Abstract
Vascular endothelial growth factor (VEGF) inhibits differentiation and maturation of dendritic cells (DC), suggesting a potential immunosuppressive role for this proangiogenic factor. Bevacizumab, sorafenib and sunitinib target VEGF-mediated angiogenesis and are active against several types of cancer, but their effects on the immune system are poorly understood. In this study, VEGF and supernatants of renal carcinoma cell lines cultured under hypoxia were found to alter the differentiation of human monocytes to DC. Resulting DC showed impaired activity, as assessed by the alloreactive mixed T-lymphocyte reaction. Bevacizumab and sorafenib, but not sunitinib, reversed the inhibitory effects of VEGF, but not of those mediated by tumour supernatants. Dendritic cells matured under the influence of VEGF expressed less human leukocyte antigen-DR (HLA-DR) and CD86, and this effect was restored by bevacizumab and sorafenib. Finally, tumour-cell supernatants decreased interleukin-12 (IL-12) production by mature DC, and such inhibition was not restored by any of the tested drugs, delivered either as single agents or in combination. The deleterious effects of tumour-cell supernatants were mainly mediated by thermostable molecules distinct from VEGF. These results indicate that inhibition of the differentiation of monocytes to DC is a multifactorial effect, and that they support the development of combinations of angiogenesis inhibitors with immunological modulators.
Keywords: dendritic cells, renal cell carcinoma, VEGF, bevacizumab, sunitinib, sorafenib
Angiogenesis is a critical step in the development of solid tumours and their metastasis, and its blockade has become a major target of cancer therapy (Kerbel and Folkman, 2002). Renal cell carcinoma (RCC) was the first human tumour in which inhibition of angiogenesis, mediated by bevacizumab, a monoclonal antibody (mAb)-neutralising vascular endothelial growth factor (VEGF), showed clinical benefit (Yang et al, 2003a). Subsequently, RCC has remained a platform for the development of antiangiogenic compounds. Sunitinib, a tyrosine kinase inhibitor of VEFG receptor (VEGFR), c-kit and platelet-derived growth factor receptor, was superior to interferon-α (IFN-α) in a randomised phase III trial as first line treatment of RCC (Motzer et al, 2007), and sorafenib, another tyrosine kinase inhibitor that targets Ras-activated factor (RAF) and VEFGR, also showed clinical benefit in a phase III trial in which it was compared with placebo as second-line treatment of this disease (Escudier et al, 2007a). Bevacizumab, in combination with IFN, was also superior to single agent IFN in another phase III trial (Escudier et al, 2007b).
Even though the main mechanism of the action of drugs that inhibit VEGF-related pathways is antiangiogenesis, an effect of VEGF inhibitors on the immune system has been reported. In an earlier study, Gabrilovich et al (1996) obtained dendritic cells (DC) from umbilical cords and described an inhibition of their ability to induce T-lymphocyte proliferation, assessed by the mixed lymphocyte reaction (MLR), when they were matured in supernatant cell cultures containing VEGF. This effect was partially reverted by anti-VEGF antibodies, showing that VEGF was probably the cause of inhibition of DC-induced proliferation. The same authors showed that VEGF inhibited the development of DC and increased B lymphocytes and immature myeloid cells in animal models (Gabrilovich et al, 1998). The proposed mechanism of action was the interference with the activation of nuclear factor-κB transcription factors (Ohm et al, 1999). Using the same model, this group also showed that the addition of anti-VEGF antibodies increased the number and activity of DC in mice implanted with subcutaneous tumours (D459 and MethA sarcoma), although without any therapeutic effect on tumour growth (Gabrilovich et al, 1999). Nevertheless, a combined treatment with anti-VEGF antibodies and DC pulsed, with p53 peptides that contained specific mutations of the implanted tumours, produced a sustained antitumour effect, indicating potential synergism between antiangiogenic drugs and DC-based immunotherapy treatments for cancer (Gabrilovich et al, 1999). Other groups have suggested that angiogenic factors at the tumour microenvironment differentiate DC that display features of vascular cells and are immunosuppressive (Conejo-Garcia et al, 2004). In addition, VEGF has been described to mediate immunosuppression (Ohm et al, 2003), which depends on the inhibition of differentiation and migration of thymic lymphocyte progenitors from the bone marrow and subsequent reduction of the numbers of CD4+ and CD8+ thymocytes, which can be reverted when exposure to VEGF ceases. Cancer therapeutic vaccines formulated with DC artificially presenting tumour antigens are being extensively tested in clinical trials in several tumour types (Vulink et al, 2008), including RCC (Dannull et al, 2005). The possibility to overcoming potential interactions of VEGF on the immune system through clinically available angiogenesis inhibitors led us to evaluate the influence of such drugs on DC differentiation from monocytes. A recent report has documented the inhibitory effects of sorafenib, but not of sunitinib, on DC maturation (Hipp et al, 2008). Maturation is the gene expression programme that renders DC capable of mediating T-cell expansion and activation (Steinman, 2008). Maturation is triggered by microbial biomolecules (such as, lipopolysaccharide (LPS) or bacterial DNA), proinflammatory cytokines and ongoing T-cell responses. In spite of the reports on the effects of VEGF on DC, little is known about the effects of these compounds on the differentiation of DC from myeloid precursors. In this study, the effects of soluble VEGF and of VEGF-containing supernatants from RCC cells were assessed on monocytes to DC differentiation cultures. The influence of bevacizumab, sorafenib and sunitinib on such cultures was defined.
Materials and methods
DC generation and maturation
Dendritic cells were generated from filter buffy coats (FBC)-derived monocytes donated by healthy human donors (Meyer et al, 2005). The protocol for obtention of FBC was approved by the Ethics Committee of our institution and was carried out on donors who gave informed consent. Isolated mononuclear cells were subjected to positive selection using anti-CD14-conjugated paramagnetic beads and purified using the AutoMACS system according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified monocytes were cultured for 7 days in AIM-V serum-free media (Gibco-BRL, Gaithersburg, MD, USA), supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) (1000 U ml−1; Leukine, Berlex, Richmond, CA, USA) and interleukin-4 (IL-4, 500 U ml−1; R&D Systems, Minneapolis, MN, USA). Cytokines were added every 2 days. Dendritic cells were matured with clinical-grade tumour necrosis factor-α (TNF-α; 50 ng ml−1; Boehringer Ingelheim, Ingelheim, Germany), IFN-α (1,000 IU ml−1; Schering-Plough, Kenilworth, NJ, USA) and poly I:C (20 μg ml−1; Ampligen, Bioclones, Tokai, South Africa) for 48 h.
Maturation was confirmed by assessing increases in the immunofluorescence of CD80, CD83, CD86 and human leukocyte antigen-DR (HLA-DR), as well as variations in CD1a. Flow cytometric analysis for immature and mature DC was performed at days 7 and 9, using a FACSCalibur Flow Cytometer (Becton Dickinson, San Diego, CA, USA). Purified anti-VEGFR-1 and VEGFR-2 mAbs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and purified anti-VEGFR-3 mAbs from R&D Systems. Indirect immunofluorescence was performed with anti-mouse IgG1-FITC (Dako, Glostrup, Denmark). Cell viability was confirmed by the trypan blue exclusion test. Recombinant human VEGF 165 (25 ng ml−1, R&D Systems), bevacizumab (rhumAb-VEGF, Avastín, 1 μg ml−1, Roche, Basel, Switzerland), sorafenib (Nexavar, 10 ng ml−1, Bayer, Berlin, Germany) and sunitinib (Sutent, 10 ng ml−1, Pfizer, New York, NY, USA) were used during DC differentiation. For experiments with supernatant of RCC line cultures, we added 1 : 5 (v/v) ratio of the RCC-10 supernatant to the culture medium of DC.
Cell culture and reagents
Parental von Hippel–Lindau (VHL)-negative RCC-10 cells, originally cultured from a clear cell renal adenocarcinoma case (Krieg et al, 2000; Cuevas et al, 2003), and a clone derived by stable transfection of the VHL gene (VHL+53), were kindly provided by Dr Luis del Peso (CSIC-UAM, Madrid, Spain). The cells were maintained in an RPMI 1640 medium with GLUTAMAX-I (Invitrogen, Carlsbad, CA, USA). The culture medium was supplemented with 100 units ml−1 penicillin, 100 μg ml−1 streptomycin and 10% foetal bovine serum. To perform hypoxic experiments, hypoxia was induced by exposing cell cultures to 1% oxygen for 24–48 h, using a hypoxic workstation (Cuevas et al, 2003).
MLR
Dendritic cells were cultured in 96-well plates in an AIM-V medium. A total of 2 × 105 lymphocytes from a distinct donor were added on day 9 at different Tcell/DC ratios (80 : 1, 40 : 1 and 20 : 1). After 3 days, the [methyl-3H]thymidine uptake was determined by the addition of 1 μCi of [methyl-3H]thymidine (25 Ci mmol−1; Amersham, Uppsala, Sweden) for 16–20 h. At the end of this labelling time, the [methyl-3H]thymidine uptake was determined by transferring cells to 96-well filter microplates (Unifilter-96 GF/C, PerkinElmer, Boston, MA, USA) and adding 25 μl of liquid scintillation (Microscint O, PerkinElmer) to measure radioactivity. Technical controls were from phytohemagglutinin (PHA)-stimulated human lymphocyte populations from a single individual.
Cytokine measurement
Renal cell carcinoma culture supernatants (106 cells/ml) were assayed for VEGF production at 24 and 48 h with an enzyme-linked immunoabsorbent assay (ELISA) kit (R&D Systems). Interleukin-6, IL-8, IL-10, TNF-α and IL-12 were simultaneously analysed by microparticle-based flow cytometry (Cytometric Bead Array) in supernatant samples of DC cultures at baseline and on day 2, according to the manufacturer's instructions (BD Bioscience, San Jose, CA, USA).
Indoleamine 2,3-Dioxygenase (IDO) activity measurement
Indoleamine 2,3-Dioxygenase (IDO) activity in DC culture supernatants was measured by high-performance liquid chromatography (HPLC). The samples were deproteinised by mixing 100 μl supernatant and 100 μl of 30% (w/v) trichloroacetic acid. After centrifugation (4 min at 10 000 g), 25 μl of the supernatant was added to a vial containing Na2HPO4 buffer of 125 μl (HPLC: Hewlett-Packard Series 1100; Agilent Technologies Inc., Santa Clara, CA, USA) using a 5 μMcolumn C18 Symmetry Shield (Waters Corporation, Milford, MA, USA). The liquid chromatography parameters were injection volume: 100 μl; flow: 0.8 ml min−1; stop time: 15 min; maximum pressure: 350 bar. The mobile phase was 15 mM sodium acetate (pH 4) with 27 ml l−1 acetonitrile. The IDO activity is proportional to the [kynurenine]/[tryptophan] ratio. Kynurenine concentration was measured at 360 nm and tryptophan at 285 nm, with a spectrophotometer coupled to HPLC. A standardised curve to measure kynurenine and tryptophan concentrations was plotted and assessed.
Arginase activity assay
Cell lysates were tested for arginase activity by measuring the production of urea derived from L-arg. A volume of 100 μl of cell lysate was treated with Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA, USA). The resulting lysate was added to 100 μl of an activation solution (Tris-HCl 25 mM, pH 7.4, MnCl2 5 mM). The mixture was heated for 10 min at 56°C, mixed with 100 μl of 0.5 M arginine and incubated for 30 min at 37°C. The reaction was interrupted with 200 μl of an acid solution (H2SO4/H3PO4/H2O; 1 : 3: 7 (v/v/v)) and the samples were boiled for 45 min with 20 μl of 9% (v/v) α-isopropiophenone in absolute ethanol. The enzymatic activity was assessed by colorimetric reaction in a spectrophotometer at 540 nm using a standard curve.
Fractions of RCC by dialysis and filtration
Renal cell carcinoma supernatant was fractionated to determine the molecular weight of the inhibitory molecules using centrifugal filters (Microcon, Millipore, Billerica, MA, USA) of 10, 30, 50 and 100 kDa. The collected fractions were tested on monocytes differentiating to DC by incubation with IL-4 and GM-CSF for 7 days. The only fraction that did not produce the inhibition of full differentiation of DC from monocytes was below 10 kDa. To know the more exact the molecular weight, the RCC supernatant was included in a 3.5 kDa dialysis cassette (Slide-A-Lycer Dialysis Cassette; 3500 MWCO; Pierce, Rockford, IL, USA) for 2 h at room temperature and overnight at 4°C under continuous stirring. Thereafter, the supernatant with moieties of molecular weight over 3.5 kDa was fractionated using the same process. To assess thermostability of the putative inhibitory factor, RCC supernatants were boiled at 100°C for 5 min.
Results
RCC culture supernatants contain abundant VEGF that is increased by hypoxia
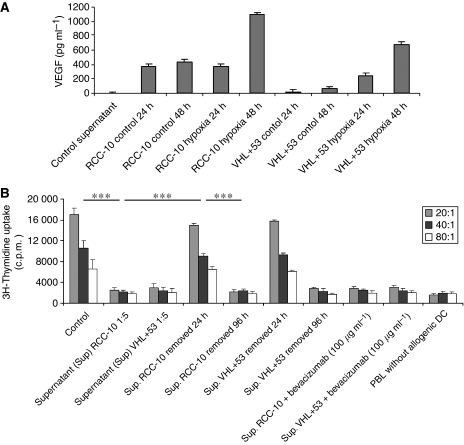
Vascular endothelial growth factor levels were measured in the culture supernatant from RCC-10 and VHL+53 tumour cell lines under normal and hypoxic (1% O2) conditions (Figure 1A). Renal cell carcinoma-10 lacks VHL expression, whereas VHL+53 is a transfected revertant for this gene that controls the hypoxia response. Exposure to hypoxia for 48 h stimulated VEGF secretion in both cell lines. Vascular endothelial growth factor production under hypoxia was higher by RCC-10 than by VHL+53, despite the absence of the VHL expression. The supernatant of RCC-10 under 48 h hypoxia was chosen for subsequent experimentation.
Figure 1.
Tissue culture supernatants from RCC cells contain VEGF and suppress differentiation of MLR-stimulating DC from monocytes. (A) Vascular endothelial growth factor concentrations measured by ELISA in the 24 and 48 h conditioned media from ∼80% confluent cultures of the indicated cell lines. Renal cell carcinoma-10 is a renal cell carcinoma with VHL deficiency. VHL+53 is a stable transfectant recovering the VHL expression. Cultures were carried out in normoxia (21% O2) or hypoxia chambers (1% O2) as indicated. HT-29 human colon carcinoma cell supernatants were used as control supernatant. (B) Lymphocyte proliferation at the indicated DC to allogenic PBL ratios, as induced by DC differentiated for 7 days from monocytes in the presence of GM-CSF and IL-4 with or without 1 : 5 (v/v) of the indicated conditioned media. After differentiation, DC were matured for 48 h with TNF-α, IFN-α and poly I:C. Conditioned media were removed when indicated by three washes. Anti-VEGF mAb (bevacizumab) was added at the indicated concentrations for the duration of the differentiation culture. Mature DC differentiated without additives were used as positive control. Data represent mean±s.d. from three experiments. ***P<0.001, statistical analyses were performed by the Kruskal–Wallis statistical test.
Human DC differentiated in the presence of VEGF or RCC supernatants show impaired ability to induce T-cell proliferation, as assessed by MLR
Dendritic cells were differentiated from CD14+ monocytes for 7 days by GM-CSF and IL-4 in the presence of either supernatants from RCC cultured under hypoxic conditions (Figure 1B) or from VEGF (Figure 2A), leading to a strong inhibition of their ability to induce allogenic T-cell proliferation, as determined by the primary MLR. This inhibition did not take place if tumour-cell supernatants were removed at 24 h, but remained if supernatants were washed after 96 h of culture (Figure 1B). The supernatants from other cell lines in Figure 1 showed similar effects (data not shown).
Figure 2.
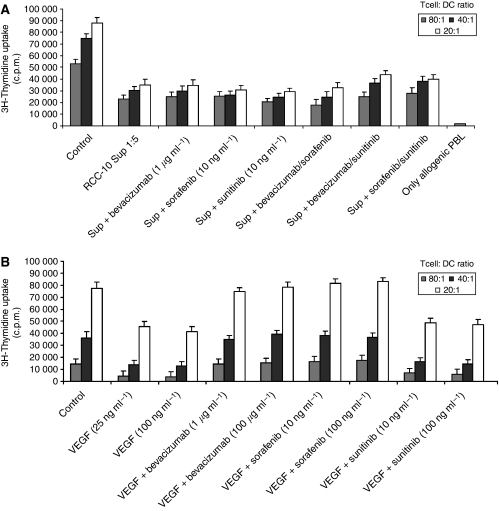
Vascular endothelial growth factor and RCC supernatants during monocyte differentiation to DC inhibit MLR-stimulating activity of resulting DC. Vascular endothelial growth factor-mediated inhibition is reversible by bevacizumab and sorafenib. (A) CD14+ monocyte cultures as in Figure 1 were set up in the presence of VEGF and the indicated VEGF inhibitors. In all the experiments, TNF-α, IFN-α and poly I:C were added to induce DC maturation for 48 h, including the control culture. (B) Anti-VEGF agents in different combinations are tested at the indicated concentrations to reverse the inhibition in the MLR from mature DC that had been differentiated in the presence of RCC supernatants. Mature DC without VEGF or RCC supernatants were used as positive controls. Microcultures of allogenic PBL without DC are plotted as negative controls. Results in panels a and b represent the mean±s.d. from four different experiments.
Bevacizumab and sorafenib reverse the effects induced by VEGF on DC activity. Neither of the drugs, as single agents or in combination, reversed the inhibitory effects of RCC culture supernatants
The addition of bevacizumab or sorafenib restored the MLR of DC differentiated in the presence of VEGF to baseline levels, whereas sunitinib did not (Figure 2A). As the activity of indoleamine 2,3-dioxygenase (Munn et al, 2002) or arginase (Ochoa et al, 2007) can decrease the ability of DC to stimulate T cells, we tested the activity of both enzymes in DC differentiated under the effects of VEGF, without finding evidence of increased activity even in the presence of pharmacological antagonists (Supplementary Figure 1).
The decrease in MLR stimulating activity caused by RCC culture supernatants could not be restored by bevacizumab, sorafenib or sunitinib, delivered either as single agent or in combination (Figures 1B and 2B). Therefore, it can be concluded that VEGF is not the only or main mediator of the deleterious effect played by RCC supernatants on DC differentiation from monocytes.
VEGF and RCC supernatants induce changes in the surface phenotype of DC
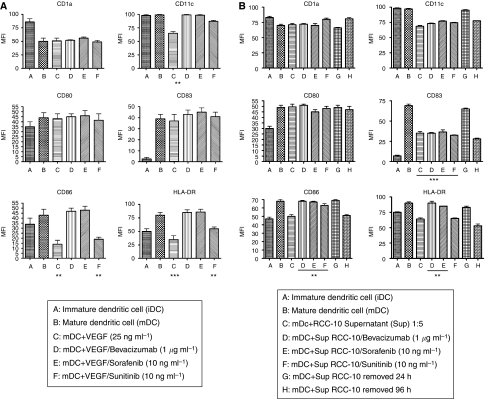
We determined the phenotype of DC differentiated with and without recombinant VEGF or RCC supernatants. Dendritic cells were maturated with a cocktail containing TNF-α, IFN-α and poly I:C (all manufactured under good manufacturing practice conditions). We assessed the surface expression of CD1a, CD11c, CD80, CD83, CD86, HLA-DR and CD14 using flow cytometry analyses (Figures 3A and B). The most relevant effects induced by VEGF on mature DC were marked decreases in the intensity of CD11c, CD86 and HLA-DR. These effects were completely reversed by the addition of bevacizumab and sorafenib. Sunitinib also restored the normal expression of CD11c, but not of CD86 and HLA-DR. Supernatants from RCC decreased the expression intensity of CD11c, CD83, CD86 and HLA-DR to some extent . In these cases, bevacizumab and sorafenib restored CD86 and HLA-DR but not CD83. Renal cell carcinoma supernatants, but not VEGF, led to DC cultures in which cells were more adherent and presented spread morphology, which was more reminiscent of macrophages under phase contrast-microscopy (Supplementary Figure 2).
Figure 3.
Vascular endothelial growth factor and RCC supernatants added to DC differentiation cultures inhibit maturation-induced surface markers on DC: effects of bevacizumab, sorafenib and sunitinib. Dendritic cells obtained from monocytes in 7-day cultures with GM-CSF and IL-4 were analysed by surface immunostaining and flow cytometry for the indicated leukocyte differentiation antigens before and after maturation in the presence of TNF-α, IFN-α and poly I:C. As indicated, recombinant VEGF (A) or RCC supernatants (B) were added during differentiation. When indicated, bevacizumab, sorafenib or sunitinib was also added during the differentiation cultures. Values are expressed as mean±s.e.m. of four different experiments. **P<0.01,***P<0.001, statistical analyses were performed by the Kruskal–Wallis statistical test. In all cultures set up with CD14+ cells, the expression of CD14 was lost, so at the end of the cultures <1% cells were CD14+.
It is of note that DC differentiated with VEGF did not repress the MLR stimulating activity when added to third-party DC/T cell cultures, thus revealing the fact that VEGF yields less-active DC rather than suppressive DC (Supplementary Figure 3).
Addition of VEGF or RCC supernatants during DC maturation did not alter the MLR-promoting activity of DC
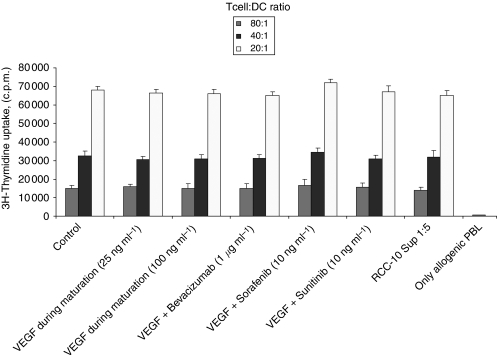
In contrast to monocyte to DC differentiation cultures, neither VEGF nor RCC supernatants altered the MLR-stimulating activity of DC if they were added to the culture during the 48 h of maturation as induced by the combined effects of TNF-α, IFN-α and poly I:C (Figure 4). This indicates that once differentiated, DC are less sensitive to the effects of VEGF or RCC supernatants with regard to activation/maturation, at least when triggered by these powerful maturation-inducing agents.
Figure 4.
Vascular endothelial growth factor and RCC supernatants, when added during maturation, cannot inhibit the MLR-stimulating activity of already differentiated DC. Vascular endothelial growth factor and RCC supernatants were added to differentiated DC during the 48 h maturation culture with TNF-α, IFN-α and poly I:C. When indicated, VEGF-inhibiting drugs were also added. The mitogenic activity on allogenic T cells of DC from the different conditions was monitored. Mature DC without further additives were used as positive control.
RCC culture supernatants, but not VEGF, decrease the production of IL-12 by mature DC
We determined concentrations of IL-12, IL-10, IL-6 and IL-8 in cultures of immature and mature DC, using microparticle-based flow cytometry assays. It is interesting that the presence or absence of VEGF during differentiation did not change the production of IL-6, IL-8, IL-10 and IL-12. It is surprising that the addition of sunitinib during DC differentiation dramatically reduced IL-12 and, to a lesser extent, IL-10 concentrations (Figure 5A and Supplementary Figure 4, showing dose dependency). Such effects did not occur when DC differentiation cultures were exposed to either bevacizumab or sorafenib, suggesting a mechanism of action that is dependent not on VEGF-R but rather on an alternative pathway (Figure 5A).
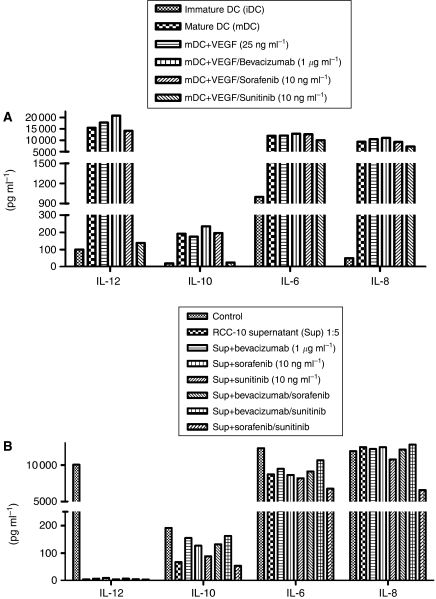
Figure 5.
Dendritic cell differentiation in the presence of RCC supernatants or sunitinib shows less IL-12 production. Cytokines were measured by CBA assays in the supernatant of the indicated DC cultures treated for the length of their differentiation from monocytes as indicated. All the experiments were performed with mature DC except when indicated as immature DC. (A) The effect of VEGF and drug inhibitors is shown, whereas the effect of RCC supernatants, along with bevacizumab, sorafenib, sunitinib or its combinations, at the indicated concentrations is shown (B).
We also measured the same cytokines on DC from cultures either conditioned or not with (1 : 5 (v/v)) hypoxic renal carcinoma cell-culture supernatant. The supernatant from the cell lines inhibited IL-12 production almost completely, and such inhibition was maintained in spite of the addition of antiangiogenic drugs. The supernatant did not alter the levels of the other assessed cytokines (Figure 5B), further indicating the functional viability of the cultures and suggesting immunomodulatory effects.
The effects of RCC culture supernatants on DC activity are mainly mediated by small thermostable molecule/s and cannot be reversed by VEGF pharmacological antagonism
As we could not revert the inhibition of MLR activity caused by RCC culture supernatants with either of the antiangiogenic compounds, we fractionated the RCC-10 supernatant to determine the molecules that could cause this effect.
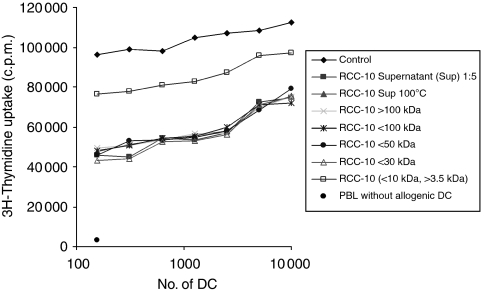
Filtration fractions showed that small molecule/s of <10 kDa suppressed the MLR activity of DC (Figure 6). Further fractionation by dialysis pre-excluding molecules <3.5 kDa showed that the activity was partially recovered when molecules >3.5 kDa were excluded. Accordingly, most of the inhibitory activity can be traced to the 3.5–10 kDa range. Moreover, such inhibitory activity persisted after heating the supernatant for 5 min at 100°C (Figure 6).
Figure 6.
The inhibitory activity of RCC-10 supernatants on the differentiation of MLR-stimulating mature DC is mainly mediated by thermostable molecules in the range of 3.5–10 kDa. RCC-10 was used as crude or fractionated by filtration and/or dialysis in molecules of the indicated molecular weight ranges. The inhibitory activity was present after treating RCC-10 supernatants for 5 min at 100°C. Mature DC without additives under identical conditions were used as positive controls for MLR activity, and microcultures of allogenic lymphocytes without DC are plotted as negative controls. The experiment shown is representative of the three similarly performed.
These results conclude that small soluble molecules distinct from VEGF (described as a moiety in the 35–45 kDa range) are the main mediators of decreased T-cell-stimulating activity in DC differentiated from monocytes in the presence of RCC culture supernatants.
Discussion
The treatment of metastatic kidney cancer has rapidly evolved during the last few years, and a large number of targeted molecules have either already shown efficacy in phase III trials or are in advanced stage of clinical development, replacing former immunotherapeutic approaches (Schrader and Hofmann, 2008). Nevertheless, immunotherapy may retain a role in the treatment of this disease. It must be remembered that the only treatment that can induce cure in some patients with metastatic renal cell cancer is high-dose intravenous IL-2 (Yang et al, 2003b; McDermott et al, 2005). Moreover, immunotherapy with vaccines is the only treatment that has shown clinical efficacy as an adjuvant treatment of resected RCC (Jocham et al, 2004). Finally, a large number of treatments based on the modulation of immunotherapy are being developed (Melero et al, 2007) and some of them are being tested in patients with metastatic RCC, suggesting that, in the near future, combinations of targeted agents with new immunotherapeutic approaches might become a reality (Coppin et al, 2008). In addition, as mentioned earlier, VEGF interferes with the development and function of DC (Gabrilovich et al, 1996, 1998), and such an effect can be blocked by anti-VEGF antibodies. Therefore, the study of potential interactions between targeted agents currently used in the treatment of RCC and the immune system is of interest.
Our data confirm that VEGF inhibits the functional differentiation of DC, and impairs their ability to induce allogenic T-cell proliferation as shown by the decrease in the allogenic MLR assay. Such an inhibition was not dependent on indoleamine 2,3-dioxygenase or on arginase activity factors, which have been described as immunosuppressive in DC and other myeloid cells (Munn et al, 2002; Ochoa et al, 2007). The addition of either sorafenib or bevacizumab reverted the effects of VEGF on DC. Sunitinib did not restore the ability of DC to induce a normal MLR, despite the fact that the concentrations used were in the range as those observed in cancer patients (31.8–65.9 ng ml−1) (Faivre et al, 2006). The immune phenomena of these kinds in RCC patients might be crucial.
To mimic in vivo conditions, we assessed the effects of differentiating DC in the presence of VEGF-containing supernatants from RCC cell lines cultured under hypoxic conditions. The supernatants induced a strong inhibition of DC differentiated in such a manner that it was not reverted by the addition of any of the anti-VEGF tested drugs. Moreover, combinations of the drugs did not have any restoring effects on the MLR decrease induced by the supernatants. Fractionation of those supernatants showed that small molecule/s >3.5 kDa and <10 kDa suppressed the MLR activity of DC, and that such molecules were thermostable. For this reason, metabolic products, such as tryptophan metabolites, can also be excluded as being responsible for the suppression. These results indicate that tumour cells exert their inhibitory function on differentiating DC through soluble factors distinct from VEGF, although VEGF could also be involved to a lesser extent.
Changes in DC differentiated in the presence of recombinant VEGF or RCC supernatants included marked decreases in CD11c, CD86 and HLA-DR expressions, which were completely reverted by bevacizumab and sorafenib, whereas sunitinib only restored normal expression of CD11c. The events in the transition from monocyte to DC are important not only because they represent a popular method of generating DC for immunotherapy (Vulink et al, 2008) but also because they might resemble the physiological differentiation of DC from myeloid precursors. The effect of VEGF on monocytes is not surprising because these leukocytes express abundant levels of the three identified receptors for this cytokine (Supplementary Figure 5). Moreover, during differentiation culture to DC, VEGFRs are downregulated.
It is interesting that, neither VEGF nor RCC supernatants were able to inhibit MLR if they had been added only during DC maturation/activation, rather than during the 7-day differentiation culture from monocytes. This indicates that mature DC might be more resistant to the effects of RCC supernatants or VEGF. Alternatively, the good manufacturing practice-compliant maturation cocktail that we use may be of such a strength that is not amenable to inhibition by these agents.
Exposure to VEGF during differentiation did not alter the secretion of several cytokines, including that of IL-12, IL-10, IL-6 and IL-8. The addition of sunitinib during DC differentiation induced a marked decrease in IL-12 concentrations and a less relevant reduction in IL-10 secretion. Bevacizumab and sorafenib did not reproduce these effects. These results suggest that the IL-12 inhibition induced by sunitinib is not mediated by the VEGF pathway, and may have relevant implications when exploring combinations of sunitinib with other immunotherapeutic strategies. Indeed, sunitinib and sorafenib are far from being specific inhibitors of a single tyrosine kinase and have been shown to inhibit various kinases with different ICs50. These included signalling pathways known to be critical in leukocyte biology (Karaman et al, 2008). Therefore, this type of VEGF-R-independent effects is not unexpected to see. Renal cell carcinoma culture supernatants also inhibited IL-12 production, and neither of the drugs tested reversed this effect, further supporting the fact that IL-12 inhibition is dependent not on the VEGF pathway but on other unidentified factors. Interleukin-12 is critical for therapeutic immunity against cancer (Mazzolini et al, 2003). Artificial enhancement of IL-12 production by DC strengthens elicited immunity and therapeutic potential (Melero et al, 1999; Mazzolini et al, 2005). Therefore, selective inhibition of IL-12 is likely to be suppressive for cellular immune responses.
Other authors have also evaluated the effects of sorafenib and sunitinib on DC (Hipp et al, 2008). They reported that sorafenib, but not sunitinib, inhibited DC function, assessed through the expression of cytokines and CD1a. The inhibitory effects were mediated by the inhibition of phosphatidylinositide 3-kinase production and mitogen-activated protein kinases, as well as by nuclear factor-κB signalling. They treated C57BL/6 mice with both drugs, and found that sorafenib significantly reduced the induction of antigen-specific T cells, whereas sunitinib reduced the number of regulatory T cells in peripheral blood. On the basis of these findings, they concluded that sorafenib does not seem to be a good candidate for combination with immunotherapeutic approaches, whereas sunitinib does. The main difference between both studies is that whereas Hipp et al (2008) investigated the effects on already differentiated DC, we focused on the transition from myeloid immature precursors to DC.
Our experimental observations join the literature on potential mechanisms accounting for immune dysfunction in RCC. Direct proapoptotic effects of RCC cells on human T cells have been reported as being mediated by the FasL expression and gangliosides released into the surrounding media (Das et al, 2008). Gangliosides are unlikely mediators of the effects on monocytes because of lower molecular weights than that of the expected factors according to Figure 6. In RCC patients, sunitinib treatment improves type I responses by T lymphocytes with decreases in regulatory T cells (Finke et al, 2008). Whether this is mediated directly on T cells within the cancer-modified environment or by indirect effects involving myeloid leukocytes, such as DC, remains to be seen. The effects of sunitinib downmodulating the ability for IL-12 production in our cultures are to be reconciled with the improvements of type I responses seen in the patients and possibly related to the complexity of the in vivo system (Finke et al, 2008).
In conclusion, our study shows that bevacizumab and sorafenib, but not sunitinib, reverse the inhibitory effects of recombinant VEGF on DC differentiation, but not of those mediated by RCC culture supernatants, which are mainly mediated by other unidentified substance(s). The changes induced by VEGF on mature DC included a reduced expression of HLA-DR and CD86, which was also reverted by bevacizumab and sorafenib. Finally, RCC culture supernatants and, surprisingly, sunitinib decreased IL-12 production by mature DC. Such inhibition was not restored by any of the tested drugs, delivered either as single agents or in combination. Moreover, our data indicate that soluble factors from RCC exert effects on monocyte to DC differentiation, which renders functionally impaired DC for T-cell stimulation. Fortunately, such altered DC by tumour-soluble factors do not interfere with the function of healthy DC differentiated apart from the influence of tumour cells. Biochemical identification of the inhibitory factors is the next step to progress in our understanding of the new described phenomena.
Acknowledgments
This study was supported by the Spanish Society of Medical Oncology (SEOM), Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri), Ministerio de Sanidad (Fondo de Investigación Sanitaria, PI060932), Ministerio de Sanidad (Fondo de Investigación Sanitaria, EC07/90133) and by Pfizer Inc. We are indebted to Leyre Urruticoechea for providing sorafenib.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G (2004) Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 10: 950–958 [DOI] [PubMed] [Google Scholar]
- Coppin C (2008) Immunotherapy for renal cell cancer in the era of targeted therapy. Expert Rev Anticancer Ther 8: 907–919 [DOI] [PubMed] [Google Scholar]
- Cuevas Y, Hernández-Alcoceba R, Aragones J, Naranjo-Suárez S, Castellanos MC, Esteban MA, Martín-Puig S, Landazuri MO, del Peso L (2003) Specific oncolytic effect of a new hypoxia-inducible factor-dependent replicative adenovirus on von Hippel–Lindau-defective renal cell carcinomas. Cancer Res 63: 6877–6884 [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115: 3623–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Sa G, Paszkiewicz-Kozik E, Hilston C, Molto L, Rayman P, Kudo D, Biswas K, Bukowski RM, Finke JH, Tannenbaum CS (2008) Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathways. J Immunol 180: 4687–4696 [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007a) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134 [DOI] [PubMed] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, AVOREN Trial investigators (2007b) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: 2103–2111 [DOI] [PubMed] [Google Scholar]
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, Bukowski R (2008) Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 14: 6674–6682 [DOI] [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248 a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24: 4–5 [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2: 1096–1103 [DOI] [PubMed] [Google Scholar]
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92: 4150–4166 [PubMed] [Google Scholar]
- Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP (1999) Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 5: 2963–2970 [PubMed] [Google Scholar]
- Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P (2008) Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111: 5610–5620 [DOI] [PubMed] [Google Scholar]
- Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, Schmitt E, Dannenberg T, Lehmacher W, von Wietersheim J, Doehn C (2004) Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 363: 594–599 [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26: 127–132 [DOI] [PubMed] [Google Scholar]
- Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2: 727–739 [DOI] [PubMed] [Google Scholar]
- Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH (2000) Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel–Lindau tumor suppressor gene loss of function. Oncogene 19: 5435–5443 [DOI] [PubMed] [Google Scholar]
- Mazzolini G, Prieto J, Melero I (2003) Gene therapy of cancer with interleukin-12. Curr Pharm Des 9: 1981–1991 [DOI] [PubMed] [Google Scholar]
- Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, Tirapu I, Arina A, Sola J, Herraiz M, Lucena F, Olagüe C, Subtil J, Quiroga J, Herrero I, Sádaba B, Bendandi M, Qian C, Prieto J, Melero I (2005) Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol 23: 999–1010 [DOI] [PubMed] [Google Scholar]
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23: 133–141 [DOI] [PubMed] [Google Scholar]
- Melero I, Duarte M, Ruiz J, Sangro B, Galofré J, Mazzolini G, Bustos M, Qian C, Prieto J (1999) Intratumoral injection of bone-marrow derived dendritic cells engineered to produce interleukin-12 induces complete regression of established murine transplantable colon adenocarcinomas. Gene Ther 6: 1779–1784 [DOI] [PubMed] [Google Scholar]
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7: 95–106 [DOI] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE (2005) Filter buffy coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods 307: 150–166 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff Jr CL, Mellor AL (2002) Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297: 1867–1870 [DOI] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 13: 721s–726s [DOI] [PubMed] [Google Scholar]
- Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101: 4878–4886 [DOI] [PubMed] [Google Scholar]
- Ohm JE, Shurin MR, Esche C, Lotze MT, Carbone DP, Gabrilovich DI (1999) Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol 163: 3260–3268 [PubMed] [Google Scholar]
- Schrader AJ, Hofmann R (2008) Metastatic renal cell carcinoma: recent advances and current therapeutic options. Anticancer Drugs 19: 235–245 [DOI] [PubMed] [Google Scholar]
- Steinman RM (2008) Dendritic cells in vivo: a key target for a new vaccine science. Immunity 29: 319–324 [DOI] [PubMed] [Google Scholar]
- Vulink A, Radford KJ, Melief C, Hart DN (2008) Dendritic cells in cancer immunotherapy. Adv Cancer Res 99: 363–407 [DOI] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003a) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA (2003b) Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21: 3127–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.