Abstract
The attenuation of food intake as induced by an increase in serotonergic (5-hydroxytryptamine, 5-HT) efficacy has been a target of antiobesity pharmacotherapies. However, the induction of tolerance and/or side-effects limited the clinical utility of the earliest serotonin-related medications. With the global prevalence of obesity rising, there has been renewed interest in the manipulation of the serotonergic system as a point of pharmacological intervention. The serotonin2C receptor (5-HT2CR), serotonin1B (rodent)/serotonin1Dβ (human) receptor (5-HT1B/1DβR) and serotonin6 receptor (5-HT6R) represent the most promising serotonin receptor therapeutic targets. Canonical serotonin receptor compounds have given way to a myriad of novel receptor-selective ligands, many of which have observable anorectic effects. Here we review serotonergic compounds reducing ingestive behaviour and discuss their clinical potential for the treatment of obesity.
Early pharmacological manipulations identified an inverse relationship between the biogenic amine neurotransmitter serotonin and food intake. More specifically, a selective reduction in serotonin bioavailability was associated with hyperphagia and subsequent weight gain, whilst diminished food intake was induced by an increase in serotonin efficacy (Saller & Stricker, 1976; Fletcher & Paterson, 1989). Researchers further sought to clarify which of the 14 distinct serotonin receptors (5-HTRs) identified in vertebrates (clustered into 7 ‘families’, 5-HT1–5-HT7, based on sequence homology and effector pathways) are critically involved in serotonin's effects on ingestive behaviour. Using pharmacological and genetic tools, the 5-HT1BR, 5-HT2CR and 5-HT6R subtypes were shown to be the principal mediators through which serotonin exerts its anorectic effects in rodents, and as such, these receptors have been investigated as pharmacotherapeutic targets for the treatment of obesity. Here we review the serotonergic compounds currently lending themselves to the treatment and investigation of obesity (Table 1).
Table 1.
Serotonergic compounds inducing hypophagia
| Compound | Mechanism | Manufacturer (clinical status) | Selected reference |
|---|---|---|---|
| Inc. bioavail. | |||
| 5-HTP | 5HT precursor | — | Fletcher & Burton (1986) |
| Fenfluramine | SSRI and releaser | Servier (Withdrawn in 1997) | Halford et al. (2007) |
| Fluoxetine | SSRI | Eli Lilly (Withdrawn from Phase III) | Yen et al. (1987) |
| Paroxetine | SSRI | GlaxoSmithKline | Konkle et al. (2003) |
| Sertraline | SSRI | Pfizer | Nielsen et al. (1992) |
| Fluvoxamine | SSRI | Solvay Pharmaceuticals | Nonogaki et al. (2007) |
| Sibutramine | SNRI | Knoll/Abbott (Registered therapy) | Connoley et al. (1995) |
| 5-HT1 | |||
| CP-93, 129 | 5-HT1BR agonist | Pfizer | Macor et al. (1990) |
| CP-94, 253 | 5-HT1BR agonist | Pfizer | Koe et al. (1992) |
| RU-24969 | 5-HT1A/1BR agonist | Organon | Kennett et al. (1987) |
| 5-HT2C | |||
| Ro 60-0175 | agonist | Organon/Hoffman-La Roche | Martin et al. (1998) |
| Ro 60-0332 | agonist | Organon/Hoffman-La Roche | Martin et al. (1998) |
| Org-12962 | agonist | Organon (Phase II as antidepressant) | Nilsson (2006) |
| Org-37684 | agonist | Organon | Schreiber & De Vry (2002) |
| VER-3323 | agonist | Vernalis/Roche | Bentley et al. (2004) |
| VER-23779 | agonist | Vernalis/Roche | Somerville et al. (2007) |
| VER-2692 | agonist | Vernalis/Roche | Adams et al. (2006) |
| VER-5584 | agonist | Vernalis/Roche | Bentley et al. (2004) |
| VER-5593 | agonist | Vernalis/Roche | Bentley et al. (2004) |
| VER-8775 | agonist | Vernalis/Roche | Dourish et al. (2004) |
| BVT.933 | agonist | Biovitrum (Withdrawn from Phase IIb) | Svartengren et al. (2003a) |
| BVT-X | agonist | Biovitrum | Lam et al. (2008) |
| YM348 | agonist | Yamanouchi Pharm. | Hayashi et al. (2004a) |
| APD-356 | agonist | Arena Pharm. (Phase IIb completed) | Thomsen et al. (2008) |
| ATHX-105 | agonist | Athersys | Nilsson (2006) |
| WAY-163909 | agonist | Wyeth | Dunlop et al. (2005) |
| WAY-161503 | agonist | Wyeth | Rosenzweig-Lipson et al. (2006) |
| WAY-629 | agonist | Wyeth | Sabb et al. 2004 |
| LY448100 | agonist | Eli Lilly | Nilsson (2006) |
| IL-639 | agonist | Bristol Myers Squibb | Nilsson (2006) |
| PNU-22394 | agonist | Pharmacia (Phase I) | McCall et al. (2001) |
| 5-HT1/2 | |||
| mCPP | 5-HT1B/2CR agonist | Now distributed by multiple vendors | Kennett & Curzon (1988) |
| TFMPP | 5-HT1B/2A/2CR agonist | Now distributed by multiple vendors | Kennett et al. (1987) |
| MK-212 | 5-HT2R agonist | Now distributed by multiple vendors | Halford et al. (1997) |
| DOI | 5-HT2R agonist | Now distributed by multiple vendors | Simansky & Vaidya (1990) |
| 5-HT6 | |||
| BVT.74316 | antagonist | Biovitrum (Phase I completed) | Heal et al. (2008) |
| BVT.5182 | antagonist | Biovitrum | Svartengren et al. (2003b) |
| E-6837 | partial agonist | Esteve | Fisas et al. (2006) |
| PRX-07034 | antagonist | Epix (Phase I completed) | Shacham et al. (2006) |
| SB-271046 | antagonist | GlaxoSmithKline | Svartengren et al. (2004) |
| Ro 04–6790 | antagonist | Organon/Hoffman-La Roche | Woolley et al. (2001) |
| SB-399885 | antagonist | GlaxoSmithKline | Perez-Garcia & Meneses (2005) |
| SB-357134 | antagonist | GlaxoSmithKline | Perez-Garcia & Meneses (2005) |
Abbreviations: SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and noradrenalin reuptake inhibitor.
Efforts to elucidate a site of action for 5-HTR-mediated ingestive behaviour highlighted the hypothalamus as a key component (Breisch et al. 1976; Fletcher & Paterson, 1989; Heisler et al. 2002). The physiological manifestations of appetite, and subsequent responses to caloric intake, involve the integration of multiple central and peripheral signals at this site. Perturbed feeding behaviour and consequential effects on body weight effectuated by surgical ablation of specific hypothalamic nuclei was the first unequivocal evidence as to the fundamentality of this brain region in modulating appetite. Much of the neurochemistry that underpins this function has begun to be elucidated. Niche populations of peptidergic neurones, principally within the arcuate (ARC), ventromedial (VMH) and paraventricular (PVH) nuclei and lateral hypothalamic area (LHA), form an intricate feeding circuit reactive to numerous appetitive signals, and initiate neuroendocrine and behavioural responses to food intake.
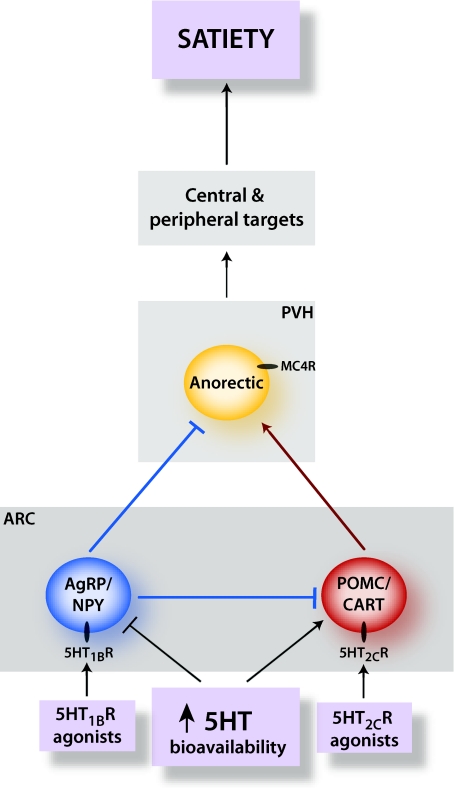
Acute administration of serotonergic compounds altered the expression of such peptidergic appetitive effectors within the hypothalamus, namely an increase in anorectic pro-opiomelanocortin (POMC) mRNA and a decrease in orexigenic neuropeptide Y (NPY) mRNA (Choi et al. 2006), both of which are synthesized within discrete neuronal populations of the ARC. Recently, it has been shown that manipulation of these first order hypothalamic POMC/cocaine and amphetamine regulated transcript (CART) and agouti-related protein (AgRP)/NPY neurones is a mechanism through which serotonergic compounds reduce food intake (Fig. 1). Specifically, the serotonin system concomitantly regulates the antagonistic functions of POMC/CART and AgRP/NPY neurones through neurotransmitter binding of two spatially distinct G-protein coupled receptor subtypes: depolarizing POMC/CART neurones via action at Gq-coupled 5-HT2CRs (Heisler et al. 2002) and hyperpolarizing AgRP/NPY neurones through action at Gi-coupled 5-HT1BRs (Heisler et al. 2006; Fig. 1). Furthermore, the anorectic effect of compounds increasing serotonergic bioavailability and 5-HT2CR and 5-HT1BR agonists is contingent upon the downstream activation of the melanocortin 4 receptors (MC4Rs) (Heisler et al. 2006; Lam et al. 2008). It is noteworthy that these serotonergic compounds, which are highly effective in reducing food intake, are rendered ineffective by pharmacological or genetic inactivation of this single downstream melanocortin receptor target (Heisler et al. 2006; Lam et al. 2008). These data elucidate that the melanocortin pathway is a key downstream target for serotonergic compound hypophagia. Recent research has further clarified that the key population of MC4Rs influencing appetite is expressed in the PVH and/or amygdala (Balthasar et al. 2005).
Figure 1. Proposed model of a serotonergic pathway modulating food intake.
An increase in serotonin bioavailability (due to food intake or pharmacological compounds such as sibutramine and fenfluramine) or direct agonism of 5HT2CRs and 5HT1BRs modulates firing of pro-opiomelanocortin (POMC)/cocaine and amphetamine regulated transcript (CART) and agouti related protein (AgRP)/neuropeptide Y (NPY) neurones within the arcuate nucleus of the of the hypothalamus (ARC). Anorectic POMC neurones expressing 5HT2CR depolarize on receptor activation and release α-melanocyte-stimulating hormone (α-MSH), which in turn activates second-order melanocortin 4 receptor (MC4R) expressing neurones, principally within the paraventricular nucleus of the hypothalamus (PVH; Balthasar et al. 2005). Concomitant activation of 5HT1BRs expressed on orexigenic AgRP/NPY neurones within the ARC causes membrane hyperpolarization and subsequent inhibition of neuropeptide release. Inhibitory 5HT1BR activation also attenuates inhibitory postsynaptic currents onto POMC/CART neurones further potentiating anorexigenesis. Subsequent downstream neuroendocrine signalling promotes satiety and the cessation of food intake.
In addition to serotonergic modulation of neurones containing the melanocortin agonist and antagonist, serotonin may also directly influence the activity of MC4R-containing cells. For example, infusion of serotonin into the PVH of rats reduces food intake (Fletcher & Paterson, 1989). Corticotropin-releasing hormone (CRH) neurones expressed within the PVH are directly innervated by serotonergic projections and both c-fos induction and CRH expression are stimulated by compounds increasing serotonergic efficacy (Liposits et al. 1986; Bovetto et al. 1996; Choi et al. 2006). A subpopulation of CRH neurones have been reported to express MC4Rs (Lu et al. 2003), and it is possible that serotonin may directly influence the activity of these CRH MC4R-expressing cells. Additional research is required to determine if this is the case. Such further research investigating the down- and upstream pathways through which serotonin influences appetite may yield additional pharmacological targets for the treatment of obesity.
Compounds increasing 5-HT bioavailability
Fenfluramine, an amphetamine derivative lacking psychostimulant properties, was synthesized in the 1970s, and followed 20 years later by the more efficacious enantiomer, dexfenfluramine. Both compounds were successfully prescribed (often in combination with phentermine) as anorectic treatments for obesity until their withdrawal from clinical use in 1997, due to corollary incidences of cardiopulmonary complications. Mechanistically, these drugs are analogous to amphetamine, causing reversal and blockade of the serotonin transporter and a consequential increase in serotonin efflux and synaptic persistence (Crespi et al. 1997). Furthermore, the major metabolite of fenfluramine, norfenfluramine, is a 5-HT2CR agonist (Gibson et al. 1993). Genetic and pharmacological studies demonstrated that action at the 5-HT2CRs and 5-HT1BRs is required for fenfluramine to influence ingestive behaviour (Lucas et al. 1998; Vickers et al. 1999, 2001; Simansky & Nicklous, 2002).
The effect of fenfluramine on body weight has been convincingly established by a number of experimental and clinical analyses (for a more extensive review see Halford et al. 2007). In obese rodents and humans, fenfluramine administration attenuated body weight gain in a manner consistent with a more rapid onset of satiety and, in the case of humans, suppressed premeal hunger (Fisler et al. 1993; Halford et al. 2007). In chronic fenfluramine trials, obese patients attained maximal weight reduction by 24 weeks, but demonstrated a considerable re-accumulation of body weight upon cessation of treatment after 3.5 years (Fernstrom & Choi, 2008). A similar effect was seen during intermittent chronic fenfluramine administration in rodents (5 days of drug followed by 5 days no drug, repeated 10 times), inasmuch as hypophagia and weight loss were only observed for the first 2 days of each treatment period (Choi et al. 2002). During no-drug periods animals regained body weight, due to increased food intake, before losing it again during the successive treatment period (Choi et al. 2002).
Administration of exogenous serotonin and its precursors also elicits a potent anorectic effect in humans and rodents. Injections of serotonin directly into the PVH of rats resulted in a significant decrease in food intake due to a reduction in meal size and duration (Fletcher & Paterson, 1989). However, serotonin itself fails to penetrate the blood–brain barrier and as such is of little therapeutic salience. By contrast, peripheral administration of 5-hyroxytryptophan (5-HTP), the carboxylated precursor of serotonin, successfully suppressed food intake (Fletcher & Burton, 1986). Within a clinical context, 5-HTP has demonstrated observable hypophagic effects in obese subjects and an associated reduction in body weight; moreover, the attenuation of caloric intake was correlated with an enhancement of within-meal satiety (Ceci et al. 1989). Despite these results, 5-HTP is not a registered antiobesity therapy. However, its amino acid precursor, tryptophan, has demonstrated anorectic effects in a clinical setting and is available as an adjunctive antidepressant medication within the UK (Halford et al. 2007).
Another class of drugs that augment serotonin bioavailability are the serotonin reuptake inhibitors. Via the blockade of monoamine transporters, these drugs promote the synaptic persistence of serotonin and thus sustained activation of postsynaptic receptors. Such compounds are more generally associated with the treatment of depression or anxiety; however, in light of their indiscriminate effect on serotonin concentrations, many also exhibit anorectic properties in rodents. For example, the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac®) significantly reduced food intake and body weight gain in the rat (Yen et al. 1987; Heisler et al. 1997; Heisler et al. 1999). Other SSRIs demonstrating therapeutic potential for obesity treatment include fluvoxamine (Nonogaki et al. 2007), paroxetine and sertraline (Halford et al. 2007).
Sibutramine (Reductil®) is a serotonin and noradrenalin reuptake inhibitor (SNRI) and a registered antiobesity treatment. Rodent studies demonstrated that sibutramine enhanced satiety (Halford et al. 1995) and induced significant hypophagia and weight loss (Connoley et al. 1995). Interestingly, studies using obese models (ob/ob mice and Zucker rats) revealed that chronic administration of sibutramine can elicit significant weight loss without a prolonged effect on food intake, suggesting that this compound also influences energy expenditure (Connoley et al. 1995; Day & Bailey, 1998; Golozoubova et al. 2006). Consistent with rodent work, clinical studies of sibutramine administration demonstrated that through the enhancement of satiety, chronic dosing leads to attenuated food intake and subsequent weight loss in the order of 10% (for review see Halford et al. 2007). Furthermore, these effects could be potentiated by a conjunctive low calorie diet, cogently demonstrating the need for life-style counselling in addition to pharmacological intervention (Apfelbaum et al. 1999). In long-term human studies, maximal weight loss was observed at 6 months followed by a stabilized body weight significantly lower than baseline levels or placebo controls (McNeely & Goa, 1998). During one such trial, food intake was still significantly reduced after 10 months of treatment, indicating the continued efficacy of the drug (Barkeling et al. 2003). At present, sibutramine represents the standard in serotonergic antiobesity therapeutics and is the yard stick by which the efficacy of newly developed compounds will be judged.
Compounds targeting serotonin receptors
5-HT1AR and 5-HT1BR compounds
5-HT1AR activation results in membrane hyperpolarization via Gi mediated inhibition of cAMP production (for review see Lam & Heisler, 2007). 5-HT1ARs, principally located on serotonergic efferents and cell bodies, are characterized autoreceptors which suppress action potential firing. Consistent with this function, administration of the 5-HT1AR agonist 8-OH-DPAT elicited an increase in rodent feeding (Hutson et al. 1988). A perhaps more critical role for the 5-HT1ARs in the serotonergic regulation of anxiety and depression has been supported by murine genetic studies (Abenhaim et al. 1996; Heisler et al. 1998; Parks et al. 1998; Ramboz et al. 1998; Gross et al. 2002), and drug discovery efforts related to this receptor have thus far not been focused on obesity.
Of the rodent 5-HT1Rs, it is the 5-HT1BR subtype that is the most intimately associated with appetitive control. The human homologue of the rodent 5-HT1BR is the 5-HT1DβR. 5-HT1BR knockout mice exhibit an approximate 9–17% elevation in body weight compared to wild-type littermates and an increase in feeding that is consistent with increased body weight (Bouwknecht et al. 2001; see Table 2). 5-HT1BR knockout mice also displayed attenuated responses to the anorectic effect of fenfluramine and the classic 5-HT1A/1BR agonist RU24969 (Lucas et al. 1998). Complementing these genetic studies, the selective 5-HT1BR pyridine agonists CP-93,129 and CP-94,253 have both demonstrated significant acute anorectic effects in rodents (Macor et al. 1990; Koe et al. 1992), effects blocked by pretreatment with 5-HT1BR antagonists (Lee et al. 2002; Heisler et al. 2006). Prolonged administration of CP-94,253 decreased food intake in rats and reduced body weight gain, although drug desensitization was apparent within a week (Koe et al. 1992). CP-93,129, despite being significantly more selective than CP-94,253, demonstrated poor blood–brain barrier penetration and thus a lack of anorectic properties when peripherally administered (Macor et al. 1990; Lee & Simansky, 1997). The anorectic effect of these 5-HT1BR agonists appears to involve hyperpolarization of ARC AgRP/NPY neurones and a disinhibition of POMC/CART neurones, and ultimately, activation of the downstream MC4Rs (Heisler et al. 2006).
Table 2.
Feeding and body weight phenotypes of 5-HTR knockout mice
| Receptor | Feeding & body weight phenotypes of mutant animals | Additional comments | References |
|---|---|---|---|
| 5-HT1A | Increased intake of sucrose solution in females (potentially sex-hormone related). No alterations in homecage feeding or body weight reported. | Increased anxiety related behaviours and reduced depression-related behaviours | Heisler et al. (1998); Parks et al. (1998); Ramboz et al. (1998); Bechtholt et al. (2008) |
| 5-HT1B | Mildly increased body weight and relative increase in food intake | Reduced sensitivity to d-fen and mCPP induced hypophagia. Compensatory reduction in 5HT2CR function | Lucas et al. (1998); Bouwknecht et al. (2001); Clifton et al. (2003); Lee et al. (2004) |
| 5-HT2A | No alterations in homecage feeding, novelty suppressed feeding or body weight reported. | Increased anxiety related behaviours | Weisstaub et al. (2006) |
| 5-HT2B | Not reported | Mutants died perinatally due to incomplete heart development | Nebigil et al. (2000) |
| 5-HT2C | Marked hyperphagia throughout life and increased body weight gain from around 12 weeks | Increased locomotor activity and subsequent age-dependent reductions in energy cost of physical activity. Dysregulation of HPA axis and reduced anxiety-related behaviour | Tecott et al. (1995); Nonogaki et al. (2003); Heisler et al. (2007a,b) |
| 5-HT3 | No observed differences in body weight or food intake | Dysregulation of the HPA axis in response to stress | Bhatnagar et al. (2004) |
| 5-HT4 | Modestly reduced weight gain in homecage environment, despite normal food intake. Food intake increased after restraint induced stress | This model of stress induced anorexia has been associated with an increase in CART expression | Compan et al. (2004); Jean et al. (2007) |
| 5-HT5 | Normal body weight. No data on food intake reported | Increased exploratory behaviour that was independent of an effect on anxiety | Grailhe et al. (1999) |
| 5-HT6 | Normal body weight. No data on food intake reported | Altered responses to ethanol | Bonasera et al. (2006) |
| 5-HT7 | Normal body weight. No data on food intake reported | Failed to exhibit the expected hypothermic response to serotonin administration | Hedlund et al. (2003) |
Abbreviations: d-fen, dexfenfluramine; HPA, Hypothalamic-Pituitary-Adrenal; CART; cocaine and amphetamine regulated transcript.
5-HT2CR compounds
In light of compelling pharmacological and genetic evidence, manipulation of 5-HT2CR signalling has been a focus of serotonin-related obesity drug discovery efforts. The established involvement of this particular receptor subtype in the broader pharmacokinetic activities of dexfenfluramine, sibutramine and 1-3(chlorophenyl)piperazine (mCPP) elegantly highlight its therapeutic pertinence. Furthermore, genetic abrogation of 5-HT2CR expression in mice engendered marked hyperphagia and middle age onset obesity (Tecott et al. 1995; see Table 2).
Two older compounds, Ro 60-0175 and Ro 60-0332, both described as full 5-HT2CR agonists, demonstrated significant anorectic properties in a paradigm of palatable food consumption in normal rats (Martin et al. 1998). Continuous subcutaneous infusion of the former revealed that hypophagia only ensued for the first 10 days of a 2 week regime. Moreover, body weight accumulation was markedly reduced compared to vehicle from 2 days postimplantation and persisted throughout the duration of the study (Vickers et al. 2000). The authors suggested that the prolonged weight-reducing effect in the absence of sustained hypophagia was due to drug-induced hyperthermia, leading to increased energy expenditure. Such a thermogenic effect was also observed by Hayashi et al. (2005) during chronic oral administration of Ro 60-0175, although interestingly in this case, attenuated weight gain and hypophagia persisted concomitantly. Consistent with these effects being transmitted via the 5-HT2CR, SB242084 antagonist pretreatment negated the hypophagic and hyperthermic properties of Ro 60-0175 (Hayashi et al. 2005). However, Ro 60-0175 also appears to have binding affinity for the 5-HT2BRs, which may limit its therapeutic potential given that action at peripheral 5-HT2BRs is thought to underlie the cardiopulmonary complications associated with some serotonergic drugs (Martin et al. 1998; Fitzgerald et al. 2000).
The Yamanouchi Pharmaceutical Company's 5-HT2CR agonist YM348 also elicited a robust dose-dependent decrease in food consumption in Zucker rats, with almost complete aphagia at some concentrations (Hayashi et al. 2004a). However, continuous infusion failed to sustain a hypophagic effect, with treated animals returning to control levels by day 10, although body weight gain remained significantly lower than controls (Hayashi et al. 2004a). This observation is potentially explained by augmented energy and calorie expenditure, as these physiological indices were not desensitized by prolonged YM348 treatment (Hayashi et al. 2004a,b). However, despite promising antiobesity properties, the affinity of this drug for the 5-HT2BR is only 2-fold lower than that for 5-HT2CR, again alluding to the potential for adverse clinical side-effects.
Vernalis, in collaboration with Hoffmann-La Roche, have synthesized a number of 5-HT2CR compounds with demonstrable anorectic activity. VER-3323, VER-5593 and VER-5348 are all indoline-based analogues similar in structure to Ro 60-175, although with greater 5-HT2CR selectivity. Both subcutaneous and oral administration of these compounds elicited hypophagia in food deprived rats (Bentley et al. 2004). A subsequent study in Siberian hamsters revealed that VER-3323, whilst decreasing food intake, had no observable effect on energy expenditure in the form of thermogenesis or locomotion (Schuhler et al. 2005). A more recent Vernalis compound, VER-2692, exhibited a greater selectivity profile than the indoline derivatives and also resulted in hypophagia when administered acutely to food deprived rats, a response blocked by 5-HT2CR antagonist pretreatment (Adams et al. 2006). A perhaps more promising Vernalis compound is VER-8775, a piperazine derivative with 15- and 476-fold selectivity for the 5-HT2CRs over 5-HT2ARs and 5-HT2BRs, respectively (Nilsson, 2006). VER-8775 significantly reduced food intake in fasted rats and mice and induced weight loss in diet-induced obese (DIO) mice during a 28-day oral regime (Dourish et al. 2004).
The Wyeth compound WAY-161503 has exhibited potent anorectic and weight reducing effects in both mouse and rat models of obesity (Rosenzweig-Lipson et al. 2006). During chronic trials in Zucker rats, drug tolerance was not observed, with both food intake and weight accumulation remaining significantly decreased throughout the 15 day study. While these results were promising, functional selectivity assays demonstrated that WAY-161503 offered no apparent selectivity over 5-HT2BRs, although SB215505 (5-HT2BR antagonist) pretreatment did not ablate the anorectic properties of this drug (Rosenzweig-Lipson et al. 2006). A more recent Wyeth compound, WAY-163909, is characterized as a full 5-HT2CR agonist and partial 5-HT2BR agonist, despite being 46-fold more selective for the former (Dunlop et al. 2005). In acute feeding paradigms, WAY-163909 reduced food intake in lean and obese rodent models. Significantly, no tolerance to this compound was observed after a 10-day regime and furthermore animals exhibited a 46% reduction in body weight as compared to controls (Dunlop et al. 2005).
Preclinical data on BVT.933 (generated by Biovitrum) indicated that chronic dosing of this compound was successful in attenuating both food intake and weight accumulation in DIO rats, and furthermore, that these results were correlated with a reduction in adiposity (Svartengren et al. 2003a). The compound was entered into clinical trials, but was withdrawn from Phase IIb. A subsequent compound developed by Biovitrum, BVT.X, has recently been characterized. Lam et al. (2008) demonstrated that acute administration of BVT.X was effective in reducing food intake in two models of murine obesity. However, chronic dosing via osmotic minipumps in leptin deficient obese ob/ob mice revealed that this anorectic effect was relatively short lived, with treated animals returning towards control levels just 2 days through the 7 day trial. Interestingly, despite desensitization to hypophagia, body weight gain was significantly attenuated at the end of the trial. Lam et al. (2008) investigated the central pathways associated with BVT.X's effect on energy balance and observed an up-regulation of POMC expression in the ARC and further determined that downstream activation of MC4Rs was required for this compound to exert its anorectic effect (see Fig. 1). The stimulation of this melanocortin anorexigenic pathway is consistent with a previously asserted model of dexfenfluramine and CP-94 253 anorexia (Heisler et al. 2002, 2006).
One of the most recently reported 5-HT2CR compounds is Arena's APD-356 (Lorcaserin®). This benzapine molecule exhibited robust functional selectivity over 5-HT2ARs and 5-HT2BRs (18- and 104-fold, respectively; Thomsen et al. 2008). Acute oral dosing of APD-356 significantly reduced cumulative food intake in non-deprived rats for up to 22 h, although post hoc analysis attributed this observation to potent hypophagia during the first 2 hours and the absence of subsequent rebound hyperphagia (Thomsen et al. 2008). This effect was readily ablated by 5-HT2CR antagonist pretreatment, suggesting the functional selectivity of the compound for the 5-HT2CR. In chronic assessments, APD-356 successfully attenuated both food intake and body weight gain in DIO rats (Thomsen et al. 2008). Although anorectic desensitization was observed at 13 days, body weight remained significantly lower than controls throughout the course of the study, indicating further effects on additional modalities of weight loss. During the 4 week withdrawal period, treated animals exhibited rebound hyperphagia and a subsequent return to control body weight (Thomsen et al. 2008). Significantly, high dosing of both Sprague–Dawley rats and cynomolgus monkeys over 3 months failed to induce any cardiopulmonary side-effects, consistent with the compound's low selectivity for peripheral 5-HT2BR (Smith et al. 2006). APD-356 is currently in Phase 3 clinical trials having successfully demonstrated weight reducing effects during a 12 week Phase IIb study. Drug treated obese patients exhibited a statistically significant 1.3 kg reduction in body weight. No valvopathies or pulmonary complications were observed in these patients or other clinical cohorts (Smith et al. 2006).
Additional 5-HT2CR compounds with observed antiobesity properties include PNU-22394, a relatively non-selective Pharmacia/Pfizer compound with anorectic effects in rodents and humans (McCall et al. 2001); ATHX-105 and ATH-188651, from Athersys; and IL639 from Bristol-Myers Squibb which demonstrated a 250-fold selectivity over 5-HT2A/2BRs and is orally efficacious in rat feeding paradigms (Nilsson, 2006).
5-HT1/2R compounds
Compounds that demonstrate a degree of 5-HT1R and 5-HT2R cross-talk have often proved highly effective in modulating feeding behaviour, most likely due to their concerted action at anorexigenic and orexigenic populations of hypothalamic neurones. For example, mCPP exhibits a preferential affinity for 5-HT1BR and 5-HT2CR subtypes and induced robust hypophagia during both acute and chronic regimes (Kennett & Curzon, 1988; Vickers et al. 2003). Significant attenuation of body weight gain was also observed during a 28 day study, with no apparent drug tolerance (Vickers et al. 2003). Furthermore, the use of pair-fed animals in this study strongly implicated the hypophagic effect of the compound as underlying the observed reduction in weight accumulation. In human subjects, mCPP was capable of reducing caloric intake at a test meal by 30% when administered to healthy women, an effect associated with a decrease in premeal hunger ratings (Halford et al. 2007). A similar decline in premeal hunger and body weight was observed in obese men receiving mCPP over a 2 week period, although assessment of food consumption was not reported (Halford et al. 2007). 1-[3-(Trifluoromethyl) phenyl]piperazine (TFMPP), a structurally related analogue of mCPP, also elicited observable hypophagic and weight reducing effects, although drug desensitization was observed during chronic administration (Kennett et al. 1987; Rouru et al. 1993). Hypophagia induced by mCPP and TFMPP is consistent with enhancement of satiety, although at higher doses both drugs have shown to induce hypoactivity (Kennett & Curzon, 1988).
5-HT6R compounds
5-HT6R distribution within the CNS includes hypothalamic regions of immediate salience to a role in appetitive control, including the ARC, PVH and VMH (Heal et al. 2008). Concordant with this expression profile, manipulation of 5-HT6R signalling has been demonstrated to have potent effects on both food consumption and body weight (for review see Heal et al. 2008). It is of note that it is the antagonism of this receptor that is generally associated with its antiobesity function (Heal et al. 2008). One of the earliest 5-HT6R antagonists reported to induce hypophagia was Ro 04–6790 (Organon/Hoffman-La Roche), which when administered to rats on three consecutive days, resulted in a suppression of body weight gain that persisted for at least 10 days postwithdrawal (Woolley et al. 2001). Interestingly, the same study also demonstrated that food consumption and weight gain were decreased following antisense knockdown of 5-HT6R, a phenotype absent in traditional 5-HT6R knockout mice (Woolley et al. 2001; Bonasera et al. 2006).
Biovitrum's BVT.5182 5-HT6R antagonist has also demonstrated significant anorectic properties during both chronic and acute studies in rat and mouse models of obesity. In DIO mice, repeated subcutaneous doses elicited a 9% and 11% decrease in food consumption and body weight, respectively (Svartengren et al. 2003b). Subsequent studies in obese ob/ob mice and non-obese rats demonstrated that the observed hypophagia was attributable to enhanced satiety (Svartengren et al. 2004). This later study also reported that acute doses of the GlaxoSmithKline 5-HT6R antagonist SB-271046 dose-dependently suppressed food consumption. However, Phase I trials of SB-271046 were apparently halted due to poor blood–brain barrier permeability. Little has been reported about Biovitrum's second 5-HT6R compound, BVT.74316, though it did complete a Phase Ia study as an antiobesity therapy (press release of Biovitrum, 19 December 2007). PRX-07034 from Epix Pharmaceuticals has also recently completed Phase I trials (press release of Epix-Pharmaceuticals, 29 October 2007). In preclinical studies, chronic administration successfully reduced food intake and body weight in DIO rats; moreover, it would appear that no drug tolerance was observed over the course of the 5 week study (Shacham et al. 2006).
E-6837 (Esteve) represents a curious pharmacological paradox in that it is classified as a 5-HT6R partial agonist, but like 5-HT6R full antagonists, it reduces body weight (for discussion on this issue, see Heal et al. 2008). Specifically, chronic oral administration of E-6837 significantly attenuated body weight by day 3 and continued to do so until reaching a plateau at day 20. At the culmination of the trial, E-6837 treated animals were approximately 6% lighter than a sibutramine control group. Furthermore, whilst the sibutramine group regained control weight by day 44, E-6837 treated animals were still significantly lighter at day 71 (Fisas et al. 2006). Interestingly, E-6837-induced hypophagia lasted for only the first 3 weeks after which significant rebound hyperphagia, commencing at week 1 of the withdrawal analysis, was observed (Fisas et al. 2006). Although no thermogenic effects were reported, further elucidation of E-6837 regulated energy expenditure and metabolism is warranted.
Conclusion
Pharmacological and genetic studies have consistently demonstrated the fundamentality of the serotonergic system in the regulation of appetite and ingestive behaviour. Moreover, they have highlighted the involvement of specific serotonin receptor subtypes in mediating these effects. Historically, pharmacological compounds that augment endogenous serotonergic signalling have proved effective in suppressing food consumption and attenuating body weight gain, physiological effects of immediate clinical salience to the ever increasing incidence of obesity. Furthermore, with many antiobesity compounds falling short of optimal therapeutic efficacy (i.e. due to minimal weight loss, adverse side-effects or long-term resistance), there is a substantial unmet clinical need for novel pharmacological therapies. Currently, the 5-HT2CR and 5-HT6R have garnered the most attention in serotonin drug discovery efforts. Both 5-HT2CR agonists and 5-HT6R compounds have entered into clinical trials. Critically, the success of any 5-HT2CR compound is contingent upon its functional selectivity, in particular over the peripherally expressed 5-HT2BRs. In this regard, 5-HT6R compounds hold an advantage, inasmuch as they demonstrate little affinity for other serotonin receptor subtypes. Although the neuro-molecular/pharmacological aspects of 5-HT6R function remain to be fully elucidated, a number of specific compounds have demonstrated significant antiobesity potential in preclinical studies. Given the apparent independence of 5-HT6R mediated appetite from that of 5-HT2CR, the possibility of combinational therapies that exploit both pathways bears considering.
Energy homeostasis is a critical balance between the intake and utilization of energy, such that a decrease in the former or an increase in the latter engenders a negative energy balance and an associated loss of weight in the form of fat. Although the weight-reducing effects of most serotonergic compounds are principally mediated via their anorectic properties, evidence from a number of pharmacological studies has indicated that augmented energy expenditure may also play a role (Connoley et al. 1995; Vickers et al. 2000; Hayashi et al. 2004b; Golozoubova et al. 2006; Lam et al. 2008). The melanocortin pathway, which is downstream of the homeostatic serotonergic system, has been demonstrated to promote energy expenditure. The necessity for intact MC4R signalling in the anorectic actions of a number of serotonergic compounds (Heisler et al. 2002, 2006; Lam et al. 2008) may also be of pertinence to the promotion of serotonin mediated energy expenditure. Murine genetic studies have reported the involvement of the MC4R in modulation of a number of expending processes, including locomotion, diet-induced thermogenesis and lipolysis (reviewed in Ellacott & Cone, 2006). Furthermore, the role of these mechanisms in regulating energy homeostasis can be spatially dissociated from the anorectic properties of MC4R (Balthasar et al. 2005). Whether the MC4Rs are a downstream component of serotonergic-mediated energy expenditure remains to be fully clarified.
In conclusion, it is apparent that the serotonergic system offers therapeutic potential in the unremitting battle against obesity. Continued pharmaceutical investment in this cause, together with more spatially refined genetic models, will afford researchers more specific tools with which to unravel the neurology of this increasingly pervasive condition.
Acknowledgments
This work was supported by the NIDDK DK065171 and the Wellcome Trust (L.K.H).
References
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- Adams DR, Bentley JM, Benwell KR, Bickerdike MJ, Bodkin CD, Cliffe IA, Dourish CT, George AR, Kennett GA, Knight AR, Malcolm CS, Mansell HL, Misra A, Quirk K, Roffey JR, Vickers SP. Pyrrolo (iso) quinoline derivatives as 5-HT (2C) receptor agonists. Bioorg Med Chem Lett. 2006;16:677–680. doi: 10.1016/j.bmcl.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long-term maintenance of weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. Am J Med. 1999;106:179–184. doi: 10.1016/s0002-9343(98)00411-2. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Barkeling B, Elfhag K, Rooth P, Rossner S. Short-term effects of sibutramine (Reductil) on appetite and eating behaviour and the long-term therapeutic outcome. Int J Obes Relat Metab Disord. 2003;27:693–700. doi: 10.1038/sj.ijo.0802298. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Smith K, Gaughan S, Lucki I. Sucrose intake and fasting glucose levels in 5-HT1A and 5-HT1B receptor mutant mice. Physiol Behav. 2008;93:659–665. doi: 10.1016/j.physbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JE, Dawson CE, Dourish CT, Duncton MA, Gaur S, George AR, Giles PR, Hamlyn RJ, Kennett GA, Knight AR, Malcolm CS, Mansell HL, Misra A, Monck NJ, Pratt RM, Quirk K, Roffey JR, Vickers SP, Cliffe IA. Indoline derivatives as 5-HT2C receptor agonists. Bioorg Med Chem Lett. 2004;14:2367–2370. doi: 10.1016/j.bmcl.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF. Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Chu HM, Brennan TJ, Tecott LH. A null mutation of the serotonin 6 receptor alters acute responses to ethanol. Neuropsychopharmacology. 2006;31:1801–1813. doi: 10.1038/sj.npp.1301030. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT1B receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001;74:507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- Bovetto S, Rouillard C, Richard D. Role of CRH in the effects of 5-HT-receptor agonists on food intake and metabolic rate. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1231–R1238. doi: 10.1152/ajpregu.1996.271.5.R1231. [DOI] [PubMed] [Google Scholar]
- Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192:382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- Ceci F, Cangiano C, Cairella M, Cascino A, Del Ben M, Muscaritoli M, Sibilia L, Rossi Fanelli F. The effects of oral 5-hydroxytryptophan administration on feeding behavior in obese adult female subjects. J Neural Transm. 1989;76:109–117. doi: 10.1007/BF01578751. [DOI] [PubMed] [Google Scholar]
- Choi S, Blake V, Cole S, Fernstrom JD. Effects of chronic fenfluramine administration on hypothalamic neuropeptide mRNA expression. Brain Res. 2006;1087:83–86. doi: 10.1016/j.brainres.2006.02.129. [DOI] [PubMed] [Google Scholar]
- Choi S, Jonak EM, Simpson L, Patil V, Fernstrom JD. Intermittent, chronic fenfluramine administration to rats repeatedly suppresses food intake despite substantial brain serotonin reductions. Brain Res. 2002;928:30–39. doi: 10.1016/s0006-8993(01)03330-3. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Somerville EM, Kennett GA, Dourish CT. 5-HT1B receptor knockout mice show a compensatory reduction in 5-HT2C receptor function. Eur J Neurosci. 2003;17:185–190. doi: 10.1046/j.1460-9568.2003.02437.x. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connoley IP, Heal DJ, Stock MJ. A study in rats of the effects of sibutramine on food-intake and thermogenesis. Br J Pharmacol. 1995;114:P388–P388. [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca2+-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C, Bailey CJ. Effect of the antiobesity agent sibutramine in obese-diabetic ob/ob mice. Int J Obes Relat Metab Disord. 1998;22:619–623. doi: 10.1038/sj.ijo.0800636. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Adams DR, Bentley J, Benwell K, Bickerdike MJ, Harrison K, Kennett GA, Knight AR, Lightowler SL, Malcolm C, Misra A, Vickers SP, Cliffe IA, Coassolo P, Frei B, Mizrahi J, Stalder H, Stephan-Guldne M. Discovery and development of selective 5-HT2C receptor agonists for obesity. Fundam Clin Pharmacol. 2004;18:127–134. SAT S1.4. [Google Scholar]
- Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel RL, Stack G, Schechter L, Harrison BL, Rosenzweig-Lipson S. WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther. 2005;313:862–869. doi: 10.1124/jpet.104.075382. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci. 2006;361:1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD, Choi S. The development of tolerance to drugs that suppress food intake. Pharmacol Ther. 2008;117:105–122. doi: 10.1016/j.pharmthera.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merce R, Holenz J, Vrang N, Sorensen RV, Heal D, Buschmann H, Pauwels PJ. Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br J Pharmacol. 2006;148:973–983. doi: 10.1038/sj.bjp.0706807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisler JS, Underberger SJ, York DA, Bray GA. d-Fenfluramine in a rat model of dietary fat-induced obesity. Pharmacol Biochem Behav. 1993;45:487–493. doi: 10.1016/0091-3057(93)90269-y. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- Fletcher PJ, Burton MJ. Dissociation of the anorectic actions of 5-HTP and fenfluramine. Psychopharmacology (Berl) 1986;89:216–220. doi: 10.1007/BF00310632. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Paterson IA. A comparison of the effects of tryptamine and 5-hydroxytryptamine on feeding following injection into the paraventricular nucleus of the hypothalamus. Pharmacol Biochem Behav. 1989;32:907–911. doi: 10.1016/0091-3057(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Kennedy AJ, Curzon G. d-Fenfluramine- and d-norfenfluramine-induced hypophagia: differential mechanisms and involvement of postsynaptic 5-HT receptors. Eur J Pharmacol. 1993;242:83–90. doi: 10.1016/0014-2999(93)90013-8. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Strauss F, Malmlof K. Locomotion is the major determinant of sibutramine-induced increase in energy expenditure. Pharmacol Biochem Behav. 2006;83:517–527. doi: 10.1016/j.pbb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT5A receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Halford JCG, Heal DJ, Blundell JE. Effects in the rat of sibutramine on food-intake and the behavioral satiety sequence. Br J Pharmacol. 1995;114:P387. [Google Scholar]
- Halford JC, Lawton CL, Blundell JE. The 5-HT2 receptor agonist MK-212 reduces food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol Biochem Behav. 1997;56:41–46. doi: 10.1016/S0091-3057(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Sonoda R, Kimura Y, Takasu T, Suzuki M, Sasamata M, Miyata K. Antiobesity effect of YM348, a novel 5-HT2C receptor agonist, in Zucker rats. Brain Res. 2004a;1011:221–227. doi: 10.1016/j.brainres.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki M, Sasamata M, Miyata K. Thermogenic effect of YM348, a novel 5-HT2C-receptor agonist, in rats. J Pharm Pharmacol. 2004b;56:1551–1556. doi: 10.1211/0022357044841. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki M, Sasamata M, Miyata K. Agonist diversity in 5-HT2C receptor-mediated weight control in rats. Psychopharmacology (Berl) 2005;178:241–249. doi: 10.1007/s00213-004-2019-z. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther. 2008;117:207–231. doi: 10.1016/j.pharmthera.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997;58:767–773. doi: 10.1016/s0091-3057(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav. 1999;63:377–385. doi: 10.1016/s0091-3057(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O'Rahilly S, Colmers WF, Elmquist JK, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007a;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes Brain Behav. 2007b;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Dourish CT, Curzon G. Evidence That the Hyperphagic response to 8-OH-DPAT is mediated by 5-HT1A receptors. Eur J Pharmacol. 1988;150:361–366. doi: 10.1016/0014-2999(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dourish CT, Curzon G. 5-HT1B agonists induce anorexia at a postsynaptic site. Eur J Pharmacol. 1987;141:429–435. doi: 10.1016/0014-2999(87)90561-9. [DOI] [PubMed] [Google Scholar]
- Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral-studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- Konkle AT, Sreter KB, Baker SL, Bielajew C. Chronic paroxetine infusion influences macronutrient selection in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2003;74:883–890. doi: 10.1016/s0091-3057(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000245. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MD, Kennett GA, Dourish CT, Clifton PG. 5-HT1B receptors modulate components of satiety in the rat: behavioural and pharmacological analyses of the selective serotonin1B agonist CP-94,253. Psychopharmacology (Berl) 2002;164:49–60. doi: 10.1007/s00213-002-1162-7. [DOI] [PubMed] [Google Scholar]
- Lee MD, Simansky KJ. CP-94,253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology (Berl) 1997;131:264–270. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG. Reduced hypophagic effects of d-fenfluramine and the 5-HT2C receptor agonist mCPP in 5-HT1B receptor knockout mice. Psychopharmacology (Berl) 2004;176:39–49. doi: 10.1007/s00213-004-1864-0. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Adrenergic innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry. 1986;84:201–205. doi: 10.1007/BF00495783. [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. Absence of fenfluramine-induced anorexia and reduced c-Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME, Nielsen JA, Ryan K, Schulz DW, Torgersen LK, et al. 3-(1,2,5,6-Tetrahydropyrid-4-yl) pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl) indole. J Med Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Kohler C, Delft AM. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- McCall RB, Franklin SR, Hyslop DK, Knauer CS, Chio CL, Haber CL, Fitzgerald LW. PNU-22394, a 5-HT2c receptor agonist, reduced feeding in rodents and produces weight loss in humans. Soc Neurosci Abstr. 2001;27:309. 302. [Google Scholar]
- McNeely W, Goa KL. Sibutramine. A review of its contribution to the management of obesity. Drugs. 1998;56:1093–1124. doi: 10.2165/00003495-199856060-00019. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Chapin DS, Johnson JL, Jr, Torgersen LK. Sertraline, a serotonin-uptake inhibitor, reduces food intake and body weight in lean rats and genetically obese mice. Am J Clin Nutr. 1992;55:185S–189S. doi: 10.1093/ajcn/55.1.185s. [DOI] [PubMed] [Google Scholar]
- Nilsson BM. 5-Hydroxytryptamine 2C (5-HT2C) receptor agonists as potential antiobesity agents. J Med Chem. 2006;49:4023–4034. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT2C receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Takahashi Y, Yamashita N, Hiraoka S, Kumano H, Kuboki T, Oka Y. Fluvoxamine, a selective serotonin reuptake inhibitor, and 5-HT2C receptor inactivation induce appetite-suppressing effects in mice via 5-HT1B receptors. Int J Neuropsychopharmacol. 2007;10:675–681. doi: 10.1017/S1461145706007206. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia G, Meneses A. Oral administration of the 5-HT6 receptor antagonists SB-357134 and SB-399885 improves memory formation in an autoshaping learning task. Pharmacol Biochem Behav. 2005;81:673–682. doi: 10.1016/j.pbb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, Stack G, Welmaker G, Barrett JE, Dunlop J. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res. 2006;1073–1074:240–251. doi: 10.1016/j.brainres.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Rouru J, Pesonen U, Isaksson K, Huupponen R, Koulu M. Effect of chronic treatment with TFMPP, a 5-HT1 receptor agonist, on food intake, weight gain, plasma insulin and neuropeptide mRNA expression in obese Zucker rats. Eur J Pharmacol. 1993;234:191–198. doi: 10.1016/0014-2999(93)90953-f. [DOI] [PubMed] [Google Scholar]
- Sabb AL, Vogel RL, Welmaker GS, Sabalski JE, Coupet J, Dunlop J, Rosenzweig-Lipson S, Harrison B. Cycloalkyl[b][1,4]benzodiazepinoindoles are agonists at the human 5-HT2C receptor. Bioorg Med Chem Lett. 2004;14:2603–2607. doi: 10.1016/j.bmcl.2004.02.100. [DOI] [PubMed] [Google Scholar]
- Saller CF, Stricker EM. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192:385–387. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- Schreiber R, De Vry J. Role of 5-HT2C receptors in the hypophagic effect of m-CPP, ORG 37684 and CP-94,253 in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:441–449. doi: 10.1016/s0278-5846(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Schuhler S, Clark A, Joseph W, Patel A, Lehnen K, Stratford E, Horan TL, Fone KC, Ebling FJ. Involvement of 5-HT receptors in the regulation of food intake in Siberian hamsters. J Neuroendocrinol. 2005;17:276–285. doi: 10.1111/j.1365-2826.2005.01303.x. [DOI] [PubMed] [Google Scholar]
- Shacham S, Heal DJ, Cheetham SC, Jackson HC, Melendez R, Rutkowski JV, Orbach P, Gannon KS. 2006 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006. PRX-07034, a potent and selective 5-HT6 receptor antagonist, reduces food intake and body weight in dietary-induced obese (DIO) rats. Program No. 62.10. [Google Scholar]
- Simansky KJ, Nicklous DM. Parabrachial infusion of D-fenfluramine reduces food intake. Blockade by the 5-HT1B antagonist SB-216641. Pharmacol Biochem Behav. 2002;71:681–690. doi: 10.1016/s0091-3057(01)00740-7. [DOI] [PubMed] [Google Scholar]
- Simansky KJ, Vaidya AH. Behavioral mechanisms for the anorectic action of the serotonin (5-HT) uptake inhibitor sertraline in rats: comparison with directly acting 5-HT agonists. Brain Res Bull. 1990;25:953–960. doi: 10.1016/0361-9230(90)90194-5. [DOI] [PubMed] [Google Scholar]
- Smith BM, Thomsen WJ, Grottick AJ. The potential use of selective 5-HT2C agonists in treating obesity. Expert Opin Invest Drugs. 2006;15:257–266. doi: 10.1517/13543784.15.3.257. [DOI] [PubMed] [Google Scholar]
- Somerville EM, Horwood JM, Lee MD, Kennett GA, Clifton PG. 5-HT2C receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice. Eur J Neurosci. 2007;25:3115–3124. doi: 10.1111/j.1460-9568.2007.05567.x. [DOI] [PubMed] [Google Scholar]
- Svartengren J, Axelsson-Lendin P, Edling N, Fhölenhag K, Isacson R, Hillegaart V, Grönberg A. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. The selective serotonin 5-HT6 receptor antagonist BVT-5182 decreases food intake and body weight in both rats and mice. Program No. 75.8. [Google Scholar]
- Svartengren J, Fholenhag K, Modiri A, Axelsson-Lendin P, Edling N, Klingström G, Larsson C, Sakariassen KS. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003a. Preclinical in vivo pharmacology of BVT-933, a selective 5-HT2c receptor agonist. Program No. 509.9. [Google Scholar]
- Svartengren J, Öhman B, Edling N, Svensson M, Fhölenhag K, Axelsson-Lendin P, Klingström G, Larsson C. The Serotonin 5-HT6 receptor antagonist BVT.5182 reduces body weight of high fat diet-induced mice. Int J Obesity. 2003b;27:T1. P1–P094. [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Benwell KR, Porter RH, Bickerdike MJ, Kennett GA, Dourish CT. Comparative effects of continuous infusion of mCPP, Ro 60-0175 and d-fenfluramine on food intake, water intake, body weight and locomotor activity in rats. Br J Pharmacol. 2000;130:1305–1314. doi: 10.1038/sj.bjp.0703443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA. Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology. 2001;41:200–209. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Webster LJ, Wyatt A, Bickerdike MJ, Dourish CT, Kennett GA. Oral administration of the 5-HT2C receptor agonist, mCPP, reduces body weight gain in rats over 28 days as a result of maintained hypophagia. Psychopharmacology (Berl) 2003;167:274–280. doi: 10.1007/s00213-002-1378-6. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC. A role for 5-HT6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology. 2001;41:210–219. doi: 10.1016/s0028-3908(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Yen TT, Wong DT, Bemis KG. Reduction of food-consumption and body-weight of normal and obese mice by chronic treatment with fluoxetine – a serotonin reuptake inhibitor. Drug Dev Res. 1987;10:37–45. [Google Scholar]