Abstract
Extensive work has shown that activation of the cAMP-dependent protein kinase A (PKA) is crucial for long-term depression (LTD) of synaptic transmission in the hippocampus, a phenomenon that is thought to be involved in memory formation. Here we studied the role of an alternative target of cAMP, the exchange protein factor directly activated by cyclic AMP (Epac). We show that pharmacological activation of Epac by the selective agonist 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT) induces LTD in the CA1 region. Paired-pulse facilitation of synaptic responses remained unchanged after induction of this LTD, suggesting that it depended on postsynaptic mechanisms. The 8-pCPT-induced LTD was blocked by the Epac signalling inhibitor brefeldin-A (BFA), Rap-1 antagonist geranylgeranyltransferase inhibitor (GGTI) and p38 mitogen activated protein kinase (P38-MAPK) inhibitor SB203580. This indicated a direct involvement of Epac in this form of LTD. As for other forms of LTD, a mimetic peptide of the PSD-95/Disc-large/ZO-1 homology (PDZ) ligand motif of the AMPA receptor subunit GluR2 blocked the Epac-LTD, suggesting involvement of PDZ protein interaction. The Epac-LTD also depended on mobilization of intracellular Ca2+, proteasome activity and mRNA translation, but not transcription, as it was inhibited by thapsigargin, lactacystin and anisomycin, but not actinomycin-D, respectively. Finally, we found that the pituitary adenylate cyclase activating polypeptide (PACAP) can induce an LTD that was mutually occluded by the Epac-LTD and blocked by BFA or SB203580, suggesting that the Epac-LTD could be mobilized by stimulation of PACAP receptors. Altogether these results provided evidence for a new form of hippocampal LTD.
Use-dependent changes in synaptic strength are thought to play an important role in learning and memory. Most attention has been given to long-term potentiation (LTP) of excitatory synaptic transmission in the hippocampus (Bliss & Lomo, 1973). Under certain conditions, the same synapses can undergo long-term depression (LTD) (Bear & Abraham, 1996). In the CA1 region of the hippocampus, LTD was first shown to depend on group I metabotropic glutamate receptors, activated by the agonist (R,S)3,5-dihydroxyphenylglycine (DHPG-LTD) (Stanton et al. 1991), or NMDA receptors activated by low frequency electrical stimulation of afferents (LFS-LTD) (Dudek & Bear, 1992). Then a large diversity of LTD mechanisms have been described that involve Ca2+ ions (Rose & Konnerth, 2001), protein phosphatases (Mulkey et al. 1993), PKA (Brandon et al. 1995; Kameyama et al. 1998), protein synthesis (Hou et al. 2006; Pfeiffer & Huber, 2006), AMPA receptor internalization (Beattie et al. 2000) and mobilization of the small GTPase Rap (Zhu et al. 2002).
Epac is a direct target for cAMP, acting as a guanine-nucleotide-exchange factor (GEF) for the small GTPases repressor-activator protein 1 (Rap1) and Rap2 (de Rooij et al. 1998; Kawasaki et al. 1998). Two genes, Epac1 and Epac2, encode Epac proteins. Both are expressed in various tissues with a predominance for Epac2 in the brain (de Rooij et al. 1998; Kawasaki et al. 1998). In the insulinoma β-cell lines (INS-1) and human pancreatic β cells, Epac induces secretion of insulin via mobilization of intracellular Ca2+ from ryanodine-sensitive and, to a lesser extent, inositol 1,4,5-inositol-trisphosphate (IP3)-sensitive Ca2+ stores (Kang et al. 2003). Only a few data are available on the role of Epac in neurons. At the calyx of Held synapse, Epac enhances neurotransmitter release via an unidentified pathway (Kaneko & Takahashi, 2004). In the medial prefrontal cortex and hippocampus, Epac potentiates synaptic transmission via a presynaptic mechanism (Huang & Hsu, 2006; Gekel & Neher, 2008; Gelinas et al. 2008). At the crayfish neuromuscular junction, Epac along with hyperpolarization-activated cyclic nucleotide (HCN) cation channels modulate neurotransmission via activation of Rap1 (Zhong & Zucker, 2005). In cultured dorsal root ganglion neurons, Epac mediates β2-adrenergic receptor stimulation of protein kinase Cε (PKCε) and mechanical hyperalgesia (Hucho et al. 2005). Finally, in cultured cerebellar granule neurons, Epac activates the extracellular signal-regulated kinase (ERK)/p38-MAPK pathway via Rap proteins and modulates postsynaptic excitability (Ster et al. 2007). At the moment, little is known about the role of Epac in synaptic plasticity. In the present study, we investigated whether Epac could participate in long-term modulation of CA1 excitatory hippocampal synapses.
The neuropeptide PACAP is a member of the vasoactive intestinal polypeptide (VIP)/secretin/glucagon family that is present in the brain in two active forms, PACAP-38 and PACAP-27. It binds to at least two types of receptors, PACAP type 1 (PAC1) and vasoactive intestinal peptide (VIP)-PACAP type 1/2 (VPAC1/2). VPAC1/2 receptors are positively coupled to adenylate cyclase, whereas PAC1 receptor stimulates both adenylate cyclase and phospholipase C (PLC) (Laburthe & Couvineau, 2002). These receptors trigger various intracellular signalling pathways and biological functions (Vaudry et al. 2000), including a PKA-independent LTD, in the hippocampal CA1 region (Kondo et al. 1997; Roberto et al. 2001). The pathway of this LTD was not identified.
Here we show that activation of Epac induces LTD in hippocampal CA1 excitatory synapses, which involves activation of p38-MAPK, intracellular Ca2+ stores, protein synthesis and PDZ ligand motif-containing AMPA receptor subunits. We found that this LTD could be triggered by stimulation of PACAP receptors.
Methods
Hippocampal slice preparation
Experiments were performed in accordance to the European Communities Council Directive of November 24, 1986, to minimize pain and discomfort of animals. Hippocampal slices were prepared from 14- to 22-postnatal-days-old Swiss mice. Animals were decapitated, and the brain quickly removed and chilled in ice-cold sucrose artificial cerebro-spinal fluid (ACSF) containing (mm): sucrose, 246; NaHCO3, 26; KH2PO4, 1.25; KCl, 2; CaCl2, 2; MgSO4, 2; glucose, 10; pH 7.4. Horizontal brain sections (380 μm thick) were cut with a vibroslicer (Vibratome, St Louis, MO, USA) and hippocampal slices dissected in cold sucrose-ACSF, bubbled with 95% O2, 5% CO2. Slices were kept at room temperature in a chamber filled with continuously oxygenated ACSF of the following composition (mm): NaCl, 124; NaHCO3, 26; KH2PO4, 1.25; KCl, 3; CaCl2, 2.5; MgSO4, 1.5; glucose, 10; sucrose, 4; pH 7.4. At least 3 h after preparation, slices were transferred to a submersion recording chamber, maintained at 30°C and perfused with oxygenated ACSF at a rate of one chamber volume (1.5 ml) per minute. In DHPG experiments, the CA1 region was separated from the CA3 region by sectioning Schaffer–commissural fibres.
Electrophysiological recordings
A bipolar twisted nickel–chromium stimulating electrode was positioned into the stratum radiatum to activate Schaffer–commissural afferents to CA1 pyramidal cells. A glass micropipette filled with ACSF (1–5 MΩ) was positioned in the apical dendritic zone of the CA1 pyramidal layer to record the dendritic field potential. The field EPSPs were evoked by 0.1 ms pulses of 10–30 V, every 60 s, in order to obtain 60–70% of the maximal response. The low-frequency stimulation (LFS) protocol consisted of 900 pulses of 0.1 ms delivered at a frequency of 1 Hz. The electrophysiological signal was amplified, filtered (1 Hz to 1 kHz) and digitized at 3 kHz. The initial linear negative-going component of the field EPSP was measured using the DASYLab software (measX GmbH & Co.KG, Moenchengladbach, Germany) after stable evoked response was obtained. Measurements were then expressed as percentage of the averaged value calculated 10 min before LTD induction. All the experiments using the pharmacological compounds and peptides were interleaved with control experiments and pooled. Significant differences between groups were determined using ANOVA performed on the last 10 min average values taken 35–45 min after the LTD induction. Probability values of P < 0.05 were considered to represent significant differences.
Biochemical assays
Hippocampal slices were prepared as described above and only the CA1 area conserved. These slices and controls were incubated each in 250 μl of lysis buffer (50 mm Hepes, 1% nonidet, 2 mm vanadate, 100 mm NaF, 10 mm PyrPO4, 4 mm EDTA, 1 mm PMSF, 1 μg ml−1 leupeptine and 1 μg ml−1 aprotinine) during 20 min at 4°C. Samples were sonicated and centrifuged at 15 000g. for 30 min at 4°C. Cyclic AMP response binding protein (CREB) phosphorylation was then assayed as follows. Proteins were separated on 12% SDS-PAGE gels and transferred to nitrocellulose membrane. The antibodies recognizing total and phosphorylated CREB were used at dilutions of 1 : 1000 and 1 : 2000, respectively, in TBS containing 5% BSA and 0.1% Tween. All antibodies were from Cell Signalling Technologies (Beverly, MA, USA). Western blots were quantified using the NIH image software. PKA activity was measured using the colorimetric EKS-390A kit from Stressgen (Ann Arbor, MI, USA).
Immunohistology for trans-activating transcriptional activator (TAT) peptide
Slices incubated with biotinylated-TAT peptides and untreated control slices were fixed in 4% paraformaldehyde in phosphate buffer (0.1 m, pH 7.4, 30 min). They were frozen in nitrogen, cut in 20 μm sections with a cryostat and mounted on slides. Sections were rinsed in PBS and incubated for 2 h with FITC-conjugated avidin (Vector Laboratories, Peterborough, UK; 1 : 200) in PBS containing 0.25% Triton X-100, washed in PBS and coverslipped. The fluorescent staining was observed with a Leitz DMRB microscope (Leica Microsystemes SAS, Rueil-Malmaison, France) equipped for epifluorescence and digitized by a 1392–1040 resolution cooled colour camera CCD (Cool Snap, Princeton Instruments, Trenton, NJ, USA) on a computer using the CoolSNAP program and transferred to Adobe Photoshop (v. 7) for image processing.
Chemicals
TAT peptide (Eurogentec, Angers, France; GenePep, Prades-le-Lez, France), (RS)-3,5-DHPG (Tocris, Bristol, UK) and 8-pCPT (Biolog, Bremen, Germany) were dissolved in distilled water. PACAP-27 (NeoSystem, Strasbourg, France) was dissolved in distilled water complemented with 1% BSA. Thapsigargin (A.G, Scientific, Inc., San Diego, CA, USA), anisomycin (Calbiochem, Fontenay-sous-Bois, France), H89, actinomycin-D, SB203580, PD98059, lactacystin, forskolin, GGTI (Sigma-Aldrich, L’isle-D’abeau, France), BFA (Epicentre Biotechnologies, Madison, WI, USA), SB415286 and GF109203X (Tocris, Bristol, UK) were dissolved in DMSO. Final DMSO dilution was always more than 1 : 2000. Drug solutions were adjusted to pH 7.4.
Results
Epac induces long-term depression of synaptic transmission
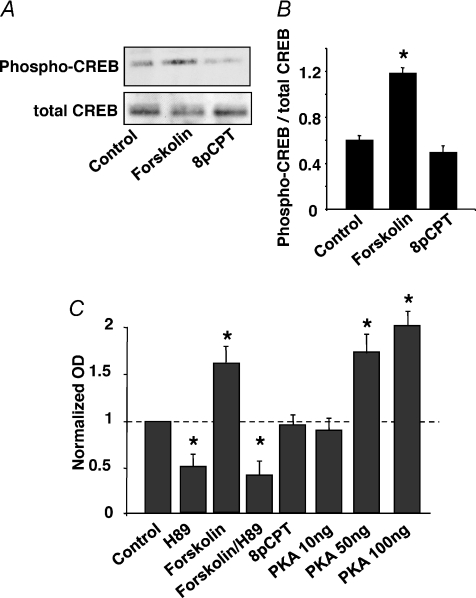
Since Epac activates the small GTPase Rap1 in cultured neurons (Maillet et al. 2003), and as a Rap–p38-MAPK signalling cascade is involved in LTD at the hippocampal Schaffer collateral–CA1 pyramidal cell synapse (Zhu et al. 2002; Moult et al. 2008), we investigated whether activation of Epac could result in long-term inhibition of this synapse in mouse hippocampal slices. First, we verified that the Epac agonist 8-pCPT (Enserink et al. 2002) did not activate PKA under our experimental conditions. In contrast to forskolin, a 15 min incubation of hippocampal slices in the presence of 8-pCPT (200 μm) did not result in significant phosphorylation of the PKA substrate CREB Fig. 1A and B). Similar results were found using a specific PKA activity test (Fig. 1C).
Figure 1. The selective agonist 8-pCPT activates Epac, but not PKA.
A, hippocampal slices were exposed to forskolin (20 μm) or 8-pCPT (200 μm) for 15 min and the CA1 region was dissected to measure phospho-CREB accumulation, using Western immunoblot analysis. Similar experiments were performed in triplicate. B, quantification of western immunoblots of phospho-CREB accumulation. C, pKA activity was measured from hippocampal CA1 region extracts, in the absence of and after 15 min exposure of the hippocampal slices to H89 (2 μm), forskolin (20 μm), forskolin + H89, or 8-pCPT (200 μm), using a colorimetric kit (EKS-390 A, Stressgen). The OD values were normalized to control value. In panels B and C, each bar represents the mean ±s.e.m. of 3 experiments. Asterisks indicate values that are significantly different from control.
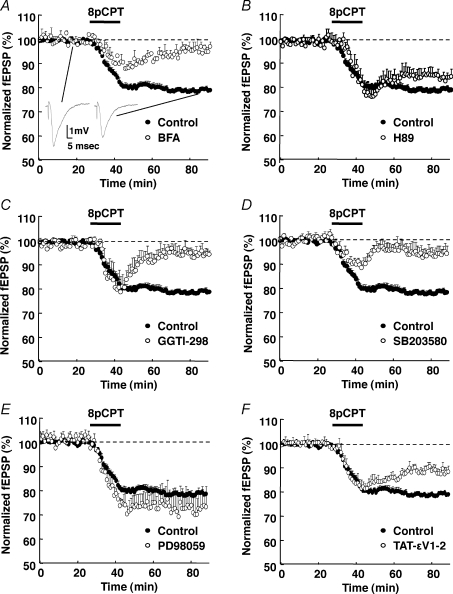
Application of 8-pCPT induced a progressive and significant decline of the Schaffer collateral–CA1 pyramidal cell synaptic response, which stabilized to 78 ± 1% (mean ±s.e.m., n= 20) of the pre-drug response, after 45 min washout of the drug (Control in Fig. 2A–F). The effect was observed whether the synaptic response was monitored by afferent stimulation (single stimulus every minute all over the experiment, Fig. 2A–F) or not (one stimulus every 15 min; data not shown) during the drug application. Brefeldin-A (BFA) was shown to inhibit GDP–GTP exchange on ADP-ribosylation factors (ARF) and vesicular traffic from Golgi apparatus. This compound was also found to antagonize 8-pCPT action at the crayfish neuromuscular junction, presumably by blocking EPAC-mediated effects (Zhong & Zucker, 2005). Here we used this drug to antagonize the action of 8-pCPT in hippocampal slices. Brefeldin-A (20 μm, 45 min) abolished the long-term inhibitory effect of the Epac agonist 8-pCPT on synaptic transmission (20 μm, 45 min; 97 ± 2% of baseline after 45 min washout of 8-pCPT, n= 5), leaving only a transient decrease of the synaptic response (Fig. 2A). Brefeldin-A alone did not affect basal synaptic transmission (supplemental data). We verified that pretreating hippocampal slices with the PKA inhibitor H89 (2 μm, 45 min) did not significantly alter synaptic transmission, nor the 8-pCPT-induced decrease of the synaptic response (84 ± 3% of pre-drug response, after 45 min wash-out of 8-pCPT, n= 5; Fig. 2B). These experiments indicated that prolonged activation of Epac resulted in a PKA-independent long-term inhibition of the Schaffer collateral–CA1 hippocampal pyramidal cell synaptic transmission, which we will tentatively name Epac-LTD.
Figure 2. Epac-mediated depression of synaptic transmission.
The initial slope of CA1 field EPSPs (inserts) recorded from hippocampal slices was measured before, during (horizontal bar) and after application of 8-pCPT (200 μm). A 45 min pretreatment of the slices with BFA (20 μm, n= 5, A), Rap1 inhibitor GGTI (10 μm, n= 5, C), p38-MAPK inhibitor SB203580 (5 μm, n= 5, D), or PKCε inhibitory peptide TAT-εV1-2 (5 μm; n= 5; F), but not PKA inhibitor H89 (2 μm, n= 5, B), or MEK1 inhibitor PD98059 (25 μm, for 1 h before recording, n= 5, E) inhibited the 8-pCPT-induced LTD. The control curve in all panels was obtained from 20 pooled interleave experiments.
As p38-MAPK is an effector of Rap1 (Sawada et al. 2001) and since Epac activates Rap1 (de Rooij et al. 1998; Kawasaki et al. 1998), we investigated whether 8-pCPT-induced LTD was Rap1 and p38-MAPK dependent. A 45 min exposure of hippocampal slices to the Rap1 inhibitor GGTI (10 μm) or the p38-MAPK inhibitor SB203580 (5 μm) left only a transient depression of the synaptic response and blocked the 8-pCPT-induced LTD (95 ± 3% of baseline after 45 min wash-out of 8-pCPT, n= 5, for both GGTI and SB203580; Fig. 2C and D, respectively), without significantly affecting baseline synaptic transmission (not shown). These results indicated that the Epac-LTD involved a Rap1–p38-MAPK-dependent pathway.
Since Epac has also been shown to activate ERK in hippocampal neurons (Lin et al. 2003), we examined whether this pathway was also required for expression of the Epac-LTD. Exposure of hippocampal slices to the MAPK ERK 1 (MEK1) kinase inhibitor PD98059 (25 μm, 60 min) did not decrease the 8-pCPT-induced LTD (72 ± 8% of baseline, n= 5, Fig. 2E). This indicated that the ERK pathway was not required for the Epac-LTD.
As Epac can modulate PKCε activity (Hucho et al. 2005), we examined whether this kinase participates in Epac-LTD. A 45 min preincubation of hippocampal slices in the presence of the cell-permeant PKCε inhibitory peptide TAT-εV1-2 (YGRKKRRQRRR-EAVSLKPT, 5 μm; see below for methodology to use TAT peptides; Bajo et al. 2008; Qi et al. 2007) partially blocked the 8-pCPT-induced LTD (88 ± 1% of baseline after 45 min washout of 8-pCPT, n= 5; Fig. 2F). This suggested the involvement of PKCε in Epac-LTD.
Epac-LTD depends on the interaction between AMPA receptor and postsynaptic PDZ protein
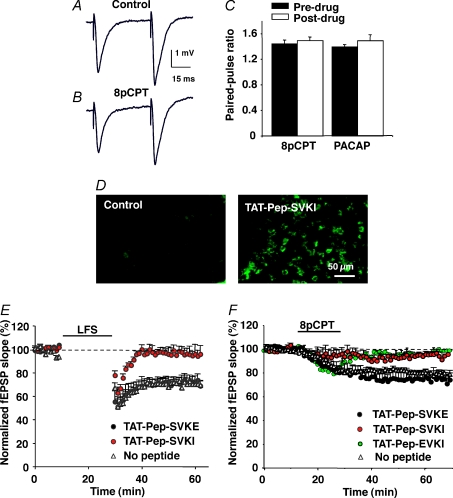
We then examined whether Epac-LTD was of pre- or postsynaptic origin. Paired-pulse facilitation (PPF) of postsynaptic response is classically interpreted as an alteration in the probability of presynaptic neurotransmitter release. We measured PPF using an interpulse interval of 50 ms, before (Fig. 3A) and 45 min after induction of LTD with 8-pCPT (Fig. 3B). No significant change in PPF was observed after induction of the Epac-LTD (44 ± 6% facilitation before, vs. 49 ± 5% facilitation after 8-pCPT-induced LTD, n= 8; Fig. 3C). This result suggested that Epac-LTD depended on postsynaptic mechanisms.
Figure 3. Epac-LTD and PACAP-LTD depend on postsynaptic mechanisms and interaction of AMPA receptors with PDZ protein.
A and B, paired-pulse-induced facilitation of CA1 field EPSP was recorded before (A) and 45 min after (B) a 15 min exposure to 8-pCPT (200 μm). C, the paired-pulse facilitation was quantified by calculating the ratio of the initial slope of the second over the first EPSP. The bar graph represents values (mean ±s.e.m., n= 8) obtained before and 45 min after application of either 8-pCPT or PACAP-27 (400 nm; 15 min application). D, immunofluorescence microscopy images obtained from the CA1 region of two hippocampal slices, 70 min after a 1 h incubation (right) or not (left) with 5 μm biotinylated TAT-Pep-SVKI peptide. The image on the right shows that the peptide was incorporated in nominally all the neurons. Similar data were obtained with the TAT-Pep-SVKE (not shown). E, a LFS protocol (900 pulses, 1Hz, 15 min, n= 5 for each curve) was applied to control hippocampal slices (no peptide) and slices preincubated for 1 h before recording, with the active (TAT-Pep-SVKI) or inactive (TAT-Pep-SVKE) peptide. Only the active peptide inhibited the LFS-induced LTD. F, the same peptides were tested on the 8-pCPT-induced LTD, with similar effects. The selective PICK1 inhibitory peptide, TAT-Pep-EVKI, also blocked the Epac-LTD.
We then tested the hypothesis that Epac-LTD resulted from internalization of postsynaptic AMPA receptors, as is the case for other forms of LTD. The dominant-negative peptide Pep2-SVKI (NVYGIESVKI) has been previously shown to block LFS-LTD at the hippocampal Schaffer collateral–CA1 pyramidal cell synapse (Daw et al. 2000). This peptide mimics the C-terminal sequence of the AMPA-receptor subunit GluR2 and prevents interaction of the subunit with PDZ proteins. The Pep2-SVKI and the inactive peptide Pep2-SVKE (NVYGIESVKE) were fused to an 11-amino-acid sequence derived from the TAT protein of the human immunodeficiency virus (HIV-1). This is an established tool to successfully deliver a variety of peptides intracellularly to neurons in vivo (Brooks et al. 2005). We adapted this in vivo method to the acute in vitro hippocampal slice preparation by incubating the preparation in oxygenated thermostated medium containing either the TAT-Pep-SVKI (YGRKKRRQRRR-NVYGIESVKI) or the TAT-Pep-SVKE peptide (YGRKKRRQRRR-NVYGIESVKE; both at 5 μm) for 1 h. To visualize their incorporation into neurons, the 21-mer TAT peptides were biotinylated and the treated slices permeabilized for subsequent labelling with FITC-conjugated avidin. Figure 3D (right) shows that a majority of CA1 cells were transduced with the TAT-Pep-SVKI peptide. A similar result was obtained with the control TAT-Pep-SVKE peptide (not shown).
Following incubation in the presence or absence of TAT peptides, the hippocampal slices were transferred to a recording chamber and a LFS protocol was applied to the Schaffer–commissural pathway. In the absence of TAT peptide treatment, LFS reliably induced LTD in all the slices (70 ± 3% of baseline 40 min after LFS, n= 5, Fig. 3E). The TAT-Pep-SVKI, but not TAT-Pep-SVKE, peptide blocked this LTD (97 ± 5% of baseline with TAT-Pep-SVKI vs. 71 ± 5% with TAT-Pep-SVKE, n= 5 in each condition), leaving only a transient depression of the synaptic response (Fig. 3E). These results corroborated previous studies (Daw et al. 2000) and further validated our method to functionally deliver peptides in our preparation.
Having established the efficacy of our method, experiments were performed in which we replaced LFS by a 15 min application of 8-pCPT in order to induce an Epac-LTD. Again, the dominant-negative TAT-Pep-SVKI peptide, but not the control TAT-Pep-SVKE peptide, significantly inhibited the 8-pCPT-induced LTD (94 ± 2% of baseline after 45 min washout of the agonist, n= 5, Fig. 3F). Taken together these results showed that the Epac-LTD required interaction of the GluR2 subunit with a PDZ protein, which has been previously shown to control internalization of AMPA receptors (Xia et al. 2000; Sheng & Kim, 2002).
The carboxyl terminus of GluR2 AMPA receptor subunit serves as a binding site for glutamate receptor interacting protein/AMPA receptor binding protein (GRIP/ABP) and protein interacting with c-kinase 1 (PICK1; Kim & Huganir, 1999; Braithwaite et al. 2000; Daw et al. 2000; Xia et al. 2000). The PICK1 protein controls the removal and synaptic insertion of GluR2-containing AMPA receptors (Terashima et al. 2004; Gardner et al. 2005). To investigate which interacting protein was important for Epac-LTD, we used the TAT-Pep-EVKI peptide (YGRKKRRQRRR-NVYGIEEVKI), which blocks PICK1, but not GRIP/ABP, binding to GluR2 (Li et al. 1999; Daw et al. 2000; Chung et al. 2000). The TAT-Pep-EVKI peptide strongly inhibited the 8-pCPT-induced LTD (98 ± 2% of baseline after 45 min washout of the agonist, n= 5, Fig. 3F). This suggested that the binding of GluR2 to PICK1, but not GRIP/ABP, is required for induction of Epac-LTD.
The Epac-LTD depends on intracellular Ca2+ stores, protein synthesis and proteasome activity
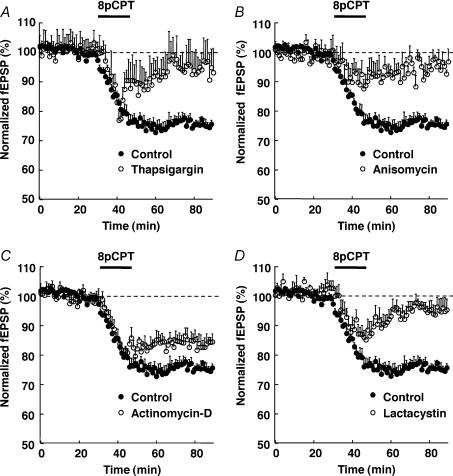
As Epac was shown to release Ca2+ from intracellular stores (Kang et al. 2003; Ster et al. 2007) and since some forms of LTD depend on mobilization of these stores (Rose & Konnerth, 2001), we examined whether depletion of intracellular Ca2+ stores could affect the Epac-LTD. Depletion of intracellular Ca2+ stores by a 75 min preincubation of the hippocampal slices with thapsigargin (5 μm) blocked the induction of Epac-LTD (93 ± 8% of baseline after 45 min wash-out of 8-pCPT, n= 5, Fig. 4A), without significantly affecting baseline synaptic transmission (not shown). This result indicated that the Epac-LTD depended on mobilization of intracellular Ca2+ stores.
Figure 4. Epac-LTD depends on mobilization of intracellular Ca2+ stores, protein synthesis and proteasome activity, but not transcription.
One hour pretreatement of hippocampal slices with the intracellular Ca2+ store depleting compound thapsigargin (5 μm, n= 5, A), or the translation inhibitor anisomycin (20 μm, n= 5, B), or the proteasome inhibitor lactacystin (10 μm, n= 5, D), but not the transcription inhibitor actinomycin-D (25 μm, n= 5, C), blocked the 8-pCPT-induced LTD, measured on the initial slope of CA1 field EPSPs. The control curve in all panels was obtained from 16 pooled interleave experiments.
As some forms of LTD also depend on protein synthesis (Pfeiffer & Huber, 2006), we investigated whether this was the case for the Epac-LTD. We incubated hippocampal slices in the presence of the mRNA translation inhibitor, anisomycin (20 μm, 45 min), before application of 8-pCPT. The anisomycin treatment had no effect on baseline synaptic transmission (not shown), but significantly impaired the establishment of the Epac-LTD (95%± 3% of baseline after 45 min washout of 8-pCPT, n= 5, Fig. 4B). We also tested whether Epac-LTD was dependent on transcription. Treatment of hippocampal slices with the transcription inhibitor actinomycin-D (25 μm, 1 h) did not significantly alter baseline synaptic transmission (not shown), nor the 8-pCPT-induced LTD (84%± 3% of baseline after 45 min wash-out of 8-pCPT, n= 5, Fig. 4C). These results indicated that the Epac-LTD also depended on mRNA translation, but not transcription.
Previous studies have shown that the ubiquitin-proteasome pathway controls some forms of synaptic plasticity (Speese et al. 2003; Zhao et al. 2003; Hou et al. 2006). We tested whether this also applied to the Epac-LTD. Pre-treatment of hippocampal slices with the proteasome inhibitor lactacystin (5 μm, 1 h) blocked the 8-pCPT-induced LTD (95 ± 3% of baseline, n= 5, Fig. 4D). This result supported the idea that the Epac-LTD also depended on protein degradation via the proteasome.
Does Epac-LTD differ from LFS-LTD and DHPG-LTD?
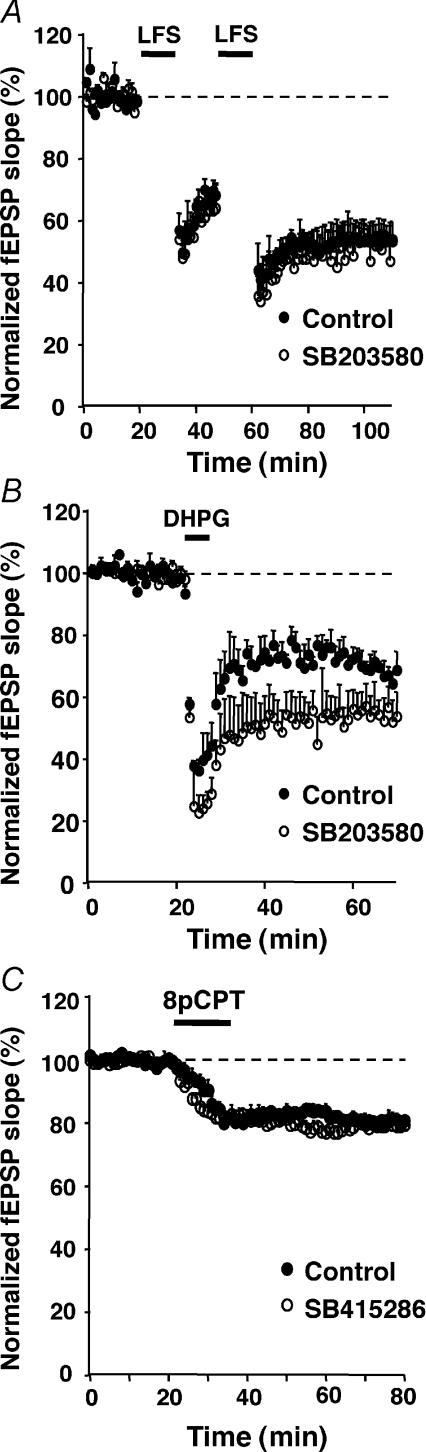
Although controversial, the LFS- and DHPG-induced LTD (DHPG-LTD) have been described as involving a p38-MAPK pathway in the CA1 region and dentate gyrus of the hippocampus (Zhu et al. 2002; Gallagher et al. 2004; Huang et al. 2004; Moult et al. 2008), as is the case for the Epac-LTD (present data). Neither the LFS-LTD (55 ± 6%vs. 54 ± 6% of baseline, 45 min after the induction protocol, in the presence vs. absence of SB203580, n= 4, Fig. 5A), nor the DHPG-LTD (54 ± 4% and 67 ± 4%, n= 4, Fig. 5B) was significantly inhibited by a pretreatment with the p38-MAPK inhibitor SB203580. These results indicated that in contrast to Epac-LTD, neither LFS- nor DHPG-LTD depended on the p38-MAPK pathway, in our preparation.
Figure 5. LFS- and DHPG-LTD do not share p38-MAPK nor GSK-3β dependency with Epac-LTD.
A and B, a 45 min pretreatment of hippocampal slices with the p38-MAPK Inhibitor SB203580 (5 μm), did not inhibit the LFS-induced LTD (n= 5, A) nor the DHPG-induced LTD (n= 5, B), in the CA1 region. C, a 60 min pretreatment with the GSK-3β inhibitor SB415286 (10 μm) did not significantly alter the Epac-LTD (n= 5).
In the CA1 hippocampal area, glycogen synthase kinase-3β (GSK-3β) inhibitors have been shown to block the NMDA-dependent LFS-LTD (Peineau et al. 2007). We therefore investigated whether EPAC-LTD involved the GSK-3β pathway. Hippocampal slices were exposed for 60 min to the cell-permeant GSK-3β inhibitor SB415286 (10 μm) (Coghlan et al. 2000) before application of 8-pCPT. The pretreatment did not significantly alter the EPAC-LTD (79 ± 2% of baseline, n= 5), suggesting that, in contrast to LFS-LTD, GSK-3β was not involved in EPAC-LTD (Fig. 5C).
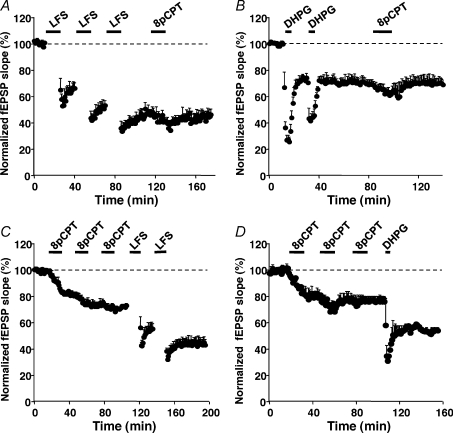
These results did not exclude the possibility that Epac-LTD could share common mechanisms with mGluR-LTD and/or LFS-LTD downstream of p38-MAPK and GSK-3β activation. We addressed this issue by performing occlusion experiments. After saturation of LFS-LTD (Fig. 6A), or full induction of a DHPG-LTD (Fig. 6B), 8-pCPT was unable to induce further depression of the synaptic response. These results indicated the existence of a common step (likely to be internalization of AMPA receptors) shared by mGluR-LTD, LFS-LTD and Epac-LTD. Saturated Epac-LTD did not occlude LFS-LTD (Fig. 6C) or DHPG-LTD (Fig. 6D), suggesting a less efficient action of Epac as compared to LFS or DHPG.
Figure 6. Occlusion experiments.
A and B, saturation of LFS-LTD (n= 5, A) or DHP-LTD (DHPG 100 μm, n= 5, B) occluded Epac-LTD. C and D, saturation of Epac-LTD (8-pCPT 200 μm) did not occlude the LFS-LTD (n= 5, C), nor DHPG-LTD (n= 5, D).
Epac-LTD can be induced by stimulation of PACAP receptors
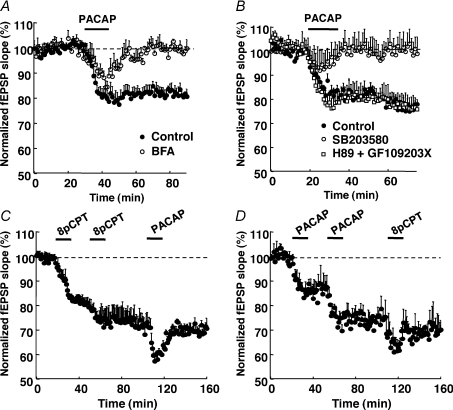
As Epac is directly activated by cAMP, we examined whether the Gs-coupled PACAP/VIP receptor mimicked the effect of 8-pCPT on synaptic transmission. The neuropeptide PACAP-27 binds to PACAP/VIP receptors and strongly increases cAMP synthesis (Spengler et al. 1993). PACAP-27 (400 nm) induced a long-term depression of synaptic transmission (80 ± 2% of baseline after 45 min wash of the agonist, n= 5) that was blocked by BFA (99 ± 3% of baseline, n= 5), leaving only a transient decrease of the synaptic response (Fig. 7A). A similar blockade was observed with the p38-MAK antagonist SB203580 (100 ± 5% of baseline after 45 min wash of the agonist, 5 μm, n= 5), but not the PKA antagonist H89 (2 μm) concomitantly applied with the PKC inhibitor GF-109203X (0.5 μm, 45 min; 76 ± 4% of baseline, n= 5, Fig. 7B).
Figure 7. PACAP-LTD properties.
A and B, application of PACAP-27 peptide (400 nm) induced an LTD that was blocked by a 45 min pretreatment of hippocampal slices with the EPAC inhibitor BFA (20 μm, n= 5, A), or the p38-MAPK inhibitor SB203580 (5 μm, n= 5, B), but not the PKA inhibitor H89 (2 μm) applied in the presence the PKC inhibitor GF109203X (0.5 μm, n= 5, B). C and D, after induction of LTD with 2 successive applications of 8-pCPT, PACAP-27 did not further decrease EPSP slope (C). Vice versa, LTD induced by 2 successive applications of PACAP was not significantly changed by a subsequent application of 8-pCPT (D). This shows mutual occlusion between Epac-LTD and PACAP-LTD (n= 5 in C and D).
As for the Epac-LTD, no significant change in PPF of the synaptic response was observed after induction of PACAP-LTD (PPF was 39 ± 3% before vs. 49 ± 9% after PACAP-27-induced LTD, n= 8; Fig. 3C).
Occlusion experiments were then performed. After full induction of Epac-LTD with 8-pCPT (74 ± 4% of baseline), a subsequent application of PACAP-27 did not further decrease significantly the synaptic response (71 ± 4% of baseline after 45 min wash out of PACAP-27, n= 5, Fig. 7C). Conversely, full induction of the PACAP-LTD (74 ± 5%) prevented subsequent 8-pCPT-induced LTD (70 ± 4% of control after 45 min wash-out of 8-pCPT, n= 5, Fig. 7D). These experiments showed mutual occlusion between Epac-LTD and PACAP-LTD. Altogether these data indicated that PACAP- and Epac-LTD shared similar features and common mechanisms.
Discussion
In the present study we identified a new LTD mechanism that involves the cAMP-dependent Epac protein and which can be induced by stimulating PACAP/VIP receptors, in the CA1 region of the hippocampus. What are the characteristics of this LTD? Paired-pulse stimulation experiments indicated that it is postsynaptic in nature. It depends on Rap-1, p38-MAPK and PKCε activation, intracellular Ca2+ stores, proteasome activity and mRNA translation, but not transcription processes and does not involve PKA or CREB activation. Importantly, the Epac-LTD depends on PICK1 PDZ interaction with the GluR2 subunit of AMPA receptors, suggesting that it is a genuine synaptic plasticity process, rather than a phenomenon independent of synaptic receptor regulation.
In some experiments we used anisomycin to block mRNA translation. This drug has been previously shown to also potently activate the p38-MAPK pathway (Shifrin & Anderson, 1999) and to induce LTD in mouse visual cortex (Xiong et al. 2006). Such effects were not likely to have occurred in our preparation, since anisomycin blocked the p38-MAPK-dependent Epac -LTD.
Cellular bases for selective activation of Epac over PKA by cAMP
Activation of PKA has been shown to result in LTP in the CA1 region of the hippocampus (Frey et al. 1993). The present findings show that in addition to this pathway, cAMP can also induce a PKA-independent LTD via activation of Epac. This raises the issue of the cellular determinants that could decide between these two cAMP-dependent pathways. Binding of cAMP to the regulatory subunit of PKA is required to dissociate the catalytic subunit from the holoenzyme complex. Similarly, cAMP binding to Epac is sufficient to trigger its GEF activity towards the small GTPase Rap (Bos, 2003). The affinity of Epac for cAMP is significantly lower (Kd= 2.9 μm) than that of the free unliganded form of the regulatory subunit of PKA (Kd= 0.9 nm; (de Rooij et al. 2000). However, the situation is quite different when considering the PKA holoenzyme complex, the affinity of which for cAMP is similar to that of Epac (Dao et al. 2006). In living cells, cAMP encounters the PKA holoenzyme complex rather than isolated regulatory PKA subunits. Therefore the most likely hypothesis is that functional compartmentalization and/or substrate availability, rather than affinity, are the cellular determinants that may decide whether Epac or PKA will be activated by cAMP in order to induce either LTD or LTP.
Is Epac-LTD mechanistically distinct from LFS- and DHPG-LTD?
We found that Epac-LTD depends on activation of p38-MAPK. This kinase is the target of the Epac-activated small GTPase Rap1 (de Rooij et al. 1998; Kawasaki et al. 1998). It is therefore likely that the Epac-LTD was mediated through mobilization of an Epac–Rap1–p38-MAPK pathway. It has been shown that activation of p38-MAPK by an unidentified Ca2+-sensitive Rap-GEF pathway is involved in hippocampal LFS-LTD (Zhu et al. 2002). In this model, Rap1–p38-MAPK receives inputs from both AMPA and NMDA receptor activity and sends its outputs to AMPA receptors. This results in synaptic removal of GluR2/3-containing AMPA receptors from the postsynaptic membrane and expression of LTD. In our hands, the LFS-LTD in the CA1 region of the hippocampus was unaffected by p38-MAPK inhibition. The discrepancy between our results and those that have led to the above model may result from differences in electrical stimulation protocols and or animal species (rat vs. mouse).
The role of p38-MAPK in the CA1 DHPG-LTD is controversial (Zhu et al. 2002; Huang et al. 2004) and in those studies where DHPG-LTD did not depend on p38-MAPK, a role of ERK was found (Gallagher et al. 2004). In our experimental conditions, the DHPG-LTD was unaffected by p38-MAPK inhibition and therefore differs from the Epac-LTD. Based on these observations, one can tentatively propose that in CA1, Epac-LTD, LFS-LTD and DHPG-LTD use distinct pathways, upstream of activation of p38-MAPK. However, all three LTD are likely to share a final step, which is a PDZ protein-mediated AMPA receptor (GluR2) internalization. Indeed, when the more effective LTD (LFS- or DHPG-LTD) was first established, this occluded the following Epac-LTD.
Similarities between Epac- and PACAP-LTD
We wondered which receptor could activate the Epac-LTD. Our occlusion experiments and sensitivity of the PACAP-LTD to Epac and p38-MAPK inhibitors suggested that Epac-LTD could be elicited by PACAP receptors. Micromolar range concentrations of PACAP-38 have been previously shown to induce a form of hippocampal LTD that requires activation of adenylate cyclase, but not PKA (Kondo et al. 1997; Roberto et al. 2001). This PACAP-LTD was also found to be independent of NMDA receptors and PKC. Our data are therefore consistent with these results and provides new insight in the mechanisms of PACAP-LTD.
In conclusion, we have demonstrated that activation of an Epac–p38-MAPK pathway can induce a postsynaptic-dependent form of LTD that is similar to the one induced by PACAP neuropeptide, in CA1 pyramidal cells. Other synaptic effects of Epac have been described and all show transient enhancement of synaptic transmission through presynaptic mechanisms (Kaneko & Takahashi, 2004; Zhong & Zucker, 2005; Huang & Hsu, 2006; Gekel & Neher, 2008; Gelinas et al. 2008). Thus it appears that Epac can modulate synaptic transmission in opposite ways, via temporally and subcellularly distinct mechanisms. These effects of Epac may play important roles in cognitive tasks, particularly learning and memory.
Acknowledgments
We would like to thank the European Community (SYNSCAFF, LSHM-CT-2004-511995) and ANR (06-NEURO-035-01) for their financial support. J.S. was supported by a FRM grant.
References
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Meyer G, Henley JM. Interactions beween AMPA receptors and intracellular proteins. Neuropharmacology. 2000;39:919–930. doi: 10.1016/s0028-3908(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Zhuo M, Huang YY, Qi M, Gerhold KA, Burton KA, Kandel ER, McKnight GS, Idzerda RL. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, Martinez A, Doskeland SO. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ, Nguyen PV. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem. 2008;15:403–411. doi: 10.1101/lm.830008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Presynaptic mechanism underlying cAMP-induced synaptic potentiation in medial prefrontal cortex pyramidal neurons. Mol Pharmacol. 2006;69:846–856. doi: 10.1124/mol.105.018093. [DOI] [PubMed] [Google Scholar]
- Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4+ neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic b-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap 1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kim JH, Huganir RL. Organization and regulation of proteins at synapses. Curr Opin Cell Biol. 1999;11:248–254. doi: 10.1016/s0955-0674(99)80033-7. [DOI] [PubMed] [Google Scholar]
- Kondo T, Tominaga T, Ichikawa M, Iijima T. Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38. (PACAP-38) Neurosci Lett. 1997;221:189–192. doi: 10.1016/s0304-3940(96)13323-1. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Li P, Kerchner GA, Sala C, Wei F, Huettner JE, Sheng M, Zhuo M. AMPA receptor–PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci. 1999;2:972–977. doi: 10.1038/14771. [DOI] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem. 2003;87:1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F. Crosstalk between Rap1 and Rac regulates secretion of sAPPa. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- Moult PR, Corrêa SAL, Collingridge GL, Fitzjhon SM, Bashir ZI. Co-activation of p38 mitogen-activated protein kinase and protein phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J Physiol. 2008;586:2499–2510. doi: 10.1113/jphysiol.2008.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R, Herron C, Malenka R. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26:7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase Cε regulates γ-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of γ2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Roberto M, Scuri R, Brunelli M. Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learn Mem. 2001;8:265–271. doi: 10.1101/lm.40501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. Stores not just for storage. Intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Nakamura K, Doi K, Takeda K, Tobiume K, Saitoh M, Morita K, Komuro I, De Vos K, Sheetz M, Ichijo H. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–1227. doi: 10.1242/jcs.114.6.1221. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Shifrin VI, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol. 2003;13:899–910. doi: 10.1016/s0960-9822(03)00338-5. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Chattarji S, Sejnowski TJ. 2-Amino-3-phosphonopropionic acid, an inhibitor of glutamate-stimulated phosphoinositide turnover, blocks induction of homosynaptic long-term depression, but not potentiation, in rat hippocampus. Neurosci Lett. 1991;127:61–66. doi: 10.1016/0304-3940(91)90895-z. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, Bockaert J, Fagni L. Exchange protein activated by cAMP. (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;26:6909–6910. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Xia J, Chung HJ, Whiler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xiong W, Kojic LZ, Zhang L, Prasad SS, Douglas R, Wang Y, Cynader MS. Anisomycin activates p38 MAP kinase to induce LTD in mouse primary visual cortex. Brain Res. 2006;1085:68–76. doi: 10.1016/j.brainres.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hegde AN, Martin KC. The ubiquitin proteasome system functions as an inhibitory constraint on synaptic strengthening. Curr Biol. 2003;13:887–898. doi: 10.1016/s0960-9822(03)00332-4. [DOI] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]