Abstract
Patterns of presynaptic activity properly timed with postsynaptic action potential output can not only increase the strength of synaptic inputs but can also increase the excitability of dendritic branches of adult CA1 pyramidal neurons. Here, we examined the role of protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) in the enhancement of dendritic excitability that occurs during theta-burst pairing of presynaptic and postsynaptic firing activity. Using dendritic and somatic whole-cell recordings in rat hippocampal slices, we measured the increase in the amplitude of back-propagating action potentials in the apical dendrite that occurs in parallel with long-term potentiation (LTP) of synaptic inputs. We found that inhibition of the MAPK pathway prevents this enhancement of dendritic excitability using either a weak or strong LTP induction protocol, while synaptic LTP can still be induced by the strong protocol. Both forms of plasticity are blocked by inhibition of PKA and occluded by interfering with cAMP degradation, consistent with a PKA-mediated increase in MAPK activity following induction of LTP. This provides a signalling mechanism for plasticity of dendritic excitability that occurs during neuronal activity and demonstrates the necessity of MAPK activation. Furthermore, this study uncovers an additional contribution of kinase activation to plasticity that may occur during learning.
Plasticity in the hippocampal CA1 region may be a key component of certain forms of learning. The occurrence of plasticity of synaptic inputs has been well established. More recently, it has emerged that neuronal membrane properties also display plasticity during neuronal activity (reviewed by Zhang & Linden, 2003; Frick & Johnston, 2005). Any modification of the membrane properties of dendrites alters their excitability, yielding a large impact on the integration of synaptic input, the propagation of these signals along the dendrites, and ultimately on the generation of action potentials (Frick & Johnston, 2005). The ability of a signal to propagate down the dendrite, or ‘dendritic excitability’, can be measured as the amplitude of a back-propagating action potential (bAP) over the dendrite. This measure of dendritic excitability is regulated by a number of ion channels, in particular, the channels that underlie the A-type K+ current (IA). The A-type K+ channels increase in density as a function of distance from the soma (Hoffman et al. 1997), and blockers of these channels enhance AP back-propagation and decrease the threshold for dendritic APs (Hoffman et al. 1997). Similarly, mitogen-activated protein kinase (MAPK) phosphorylates A-type K+ channels and decreases IA, thereby enhancing dendritic excitability (Yuan et al. 2002; Schrader et al. 2006). Conversely, MAPK blockers reduce dendritic excitability (Watanabe et al. 2002). In addition, protein kinase A (PKA) and protein kinase C (PKC) have been shown to converge on MAPK in mediating this reduction in dendritic K+ current (Yuan et al. 2002).
Previous studies demonstrate that modifications of dendritic membrane properties can also occur in parallel with long-term potentiation (LTP) of synaptic transmission (Wang et al. 2003; Frick et al. 2004; Fan et al. 2005; Xu et al. 2005; Kim et al. 2007; Narayanan & Johnston, 2007; Losonczy et al. 2008). Certain activity patterns that induce LTP also trigger the phosphorylation of MAPK (English & Sweatt, 1996) by MAP-ERK kinase (MEK). The synaptic changes that occur during these forms of LTP rely upon MAPK (English & Sweatt, 1997; Kanterewicz et al. 2000). In addition, after LTP the channels that underlie IA (Kv4.2) are internalized (Kim et al. 2007), leading to a localized decrease of IA and a concomitant increase in the amplitude of bAPs (Frick et al. 2004). Thus, activation of MAPK and induction of LTP both lead to a reduction of IA and an increase of dendritic excitability. Given the parallels between the effects of MAPK on dendritic excitability and the changes of dendritic excitability that occur during LTP, it has been hypothesized that the change of dendritic excitability that occurs during LTP is dependent upon MAPK. However, this has not been directly tested. In addition, it is unknown whether PKA, a kinase that can initiate cascades that phosphorylate MAPK (Yuan et al. 2002), is involved in the dendritic changes that occur during LTP. This study uses in vitro dendritic and somatic whole-cell recordings to test whether the changes of synaptic input and dendritic excitability that occur during theta-burst pairing LTP are dependent upon MAPK.
Methods
Ethical approval
All procedures were performed in accordance with the Institutional Animal Care and Use Committee at The University of Texas at Austin and Baylor College of Medicine, and followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Slice preparation
Male Sprague–Dawley rats (aged 6–9 weeks, 126 animals) were anaesthetized with an intraperitoneal injection of a combination of ketamine (42 mg ml−1) and xylazine (8 mg ml−1). Once anaesthetized, rats were perfused intracardially with an ice-cold solution containing (mm): 210 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 dextrose, 1.3 ascorbic acid and 3 pyruvic acid (all drugs were from Sigma, St Louis, MO, USA unless otherwise specified). The solution was saturated with a 95% O2–5% CO2 gas mixture. After perfusion, the rats were quickly decapitated; the brain was removed, and sliced in 300–350 μm sections in the same solution (Vibratome Series 1000, Vibratome, St Louis, MO, USA). Brain slices were then incubated at 37°C for 20–30 min in a solution saturated with 95% O2-5% CO2 gas mixture, containing (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 10 dextrose, 1.3 ascorbic acid and 3 pyruvic acid. After incubation, brain slices were allowed to stabilize for at least 50 min at room temperature prior to transfer to the recording chamber. Immediately prior to transferring the slice to the recording chamber, a small cut was made between CA1 and CA3 to diminish the possibility of epileptiform activity.
Whole-cell recordings
Brain slices were transferred to a recording chamber perfused with a solution saturated with 95% O2-5% CO2 gas mixture, containing (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2 and 10 dextrose. Pyramidal neurons in CA1 were visually identified using infrared differential interference contrast microscopy. Pyramidal neurons were selected based on the protrusion of a long apical dendrite and a non-stellate appearance. Patch electrodes were constructed from borosilicate glass with an outer diameter of 1.65–2.0 mm. Electrodes were pulled using a Flaming/Brown micropipette puller (Model P-97, Sutter Instruments, Novato, CA, USA). Electrodes were filled with (mm): 120 potassium gluconate, 20 KCl, 0.2 EGTA, 10 HEPES and 2 MgCl2, with a pH of 7.3. On the day of recordings, 4 Na2-ATP, 0.3 Tris-GTP and 7 phosphocreatine were added to the internal recording solution (yielding an osmolality of approximately 290–295 mosmol kg−1). Whole-cell recordings were performed at approximately 34°C. The electrode junction potential was corrected in the bath, but the diffusion potential was not compensated after seal formation and membrane rupture. Prior to formation of a seal, the electrode tip potential was negated, and the open-tip resistance measured (typically 4–6 MΩ). Upon successful transition to whole-cell configuration, the neuron was given at least 5 min to stabilize before data were collected. Series resistance was monitored and compensated throughout the experiment using built-in bridge circuitry.
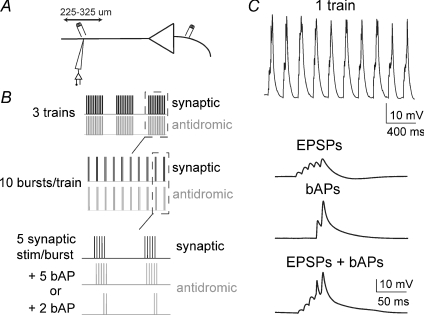
Synaptic EPSPs were evoked by electrical stimulation (0.1 ms, 0.01–0.2 μA) along the apical dendrite 225–325 μm from the soma and 15–25 μm lateral to the apical dendrite. Back-propagating APs were evoked by electrical stimulation (0.1 ms, 0.01–0.2 μA) 5–20 μm from the initial segment of the axon of the same neuron. Two types of theta-burst pairing (TBP) protocols were used to induce LTP. In both instances, five EPSPs were evoked in bursts of 100 Hz, repeated in 10 trains of 5 Hz (theta-frequency; Fig. 1). These trains were repeated three times at 0.1 Hz. The two forms of TBP protocols differed in the number of bAPs paired with the EPSPs (see, e.g. Watanabe et al. 2002; Frick et al. 2004). In the ‘strong protocol’ (5 bAP protocol), a single bAP was evoked for each EPSP (5 bAPs per burst). In the ‘weak protocol’ (2 bAP protocol), a single bAP was evoked for each of the last two EPSPs in the burst (2 bAPs per burst; Fig. 1). In both conditions, the bAP was timed to occur close to the peak of the EPSP. After LTP induction, a single test bAP or EPSP was evoked, alternating every 15 s for 45 min. If the membrane potential deviated from the resting membrane potential (Vrest) after LTP induction, it was brought close to the original value with DC injection. Series resistance was monitored throughout the experiment with test current injections (−40 pA, 500 ms). Experiments were only included in analysis if the series resistance remained relatively stable (< 15% change) and was below 25 MΩ for dendritic recordings and 15 MΩ for somatic recordings. Additionally, data were not included if the bAP appeared to be evoked by synaptic stimulation instead of antidromic stimulation (i.e. if the bAP was riding on top of an EPSP and/or it had a latency > 2 ms).
Figure 1. Theta-burst pairing protocol.
During dendritic recordings, at variable distances from the soma, inputs proximal to the recording site were stimulated (A) in a TBP pattern (B) to induce synaptic and dendritic plasticity. C, in a train of EPSP and bAP pairings, 5 EPSPs were evoked, and paired with 5 or 2 bAPs. Black traces represent baseline conditions and grey traces represent post-LTP. All traces are overlays of 4 or 5 consecutive sweeps.
Drug application
In all experiments, 10 μm bicuculline (in DMSO, Tocris Bioscience, Ellisville, MO, USA), 10 μm picrotoxin (in ethanol, Sigma) and 2 μm CGP55845 (in DMSO, Tocris Bioscience) were included in the bath. In some experiments, brain slices were pre-incubated with the MEK inhibitors U0126 (10 μm, Tocris Bioscience) or PD098059 (50 μm, Sigma) for approximately 30–45 min prior to LTP induction, and were present throughout the experiment. This allowed ample time for the MEK blockers to reach a plateau of effect (Watanabe et al. 2002; and see Fig. 4C). The PKA inhibitor KT5720 (200 nm, Tocris Bioscience) or the phosphodiesterase (PDE) inhibitor Ro 20-1724 (10 μm, Tocris Bioscience) were likewise pre-applied in some experiments. In a group of experiments, the PKA inhibitory peptide fragment (10 μm, PKAi fragment 6–22, Tocris Bioscience) was applied intracellularly in the recording pipette. In a separate group of experiments, these drugs were applied after baseline data had been obtained, to determine their effects on bAP parameters. The amount of DMSO in the bath was maintained at or below 0.1%.
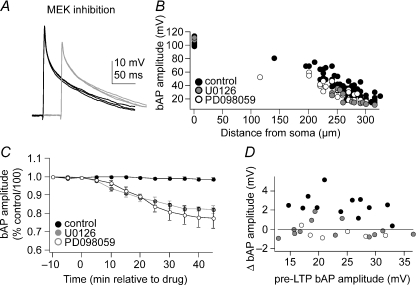
Figure 4. Inhibition of MAPK reduces the bAP amplitude.
Application of the MEK inhibitor U0126 (10 μm) or PD098059 (50 μm) reduces the amplitude of the bAP (A; in this example U0126 was used). Black traces represent baseline conditions and grey traces represent post-treatment. All traces are overlays of 4 or 5 consecutive sweeps. This is observed when comparing across groups that were pre-incubated in MEK inhibitors [B; each data point represents the bAP amplitude of a single neuron during the first 10 min of patching in control conditions (filled circles), or after pre-incubation with U0126 (grey circles) or PD098059 (open circles)], or within neurons when the effects of MEK inhibition were measured over time (C; drug application occurs at time = 0 min). Data points in C represent the means ±s.e.m. of bAP amplitudes; distances ranged from 250 to 310 μm. D, an increase in bAP amplitude after TBP was seen in control conditions (filled circles), while in the presence of PD098059 (open circles) or U0126 (grey circles) no change in bAP amplitude was observed over a range of baseline bAP amplitudes (displayed are the bAP amplitudes from the experiments utilizing the 2 bAP LTP protocol).
Data collection and analysis
Voltage signals were amplified and filtered in most experiments at 10 kHz (SEC-05L, npi electronic GmBH, Tamm, Germany; AxoClamp 2A, Axon Instruments, Union City, CA, USA), and collected and digitized at 50 kHz via an ITC-18 interface board (Instrutech Corp., Port Washington, NY, USA), transmitting to an Apple computer (PowerPC G5, Cupertino, CA, USA) running Igor Pro software (WaveMetrics Inc., Lake Oswego, OR, USA).
The action potential rise time was quantified as the peak of the first derivative (dV/dt) of the action potential waveform and the initial dV/dt. The bAP amplitude was defined as the difference between the action potential peak voltage and the initial membrane potential. The EPSP slope was quantified from 10 to 90% of peak amplitude.
All planned comparisons were made with Student's paired t tests, with an α-level of 0.05 considered significant. Further post hoc comparisons were made with Student's unpaired t tests, unless otherwise noted, with Bonferroni corrections of P values. In all plots, markers indicate the mean value ±s.e.m.
Results
A total of 165 neurons were analysed for this study. Twenty-eight recordings were made from the soma and 137 were dendritic recordings (225–325 μm from soma, median 275 μm). The neurons displayed properties similar to previous reports of CA1 pyramidal neurons at this age (somatic Vrest, −67.4 ± 1.1 mV; somatic neuronal input resistance, 69.7 ± 4.6 MΩ; somatic AP amplitude, 101.0 ± 0.5 mV; dendritic Vrest, −63.8 ± 1.4 mV; and dendritic neuronal input resistance = 58.5 ± 8.2 MΩ). The amplitude of the dendritic bAP was distance dependent.
Theta-burst pairing induced dendritic plasticity
Excitatory postsynaptic potentials with baseline amplitudes of 2–6 mV (as measured at the dendritic recording site) were evoked by electrical stimulation close to the apical dendrite, at distances of 225–325 μm (see Methods). The recording patch electrode was at the trunk of the adjacent apical dendrite (Fig. 1A, Frick et al. 2004). Long-term potentiation was induced by one of two previously described theta-burst pairing protocols: repeated pairing of five EPSPs with five bAPs (5 bAP protocol, ‘strong protocol’), or pairing five EPSPs with two bAPs (2 bAP protocol, ‘weak protocol’; Fig. 1B and C; see Methods for more details; Watanabe et al. 2002). When recording in the dendrite, the standard five bAP stimulation protocol induced a strong increase in the amplitude of the evoked EPSP and in the slope of the evoked EPSP rise time (Fig. 2A and C; 250.1 ± 32.2% of normalized slope; n= 11, t= 4.66, P < 0.001, one sample t test significantly different from 100), and an ∼20% increase in the bAP amplitude (Fig. 2A and C; baseline, 26.4 mV ± 2.1 mV; post-LTP, 31.7 ± 2.7 mV; n= 11, t= 4.82, P < 0.01, Student's paired t test). The two bAP protocol induced a smaller LTP of EPSPs (Fig. 2B and C; 164.4 ± 25.4% of normalized slope of the rise time; n= 12, t= 2.54, P= 0.026, one sample t test significantly different from 100), and a smaller (∼10%) increase of the bAP (Fig. 2B and C; baseline, 24.6 ± 2.3 mV; post-LTP, 27.4 ± 2.7 mV; n= 12, t= 2.91, P= 0.016, Student's paired t test). There was no significant difference in the peak dV/dt or the initial dV/dt in the bAP after LTP (5 bAP LTP: baseline peak dV/dt, 41.4 ± 5.3 mV ms−1; post-LTP, 43.0 ± 6.6 mV ms−1; P > 0.05, t= 2.02, Student's paired t test; baseline initial dV/dt, 39.4 ± 5.6 mV ms−1; post-LTP, 44.8 ± 5.8 mV ms−1; P > 0.05, t= 2.09, Student's paired t test; and 2 bAP LTP: baseline peak dV/dt, 43.3 ± 6.9 mV ms−1; post-LTP, 45.7 ± 7.0 mV ms−1; P > 0.05, t= 2.14, Student's paired t test; baseline initial dV/dt, 36.5 ± 6.1 mV ms−1; post-LTP, 38.1 ± 6.3 mV ms−1; P > 0.05, t= 2.12, Student's paired t test).
Figure 2. Long-term potentiation of EPSP and dendritic excitability after TBP.
The TBP protocol increases the amplitude of the EPSP and bAP. This effect is greater in the 5 bAP protocol (A) than in the 2 bAP protocol (B). Overall, the 5 bAP TBP protocol caused almost double the amount of increase of the EPSP and bAP compared with the 2 bAP protocol (C). Black traces represent baseline conditions and grey traces represent post-LTP. All traces are overlays of 4 or 5 consecutive sweeps. The LTP induction protocol occurs at time = 0 min. Data points represent the means ±s.e.m.
These two TBP protocols were also examined using somatic recordings. As observed previously (Watanabe et al. 2002), and in accordance with our results obtained with dendritic recordings, both protocols induced LTP of the EPSP recorded at the soma (5 bAP protocol, 233.8 ± 27.5% of normalized EPSP rise time slope, n= 6; 2 bAP protocol, 155.2 ± 31.9% of normalized EPSP rise time slope, n= 11).
Inhibition of MAPK blocked dendritic plasticity
We next probed the role of the MAPK pathway in mediating synaptic and dendritic plasticity induced with either the ‘strong’ or ‘weak’ TBP protocol. Specific inhibitors of MEK (10 μm U0126 or 50 μm PD098059) were bath applied 30–45 min before LTP induction. As shown in the previous subsection, in control conditions LTP was accompanied by an increase in bAP amplitude by ∼10–20% (depending on the protocol used); however, in the presence of MEK inhibitors the bAP amplitude did not change after the TBP protocols (Fig. 3) regardless of whether the five bAP protocol (Fig. 3A and C, bottom panel; in PD098059, baseline bAP amplitude, 28.4 ± 3.1 mV; post-pairing bAP amplitude, 27.8 ± 2.9 mV; P= 0.48, t= 0.72, n= 12, Student's paired t test; and in U0126, baseline bAP amplitude, 22.9 ± 2.7 mV; post-pairing bAP amplitude, 22.2 ± 2.5 mV; P= 0.09, t= 1.90, n= 10, Student's paired t test) or two bAP protocol was used (Fig. 3B and C, bottom panel; in PD098059, baseline bAP amplitude, 23.5 ± 2.4 mV; post-pairing bAP amplitude, 22.8 ± 2.2 mV; P= 0.25, t= 21.30, n= 6, Student's paired t test; and in U0126 baseline bAP amplitude, 22.6 ± 2.1 mV; post-pairing bAP amplitude, 21.9 ± 2.2 mV; P= 0.22, t= 2.63, n= 13, Student's paired t test). The blockade of the TBP-induced increase of the bAP amplitude by MEK inhibitors is consistent with the hypothesis that these activity patterns activate MAPK, and that this mechanism is responsible for the change of dendritic excitability after LTP induction.
Figure 3. Inhibition of MAPK blocks dendritic plasticity.
Application of the MEK inhibitor U0126 (10 μm) or PD098059 (50 μm) blocks the increase of the dendritic bAP after TBP in both the 5 bAP (A and C) and the 2 bAP protocols (B and C); both A and B display examples using U0126. However, the MEK inhibitors only block LTP of the EPSP in the 2 bAP protocol (B and C) and not the 5 bAP protocol (A and C), indicating an activity-dependent dissociation of the signalling pathways that modulate plasticity. Black traces represent baseline conditions and grey traces represent post-LTP. All traces are overlays of 4 or 5 consecutive sweeps. The LTP induction protocol occurs at time = 0 min. Data points represent the means ±s.e.m.
However, the MAPK pathway appears to have a more limited role in synaptic plasticity. Thus, MEK inhibitors blocked LTP of the EPSP only in the two bAP protocol (Fig. 3B and C, top panel; in U0126 102 ± 11% of normalized EPSP rise time slope; P= 0.63, t= 0.50, n= 12, one-sample t test; and in PD098059 98 ± 13% of normalized EPSP rise time slope; P= 0.86, t= 0.181, n= 6) and not in the five bAP protocol (Fig. 3A and C, top panel; in U0126 190 ± 18% of normalized EPSP rise time slope; P= 0.006, t= 5.11, n= 10, one-sample t test; and in PD098059 228 ± 27% of normalized EPSP rise time slope; P= 0.0017, t= 4.40, n= 10). This effect of MEK inhibitors on LTP of the EPSP was also apparent when recording LTP at the soma, as previously described (Watanabe et al. 2002), and replicated here (U0126, 5 bAP protocol, 211 ± 38% of normalized EPSP rise time slope; P= 0.0034, t= 4.02, n= 6; 2 bAP protocol, 113 ± 29% of normalized EPSP rise time slope, P > 0.05, t= 1.01, n= 5; data not shown).
Inhibition of MEK by itself (without an LTP protocol) decreased the amplitude of the bAP (Fig. 4). When slices were pre-incubated in U0126 or PD098059 (30 min), the bAP amplitude tended to be smaller, compared with control groups (Fig. 4B). When applied during the recording, U0126 or PD098059 decreased the amplitude of the bAP over time, reaching a plateau after approximately 30 min (Fig. 4A and C; baseline bAP amplitude, 28.5 ± 4.1 mV; post U0126 bAP amplitude, 23.6 ± 4.4 mV; P < 0.001, t= 4.81, n= 8, Student's paired t test; and baseline bAP amplitude, 32.9 ± 5.6 mV; post PD098059 bAP amplitude, 26.0 ± 6.3 mV; P > 0.001, t= 5.12, n= 9, Student's paired t test). However, this reduction in bAP amplitude alone does not account for the effects of these drugs on TBP-induced changes in dendritic excitability, as supported by the following finding. A range of bAP amplitudes was observed both in control conditions and in conditions when MAPK was blocked (Fig. 4B), and even in the presence of larger bAP amplitudes the MEK inhibitors still blocked the increase of the bAP amplitude (Fig. 4D) and LTP when the two bAP protocol was used. Furthermore, the reduction in the amplitude of the bAP after MEK inhibition is unlikely to be the result of time-dependent cellular rundown, since control groups that were not exposed to MEK inhibitors did not show this decrease of bAP amplitude (Fig. 4C).
We can also exclude the possibility that the lack of a significant change in the bAP amplitude (after TBP in the presence of MEK inhibitors) was due to an insufficient duration of pre-application of the MEK inhibitors. The neurons were pre-exposed to the MEK inhibitors for at least 30 min prior to TBP (see Fig. 4C and Methods). Thus, it is unlikely that the reduction of bAP amplitude by MEK inhibition and its increase following TBP activity could offset each other.
Role of cAMP and PKA in dendritic plasticity
In CA1 neurons, PKA can modulate IA and bAP amplitude through effects on MAPK (Yuan et al. 2002). If the changes of bAP amplitude after TBP truly necessitate PKA-induced activation of MAPK, blockade of PKA should also disrupt the effect of TBP on the bAP amplitude. Consistent with this idea, when the PKA inhibitor KT5720 (200 nm) was pre-applied, TBP did not cause changes of bAP amplitude (Fig. 5A and C, bottom panel). This effect of KT5720 on TBP-induced bAP changes was observed when either the five bAP protocol or the two bAP protocol was used (5 bAP protocol: baseline bAP amplitude, 23.9 ± 2.1 mV; postpairing, 22.8 ± 2.0 mV; P > 0.05, t= 1.48, n= 7, Student's paired t test; and 2 bAP protocol: baseline bAP amplitude, 22.5 ± 1.8 mV; postpairing, 21.7 ± 1.9 mV; P > 0.05, t= 1.77, n= 7, Student's paired t test). In addition, KT5720 blocked LTP of the EPSP (Fig. 5A and C, top panel; 2 bAP protocol, 103 ± 3% of normalized EPSP rise time slope, P > 0.05, n= 7, Student's paired t test; 5 bAP protocol 106 ± 8% of normalized EPSP rise time slope, P > 0.05, n= 7, Student's paired t test). These effects are consistent with a crucial role for PKA in TBP-induced synaptic and dendritic plasticity. These experiments were replicated using another specific PKA inhibitor, the PKA inhibitor peptide PKAi (10 μm; Glass et al. 1989), added to the recording pipette. Similar to the effects of PKA blockade with KT5720, in the presence of intracellular PKAi peptide, TBP did not induce changes in the bAP amplitude (Fig. 5C, bottom panel; 5 bAP protocol, baseline bAP amplitude, 24.3 ± 2.8 mV; postpairing, 26.8 ± 2.8 mV; P > 0.05, t= 1.83, n= 5, Student's paired t test; and 2 bAP protocol, baseline bAP amplitude, 21.0 ± 2.4 mV; postpairing, 22.5 ± 2.7 mV; P > 0.05, t= 1.36, n= 5, Student's paired t test). Also similar to the effects of KT5720 on synaptic plasticity, PKAi blocked LTP of the EPSP after both the two bAP protocol and the five bAP protocol (Fig. 5C, top panel; 2 bAP protocol, 107 ± 7% of normalized EPSP rise time slope, P > 0.05, n= 5, Student's paired t test; 5 bAP protocol, 115 ± 11% of normalized EPSP rise time slope, P > 0.05, n= 5, Student's paired t test).
Figure 5. Interference with the PKA signalling system disrupts TBP-induced plasticity.
Pre-application of the PKA inhibitor KT5720 (200 nm) blocks LTP of the EPSP and facilitation of the dendritic bAP (A and C). Inhibition of PDE IV with Ro 20-1724 (10 μm) also blocks LTP of the EPSP and facilitation of the dendritic bAP (B and C). Displayed are the results from the experiments using the 2 bAP LTP protocol. Black traces represent baseline conditions and grey traces represent post-LTP. All traces are overlays of 4 or 5 consecutive sweeps. Data points in C represent the means ±s.e.m.
Next we tested whether the PKA pathway modulates dendritic excitability under basal conditions. Inhibition of PKA with KT5720 (200 nm) in the absence of TBP protocols had little apparent effect on the amplitude of the bAP (Fig. 6A and D; baseline bAP amplitude, 23.4 ± 3.3 mV; post-KT5720 application, 22.1 ± 3.4 mV; P > 0.05, t= 0.79, n= 4, Student's paired t test). This indicates that the lack of a significant effect of TBP on the bAP amplitude during PKA inhibition is not caused by an action of KT5720 on bAP amplitude. Furthermore, these results indicate that, unlike MAPK, there is minimal tonic influence of PKA on dendritic excitability. Similarly, addition of PKAi (10 μm) did not exert significant time-dependent effects on the bAP amplitude (Fig. 6D), indicating that the effects of PKAi are not due to offsetting actions of PKAi on basal bAP amplitude and TBP-induced changes.
Figure 6. Modulation of bAP amplitude by interference with cAMP and PKA signalling.
Inhibition of PKA with KT5720 (200 nm) or PKAi (10 μm) had minimal effect on the bAP amplitude (A and D; KT5720 administered at time = 0; PKAi present in the pipette from first point, t = −10). In contrast, inhibition of PDE IV with Ro 20-1724 (10 μm) increased the amplitude of the bAP (B and D). Pre-application of the MAPK inhibitor U0126 (10 μm) blocked the effect of Ro 20-1724 (C and D), indicating that the effects of PDE IV on bAP amplitude require MAPK activity. Black traces represent baseline conditions and grey traces represent post-LTP. All traces are overlays of 4 or 5 consecutive sweeps. Data points in D represent the means ±s.e.m.
If the effects of TBP are mediated by activation of the PKA–MAPK signalling cascade, then when this cascade is already potently activated, TBP is expected to have minimal effects. Since the effects of the pairing protocol that uses two bAPs has complete reliance on MAPK (in the presence of MAPK blockers, the changes of bAP and EPSP amplitudes are suppressed) and PKA, we used this TBP protocol to examine the effects of facilitation of the PKA–MAPK cascade. Protein kinase A is activated by cAMP. Cyclic AMP, in turn, is degraded by phosphodiesterases, in particular PDE IV in the brain, thereby limiting its actions on PKA. Therefore, PDE inhibition is expected to lead to an accumulation of cAMP and to result in increased activation of PKA and MAPK. To test whether PDE inhibition (and cAMP accumulation) occludes the effects of the two bAP TBP protocol on the bAP amplitude, the specific PDE IV blocker Ro 20-1724 (10 μm) was applied before TBP. In contrast to control conditions, in the presence of Ro 20-1724 there was no significant change in the EPSP rise time slope (Fig. 5B and C, top panel; 109 ± 4% of normalized EPSP rise time slope, P > 0.05, n= 7) or the amplitude of the bAP after TBP (Fig. 5B and C bottom; baseline bAP amplitude, 24.8 ± 2.3 mV; postpairing bAP amplitude, 25.2 ± 2.8 mV; P= 0.55, t= 0.67, n= 7, Student's paired t test). Several features make this consistent with occlusion and not blockade of LTP by PDE inhibition, as follows: (1) application of Ro 20-1724 alone during dendritic recordings, without TBP, increased the amplitude of the bAP (Fig. 6B and D; baseline bAP amplitude, 25.8 ± 3.3 mV; post Ro 20-1724, bAP amplitude, 31.2 ± 3.2 mV; P > 0.001, t= 3.68, n= 6, Student's paired t test); (2) this effect of Ro 20-1724 on the bAP amplitude was blocked by the MAPK inhibitor U0126 (Fig. 6C and D; U0126, bAP amplitude, 22.9 ± 3.5 mV; and U0126 + Ro 20-1724, bAP amplitude, 22.7 ± 3.1 mV; P < 0.05, t= 0.79, n= 4, Student's paired t test); and (3) LTP could not be induced even with the strong five bAP protocol (104 ± 9% of normalized EPSP rise time slope, P > 0.05, n= 7), but LTP could be induced using the strong protocol when MAPK was blocked (see previous subsection). This is consistent with a PKA-mediated increase in MAPK during LTP, and that this effect can be occluded if PKA activity is already amplified before LTP.
Discussion
The major conclusion from this study is that specific patterns of pre- and postsynaptic activity (TBP) are linked to an increase in dendritic excitability via activation of MAPK. Specifically, we found that blocking MAPK prevents changes in dendritic excitability, while LTP of synaptic efficacy can still be induced by more robust TBP protocols. Furthermore, activation of MAPK during LTP may rely upon concurrent activation of PKA, since inhibition of PKA also suppresses synaptic and dendritic plasticity. Expanding on our previous finding that TBP can induce synaptic plasticity and change the integrative properties of dendritic subunits, our results suggest a mechanism by which both forms of plasticity can be induced, either together or separately, depending on the activity patterns used. While previous studies have demonstrated a significant role for PKA-activated MAPK in LTP, these findings demonstrate the novel role of these protein kinases in the plasticity of dendritic excitability that occurs during TBP activity, and strongly support the idea that synaptic and dendritic plasticity are not necessarily linked in the same signalling cascade.
Dendritic excitability regulates neuronal function and plasticity. The A-type K+ channels are important regulators of dendritic excitability in hippocampal CA1 pyramidal neurons, by reducing synaptic integration, the initiation of dendritic spikes and the back-propagation of action potential output (Hoffman et al. 1997; Ramakers & Storm, 2002; Cai et al. 2004; Frick et al. 2004; Losonczy & Magee, 2006; Kim et al. 2007). The impact of A-type K+ channels on dendritic excitability can be modified by many factors, including phosphorylation by MAPK activity. Dendritic excitability can also be shifted in response to changes in synaptic and intrinsic activity, such as during LTP-inducing activity patterns. This study demonstrates a link between activity and dendritic excitability due to MAPK activation occurring during TBP. Given the role of A-type K+ channels in regulating dendritic excitability, and their modulation by MAPK and periods of robust neuronal and synaptic activity, it is likely that the results observed in this study are due to a MAPK-mediated modulation of A-type K+ channels. Future studies will determine whether MEK inhibition can block the effects of neuronal activity on IA.
For the reasons described in the previous paragraph, we favour a role for kinase-dependent modulation of A-type K+ channels in the effects of TBP on the bAP amplitude. However, it is possible that other mechanisms may underlie this bAP change. For instance, an increase of Na+ current has been observed after similar LTP-inducing protocols (e.g. Xu et al. 2005), and blockade of MEK can reduce activity-dependent increases of Na+ channel activity (Loftis et al. 2003). Our data, however, argue against a major contribution of Na+ channels to the increase in bAP amplitude, because the initial rate of rise of the bAPs, a parameter that reflects the fast activation of Na+ channels (Hodgkin & Katz, 1949; Colbert et al. 1997), was not changed following TBP (see also Frick et al. 2004). Another possibility is that the role of kinases is related to modulation of protein synthesis and post-translation modifications (Klann & Sweatt, 2008), in which case the TBP protocols may cause MAPK-dependent phosphorylation of factors that regulate the synthesis of proteins that modulate dendritic excitability.
The increase of dendritic excitability during LTP requires PKA activation, as well as MEK-induced activation of MAPK. Interestingly, both a blocker of PKA and of PDE (which should increase PKA activity) suppressed all changes during TBP. One interpretation of this finding is that PKA blockade will prevent LTP by discontinuity in the signalling cascades that promote LTP, while increased levels of PKA will disrupt LTP by occlusion of changes of PKA levels that usually occur during LTP, resulting in diminished changes of MAPK. Consistent with this interpretation, the PDE inhibitor increased dendritic excitability, and a MEK inhibitor blocked this effect. An essential role for PKA in LTP has been seen before (Huang & Kandel, 1994; Nguyen & Kandel, 1996), and is extended by these studies to include its role in dendritic plasticity.
The increase of dendritic excitability during LTP may help facilitate synaptic changes, a phenomenon referred to as metaplasticity (Abraham & Bear, 1996). However, the MAPK-dependent change of excitability is not always directly tied to LTP of synaptic inputs. In more robust forms of LTP (5 bAP protocol), synaptic changes persist in the absence of dendritic changes when MAPK is blocked. In less robust forms of LTP (2 bAP protocol), the synaptic changes are more closely related to changes of dendritic excitability, and MAPK inhibition blocks both. However, inhibition of PKA blocks dendritic and synaptic plasticity in both stronger and weaker forms of LTP, which might not be expected if PKA is modulating plasticity entirely through MAPK. Perhaps in the weaker forms of plasticity, PKA activation induces MAPK activity, resulting in synaptic LTP and increased dendritic excitability (Fig. 7), whereas in more robust forms of LTP, PKA is activated to such an extent that it exerts MAPK-dependent effects on dendritic excitability and MAPK-independent effects on synaptic plasticity (Fig. 7). In fact, it has been demonstrated that PKA can exert direct effects on AMPA and NMDA receptors and small conductance Ca2+-activated K+ (SK) channels during synaptic plasticity (Nayak et al. 1998; Westphal et al. 1999; Banke et al. 2000; Tavalin et al. 2002; Esteban et al. 2003; Skeberdis et al. 2006; Lin et al. 2008), and the degree of PKA activation may depend upon the potency of the induction protocol (Huang & Kandel, 1994; Roberson & Sweatt, 1996; Woo et al. 2000). Coupled with this, more potent LTP induction protocols are likely to induce greater postsynaptic Ca2+ influx, which may also influence PKA targets involved in the expression of synaptic plasticity. Other protein kinases and signalling molecules are also likely to be involved in plasticity to differing degrees and in an activity-dependent manner, thereby influencing synaptic plasticity when MAPK is blocked; however, this has yet to be demonstrated. Protein kinase C, in particular, may play a significant role in the plasticity of the bAP (Yuan et al. 2002). However, blockade of PKA alone does not uncover residual bAP plasticity that might be induced by PKC activation (Fig. 5). Perhaps the TBP protocol does not activate PKC, or coactivation of PKA and PKC may be needed, and blockade of either alone may minimize bAP plasticity.
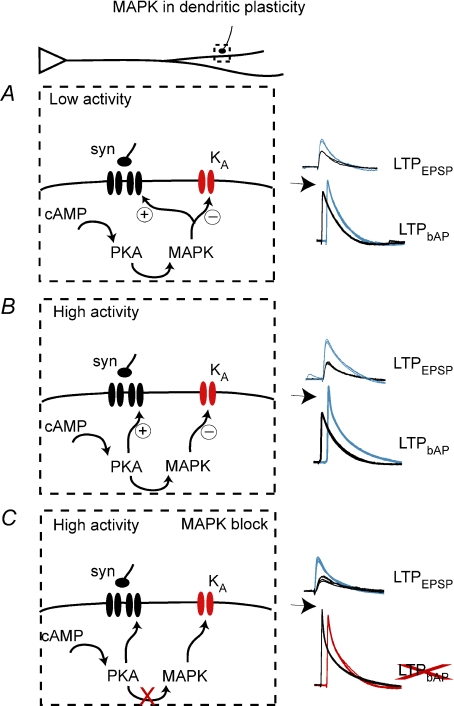
Figure 7. Schematic diagram of divergent activity-dependent roles of PKA and MAPK in synaptic and dendritic plasticity.
A, in conditions of low paired pre- and postsynaptic activity (2 bAP TBP), PKA activates MAPK, which is necessary for synaptic and dendritic plasticity. B, when there is a higher degree of paired activity (5 bAP TBP), dendritic plasticity still requires MAPK, while synaptic plasticity does not. C, when MAPK is blocked and there is a high degree of paired activity, synaptic plasticity can still occur, even in the absence of dendritic plasticity. It is important to note that this model describes only the role of MAPK in plasticity and not its role in modulation of basal synaptic transmission, which has been studied elsewhere (English & Sweatt, 1997).
In summary, this study demonstrates the crucial role for MAPK in the effects of synaptic and intrinsic activity on dendritic signal propagation. Furthermore, it demonstrates the existence of convergent and separate pathways leading to the induction of synaptic plasticity and plasticity of dendritic excitability. The dissociation between forms of plasticity is dependent upon the amount of pre- and postsynaptic activity, and is the result of the recruitment of different signalling pathways. One important ramification is that there are conditions in which synaptic plasticity can occur in the absence of dendritic plasticity, or perhaps vice versa. This novel information may also help to differentiate the roles of dendritic and synaptic plasticity, and of PKA and MAPK, in different forms and strengths of learning.
Acknowledgments
The authors wish to thank Dr Randy Chitwood for experimental suggestions and valuable discussion and Dr Rick Gray for programming and assistance with software. This work was supported by National Institutes of Health (NIH) Grant MH48432 (D.J.) and the Wodecroft Foundation and National Alliance for Research on Schizophrenia and Depression (J.A.R.).
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis S. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager OH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Glass DB, Cheng HC, Mende-Mueller L, Reed J, Walsh DA. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J Biol Chem. 1989;264:8802–8810. [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387(6636):869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Kanterewicz BI, Urban NN, McMahon DB, Norman ED, Giffen LJ, Favata MF, Scherle PA, Trzskos JM, Barrionuevo G, Klann E. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci. 2000;20:3057–3066. doi: 10.1523/JNEUROSCI.20-09-03057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral–CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JL, King DD, Colbert CM. Kinase-dependent loss of Na+ channel slow-inactivation in rat CA1 hippocampal pyramidal cell dendrites after brief exposure to convulsants. Eur J Neurosci. 2003;18:1029–1032. doi: 10.1046/j.1460-9568.2003.02832.x. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452(7186):436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature. 1998;394(6694):680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GM, Storm JF. A postsynaptic transient K+ current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci USA. 2002;99:10144–10149. doi: 10.1073/pnas.152620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271:30436–30441. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290:C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu C-P, Duan S, Poo M-M. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron. 2003;37:463–472. doi: 10.1016/s0896-6273(02)01189-3. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2002;99:8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285(5424):93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Genetic and pharmacological demonstration of differential recruitment of cAMP-dependent protein kinases by synaptic activity. J Neurophysiol. 2000;84:2739–2745. doi: 10.1152/jn.2000.84.6.2739. [DOI] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]