Abstract
Some of the neurones controlling sleep, appetite and hormone release act as specialized detectors of ambient glucose. Their sugar sensing is conventionally thought to involve glucokinase-dependent metabolism of glucose to ATP, which then alters membrane excitability by modulating ATP-dependent channels or transporters, such as ATP-inhibited K+ channels (KATP). However, recent studies also provide examples of both glucose-excited (GE) and glucose-inhibited (GI) neurones that sense glucose independently of such metabolic pathways. Two-thirds of hypothalamic GE neurones in primary cultures are also excited by the non-metabolizable glucose analogue α-methylglucopyranoside (α-MDG), which acts as a substrate for electrogenic (depolarizing) sodium–glucose cotransporter (SGLT). The excitatory responses to both glucose and α-MDG are abolished by arresting SGLT activity by sodium removal or the SGLT inhibitor phloridzin. Direct depolarization and excitation by glucose-triggered SGLT activity may ensure that GE neurones continue to sense glucose in ‘high-energy’ states, when KATP channels are closed. A major class of hypothalamic GI neurones, the orexin/hypocretin cells, also appear to use a non-metabolic sensing strategy. In these cells, glucose-induced hyperpolarization and inhibition are unaffected by glucokinase inhibitors such as alloxan, d-glucosamine, and N-acetyl-d-glucosamine, and mimicked by the non-metabolizable glucose analogue 2-deoxyglucose, but not by stimulating intracellular ATP production with lactate. The dissociation between sensing and metabolism of sugar may allow the brain to predict and prevent adverse changes in extracellular glucose levels with minimal impact on the flow of intracellular fuel.
Brain glucose sensors: what, where and why?
Unlike most tissues, the brain becomes profoundly damaged if deprived of glucose for just a few minutes. To anticipate and prevent this, it varies behaviour and metabolism according to glucose levels. To measure glucose levels, the brain contains its own glucose sensors. This is presumably because brain glucose levels generally differ from those in the blood, and may be region-specific, thereby making it unreliable to use information from the peripheral glucose sensing cells as an estimate of glucose supply. Specifically, in many brain regions, glucose levels have been measured to be 10–30% of those in the blood (e.g. Silver & Erecinska, 1994; Routh, 2002; de Vries et al. 2003), and even for areas near ‘windows’ in the blood–brain barrier, such as the hypothalamic arcuate nucleus, it remains controversial whether neuronal cell bodies ‘see’ plasma-like glucose concentration since this depends on factors such as the direction of interstitial fluid flow and local tanycyte barriers (e.g. see discussion in Peruzzo et al. 2000).
Brain glucose sensors are specialized neurones that respond to fluctuations in local extracellular glucose concentration with changes in their firing rate in a manner that is different from the non-specific ‘out-of-fuel’ effects of low glucose (Anand et al. 1964; Oomura et al. 1969; Yang et al. 1999; Mobbs et al. 2001; Routh, 2002). These glucose sensing neurones have so far been found only in a few brain regions, in particular the hypothalamus and brainstem (Anand et al. 1964; Oomura et al. 1969; Ritter et al. 1981; Balfour et al. 2006). This review concentrates on hypothalamic glucose sensing neurones, which are found in the lateral, arcuate and ventromedial hypothalamic regions, and whose firing rate either increases (glucose-excited, GE, neurones) or decreases (glucose-inhibited, GI, neurones) in response to extracellular glucose (Anand et al. 1964; Oomura et al. 1969; Routh, 2002; Wang et al. 2004).
Recent research has provided important information about the neurochemical identities and physiological significance of hypothalamic glucose sensing neurones, in particular in sleep- and appetite- regulating circuits. For example, hypothalamic GI neurones have been reported to correspond to a neurochemically diverse group of cells including wakefulness-promoting hypocretin/orexin neurones in the lateral hypothalamus (Sakurai, 2007), and a subpopulation (40–80%) of appetite-stimulating NPY neurones in the hypothalamic arcuate nucleus (Fioramonti et al. 2007; Mountjoy et al. 2007). In turn, GE neurones may correspond to cells containing melanin-concentrating hormone in the lateral hypothalamus (Burdakov et al. 2005a), and POMC-containing neurones in the arcuate nucleus (Ibrahim et al. 2003; Parton et al. 2007) (although some groups also report that the majority of POMC neurones do not sense glucose, e.g. see Fioramonti et al. 2007).
Although orexin, NPY and POMC neurones are key regulators of feeding behaviour, the relative physiological importance of hypothalamic glucose sensing for feeding behaviour versus the regulation of counter-regulatory hormone release (e.g. McCrimmon et al. 2005) and control of hepatic glucose production (e.g. Lam et al. 2007) is currently unclear, and will not be discussed further here. Instead, this short review will comment on the mechanisms used by glucose sensing neurones to translate glucose levels into changes in their firing rate, specifically focusing on a small number of recent studies suggesting that intracellular glucose metabolism, while acting as a general permissive process for cellular reactions since it provides them with energy, does not always directly participate in the specialized glucose sensing responses of hypothalamic neurones.
Comments on ‘metabolic’ models of neuronal glucosensing
Inspired by the pancreatic β-cell, a GE cell sometimes called an ‘honorary neuron’ for its ability to fire action potentials, metabolic models of glucose sensing have for many years held centre-stage in publications on both GE and GI neurones. Here we will consider the two separately.
In GE neurones, the metabolic sensing model postulates that glucose enters the cell, is phosphorylated by glucokinase, and is then metabolized to give ATP. The subsequent increase in the cytosolic ATP : ADP ratio then closes membrane KATP channels, thereby causing depolarization and increased electrical activity (reviewed in the context of glucose sensing neurons in Routh, 2002; Burdakov et al. 2005b). Some experimental observations are indeed consistent with this model. For example, pharmacological blockade of glucokinase has been reported to decrease the activity of GE neurones (Kang et al. 2004). In turn, reducing glucose levels around mediobasal hypothalamic neurones in vitro activated channels with biophysics and pharmacology consistent with KATP channels (Ashford et al. 1990; Wang et al. 2004).
Studies of GE neurones in mice with deficient KATP channels have also been carried out. In mice with global knockout of the Kir6.2 subunit of the KATP channel, the firing rate of the hypothalamic ventromedial neurones no longer increased in response to a switch from 2.5 to 25 mm extracellular glucose (Miki et al. 2001). Similarly, glucose no longer modulated POMC cell firing or α-MSH release in mice that expressed mutant, ATP-insensitive, KATP channels in arcuate POMC neurones (Parton et al. 2007).
While these elegant genetic studies clearly illustrate the importance of KATP channels for normal function of key neuronal populations, their meaning in the context of physiological glucose sensing is complicated by the fact that the intrinsic electrical properties of GE neurones were substantially altered by these genetic manipulations. Specifically, in the Kir6.2-null mice, disruption of KATP channels per se elevated the firing rate of ventromedial neurones 3-fold relative to wild-type neurones, an increase roughly 2-fold greater than the maximal response to glucose in these cells (see Fig. 2B in Miki et al.). It is therefore possible that, through a saturation effect, the already high basal firing rate in the Kir6.2-null mice masked the more subtle firing rises due to glucose.
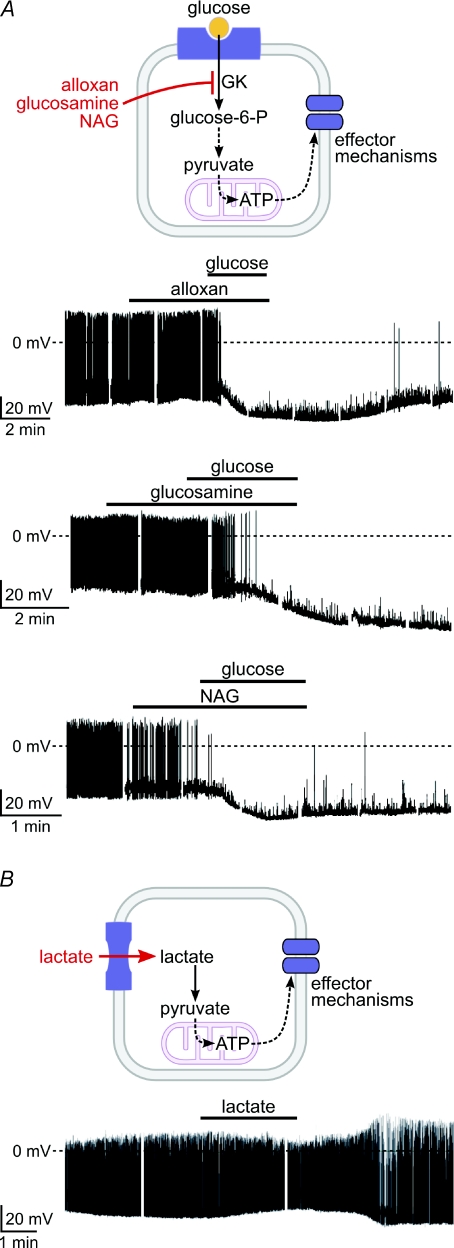
Figure 2. Pharmacological properties of glucosensing in GI orexin neurones.
A, top, cartoon of protocol. Bottom, patch-clamp recordings from orexin neurones; glucokinase inhibitors (at 10 mm) do not prevent glucose responses (5 mm glucose, baseline glucose = 1 mm). B, top, cartoon of protocol. Bottom, recording from an orexin neuron showing that lactate (5 mm) does not mimic the effects of glucose. From Gonzalez et al. (2008). Copyright © 2008 American Diabetes Association. From Diabetes®-http://dx.doi.org/10.2337/db08-0548. Reprinted with permission from The American Diabetes Association.
In turn, in the POMC/Kir6.2-mutant mice, POMC neurones had more negative resting membrane potentials and lower firing rates than wild-type POMC neurones, presumably due to increased basal activity of the mutant KATP channels (see Fig. 1S in Parton et al.). If the currents that cause glucose excitation are small, the increase in inhibitory conductance in KATP-mutant neurones would prevent these currents from influencing the membrane potential irrespective of whether they flow through KATP channels or not. This follows from Ohm's law: V=IR = I/g (where V is the membrane potential, and I, R and g are current, resistance and conductance, respectively), which states that the greater the background membrane conductance (g), the smaller the impact of current (I) on the membrane potential (V).
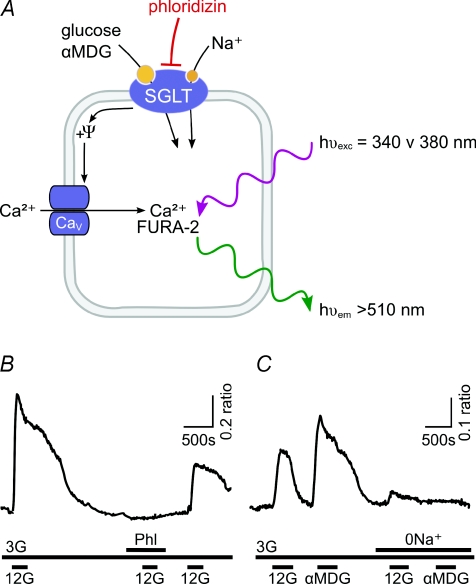
Figure 1. Evidence for SGLT-mediated glucosensing in cultured GE neurones.
A, cartoon of the experimental set up; Ψ represents the membrane potential. B, stimulation of activity (calcium signal) of a GE neuron by glucose (G, numbers are concentrations in mm) is blocked by SGLT inhibitor phloridzin (Phl, 100 nm). C, stimulation of a GE neuron by a non-metabolizable sugar, α-MDG, and block of responses to both glucose and α-MDG by removal of extracellular sodium (0 Na+). B and C are from O’Malley et al. (2006). Copyright © 2006 American Diabetes Association. From Diabetes®, Vol. 55, 2006; 3381–3386. Reprinted with permission from The American Diabetes Association.
The universality of the β-cell model in GE neurones is also questioned by a number of other observations. For example, Ainscow et al. (2002) failed to detect any rises in cytosolic ATP levels in hypothalamic neurones exposed to physiological elevations in glucose concentration. Moreover, KATP channels are expressed in many places in the brain, whereas GE neurones are only found in a few restricted locations (Karschin et al. 1997; Dunn-Meynell et al. 1998). Furthermore, both SUR1, a subunit of the pancreatic β-cell KATP channel, and glucokinase have been detected in some but not all glucose sensing neurones (Kang et al. 2004). The less than complete overlap of glucose sensitivity and glucokinase expression in neurones suggests that this enzyme may not be the ‘glucose sensor’, but may instead provide general metabolic support to some glucose sensing (as well as other) neurones. For example, glucokinase may act as a general ‘booster’ of energy supply to mediobasal hypothalamic neurones irrespective of whether they are glucose sensing, which may help fuel the spontaneous firing typical of these cells (Minami et al. 1986; Burdakov & Ashcroft, 2002).
For GI neurones, much less information exists, but ‘metabolic’ sensing theories have also been proposed, such as ATP-mediated activation of the hyperpolarizing Na+/K+ ATPase (Oomura et al. 1974), ATP-dependent opening of CFTR Cl– channels (Fioramonti et al. 2007), and a critical role for glucokinase (Dunn-Meynell et al. 2002). However, not all GI neurones express glucokinase (Kang et al. 2004), and stimulation of ATP production with lactate does not mimic the inhibitory glucose responses in ventromedial hypothalamic GI neurones (Song & Routh, 2005).
In addition to the classical glucokinase-KATP pathway, another metabolic sensing element has been recently proposed to be critical for hypothalamic glucose sensing, the AMP-activated protein kinase (AMPK), an enzyme regulated by AMP/ATP levels (Minokoshi et al. 2004; Hardie, 2007). For example, Mountjoy et al. (2007) reported that pharmacological AMPK activation reversed the inhibitory effects of high glucose in GI neurones, but did not reverse the excitatory effects of high glucose in GE neurones. In turn, inhibiting AMPK with compound C suppressed the activity of GI neurones and their ability to be stimulated by low [glucose], but had no consistent effects on GE cell activity (Mountjoy et al. 2007). These results suggest that AMPK activity has an excitatory effect on GI neurones, and may participate in their glucose sensing responses (see models in Mountjoy et al. 2007 and Canabal et al. 2007). In turn, Claret et al. (2007) reported that knockout of the α2 isoform of AMPK abolished glucose-induced excitation of arcuate POMC and NPY/AgRP neurones (although described by others as GI, a subset of NPY/AgRP cells were GE in this study), thus also implicating AMPK in glucose sensing in GE neurones. However, how changes in AMPK activity are coupled to membrane excitability in glucose sensing cells remains to be determined experimentally.
As in the case of glucokinase, it is also currently unclear whether AMPKs act as specific ‘glucose sensors’ in glucose sensing neurones or as indirect permissive elements for general cellular adaptations to changes in energy levels, since these enzymes are not specific to glucosensing neurones, but maintain energy balance in all eukaryotic cells (Hardie, 2007). Interestingly, Claret et al. (2007) reported that AMPKα2 deletion disrupted glucose responses but did not affect responses to insulin and leptin, suggesting that hypothalamic integration of metabolic and hormonal signals does not converge at the level of AMPK.
In summary, although elements of the classical β-cell model, as well as other metabolic sensing pathways such as AMPK, remain popular in explaining hypothalamic glucosensing, some of the above-mentioned caveats recently led several laboratories to examine possible alternative models.
Evidence for ‘non-metabolic’ glucosensing in glucose-excited neurones
A concrete ‘metabolism-independent’ alternative to the classical KATP sensing strategy in GE neurones is offered by sodium–glucose cotransporters (SGLTs). These transmembrane proteins operate as sodium-dependent secondary active transporters of glucose and certain other sugars, coupling the uptake of a sugar molecule to the influx of one or two Na+ ions (Wright, 2001). Because glucose is electroneutral, SGLT activity thus generates a net inward current of sufficient size to cause direct membrane depolarization and increased electrical activity without the need for glucose metabolism (Gribble et al. 2003). The possibility that SGLTs may be involved in neuronal glucose sensing was initially suggested by the observation that the SGLT antagonist phloridzin enhanced food intake in rats when given intracerebroventricularly (Tsujii & Bray, 1990), and inhibited glucose-induced excitation of GE neurones in the ventromedial hypothalamus (Yang et al. 1999). More recently, it has been suggested that human SGLT3 may function like a glucose-activated Na+ channel, and is expressed in peripheral cholinergic neurones (Diez-Sampedro et al. 2003).
To assess the contribution of SGLTs to the operation of GE neurones separately from the classical metabolic sensing pathways, O’Malley et al. (2006) examined the effects of non-metabolizable glucose analogues on primary cultures of rat hypothalamic neurones, using Ca2+ imaging to monitor the activity of single cells. They found that 67% of glucose-excited neurones were also excited by α-methylglucopyranoside (α-MDG), a non-metabolizable substrate of SGLTs. The effects of both glucose and α-MDG were abolished by phloridzin or by the removal of extracellular Na+ (Fig. 1), suggesting that they were mediated predominantly by SGLTs. These experiments indicate that generation of ATP is not an essential prerequisite for sugar sensing in many hypothalamic GE neurones, which instead can be non-metabolically excited by glucose via SGLTs. It remains to be elucidated which SGLT isoform(s) mediate these effects: SGLT1-selective substrates activated a smaller proportion of GE neurones (37–45%) than SGLT1/3-non-selective sugars (67%), suggesting that SGLT1 is important but other SGLTs may also contribute (O’Malley et al. 2006). RT-PCR analysis showed expression of mRNA for SGLT1 and 3 isoforms in both cultured hypothalamic neurones and adult hypothalamus (O’Malley et al. 2006).
Interestingly, the amplitude of Ca2+ responses to α-MDG was greater than that of glucose responses, even though the two sugars are not distinguished by SGLTs, and α-MDG was less effective in activating GE neurones in the presence of lactate (O’Malley et al. 2006). The explanation for this is currently unclear but it is possible that energy metabolites inhibit SGLTs via a negative feedback loop, thereby linking non-metabolic glucose sensing with the energy status of the cell. This theory is yet to be tested experimentally.
The role of SGLTs in hypothalamic glucose sensing remains to be explicitly examined by direct measurements of glucose-triggered changes in the membrane potential of neurochemically defined GE neurones. But it is noteworthy that some existing patch-clamp data on GE neurones may already be interpreted in support of the SGLT model. For example, functional GE neurones are still present in the hypothalamic arcuate nucleus of KATP channel knockout (Kir6.2 null) mice, and their glucose-induced excitation involves activation of an inward current with a reversal potential of around –20 mV (Fioramonti et al. 2004). It can be shown using electrochemical equilibrium equations that such reversal potential could be consistent with either non-selective cation channels or electrogenic cation transporters such as SGLTs.
Evidence for ‘non-metabolic’ glucosensing in glucose-inhibited neurones
The involvement of conventional glucose metabolism in GI neuron glucosensing was recently questioned by experiments on orexin/hypocretin neurones, widely projecting hypothalamic cells essential for normal wakefuness and reward-seeking behaviour (de Lecea et al. 2006; Sakurai, 2007). Using whole-cell patch-clamp recordings from GFP-labelled orexin cells in acute mouse brain slices, Gonzalez et al. (2008) found that high concentrations of three different glucokinase inhibitors did not prevent glucosensing responses (Fig. 2A). Like in GI neurones from the ventromedial hypothalamus (Song & Routh, 2005), stimulating ATP production with lactate did not mimic glucose-induced hyperpolarization in these orexin cells, but was instead excitatory (Fig. 2B), as expected from the ubiquitous facilitation of neuronal activity by high energy levels (Mobbs et al. 2001). Furthermore, 2-deoxyglucose, a non-metabolizable glucose analogue, had similar effects to glucose on the membrane potential of orexin cells.
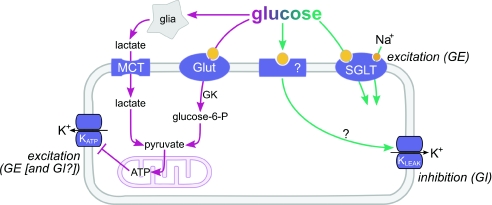
The inhibitory response of orexin neurones to physiological rises in glucose involves opening of leak-like K+ channels in the plasma membrane (Burdakov et al. 2006), but it is not yet understood how glucose opens these channels. The above experiments suggest that this process does not require intracellular metabolism of glucose in these neurones. Based on current evidence, it seems likely that glucose entry is not required at all, since intracellular infusion of glucose via the pipette tip does not initiate the inhibitory response while the same cell still responds to extracellularly applied glucose (Gonzalez et al. 2008). The pharmacology of sugar sensing in orexin cells also hints at dissociation between sensing and uptake, since the sugar transporters GLUT1 and SGLT1 transport fructose and galactose, respectively, yet these sugars do not inhibit orexin cells (Gonzalez et al. 2008). While further work remains to be done on the molecular characterization of GI cell glucosensing, based on current evidence we therefore hypothesize that it belongs in the non-metabolic category (Fig. 3).
Figure 3. Metabolic (purple) and non-metabolic (green) ways through which glucose can alter neuronal electrical activity.
We propose that KATP channels play a general neuroprotective role in both GE and GI neurones (i.e. these channels open and hyperpolarize the cell during energy depletion to stop the cell from using more energy on firing). In addition, in GE neurones, KATP channels may have another way of regulation that allows them to act as more specific glucose sensors. New pathways, such as SGLT and leak K+ channels, may control the activity of GE and GI neurones, respectively, in a ‘non-metabolic’ manner.
Theoretical advantages of ‘metabolic’vs. ‘non-metabolic’ glucose detection
How might the brain benefit from non-metabolic sugar sensing? It seems advantageous to ‘uncouple’ glucose sensing from the energy status of the cell in a variety of scenarios. For example, as well as glucose, a major and perhaps even predominant source of metabolic energy for central neurones is lactate, which they take up via monocarboxylate transporters (MCT, see Fig. 3) (Ainscow et al. 2002; Pellerin & Magistretti, 2004). This lactate is provided by astrocytes, which are capable of using their glycogen stores to generate lactate when glucose levels in the brain are low (Brown & Ransom, 2007). Thus neuronal ATP levels can presumably be maintained, for short periods of time at least, irrespective of ambient glucose levels. In this situation, sensing intracellular metabolic indicators would clearly not be a reliable indicatory of changes in glucose levels, since neuronal metabolism would be protected from glucose fluctuations by astrocyte-derived lactate. Non-metabolic sensing may also be advantageous in an opposite scenario, that of rapid ATP depletion inside the glucose sensing neurone, caused, for example, by brief periods of intense firing such as those seen in in vivo recordings (Mileykovskiy et al. 2005). It may not be advantageous for this type of [ATP] changes to influence the firing of glucose sensing neurone, since such small local depletions of fuel can be more efficiently replenished by uptake of local lactate, rather than by sending an order, via glucosensor cell firing, to engage global, energetically expensive, processes such as food seeking or glucose release from the liver.
Non-metabolic sensing would thus enable behaviour and hormone release to be controlled by true glucose levels rather than by local metabolic environment. Presumably, non-metabolic sensing would also be more versatile than metabolic, since it can still operate in high glucose levels such as in obesity and diabetes, where metabolism may be saturated. For example, SGLTs in GE neurones would still operate at high substrate levels because they can take up glucose against its concentration gradient, while non-metabolic GI sensors can operate under a variety of glucose baselines through a process of adaptation (Williams et al. 2008). This may allow the brain to sense trends in glucose levels irrespective of baseline levels of glucose.
Metabolic sensing may also have specific advantages. Measuring intracellular fuel (e.g. ATP) rather than extracellular substrate (glucose) could be useful when metabolism does not function normally despite normal glucose levels, e.g. if there is a reduced expression of glucose transporters, and so more glucose needs to be ‘ordered’ to compensate. A sensor based on general metabolic intermediaries would also be able to integrate information from different fuel supplies into its measurements – for example showing reduced responses to glucose during abundance of other fuel substrates, e.g. fatty acids. However, this may perhaps be more useful for peripheral tissues, since the brain preferentially uses glucose as its energy fuel.
In conclusion, both metabolic and non-metabolic glucose sensors may have distinct advantages for brain function. Combining the two may enable the brain to monitor all aspects of its energy supply. We would like to hypothesize that metabolic sensors act as ‘emergency alarms’, detecting large abnormalities in energy levels which break down the metabolic buffer provided by the astrocytic generation of lactate. In turn, non-metabolic sensors could be better placed to carry out ‘predictive’ monitoring of glucose in the more physiological range, treating glucose as a signal while using astrocytic lactate as fuel.
References
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K+ channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005a;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005b;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–R600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A. 2003;100:11753–11758. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Lorsignol A, Taupignon A, Penicaud L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes. 2004;53:2767–2775. doi: 10.2337/diabetes.53.11.2767. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K (ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes. 2005;54:3169–3174. doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Sugimori M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. J Physiol. 1986;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Kow LM, Yang XJ. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab. 2001;281:E649–E654. doi: 10.1152/ajpendo.2001.281.4.E649. [DOI] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes. 2006;55:3381–3386. doi: 10.2337/db06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Bray GA. Effects of glucose, 2-deoxyglucose, phlorizin, and insulin on food intake of lean and fatty rats. Am J Physiol Endocrinol Metab. 1990;258:E476–E481. doi: 10.1152/ajpendo.1990.258.3.E476. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM. Renal Na+-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor: similarities to and differences from pancreatic b-cell mechanisms. Diabetes. 1999;48:1763–1772. doi: 10.2337/diabetes.48.9.1763. [DOI] [PubMed] [Google Scholar]