Abstract
Sleep disturbances are associated with hormonal imbalances and may result in metabolic disorders including obesity and diabetes. Therefore, circuits controlling both sleep and metabolism are likely to play a role in these physiopathological conditions. The hypocretin (Hcrt) system is a strong candidate for mediating both sleep and metabolic imbalances because Hcrt neurons are sensitive to metabolic hormones, including leptin and ghrelin, and modulate arousal and goal-orientated behaviours. This review discusses the role of Hcrt neurons as a sensors of energy balance and arousal and proposes new ways of probing local hypothalamic circuits regulating sleep and metabolism with unprecedented cellular specificity and temporal resolution.
Although the neuronal substrates of the sleep/wake cycle have been extensively described (Pace-Schott & Hobson, 2002), the function of sleep remains unknown and may include cortical reorganization and processing associated with learning and memory, brain development and neurogenesis, cellular repair and replenishment of energy stores (Hobson & Pace-Schott, 2002; Frank, 2006; Cirelli & Tononi, 2008). Although a role for sleep in energy conservation is still a matter of debate (Zepelin & Rechtschaffen, 1974; Knutson & Van Cauter, 2008), sleep duration and the length of a sleep–wake cycle are inversely correlated with brain metabolic rate across species (Savage & West, 2007), suggesting that availability of metabolic fuels conditions sleep architecture. In addition, sleep restriction has been associated with alteration of endocrine functions and increased risk of obesity, diabetes and cardiovascular disease (Knutson & Van Cauter, 2008). Chronic sleep reduction in humans results in higher plasma concentration of leptin and lower levels of ghrelin, which ultimately may increase appetite, body weight and metabolic imbalances (Taheri et al. 2004; Chaput et al. 2007; Penev, 2007; Knutson & Van Cauter, 2008). Interestingly, short sleep duration results in increased levels of cortisol (Spiegel et al. 2004).
These studies suggest the existence of neuronal circuits regulating both sleep and metabolism. This review highlights a role for the hypothalamic hypocretin (Hcrt), also known as orexin, system as a sensor for metabolism and arousal in the modulation of wakefulness, sleep and comsumatory behaviours. Hcrt neurons and other hypothalamic neuronal populations are strongly interspersed, which makes the study of their physiological functions extremely difficult. Use of newly developed optogenetic methods to selectively interrogate neuronal circuits has been proposed to gain a better understanding of the neuronal circuits underlying sleep and metabolism imbalances.
Hypothalamic regulation of metabolism
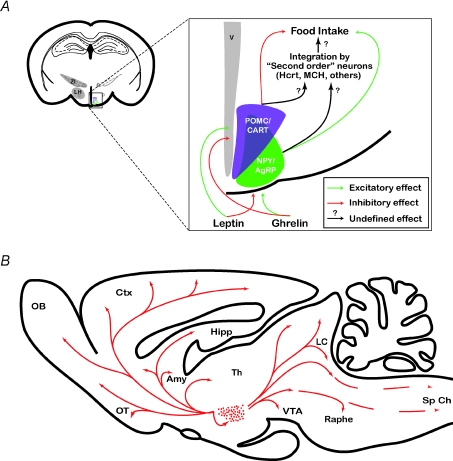
Appetite results from the integration of metabolic and hormonal signals within the central nervous system. The hypothalamus is an important brain area regulating several homeostatic processes including energy balance (Saper, 2006). Neurons in the arcuate nucleus of the hypothalamus act as sensors of circulating hormones (Fig. 1A). The satiety hormone leptin is produced by adipose tissues and inhibits arcuate neurons that coexpress neuropeptide Y (NPY) and agouti-related peptide (AGRP) and activates proopiomelanocortin (POMC) neurons that also coexpress cocaine- and amphetamine-related transcripts (CART). In contrast the appetite-stimulating hormone ghrelin is produced in the gut and has the opposite effect (Abizaid & Horvath, 2008). Thus, activation of POMC/CART and NPY/AGRP neurons have anorexigenic and orexigenic properties, respectively. In addition, leptin also increases energy expenditure, possibly via increased thermogenesis, whereas ghrelin decreases locomotor activity and thus promotes energy conservation (Knutson & Van Cauter, 2008). These neurons convey feeding signals to the lateral hypothalamus (LH) that are further processed together with autonomic, endocrine and environmental inputs into appropriate behaviours (Fig. 1A) (Saper, 2006). Thus, in regard to their hierarchical position in the integration process (Fig. 1A), NPY/AgRP- and POMC-expressing neurons have been termed ‘first order’ (or ‘sentinel’) neurons; neurons in the medial and lateral hypothalamus producing hypocretins/orexins (Hcrt/ox), melanin-concentrating hormone (MCH), CART, corticotropin-releasing factor (CRF) and endocannabinoids (Abizaid & Horvath, 2008) have been termed ‘second order neurons’.
Figure 1. Hypothalamic circuits regulate energy homeostasis.
A, schematic drawing of a coronal section through the entire rat brain showing the lateral hypothalamus (LH) and the zona incerta (ZI) and a magnification of the arcuate nucleus showing neuropeptide Y (NPY)/agouti-related protein (AgRP) and proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons. Peripheral signals (leptin, ghrelin) directly regulate the activity of arcuate nucleus through the median eminence, which lacks a brain blood barrier. Leptin inhibits NPY/AgRP neurons and activates POMC/CART neurons whereas ghrelin has the opposite effect (Abizaid & Horvath, 2008). These neurons project to mutiple brain regions including the lateral hypothalamus where signal is further processed and integrated into coherent feeding behaviour. B, schematic drawing of a saggital section through the rat brain showing the neuroanatomical organization of the Hcrt system. Dots indicate the relative location and abundance of Hcrt-expressing cell bodies. Arrows point out some of the more prominent terminal fields including the (noradrenergic cells of the LC), histaminergic neurons of the posterior hypothalamus, cholinergic cells of the basal forebrain, serotonine-procuding neurons of the raphe and dopaminergic neurons of the VTA. Abbreviations used: Amy, amygdala; Ctx, cortex; Hipp, hippocampus; LC, locus coeruleus; ME, median eminence; OB, olfactory bulb; OT, olfactory tubercule; PP, posterior pituitary; Sp Ch, spinal chord; Th, thalamus; VTA, ventro-tegmental area; LH, lateral hypothalamus; V, 3rd ventricle; ZI, zona incerta.
Hcrt, sleep and metabolism: identical circuits, new functions
Hcrt-1 and 2 are neuroexcitatory peptides produced in approximately ∼6700 neurons in rat restricted to the LH (Peyron et al. 1998; Modirrousta et al. 2005) (Fig. 1B). Hcrt peptides bind to two specific Hcrt receptors (Hcrt-1R and Hcrt-2R) that results in increased neuronal excitability (Sakurai et al. 1998). Hcrt neurons have widespread projections throughout the brain including the cortex, hippocampus, amygdala, nucleus accumbens, hypothalamus, thalamus, ventral tegmental area (VTA), locus coeruleus (LC) and the raphe (Sakurai, 2007) (Fig. 1B). In turn, these neurons are the target of multiple neuronal cell types including those regulating the sleep–wake cycle, energy homeostasis and motivation (reviewed in Sakurai, 2007). The resulting neuronal circuit supports Hcrt function integrating arousal, energy homeostasis and goal-orientated behaviours (Sakurai, 2007; Boutrel & de Lecea, 2008).
Deficiency in the Hcrt system has been linked to narcolepsy in humans, dogs and mice, a chronic neurological disorder resulting in excessive daytime sleepiness, cataplexy and fragmented sleep (Chemelli et al. 1999; Lin et al. 1999; Peyron et al. 2000). Cerebrospinal fluid (CSF) Hcrt-1 content is maximal during the wakefulness period and infusion of Hcrt peptides promotes wakefulness (Sakurai, 2007). Collectively, these results support the hypothesis that the Hcrt system controls behavioural state boundaries by lowering the arousal threshold (Sutcliffe & de Lecea, 2002). Accordingly, Hcrt neurons mediate stress-induced arousal since they are activated by CRF, a neurotransmitter that initiates the central stress response (Winsky-Sommerer et al. 2004), and the flight-or-fight response is impaired in Hcrt knockout (KO) animals (Kayaba et al. 2003). Thus, Hcrt neurons may integrate CRF-mediated stress response with homeostatic needs and environmental factors to promote arousal. Furthermore, recent studies suggest that stress-induced activation of Hcrt neurons may lead to ‘hyper-arousal’– a state of physiological and psychological tension characterized by anxiety, insomnia and fatigue, which is often associated with post-traumatic stress disorders and drug abuse. In rodents, activation of Hcrt neurons has been linked to cues associated with drug and food reward and the development of opioid drug dependence and withdrawal (reviewed in Boutrel & de Lecea, 2008). In contrast, morphine withdrawal symptoms, morphine-induced place preference and hyperlocomotion are diminished in Hcrt KO animals (Boutrel & de Lecea, 2008). More importantly, the Hcrt system mediates stress-induced reinstatement of cocaine-seeking behaviour (Boutrel et al. 2005). These recent data demonstrate Hcrt's ability to modulate motivation and reward pathways of the brain possibly via an increased sensitivity to glutamate in VTA synapses (Borgland et al. 2006)
These modulations of arousal and reward by the Hcrt system suggest that the mild orexigenic effect induced by infusion of the peptides in the brain of rat (Sakurai et al. 1998) may result from increased arousal and possibly hedonic feeding. This hypothesis is supported by the fact that activation of Hcrt neurons correlates with opioid-induced palatable food intake in rats (Zheng et al. 2007), possibly through Hcrt peptide action on the VTA (Borgland et al. 2006). In addition, an Hcrt-R1 antagonist attenuated high-fat pellet self-administration in rats (Nair et al. 2008).
The hypothalamic Hcrt system shares common brain circuits regulating arousal, metabolism and reward. How the Hcrt system modulates the activity of these circuits upon specific physiological inputs remains to be determined. According to Sutcliffe & de Lecea (2002), the cortical and physiological arousal associated with Hcrt neurons activation may facilitate consummatory behaviours and other goal-orientated behaviours including stress response and reproductive behaviours (Muschamp et al. 2007; Boutrel & de Lecea, 2008).
The Hcrt system: a sensor for metabolic changes
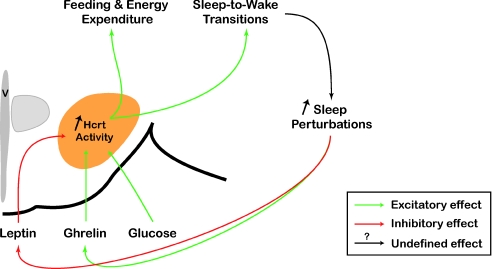
In humans, chronic short sleep decreases the concentration of leptin and increases glucose and ghrelin levels (Taheri et al. 2004; Penev, 2007; Tasali et al. 2008). These hormonal changes are known to inhibit POMC/CART neurons and excite NPY/AgRP neurons resulting in a net feeding signal form the arcuate nuclei. This may lead to the increased body mass index and higher risk of developing obesity and type 2 diabetes associated with chronic sleep restriction. Although experimental evidence has shown that NPY/AgRP and POMC/CART neurons set up the initial feeding response, recent studies suggest that Hcrt neurons may also act as a ‘primary’ sensor of metabolism (which measures or detects a changing condition from neurotransmitters, neuromodulators, or metabolic factors and converts it into an output signal) (Fig. 2). Decreased circulating leptin levels and increased glucose and ghrelin levels directly modulate Hcrt activity by increasing Hcrt neuron excitability (Burdakov et al. 2005, 2006; Sakurai, 2007; Williams et al. 2008). Thus, enhanced Hcrt neuronal activity may promote consummatory behaviours, arousal and increased energy expenditure (via higher locomotor activity and metabolic rate) by activation of NPY/AgRP neurons and indirect inhibition of POMC/CART cells (Ma et al. 2007; Sakurai, 2007), by activation of arousal centres of the brain (Sakurai, 2007) and by increased sympathetic tone (Sakurai, 2007). Sensitivity of Hcrt neurons to triglycerides, carbon dioxide and pH (Williams et al. 2007) further supports a role for Hcrt neurons in sensing metabolic factors. A direct consequence of this increase in Hcrt system activity is an instability of the sleep–wake cycle, since selective stimulation of Hcrt neurons in vivo increases the probability of NREM and REM sleep-to-wake transitions (Adamantidis et al. 2007). This positive feedback may result in extended periods of sleep and metabolism perturbation that may be counterbalanced by the sleep-promoting effect of accumulated adenosine (Liu & Gao, 2007) and the disinhibition of sleep-promoting circuits such as GABAergic/glycinergic cells of the ventrolateral preoptic area (VLPO) (Lu & Greco, 2006).
Figure 2. Hcrt system as a sensor of metabolism.
Hcrt neurons are inhibited by leptin and activated by ghrelin and glucose. In addition, they received indirect circadian inputs that are integrated with metabolic signals to modulate sleep and metabolism. Chronic short sleep perturbation induces metabolic changes including lowering leptin levels and increasing ghrelin levels that directly increase the activity of the Hcrt system. This increase in Hcrt activity promotes consummatory behaviours (food, drug), energy expenditure (via higher locomotor activity and metabolic rate) and sleep-to-wake transitions. Consequently, activation of the Hcrt system inhibits sleep and energy conservation. Triggering activity of the Hcrt neuronal circuit by circulating metabolic factors may then result in a positive feedback loop, which worsens existing sleep perturbation symptoms. This positive loop is inhibited by increasing sleep pressure (a consequence of sleep demand) and may result in a stabilization of the sleep–wake cycle. V: 3rd ventricle.
Thus, the Hcrt system provides intra- and extrahypothalamic target circuits with a local sensor circuit that detects subtle changes in metabolism and arousal. One of those is the dopaminergic mesolimbic pathway (Borgland et al. 2006), whose activation is associated with motivation and incentive salience. Interestingly, dopaminergic cells are sensitive to metabolic factors and have been identified as crucial brain regulatory circuits of energy homeostasis (Abizaid et al. 2006; Fulton et al. 2006; Hommel et al. 2006). Direct administration of leptin into the VTA decreases food intake (Hommel et al. 2006), possibly through modulating dopamine release in the nucleus accumbens (Fulton et al. 2006), whereas VTA administration of ghrelin has the opposite effect (Abizaid et al. 2006). Moreover, leptin reduces the firing rate of VTA neurons, whereas ghrelin and Hcrt have the opposite effect (Abizaid et al. 2006; Hommel et al. 2006; Muschamp et al. 2007). Finally, dopamine is necessary for appropriate occurrence and electroencephalogram spectral quality of REM sleep during the sleep–wake cycle (Dzirasa et al. 2006), and REM sleep occurrence correlates with a change of firing patterns of dopaminergic cells in the VTA (Dahan et al. 2007).
In conclusion, Hcrt neurons act as ‘first order’, instead of ‘second order’, sensors of subtle fluctuations of metabolic factors, as suggested by the recent studies described in this review. These signals are integrated with others inputs (internal, external) by hypothalamic local circuits and brain reward pathways to regulate brain states and consummatory behaviours. However, the underlying molecular and cellular mechanisms by which Hcrt neuronal activity is relayed to brain circuits regulating arousal, metabolism and reward remain unknown. Such goals are now being made possible by newly developed optical methods to selectively manipulate neuronal circuits with unprecedented cellular specificity and temporal resolution.
Next-generation tools for manipulating neuronal circuit activity
Hcrt-containing neurons account for only 10–30% of all neurons in the LH region, and are surrounded by multiple neuronal subtypes with excitatory, inhibitory and complex modulatory properties (e.g. NPY/AgRP, POMC/CART, Hcrt, MCH) (Gerashchenko & Shiromani, 2004). Therefore, correlations between cellular activity and specific behaviour using classical methods of manipulation of brain function, including lesions and electrical stimulations, may be difficult to interpret. Some of these limitations have been partially overcome by the use of mouse genetics including gene knockouts, mutations and targeted neuronal ablations. To overcome the lack of specificity and low temporal resolution of these approaches, optical tools to manipulate membrane potential of defined cell populations have been recently developed. Such techniques are classified on their molecular mechanisms and have different temporal and spatial resolutions (reviewed in Zhang et al. 2006; Sjulson & Miesenbock, 2008). Optogenetics uses light-sensitive cations channels (channelrhodopsin-2, VChR1; Zhang et al. 2008) or an ionic pump (halorhodopsin or NpHR (Natronomonas pharaonis halorhodopsin); Zhang et al. 2007) to selectively depolarize or hyperpolarize, respectively, specific neuronal cell groups with millisecond time scale temporal resolution. We recently used optogenetic stimulation to manipulate Hcrt neuronal activity in vitro and in vivo using a fibre optic-based system (Aravanis et al. 2007; Gradinaru et al. 2007) to deliver light in the hypothalamus in freely moving animals (Adamantidis et al. 2007). We found that optical stimulation of ChR2-expressing Hcrt neurons increases the probability of NREM and REM sleep-to-wake transitions in vivo (Adamantidis et al. 2007).
This approach has been used successfully for optical interrogation of the role of intact neural circuits on specific behaviours in mamalian and non-mamalian species (Lima & Miesenbock, 2005; Schroll et al. 2006; Adamantidis et al. 2007; Zhang et al. 2007; Huber et al. 2008). Genetic targeting of ChR2 or NpHR into defined classes of hypothalamic neurons allows bimodal manipulation of specific circuit activity and avoids the inadvertent stimulation of neighbouring neurons that occurs with electrical stimulation.
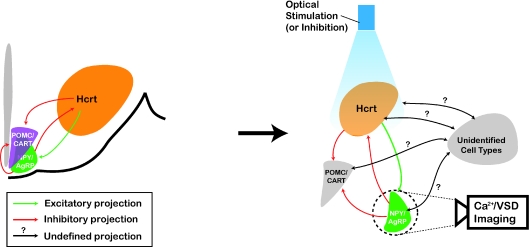
Thus, optogenetics may be use to deconstruct the connectivity and manipulate the plasticity of intermingled hypothalamic circuits (e.g. Hcrt, MCH, NPY, POMC, etc.) (Fig. 3). The possibility of inhibiting single action potentials in cells targeted with NpHR opens new questions about the neuronal encoding of information by hypothalamic cell populations. Whereas electrophysiological recordings of neurons tagged with fluorescent markers have generated valuable information about the cellular properties of hypothalamic cells, genetic tools allow manipulation (stimulation and inhibition) of entire targeted neuronal population with temporal and spatial resolutions relevant to physiological conditions. Combination of optogenetic stimulation and inhibition with voltage sensitive dyes and calcium sensors will allow non-invasive all-optical interrogation of neuronal circuit function and plasticity associated with specific behaviour (Zhang et al. 2006) (Fig. 3). Eventually, it will define the dynamics and functions of hypothalamic circuits in arousal, sleep and compulsive consummatory behaviours. Elucidating the responsible mechanism and the circuits involved will define new treatments for associated metabolic and arousal disorders.
Figure 3. Optical deconstruction of Hypothalamus local circuits.
Schematic drawing of a coronal section through the rat showing hypothalamic neuronal populations involved in sleep and metabolism. Hcrt neurons are the targets of projections from multiple brain areas, including NPY/AgRP and POMC/CART neurons of the arcuate nucleus. Although the interplay between hypothalamic nuclei in regulating energy homeostasis remains unclear, NPY/AgRP neurons inhibit Hcrt cells, which in turn activate NPY/AgRP cells and inhibit POMC/CART neurons. Complex hypothalamic circuits can be functionally deconstructed with high temporal and spatial resolution using optogenetics. Genetic targeting of ChR2 or NpHR into defined classes of hypothalamic neurons (e.g. Hcrt as shown in the figure) allow bimodal manipulation of specific circuit activity without inadvertent activation/inhibition of neighbouring cells (e.g. grey neuronal populations as shown in the figure). Combination of optogenetics with imaging of fluorescent calcium sensors (Ca2+) or voltage sensitive dyes (VSD) of identified neuronal populations (e.g. NPY/AgRP as shown in the figure) in brain slices will reveal synaptic function and plasticity associated with metabolism and arousal. In addition, bath application of metabolic factors (leptin, ghrelin, glucose), variation of environmental parameters (temperature, pH, CO2) and the use of animal models for neurological disorders (narcoleptic mice, ob/ob obese mice, etc.) may model pathophysiological conditions.
Summary
In this review, we proposed that the Hcrt system has a primary sensory function for metabolism that is integrated with additional physiological inputs (circadian, motivation, environment) into appropriate goal-orientated behaviours. The Hcrt system also acts as an arousal sensor through its sensitivity to circulating metabolic factors (leptin, ghrelin, glucose) since their concentration correlates with modification of arousal. We hypothesize that plasticity of this functional circuit results in a positive feedback loop between Hcrt sensory and effector properties that may be responsible for the metabolic disorders associated with sleep perturbation. Optogenetic tools now allow functional deconstruction of hypothalamic neuronal circuits with unprecedented spatial and temporal resolution relevant to in vivo physiological conditions. Such synergy between genetic tools to manipulate and monitor neuronal activity will definitely help to decipher the function of each hypothalamic circuit in regulating homeostatic processes of arousal, metabolism, motivation and reward.
Acknowledgments
A. Adamantidis is supported by the Fonds National de la Recherche Scientifique (FRS-FNRS –‘Charge de Recherche’), NARSAD and the Fondation Leon Fredericq. L. de Lecea is supported by NIDA DARPA and NARSAD. We are grateful to J. Schaich-Borg and M. Carter for their helpful discussions and feedback.
References
- Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept. 2008;149:3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, de Lecea L. Addiction and arousal: The hypocretin connection. Physiol Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–2304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG. The mystery of sleep function: current perspectives and future directions. Rev Neurosci. 2006;17:375–392. doi: 10.1515/revneuro.2006.17.4.375. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med. 2006;2:S19–S26. [PubMed] [Google Scholar]
- Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci. 2007;27:1529–1533. doi: 10.1523/JNEUROSCI.3583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Penev PD. Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes. 2007;14:374–381. doi: 10.1097/MED.0b013e3282be9093. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–252. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- Savage VM, West GB. A quantitative, theoretical framework for understanding mammalian sleep. Proc Natl Acad Sci U S A. 2007;104:1051–1056. doi: 10.1073/pnas.0610080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Sjulson L, Miesenbock G. Photocontrol of neural activity: biophysical mechanisms and performance in vivo. Chem Rev. 2008;108:1588–1602. doi: 10.1021/cr078221b. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain Behav Evol. 1974;10:425–470. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]