol 110: R1–R6 [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ (2007) Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 21: 2855–2863 [DOI] [PubMed] [Google Scholar]
- Dennis S, Aikawa M, Szeto W, d’Amore PA, Papkoff J (1999) A secreted frizzled related protein, FrzA, selectively associates with Wnt-1 protein and regulates wnt-1 signaling. J Cell Sci 112(Part 21): 3815–3820 [DOI] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS (1997) Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA 94: 6770–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, Widhopf II GF, Rassenti LZ, Cantwell MJ, Prussak CE, Carson DA, Kipps TJ (2008) Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci USA 105: 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT (2004) Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res 19: 1873–1881 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M (2002) The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129: 5227–5239 [DOI] [PubMed] [Google Scholar]
- Hoang B, Moos Jr M, Vukicevic S, Luyten FP (1996) Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem 271: 26131–26137 [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang YL, Teng XM, Lin Y, Zheng DL, Yang P, Han ZG (2007) Down-regulation of SFRP1 as a putative tumor suppressor gene can contribute to human hepatocellular carcinoma. BMC Cancer 7: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong BC, Hong CY, Chattopadhyay S, Park JH, Gong EY, Kim HJ, Chun SY, Lee K (2004) Androgen receptor corepressor-19 kDa (ARR19), a leucine-rich protein that represses the transcriptional activity of androgen receptor through recruitment of histone deacetylase. Mol Endocrinol 18: 13–25 [DOI] [PubMed] [Google Scholar]
- Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC (2005) Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res 65: 10423–10430 [DOI] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440: 692–696 [DOI] [PubMed] [Google Scholar]
- Kassen AE, Sensibar JA, Sintich SM, Pruden SJ, Kozlowski JM, Lee C (2000) Autocrine effect of DHT on FGF signaling and cell proliferation in LNCaP cells: role of heparin/heparan-degrading enzymes. Prostate 44: 124–132 [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kitaoka M, Hamada Y, Walker MM, Waxman J, Kypta RM (2006) Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene 25: 6528–6537 [DOI] [PubMed] [Google Scholar]
- Klopocki E, Kristiansen G, Wild PJ, Klaman I, Castanos-Velez E, Singer G, Stohr R, Simon R, Sauter G, Leibiger H, Essers L, Weber B, Hermann K, Rosenthal A, Hartmann A, Dahl E (2004) Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol 25: 641–649 [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88: 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, Li J, Nishita M, Minami Y, Minoo P (2008) Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Wang S, Julius MA, Kitajewski J, Moos Jr M, Luyten FP (1997) The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA 94: 11196–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo PK, Mehrotra J, D’Costa A, Fackler MJ, Garrett-Mayer E, Argani P, Sukumar S (2006) Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol Ther 5: 281–286 [DOI] [PubMed] [Google Scholar]
- Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H (2005) Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res 65: 4218–4227 [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L (2002) Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol 12: 876–884 [DOI] [PubMed] [Google Scholar]
- Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM (2004) Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene 23: 7882–7892 [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC (2002) The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 277: 17933–17943 [DOI] [PubMed] [Google Scholar]
- Muller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schule R (2000) FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J 19: 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schule R (2002) The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J 21: 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima M, Suzuki H, Toyota M, Watanabe Y, Maruyama R, Sasaki S, Sasaki Y, Mita H, Nishikawa N, Yamaguchi K, Hirata K, Itoh F, Tokino T, Mori M, Imai K, Shinomura Y (2007) Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene 26: 4699–4713 [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8: 645–654 [DOI] [PubMed] [Google Scholar]
- Pousette A, Carlstrom K, Henriksson P, Grande M, Stege R (1997) Use of a hormone-sensitive (LNCaP) and a hormone-resistant (LNCaP-r) cell line in prostate cancer research. Prostate 31: 198–203 [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P (2005) SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci 8: 1301–1309 [DOI] [PubMed] [Google Scholar]
- Sala CF, Formenti E, Terstappen GC, Caricasole A (2000) Identification, gene structure, and expression of human frizzled-3 (FZD3). Biochem Biophys Res Commun 273: 27–34 [DOI] [PubMed] [Google Scholar]
- Sato N, Sadar MD, Bruchovsky N, Saatcioglu F, Rennie PS, Sato S, Lange PH, Gleave ME (1997) Androgenic induction of prostate-specific antigen gene is repressed by protein–protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem 272: 17485–17494 [DOI] [PubMed] [Google Scholar]
- Shih YL, Hsieh CB, Lai HC, Yan MD, Hsieh TY, Chao YC, Lin YW (2007) SFRP1 suppressed hepatoma cells growth through Wnt canonical signaling pathway. Int J Cancer 121: 1028–1035 [DOI] [PubMed] [Google Scholar]
- Shih YL, Shyu RY, Hsieh CB, Lai HC, Liu KY, Chu TY, Lin YW (2006) Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer 107: 579–590 [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT (1997) Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 182: 114–120 [DOI] [PubMed] [Google Scholar]
- Stoehr R, Wissmann C, Suzuki H, Knuechel R, Krieg RC, Klopocki E, Dahl E, Wild P, Blaszyk H, Sauter G, Simon R, Schmitt R, Zaak D, Hofstaedter F, Rosenthal A, Baylin SB, Pilarsky C, Hartmann A (2004) Deletions of chromosome 8p and loss of sFRP1 expression are progression markers of papillary bladder cancer. Lab Invest 84: 465–478 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Aida S, Akimoto S, Igarashi T, Yatani R, Shimazaki J (1994) State of adenomatous polyposis coli gene and ras oncogenes in Japanese prostate cancer. Jpn J Cancer Res 85: 847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB (2002) A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet 31: 141–149 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB (2004) Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36: 417–422 [DOI] [PubMed] [Google Scholar]
- Takada T, Yagi Y, Maekita T, Imura M, Nakagawa S, Tsao SW, Miyamoto K, Yoshino O, Yasugi T, Taketani Y, Ushijima T (2004) Methylation-associated silencing of the Wnt antagonist SFRP1 gene in human ovarian cancers. Cancer Sci 95: 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truica CI, Byers S, Gelmann EP (2000) Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res 60: 4709–4713 [PubMed] [Google Scholar]
- Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adelaide J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, Pebusque MJ (2001) WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene 20: 5810–5817 [DOI] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS (2000) Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 275: 4374–4382 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H (2003) Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeck J, Niederacher D, An H, Klopocki E, Wiesmann F, Betz B, Galm O, Camara O, Durst M, Kristiansen G, Huszka C, Knuchel R, Dahl E (2006) Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene 25: 3479–3488 [DOI] [PubMed] [Google Scholar]
- Verras M, Brown J, Li X, Nusse R, Sun Z (2004) Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res 64: 8860–8866 [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP (1998) Beta-catenin mutations in human prostate cancer. Cancer Res 58: 2520–2523 [PubMed] [Google Scholar]
- Watanabe M, Kakiuchi H, Kato H, Shiraishi T, Yatani R, Sugimura T, Nagao M (1996) APC gene mutations in human prostate cancer. Jpn J Clin Oncol 26: 77–81 [DOI] [PubMed] [Google Scholar]
- Wawrzak D, Metioui M, Willems E, Hendrickx M, de Genst E, Leyns L (2007) Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun 357: 1119–1123 [DOI] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C (2007) Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA 104: 12679–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y (2007) Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells 12: 1215–1223 [DOI] [PubMed] [Google Scholar]
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z (2002) Linking beta-catenin to androgen-signaling pathway. J Biol Chem 277: 11336–11344 [DOI] [PubMed] [Google Scholar]
- Yardy GW, Bicknell DC, Wilding JL, Bartlett S, Liu Y, Winney B, Turner GD, Brewster SF, Bodmer WF (2008) Mutations in the AXIN1 gene in advanced prostate cancer. Eur Urol (in press) [DOI] [PubMed]
- Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET (2001) Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest 107: 1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Mazor M, Kawano Y, Walker MM, Leung HY, Armstrong K, Waxman J, Kypta RM (2004) Analysis of Wnt gene expression in prostate cancer: mutual inhibition by WNT11 and the androgen receptor. Cancer Res 64: 7918–7926 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.