Abstract
Binding of γ-aminobutyric acid (GABA) to its receptor initiates a conformational change to open the channel, but the mechanism of the channel activation is not well understood. To this end, we scanned loop F (K210–F227) in the N-terminal domain of the ρ1 GABA receptor expressed in Xenopus oocytes using a site-specific fluorescence technique. We detected GABA-induced fluorescence changes at six positions (K210, K211, L216, K217, T218 and I222). At these positions the fluorescence changes were dose dependent and highly correlated to the current dose–response, but with lower Hill coefficients. The competitive antagonist 3-aminopropyl(methyl)phosphinic acid (3-APMPA) induced fluorescence changes in the same direction at the four middle or lower positions. The non-competitive antagonist picrotoxin blocked nearly 50% of GABA-induced fluorescence changes at T218 and I222, but only <20% at K210 and K217 and 0% at K211 and L216 positions. Interestingly, the picrotoxin-blocked fraction of the GABA-induced fluorescence changes was highly correlated to the Hill coefficient of the GABA-induced dose-dependent fluorescence change. The PTX-insensitive mutant L216C exhibited the lowest Hill coefficient, similar to that in binding. Thus, the PTX-sensitive fraction reflects the conformational change related to channel gating, whereas the PTX-insensitive fraction represents a binding effect. The binding effect is further supported by the picrotoxin resistance of a competitive antagonist-induced fluorescence change. A cysteine accessibility test further confirmed that L216C and K217C partially line the binding pocket, and I222C became more exposed by GABA. Our results are consistent with a mechanism that an outward movement of the lower part of loop F is coupled to the channel activation.
GABA-gated ion channels (GABAA/C receptors) belong to the cys-loop receptor family of ligand-gated ion channels, which also includes nicotinic receptors, serotonin receptors, glycine receptors and zinc-activated receptors (Lester et al. 2004). The best structural models available for the cys-loop receptor family are pentameric crystal structures of several homologous soluble proteins: acetylcholine binding proteins (AChBP) (Brejc et al. 2001; Celie et al. 2004, 2005; Hansen et al. 2005). Experimentally determined binding residues contributed from six segments (designated loops A through F) of the N-terminal domains of the cys-loop receptor subunits can be mapped to the 3-D structure of AChBP to form an agonist/antagonist binding pocket in the subunit interface. Crystal structures of AChBPs in the presence or absence of its agonists/antagonists have also revealed that agonists and antagonists induce structural changes mainly in loop C and loop F (Celie et al. 2004, 2005; Hansen et al. 2005). However, since these proteins have no transmembrane channel domains, the putative coupling loop (loop 2) did not show significant agonist-induced change. Thus, detecting conformational changes in an intact receptor is necessary to directly correlate structural change to channel function.
With the aid of the AChBP structure and accumulated data in the past two decades, the structural models for the binding pockets of the αβγ GABAA receptor (Sigel et al. 1992; Amin & Weiss, 1993; Smith & Olsen, 1994; Westh-Hansen et al. 1997, 1999; Boileau et al. 1999, 2002; Newell & Czajkowski, 2003; Holden & Czajkowski, 2004) and ρ1 GABAC receptor (Amin & Weiss, 1994; Lummis et al. 2005; Sedelnikova et al. 2005; Harrison & Lummis, 2006) have rapidly emerged. In the GABAA receptor, six loops (A through F) have been identified that form the agonist/antagonist binding pocket in the interface between β and α subunits. The agonist/antagonist binding pocket for the ρ1 GABAC receptor is formed in the subunit interface between two ρ1 subunits with five binding loops (A–E) identified (Sedelnikova et al. 2005).
The cys-loop receptors are allosteric proteins (Changeux & Edelstein, 1998). Binding of an agonist to its receptor induces a conformational change that propagates to the distant gating machinery to open the channel (Changeux & Edelstein, 1998; Grosman et al. 2000; Karlin, 2002; Lester et al. 2004), potentially via an interconnected allosteric network (Chen et al. 2006b). While binding loops are relatively well defined, conformational changes that couple the agonist binding to the channel gate are still not fully understood. GABA receptor activation clearly involves conformational changes in the gating machinery (channel opening) and in the agonist-binding pocket (increase in binding affinity) (Chang & Weiss, 1999a,b). In fact, conformational changes in loops C and E of the agonist binding pocket have been detected with cysteine scanning or fluorescence techniques (Wagner & Czajkowski, 2001; Chang & Weiss, 2002; Muroi et al. 2006). The coupling between the N-terminal binding domain and transmembrane gating machinery is through charge interactions (Kash et al. 2003, 2004; Wang et al. 2007). Coupling between two N-terminal loops also makes a significant contribution to the channel gating (Venkatachalan & Czajkowski, 2008). Since conformational changes are initiated from agonist binding, studying structural dynamics in the binding loops will provide clues to understand the initiation of the gating mechanism.
To detect conformational change of ion channels in intact cells, two major techniques were successfully used: substituted cysteine accessibility method (SCAM) and site-specific fluorescence reporting (SSFR). SCAM is based on the functional consequence of a thio-reactive reagent attached to the substituted cysteine (Akabas et al. 1992). However, a limitation of SCAM is that the functional consequence cannot be monitored in real time for dynamic information. SSFR was first used to study conformational changes in voltage-gated ion channels (Mannuzzu et al. 1996; Cha & Bezanilla, 1997). With SSFR, an environmentally sensitive fluorescent reporter is attached to a substituted cysteine, and conformational changes of the channel can be detected and monitored in real time by observing fluorescence changes related to channel function. We have previously successfully adapted this technique to detect conformational changes of a GABA receptor (Chang & Weiss, 2002). A similar technique with smaller fluorophores (sulforhodamine methanethiosulphonate, MTSR, or tetramethylrhodamine maleimide, TMRM) has been successfully used in detecting conformational changes in nicotinic receptors (Dahan et al. 2004; Mourot et al. 2008), a GABAA receptor (Muroi et al. 2006), and a glycine receptor (Pless et al. 2007).
In this study, using SSFR with MTSR labelling, we scanned 18 residues (K210–F227) of loop F in the ρ1 GABAC receptor, and detected fluorescence changes at six positions. To correlate the fluorescence changes to channel function, we compared time courses and dose dependence of the GABA-induced fluorescence changes and currents. We also tested the effect of the GABAC receptor competitive antagonist 3-aminopropyl(methyl)phosphinic acid (3-APMPA) on fluorescence. To further evaluate functional significance of the fluorescence changes, we examined the sensitivity of the GABA- or 3-APMPA-induced fluorescence changes to the non-competitive antagonist, picrotoxin. Finally, to aid interpretation of the fluorescence scanning results for positions near the binding pocket, we also performed SCAM at these positions. A potential mechanism relating the conformational change to channel gating is discussed in the context of the homology model of the N-terminal domain of the ρ1 GABAC receptor and crystal structures of an AChBP.
Methods
Mutagenesis and cRNA preparation
The cDNAs encoding wild-type and 12 cysteine mutants (L216C–F227C) in loop F of the human ρ1 subunit were kindly provided by Dr David S. Weiss's laboratory. These cDNA clones are in pGEMHE vector with T7 orientation. The remaining residues in loop F (K210–S215) were mutated to cysteine, one at a time, using the PCR-based QuickChange method of the site-directed mutagenesis (Stratagene, Hercules, CA, USA) with pfuUltra DNA polymerase. The mutations were confirmed by automated DNA sequencing. The wild-type and mutant cDNAs were then linearized by NheI digestion. The cRNAs were transcribed by standard in vitro transcription using T7 RNA polymerase. After degradation of the DNA template by RNase-free DNase I, the cRNAs were purified and resuspended in diethyl pyrocarbonate (DEPC)-treated water. cRNA yield and integrity were examined on a 1% agarose gel.
Oocyte preparation and injection
Oocytes were harvested from female Xenopus laevis (Xenopus I, Ann Arbor, MI, USA) by an IACUC-approved protocol for Xenopus care and use. Briefly, the frog was anaesthetized by 0.2% MS-222. The ovarian lobes were surgically removed and placed in the incubation solution consisting of (in mm): 82.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 1 Na2HPO4, 0.6 theophylline, 2.5 sodium pyruvate and 5 Hepes; 50 μg ml−1 gentamycin, 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin, pH 7.5. The frog was given analgesic xylazine hydrochloride (10 mg kg−1, i.p.) and then allowed to recover from the surgery before being returned to the incubation tank. After the third surgery, the frog was killed under anaesthesia. The ovarian lobes were cut into small pieces and digested with 1 Wunsch units per ml liberase blendzyme 3 (Roche Applied Science, Indianapolis, IN, USA) with constant stirring at room temperature for 1.5–2 h. The dispersed oocytes were thoroughly rinsed with the above solution. The stage VI oocytes were selected and incubated at 16°C before injection. Micropipettes for injection were pulled from borosilicate glass on a Sutter P87 horizontal puller, and the tips were cut with forceps to ∼40 μm in diameter. The cRNA was drawn up into the micropipette and injected into oocytes with a Nanoject microinjection system (Drummond, Broomall, PA, USA) at a total volume of 20–60 nl.
Two-electrode voltage-clamp
The oocytes expressing a single cysteine mutant of the GABA receptor were labelled with MTSR (see below) and then placed in a custom made small volume chamber with continuous perfusion with oocyte Ringer solution (OR2), which consisted of (in mm): 92.5 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2 and 5 Hepes, pH 7.5. The chamber was grounded through an agar KCl bridge. The oocytes were voltage-clamped at −60 mV to measure GABA-induced currents using a GeneClamp 500B amplifier (Axon Instruments, Union City, CA, USA). The current signal was filtered at 10 Hz with the built-in 4-pole low-pass Bessel filter in the GeneClamp 500B and digitized at 20 Hz.
Fluorescence labelling and detection
Thio-reactive sulforhodamine methanethiosulphonate (MTSR) was purchased from Toronto Research Chemicals (Toronto, Canada). The entire bottle was dissolved in methanol, aliquotted and then desiccated with a Savant SpeedVac, and stored at −80°C in single vials wrapped with aluminium foil. Each single-use aliquot was dissolved in methanol to 5 mm concentration and diluted to 100 μm final concentration with the OR2 immediately before use. Tetramethylrhodamine-6-maleimide (TMRM) was from Invitrogen (Carlsbad, CA, USA) and was dissolved in dimethyl sulfoxide to 5 mm concentration and aliquotted and stored at −80°C. One to 7 days after injection, oocytes expressing single cysteine mutants of the GABA receptor were labelled with MTSR for 10 min, or TMRM (5 μm) for 30 min, at 18°C, and rinsed 5 times before electrophysiological or fluorescence recording. However, for the I222C mutant with MTSR labelling, in our preliminary data we noticed that the GABA dose–response curve of the current had a lower Hill coefficient and a nearly 2-fold lower EC50 than that for fluorescence change of the same mutant. This is probably due to incomplete labelling, which resulted in mixed populations of the receptors with two different affinities. Thus, the I222C mutant was labelled in the presence of 10 μm GABA, which resulted in almost superimposed dose-dependent change in current and fluorescence. Higher labelling efficiency in the presence of GABA also suggests that I222C becomes more exposed when the channel is activated. However, the Hill coefficient of the current dose–response relationship for this mutant was still less than 2, suggesting the labelling was still slightly incomplete. This may result in a small leftward shift of the current dose–response curve and influence the interpretation of binding and gating relationships. However, it does not influence our interpretation of the picrotoxin effect on GABA-induced fluorescence change at this position. It should be noted that after labelling, newly unlabelled receptors are still continuously incorporated into the plasma membrane. Thus, in a strict sense, all mutants were incompletely labelled slightly. Fluorescence recording was performed similarly to previously described (Chang & Weiss, 2002). Briefly, a MTSR-labelled oocyte was placed in a custom made two-compartment chamber with the animal pole facing down and exposed to the lower compartment through a ∼0.8 mm hole. The oocyte was perfused continuously through the lower compartment with OR2 recording solution, which was then switched to various test solutions by a Valve-link 8 computer-controlled perfusion system (AutoMate Scientific, Oakland, CA, USA). The chamber was mounted on a Zeiss Axiovert 200 inverted microscope. The portion of the oocyte exposed to ligand was entirely within the visual field when viewed through a ×20 objective. A set of fluorescence filters (HQ 545/30x excitation, Q570LP dichroic, and D580/25m emission; Chroma Technology Corp., Brattleboro, VT, USA) was used in the microscope. The fluorescence signal was detected and amplified by an H9306-03 PhotoSensor module (Hamamatsu Corp., Bridgewater, NJ, USA) mounted on the camera port of the microscope. The signal was low-pass filtered at 1 Hz by an 8-pole Bessel filter (Frequency Devices, Haverhill, MA, USA) and digitized at 10 Hz using an Axon Digidata 1322A computer interface (Molecular Devices Corp., CA, USA) attached to a Dell computer (Dell, Austin, TX, USA). To minimize photobleaching, the dose-dependent fluorescence change was performed with sequential applications of different GABA concentrations in a single sweep. Minimal current desensitization of the ρ1 GABAC receptors also made it possible to use the same sequential GABA concentration steps for the current dose–response relationship measurement. In the case of traces with detectable photobleaching, the measurement was made with a corrected baseline to compensate for the photobleaching effect.
SCAM
The cysteine accessibility test for each mutation was performed with application of the long half-life (370 min) 2-sulphonatoethyl-methanethiosulphonate (MTSES; Karlin & Akabas, 1998; Toronto Research Chemicals, Toronto, Canada) with an empirically determined optimal concentration for the reaction rate determination of each mutant. The current induced by GABA with an ∼EC50 concentration was recorded before and repeatedly after each 10 s exposure to MTSES. The current was altered by MTSES, depending on the cumulated exposure time, and exponentially reached a plateau. The pseudo-first-order rate was derived by least-squares fitting the data with a single exponential function using Prism 4.0 (GraphPad Software, Inc., San Diego, CA, USA). The second-order rate was calculated from the pseudo-first-order rate divided by the MTSES concentration.
Drug preparation
GABA (Sigma-Aldrich, St Louis, MO, USA) stock solution (100 mm) was prepared daily from the solid. 3-APMPA (100 mm; SKF97541, Tocris, Ellisville, MO, USA) and picrotoxin (4 mm; Sigma-Aldrich) stock solutions were prepared and stored at −20°C in aliquots. Working concentrations of these drugs were prepared from stock solutions immediately before use.
Data analysis
The dose–response relationship of the GABA-induced current or fluorescence in recombinant GABAC receptors was least-squares fitted to a Hill equation with Prism 4.0 to derive the EC50 (the GABA concentration required for inducing a half-maximal change), Hill coefficient (the slope factor), and maximum current/fluorescence, which was then used to normalize the dose–response curve from individual oocytes. The average of the normalized currents or fluorescence for each GABA concentration was used to plot the data. All the data were presented as means ± standard error of the mean (s.e.m.). Statistical comparison between control and multiple groups was performed with one-way ANOVA with post hoc Dunnett's test using Prism 4.0. Statistical comparison for logEC50 values and Hill coefficients between current and fluorescence was performed with two tailed, unpaired Student's t test with Microsoft Excel. Correlation analysis between parameters was performed using Prism 4.0.
Homology modelling
Three-dimensional models of the pentameric extracellular domains of the ρ1 GABA receptor were made using Discovery Studio 1.7 software (Accelrys, San Diego, CA, USA) running in a Dell Precision 690 computer. Five N-terminal domains, each ranging from L51 to I260, of the human ρ1 GABA receptor subunit were individually modelled using five chains (A–E) of an AChBP (PDB file of 1I9B) as templates. Briefly, the N-terminal domain of the ρ1 subunit was aligned to the AChBP sequence from the structural model in the Discovery Studio using ‘Align Sequence with Structure’ protocol with blosum62 scoring matrix, gap open penalty of −200, gap extension penalty of −10, and default 2-D gap weights. Five homology models were then built individually using the ‘Building Homology Models’ protocol with a predefined disulphide bridge in each cysteine loop. The resulting models of five individual subunits were merged into a single layer to form a pentameric structure. The pentameric model was further energy minimized for 400 steps of Steepest Descent method followed by 1000 steps of Conjugated Gradient method using the ‘Minimization’ protocol with CHAMm force field. Two adjacent subunits with an interface were chosen for presentation. 3-D presentation was made using the DEEPVIEW/SwisspdbViewer v3.7 (http://us.expasy.org/spdbv/) (Guex & Peitsch, 1997). The resulting image was saved as a POV-Ray 3.5 scene file. The final image of the model was rendered by POV-Ray 3.6 software (http://www.povray.org/download/).
Superimposition of two AChBP subunits in the presence or absence of nicotine was performed by using DEEPVIEW/SwisspdbViewer v3.7. Crystal structures of AChBP in the Hepes bound (1UX2) and nicotine bound (1UW6) states were downloaded from the protein data bank. A single subunit (chain A in both cases) was saved individually. Two subunits in different states were loaded to the same file, and then they were superimposed by ‘Magic fit’ function. The resulting image was saved as a POV-Ray 3.5 scene file and rendered by POV-Ray 3.6 software.
Results
Fluorescence scanning region
Crystal structures of AChBPs have revealed that agonist-induced conformational changes are mainly observed in loop C and loop F. In this study, we focused on the structural dynamics of loop F. We made 18 single cysteine mutants of the ρ1 GABA receptor in the region of 210–227. Figure 1 shows the sequence alignment of the GABA receptor subunits ρ1, α1 and β2 and AChBP in this region. Clearly, this is a highly diversified region. Even within the GABA receptor subfamily, there are insertions or deletions in this region.
Figure 1. Fluorescence scanning region of loop F in the ρ1 GABA receptor subunit aligned to the homologous region of other subunits and AChBP.
The scanned residues are marked with ‘c’ for cysteine substitution. In the ρ1 subunit, bold residues represent the location with detectable GABA-induced fluorescence change. In the α1 subunit, bold residues are the previously identified binding pocket-lining residues (Newell & Czajkowski, 2003).
Activation properties of the cysteine mutants
All the single cysteine mutant receptors were functional. Activation properties of individual cysteine mutants at positions of 216–227 have been reported previously (Sedelnikova et al. 2005). Their EC50 values are re-listed in Table 1 along with those from the new mutants in the 210–215 region for easy reference. Note that except for S215C and Q226C with ∼20-fold decrease in sensitivity to GABA, there is no major change in the GABA response. Expression level (maximum GABA-induced current) also showed no dramatic variation. No abnormal gating behaviour was observed, except for S225C, which became spontaneously opening after MTSET modification (Sedelnikova et al. 2005), indicating no major perturbation to the receptor by mutation of most residues. We then proceeded to do fluorescence labelling and recording.
Table 1.
EC50 values of the GABA-induced currents of the 18 mutants in loop F of the ρ1 GABA receptor expressed in Xenopus oocytes
| Constructs | EC50 (μm) | Constructs | EC50 (μm) |
|---|---|---|---|
| WT | 0.81 ± 0.04‡ | ||
| K210C | 1.40 ± 0.09 | D219C | 3.02 ± 0.25† |
| K211C | 0.93 ± 0.11 | E220C | 1.46 ± 0.11† |
| G212C | 1.39 ± 0.04 | R221C | 0.51 ± 0.02† |
| N213C | 4.21 ± 0.29 | I222C | 0.5 ± 0.03† |
| D214C | 1.75 ± 0.13 | S223C | 0.66 ± 0.01† |
| S215C | 20.34 ± 1.71 | L224C | 3.38 ± 0.36† |
| L216C | 0.60 ± 0.03† | S225C | 0.53 ± 0.02† |
| K217C | 0.75 ± 0.06† | Q226C | 17.60 ± 3.58† |
| T218C | 1.03 ± 0.04† | F227C | 0.38 ± 0.32† |
Values from a previous publication (Sedelnikova et al. 2005).
Value from another publication (Chang & Weiss, 1999b). WT: wild-type ρ1 GABA receptor.
Agonist-induced fluorescence changes
The wild-type (for negative control) and individual cysteine mutants were separately expressed in oocytes and labelled with the fluorescent thio-reactive reagent MTSR. A saturating concentration of GABA was applied to the mutants to screen for the GABA-induced fluorescence change. Six out of 18 cysteine mutants exhibited detectable GABA-induced fluorescence changes (Fig. 2A). These mutants were K210C, K211C, L216C, K217C, T218C and I222C. However, the degree of fluorescence intensity change varies with different mutants. In the wild-type receptor-expressing oocytes labelled with MTSR, no fluorescence change was observed (data not shown), suggesting that the detected fluorescence changes are mutant specific. Furthermore, switching to a control solution in these six mutants did not produce a detectable fluorescence change (data not shown) for the reported oocytes. However, when the oocyte condition was compromised, switching artifact became apparent, and the data were discarded for these instances. One salient feature of fluorescence change in the six mutants is the direction of the fluorescence change. Mutants of L216C, K217C, T218C and I222C exhibited a decrease in fluorescence intensity in response to GABA, whereas mutants K210C and K211C demonstrated an increase in fluorescence intensity. The time course of fluorescence traces roughly followed the time course of current traces. A relatively small fluorescence signal precludes precise comparison of time courses between fluorescence and current. However, in the case of K217C, an earlier onset of fluorescence signal than the current signal in response to GABA application is evident. In addition, deactivation rate of current was slightly slower than the fluorescence recovery rate in several cases. This could be due to the possibility that switching impact causes back-flow of a trace amount of GABA into the upper chamber. Figure 2B represents the average fluorescence changes for the six mutants, and Fig. 2C depicts significantly higher total fluorescence in the labelled oocytes expressing these six mutants when compared to the labelled oocytes expressing the wild-type receptor.
Figure 2. Six cysteine mutants labelled with MTSR showed detectable GABA-induced fluorescence changes.
A, examples of fluorescence changes (grey and continuous lines) and currents (black and dotted lines) induced by a saturation concentration of GABA for the cysteine mutants as indicated. Note that the current traces are normalized to the same amplitude of the fluorescence traces. In the case of K210C and K211C, the current traces are also reversed for easy comparison. B, averages of the GABA-induced fluorescence changes in labelled oocytes expressing individual cysteine mutants (n= 3). C, averages of total fluorescence intensities in the labelled oocytes expressing wild-type or mutant GABAC receptors (n= 3). All six cysteine mutants exhibited significantly higher total fluorescence intensities compared to the wild-type (P < 0.001).
To aid interpretation of the fluorescence changes, we measured fluorescence intensity of MTSR in pure water and in ethanol. With our fluorescence detection system, the same concentration of MTSR exhibited a 5.8-fold higher fluorescence signal in ethanol than in water, demonstrating that MTSR emits more fluorescent light in the medium with lower dielectric constant. This is consistent with the interpretation of a previous study using MTSR (Dahan et al. 2004). Higher fluorescence in a lower dielectric constant environment suggests that during channel activation, residues L216C, K217C, T218C and I222C may become more exposed to aqueous solution and residues K210C and K211C become more buried in the protein hydrophobic core. It is also possible that an amino acid side-chain may cause quenching. In fact, quenching of tryptophan fluorescence by aromatic residues has been used to study ligand-induced conformational changes in an acetylcholine binding protein (Hansen et al. 2002). However, aromatic amino acids have absorption spectra in the UV range (<400 nm), which is far away from our emission detection range (567.5–592.5 nm). We specifically selected this emission filter to maximize detection sensitivity for emission spectral change of MTSR as observed in a nicotinic receptor due to a conformational change (Dahan et al. 2004). In addition, given that the size of the fluorescent dye is significantly larger than any amino acid side-chain, in the vicinity of the binding pocket, we cannot exclude the possibility of a binding effect on fluorescence because of ligand quenching/dequenching, although we did not see an obvious (dynamic) quenching effect of these ligands (100 μm GABA, 500 μm 3-APMPA, and 1 mm picrotoxin) in pure water or ethanol (data not shown).
To further explore the relationship of channel activation and fluorescence change, we constructed dose–response curves for both current (after labelling) and fluorescence change. Figure 3 shows the dose–response relationships of the GABA-induced currents and fluorescence changes. EC50 values and Hill coefficients for GABA-induced currents and fluorescence changes for all six mutants are listed in Table 2. Note that except for K211C, the five other mutants exhibited discernable dose–fluorescence change relationships that are closely correlated to the dose–response relationships of the current, but dose–fluorescence changes tend to have lower Hill coefficients, similar to the binding. However, we also noticed that unlike our previous study, which shows a slightly higher apparent binding affinity than the EC50 of gating in the wild-type ρ1 GABAC receptor (Chang & Weiss, 1999b), the fluorescence changes here had similar or slightly higher EC50 than that of current signals. There are two possible reasons: (1) the Hill coefficients of the current dose–response in all mutants were slightly lower than that for the wild-type receptor. This could be due to the gating free energy landscape being altered by the mutation, making the receptor slightly easier to open when two agonists bound and shifting the current dose–response curve to the left. Alternatively, after labelling, new unlabelled receptors can be slowly added to the plasma membrane repertoire, resulting in slightly incomplete labelling. This will also cause a small left shift of current dose dependence. (2) In some mutants, such as T218C and I222C, the fluorescence change represents the mixed effects of binding and gating (see Discussion). The gating component will further make the fluorescence data closer to the current data.
Figure 3. GABA-induced fluorescence changes were dose dependent and closely correlated to channel function.
The figure shows GABA activated currents and fluorescence changes in 6 mutants of the ρ1 GABA receptor, K210C, K211C, L216C, K217C, T218C and I222C, in a concentration-dependent manner. For each mutant, the averaged data from at least three oocytes are plotted with GABA concentration agonist normalized amplitudes. The continuous lines are best fits to a Hill equation. The resulting EC50 values and Hill coefficients are listed in Table 2. Note that in the K211C mutant, the fluorescence signal was relatively small. Therefore, the dose–response relationship of fluorescence change at this position could not be reliably resolved.
Table 2.
EC50 values and Hill coefficients of GABA-induced currents and fluorescence changes in six fluorescently labelled cysteine mutants of the ρ1 GABA receptor expressed in Xenopus oocytes
| Current | Fluorescence change | |||||
|---|---|---|---|---|---|---|
| Construct | EC50(μm) | nH | n | EC50(μm) | nH | n |
| WT | 0.81 ± 0.04‡ | 2.83 ± 0.07‡ | ND | ND | ||
| K210C | 1.95 ± 0.17 | 2.42 ± 0.03 | 3 | 1.89 ± 0.04 | 1.86 ± 0.04* | 3 |
| K211C | 0.47 ± 0.02 | 2.84 ± 0.04 | 4 | ND | ND | |
| L216C | 6.50 ± 0.32 | 2.78 ± 0.06 | 4 | 7.66 ± 0.35 | 1.50 ± 0.36* | 4 |
| K217C | 2.16 ± 0.09 | 2.37 ± 0.03 | 4 | 2.51 ± 0.03* | 1.63 ± 0.12** | 4 |
| T218C | 2.17 ± 0.24 | 2.48 ± 0.09 | 4 | 3.18 ± 0.09* | 1.97 ± 0.10* | 3 |
| I222C | 2.74 ± 0.09 | 1.91 ± 0.04 | 7 | 3.14 ± 0.10* | 2.12 ± 0.23 | 6 |
WT: wild-type ρ1 GABA receptor; ND: not determined; nH: Hill coefficient.
Value from a previous publication (Chang & Weiss, 1999b).
P < 0.05 when compared to parameters for current;
P < 0.01 when compared to parameters for current.
Competitive antagonist-induced changes
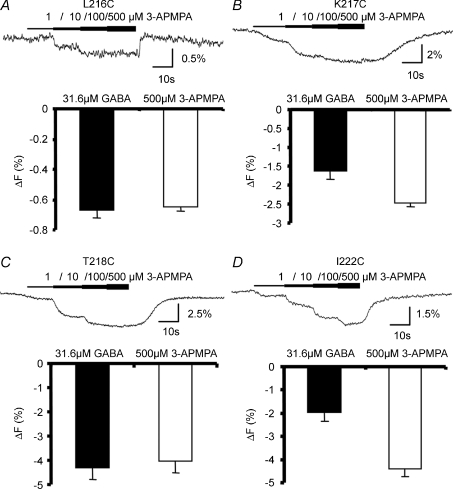
A structural analogue of GABA, 3-aminopropyl (methyl)phosphinic acid (3-APMPA), is a competitive antagonist of the GABAC receptor, occupying the GABA-binding pocket without gating the channel. In this study, we found that 3-APMPA induced fluorescence changes in four labelled mutants, L216C, K217C, T218C and I222C (Fig. 4). In these mutants, 3-APMPA induced fluorescence changes with the same direction as GABA-induced ones. Similar changes induced by both agonist and competitive antagonist suggest that observed fluorescence change could represent binding. The differential sensitivities of these agonist/competitive antagonist-induced fluorescence changes to the non-competitive antagonist picrotoxin would further help to evaluate the functional significance of the fluorescence changes (see below and Supplemental Fig. 1.
Figure 4. The competitive antagonist 3-APMPA induced fluorescence changes in four mutants, L216C, K217C, T218C and I222C.
The figure shows raw fluorescence traces induced by 3-APMPA and their averages compared to the GABA-induced fluorescence changes in the same sets of oocytes. Bar graph of each mutant represents the average fluorescence change induced by a given concentration of GABA or 3-APMPA from at least 3 different oocytes. Note that in these mutants, 3-APMPA-induced fluorescence changes were in the same direction as GABA-induced changes.
Non-competitive antagonist effect
The non-competitive antagonist picrotoxin antagonizes GABA action by binding to a site in the channel pore, distant from the GABA binding site. The mechanism for picrotoxin antagonism is not fully understood. We previously revealed that picrotoxin can antagonize GABA-induced conformational changes at two interface residues in N-terminal domains that are distant from its binding site (Chang & Weiss, 2002). Thus, picrotoxin can allosterically antagonize a GABA-induced conformational change. Here we examined this allosteric antagonizing effect of picrotoxin on the GABA-induced fluorescence changes in another important part of the receptor. As shown in Fig. 5A–G, picrotoxin blocked the GABA-induced current in all six mutants dose-dependently with nearly complete block at the highest concentration. However, it only partially blocked the GABA-induced fluorescence changes in K210C, K217C, T218C and I222C mutant receptors but did not block the fluorescence change in K211C and L216C mutants. Note that when normalized to the fraction of picrotoxin blocked current, the picrotoxin blocked fractions of fluorescence changes for T218C and I222C were higher. The picrotoxin sensitivity of the GABA-induced fluorescence change is not due to a direct effect of picrotoxin on fluorescence, because picrotoxin did not influence fluorescence in all mutants in a similar way, and picrotoxin alone did not induce detectable fluorescence change in these mutants (data not shown). Interestingly, the fraction of picrotoxin (at 1 mm)-blocked fluorescence change was highly correlated to the Hill coefficient of the GABA dose-dependent fluorescence change (Fig. 5H). These results further help us to evaluate functional significance of the observed fluorescence changes in loop F of the ρ1 GABA receptor.
Figure 5. GABA-induced fluorescence changes in 6 mutants exhibited differential sensitivity to GABA receptor non-competitive antagonist, picrotoxin.
A–F, GABA-induced fluorescence changes were partially blocked by picrotoxin in four mutants: K210C, K217C, T218C and I222C. This is in contrast to the GABA-induced currents, which were nearly completely blocked by 1 mm picrotoxin in these mutants. In the mutants K211 and K216, GABA-induced fluorescence changes were insensitive to picrotoxin (up to 1 mm). G, averages of the fraction of inhibition by 1 mm picrotoxin of the GABA-induced currents and fluorescence changes for all 6 mutants. Each bar represents the averaged data from at least 3 oocytes. H, correlation analysis of the fraction of inhibition by 1 mm picrotoxin of the GABA-induced fluorescence changes (normalized to the fraction of inhibition for current) and Hill coefficient of the GABA-induced dose-dependent fluorescence changes.
Cysteine accessibility
Although we previously did not identify a major binding site in loop F (Sedelnikova et al. 2005) for residues located in the vicinity of the ligand binding pocket, we cannot completely exclude the possibility that the detected fluorescence changes could be due to ligand quenching, especially when we attached a fluorophore with severalfold higher relative molecular mass than the largest side chain of a residue. To test this, we performed the SCAM for several cysteine mutants. The change in current amplitude of K210C, K211C and I222C after MTSES treatment was too small for reliable determination of the reaction rate. Thus, we measured the reaction rate of MTSES only for mutants L216C, K217C and T218C. Figure 6 shows the functional modification of these three mutants by MTSES. The second order reaction rates derived from these data are listed in Table 3. The reaction rate was slowed by GABA for L216C and K217C. The reaction rate for L216C and K217C was also clearly slowed by 3-APMPA, although the changes did not reach statistical significance. Thus, in the presence of GABA or 3-APMPA, these two positions, especially L216C, became less accessible to MTSES. That is, L216 and K217 residues could partially line the binding pocket, although they make no contribution to binding as indicated by no EC50 changes in their cysteine mutants. For I222C, in the absence of GABA, 1 mm MTSES modification for 1 min only resulted in 9.7 ± 4.3% (n= 4) current reduction. However, in the presence of 10 μm GABA, MTSES treatment (1 mm for 1 min) resulted in a current reduction by 36.0 ± 1.1% (n= 4, P < 0.01), suggesting an increase in accessibility when the receptor was activated. In addition, we also noticed that the difference in accessibility of K217C to MTSET (Sedelnikova et al. 2005) and MTSES, and reconfirmed the MTSET effect in the current study (data not shown). This difference could be due to opposite charges carried by these two thio-reactive modulators.
Figure 6. Influence of accessibility to MTSES of three cysteine mutants by GABA or 3-APMPA.
A, raw traces for an EC50 (0.6 μm) GABA-induced current in the oocytes expressing L216C mutant. Note that repeated exposure of MTSES progressively reduced the current to < 20% of original current level. However, in the presence of GABA or 3-APMPA, the rate for current reduction was slowed. B, the normalized and averaged currents for the mutant L216C were plotted against cumulative MTSES exposure time. Continuous lines are best fits of a single exponential function to the data sets. The resulting rate constants were used to calculate second order reaction rates, which are listed in Table 3. C and D, similar to B, but for mutants K217C (the testing GABA concentration was 0.6 μm for the effect of 4 μm MTSES, or MTSES + 1.8 μm GABA/500 μm 3-APMPA) and T218C (the testing GABA concentration was 1 μm for the effect of 50 μm MTSES, or MTSES + 2.5 μm GABA/500 μm 3-APMPA).
Table 3.
Second order reaction rate of MTSES of three cysteine mutants (m−1 s−1)
| Control |
3-APMPA |
GABA |
||||
|---|---|---|---|---|---|---|
| Receptor | k2 | n | k2 | n | k2 | n |
| L216C | 3381 ± 98 | 4 | 1802 ± 712 | 4 | 1480 ± 374* | 3 |
| K217C | 11424 ± 696 | 4 | 9778 ± 489 | 3 | 3725 ± 1101** | 3 |
| T218C | 545 ± 31 | 3 | 985 ± 130 | 4 | 850 ± 121 | 3 |
P < 0.05;
P < 0.01 (compared to control).
Discussion
To explore the potential role of loop F in the ρ1 GABA receptor function, we fluorescently scanned loop F and detected GABA-induced fluorescence changes at six positions. At these positions, the directions of the GABA-induced fluorescence changes were not the same. At positions 210 and 211, fluorescence increased during channel activation. In contrast, at positions 216, 217, 218 and 222, fluorescence intensity decreased. This difference in the direction of fluorescence change may suggest different directions of movement. However, the cysteine accessibility test suggests that fluorescence change at 216 and 217 are more likely to be due to ligand quenching. Thus, the fluorescence change at these two positions may reflect binding. The detected fluorescence changes were GABA concentration dependent and closely correlated to the channel function, but with lower Hill coefficients. Finally, the GABA-induced fluorescence changes at four positions, especially at 218, and 222 of the lower part of loop F, were partially blocked by the non-competitive GABA receptor antagonist picrotoxin. Interestingly, the picrotoxin blocked fraction was highly correlated to the Hill coefficient of the GABA dose-dependent fluorescence change, suggesting fluorescence changes at these positions of loop F represent a mixed effect of binding and gating. The binding effect is picrotoxin insensitive, and the gating effect is picrotoxin sensitive. Thus, loop F, especially the lower part, is partially coupled to channel gating.
To understand the potential mechanism of loop F in channel gating, we identified amino acid residues in the homology model of the N-terminal domain of ρ1 GABA receptor (Fig. 7A). Mapping the residues with detectable GABA-induced fluorescence changes onto the 3-D structure revealed that residues K210 and K211 are in the upper part of loop F, whereas residues L216, K217, T218 and I222 are located in the middle and lower part of loop F.
Figure 7. 3-D views of loop F in the ρ1 GABA receptor N-terminal domain and AChBP.
A, location of the 6 residues of loop F in the homology model of the ρ1 GABA receptor (side view). Six residues with detectable agonist/antagonist-induced fluorescence change in loop F (yellow ribbon) are presented as a space fill model and colour coded. Loop C is also presented with a space fill model for easy comparison of its special relationship to loop F. Note that loop 2 and the upper part of one arm in loop 7 of the left (principal) subunit is in close proximity to the bottom part of loop F of the right (complementary) subunit. B, superimposed crystal structures of AChBP subunits in Hepes bound state (from pdb 1UX2) and nicotine bound state (from pdb 1UW6). Upon nicotine binding, major structural changes in loop F are obvious. Note that the loops F from the two subunits are colour-coded: yellow for Hepes bound state, blue for nicotine bound state. Green arrows indicate the major nicotine-induced conformational changes and their directions in loop F. Six residues with side-chains in an AChBP are colour-coded to match homologous residues in the GABA receptor in A. C, loop F is close to loop 2 and loop 7 in the principal face in the ρ1 N-terminal domain homology model (viewed from bottom and slightly outside).
The increase in fluorescence for two upper residues (K210C, K211C) during channel activation suggests that they become more buried. This could result from the inward (toward the central vestibule) movement of upper part of loop F. Interestingly, the interpretation of our result in the GABA receptor is similar to the movement observed in the crystal structure of AChBP (Celie et al. 2004) (Fig. 7B), whose agonist also induces an inward movement in the upper part of the loop. The decrease in fluorescence for four lower residues suggests that these residues could become more exposed (outward movement) during channel activation. Alternatively, in the vicinity of the binding site, observed fluorescence change could be the result of direct quenching by the ligand. For the quenching effect, we can expect that a fluorophore with a shorter linker would protrude less into the binding pocket and experience less quenching. In fact, using TMRM, we have recorded the GABA-induced fluorescence changes in the same direction as those using MTSR for all six mutants, but with smaller amplitude in the lower four mutants (Supplemental Fig. 2). The result suggests that two rhodamines (MTSR and TMRM) are qualitatively similar. Since TMRM has a shorter linker, it may explain the smaller fluorescence signal at the lower four locations.
Furthermore, our SCAM result indicates that L216C and K217C in the middle part of loop F partially line the binding pocket. This is strengthened by a substantially lower Hill coefficient of the dose-dependent fluorescence in these two mutants than the Hill coefficient for the current, similar to the observed Hill coefficient for binding (nH= 1.4) relative to activation (nH= 2.8) for the ρ1 GABA receptor (Chang & Weiss, 1999b). Since the ρ1 GABA receptor requires three bindings to open (Amin & Weiss, 1996), at low GABA concentrations most of the receptors will have only one or two bindings and will not be open but with detectable binding. At higher GABA concentrations current saturates faster than binding, possibly because the fourth and fifth bindings make much less further contribution to the channel gating. Thus, the observed fluorescence changes at L216C and K217C positions could mainly reflect binding rather than gating. Slightly earlier onset in the time course of the fluorescence trace compared to the current trace for K217C (Fig. 2A) further supports this notion.
For T218C, greater exposure with activation is supported by slightly increased accessibility to MTSES by GABA (not statistically significant but in the right direction). A higher Hill coefficient for dose-dependent fluorescence change of T218C also suggests that fluorescence changes are more likely to have a component of conformational changes related to channel gating. For I222C, it is supported by the increased accessibility of MTSES (in Results) or MTSR (see Methods) to I222C in the presence of GABA and the highest Hill coefficient of the dose-dependent fluorescence changes among all six mutants. Interestingly, crystal structure of AChBP (Fig. 7B) reveals that in the AChBP the residue homologous to T218 of the ρ1 GABA receptor exhibited similar outward movement. However, in the AChBP the residue homologous to I222 does not move as I222C in the GABA receptor. This difference could reflect the distinction between a soluble protein and a ligand-gated ion channel. Coupling between N-terminal agonist/antagonist binding domains and channel forming domains must alter the conformation of the protein in the vicinity of the coupling region.
To further evaluate the functional significance of the observed fluorescence changes at residues in loop F, we measured the effect of picrotoxin on the GABA-induced fluorescence changes. Our results demonstrated that while the GABA-induced fluorescence changes in the upper and middle parts of loop F are relatively resistant to picrotoxin, the GABA-induced fluorescence changes in the lower part of loop F, mainly at the level of T218C and I222C, are partially sensitive to picrotoxin. More interestingly, the picrotoxin-blocked fraction of the GABA-induced fluorescence changes was positively correlated to the Hill coefficient of the GABA-induced dose-dependent fluorescence changes. Picrotoxin resistant mutants exhibited a Hill coefficient for fluorescence changes similar to that for binding, whereas picrotoxin-sensitive mutants had a Hill coefficient for fluorescence closer to that for channel activation. Thus, the detected fluorescence changes in the lower part of loop F are likely to be a mixed effect of binding and gating. The picrotoxin-insensitive fraction would represent a binding effect, whereas the picrotoxin-sensitive fraction would suggest a conformational change related to channel gating. This conclusion is further supported by the observation that the 3-APMPA-induced fluorescence changes in T218C and I222C were insensitive to picrotoxin (Supplemental Fig. 1). Thus, 3-APMPA and GABA both induced fluorescence changes at these two positions. However, GABA-induced fluorescence changes have binding and gating components, whereas 3-APMPA-induced changes mainly reflect binding. The picrotoxin resistant GABA-induced fluorescence changes are also observed in a binding residue (Y241C) of the ρ1 GABAC receptor (Chang & Weiss, 2002) and two residues in loop E of the GABAA receptor binding pocket (Muroi et al. 2006).
The picrotoxin binding site is believed to be located in the pore (Gurley et al. 1995; Xu et al. 1995; Buhr et al. 2001; Chen et al. 2006a; Olsen, 2006). However, its antagonist mechanism is still not fully understood. A recent study further reveals that after agonist removal, the picrotoxin blocked channel appears to be in a closed state (Bali & Akabas, 2007). Thus, with picrotoxin trapped in the pore, the pore-lining domains do not stay in the open conformation in the absence of agonist. In addition, we have previously demonstrated that single mutations at the 9′ position of the M2 domain can nearly completely knock out picrotoxin sensitivity (Chang & Weiss, 1998), suggesting that picrotoxin has only one binding site located near the gate for the ρ1 GABA receptor. Thus, the picrotoxin antagonizing effect on the fluorescence far away from its presumed binding pocket must be an allosteric effect, unless the cysteine mutation and fluorescence labelling created another binding pocket for picrotoxin in all single cysteine mutants that are sensitive to picrotoxin block. This seems highly unlikely. Thus, picrotoxin not only blocks the pore, but also induces global conformational changes to actively antagonize the GABA-induced conformational changes. In a glycine receptor, at the 19′ position of the second transmembrane domain (M2), the agonist-induced fluorescence change is picrotoxin resistant (Pless et al. 2007). The conformational change at the 19′ position of the M2 in a nicotinic receptor, however, is not tightly coupled to the gating (Dahan et al. 2004).
With the above findings and discussion, we can now speculate on the potential role of loop F in the ρ1 GABA receptor activation. In our model, the S223–Q226 region of loop F is close to loop 2 and inner arm of loop 7 of the principal face (Fig. 7C). Interestingly, S225C spontaneously opened when modified by the positively charged MTSET (Sedelnikova et al. 2005), potentially interacting with a negatively charged residue, such as D94, in loop 2. Moreover, Q226C exhibited 20-fold reduction in sensitivity to GABA. This residue is clearly not in the binding pocket. Instead, it is close to V93 and D94 in loop 2. Thus, the reduction of GABA sensitivity by Q226C can be only explained by a gating effect. That is, the bottom part of loop F contributes to channel gating. It is possible that in the resting state, the M2 domain in the closed conformation would pull loop 2 toward the central vestibule through the M2–M3 linker. Since loop F is potentially coupled to loop 2 in a neighbouring subunit, it may also have an inward movement in the resting state. However, upon agonist binding, adaptive fitting of loop F to the bound agonist would cause outward movement of the lower part of loop F in the complementary face, which is then coupled to the outward movement of the nearby loop 2 and one arm of loop 7 in the principal face for channel activation. In this way, adaptive fitting of loop F not only contributes to the increased binding affinity, but also would create an outward motion in its lower portion during channel activation. This outward motion is then coupled to loop 2 and loop 7 in the principal face to facilitate channel activation.
In summary, using site-specific fluorescence scanning of loop F supplemented with SCAM, we have detected GABA-induced fluorescence changes of at six positions of loop F in the ρ1 GABAC receptor. Our results suggest that during channel activation, the upper part of loop F became more buried, whereas the lower part of the loop became more exposed. The middle part of the loop could partially line the binding pocket. With the aid of a homology model, we propose a novel mechanism for channel activation. The outward movement of the lower part of loop F, probably due to adaptive fitting to the bound agonist, is coupled to channel activation, potentially through the coupling to loop 2 and inner arm of loop 7 in the principal face. While a complete picture of conformational change underlying the mechanism of GABA receptor gating still awaits investigation, the current study represents one step further toward that goal and also sheds light on the activation mechanism of other members of this receptor family.
Acknowledgments
This study was supported by Arizona Biological Research Commission grant (ABRC0702) and by Barrow Neurological Foundation (to Y.C.). We thank Dr David S. Weiss and Ms Anna Sedelnikova in the Department of Physiology, University of Texas Health Science Center at San Antonio for kindly providing the cDNAs of wild-type and 12 cysteine mutants of the human ρ1 GABA receptor subunit. We also thank Dr Alan Gibson in the Barrow Neurological Institute for his help in proofreading the manuscript.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.160093/DC1
References
- Akabas M, Stauffer D, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss D. GABAA receptor needs two homologous domains of the β subunit for activation by GABA, but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss D. Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Amin J, Weiss D. Insight into the activation mechanism of ρ1 GABA receptors obtained by coexpression of wild type and activation impaired subunits. Proc Biol Sci. 1996;263:273–282. doi: 10.1098/rspb.1996.0042. [DOI] [PubMed] [Google Scholar]
- Bali M, Akabas M. The location of a closed channel gate in the GABAA receptor channel. J Gen Physiol. 2007;129:145–159. doi: 10.1085/jgp.200609639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau A, Evers A, Davis A, Czajkowski C. Mapping the agonist binding site of the GABAA receptor: evidence for a β-strand. J Neurosci. 1999;19:4847–4854. doi: 10.1523/JNEUROSCI.19-12-04847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau A, Newell JG, Czajkowski C. GABAA receptor β2 Tyr97 and Leu99 line the GABA-binding site: Insights into mechanisms of agonist and antagonist actions. J Biol Chem. 2002;277:2931–2937. doi: 10.1074/jbc.M109334200. [DOI] [PubMed] [Google Scholar]
- Brejc K, Dijk W, Klaassen R, Schuurmans M, Oost J, Smit A, Sixma T. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Buhr A, Wagner C, Fuchs K, Sieghart W, Sigel E. Two novel residues in M2 of the γ-aminobutyric acid type A receptor affecting gating by GABA and picrotoxin affinity. J Biol Chem. 2001;276:7775–7781. doi: 10.1074/jbc.M008907200. [DOI] [PubMed] [Google Scholar]
- Celie P, Klaassen R, Rossum-Fikkert S, Elk R, Nierop P, Smit A, Sixma T. Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J Biol Chem. 2005;280:26457–26466. doi: 10.1074/jbc.M414476200. [DOI] [PubMed] [Google Scholar]
- Celie P, Rossum-Fikkert S, Dijk W, Brejc K, Smit A, Sixma T. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Cha A, Bezanilla F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 1997;19:1127–1140. doi: 10.1016/s0896-6273(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Chang Y, Weiss D. Substitutions of the highly conserved M2 leucine create spontaneously opening ρ1 γ-aminobutyric acid receptors. Mol Pharmacol. 1998;53:511–523. doi: 10.1124/mol.53.3.511. [DOI] [PubMed] [Google Scholar]
- Chang Y, Weiss D. Allosteric activation mechanism of the α1β2γ2 γ-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys J. 1999a;77:2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss D. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci. 1999b;2:219–225. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- Chang Y, Weiss D. Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat Neurosci. 2002;5:1163–1168. doi: 10.1038/nn926. [DOI] [PubMed] [Google Scholar]
- Changeux J, Edelstein S. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- Chen L, Durkin K, Casida J. Structural model for γ-aminobutyric acid receptor noncompetitive antagonist binding: Widely diverse structures fit the same site. Proc Natl Acad Sci U S A. 2006a;103:5185–5190. doi: 10.1073/pnas.0600370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Reilly K, Chang Y. Evolutionarily conserved allosteric network in the cys-loop family of ligand-gated ion channels revealed by statistical covariance analyses. J Biol Chem. 2006b;281:18184–18192. doi: 10.1074/jbc.M600349200. [DOI] [PubMed] [Google Scholar]
- Dahan D, Dibas M, Petersson E, Auyeung V, Chanda B, Bezanilla F, Dougherty D, Lester H. A fluorophore attached to nicotinic acetylcholine receptor M2 detects productive binding of agonist to the site. Proc Natl Acad Sci U S A. 2004;101:10195–10200. doi: 10.1073/pnas.0301885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch M. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Gurley D, Amin J, Ross P, Weiss D, White G. Point mutations in the M2 region of the α, β, or γ subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- Hansen S, Radic Z, Talley T, Molles B, Deerinck T, Tsigelny I, Taylor P. Tryptophan fluorescence reveales conformational changes in the acetylcholine binding protein. J Biol Chem. 2002;277:41299–41302. doi: 10.1074/jbc.C200462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N, Lummis S. Locating the carboxylate group of GABA in the homomeric rho GABAA receptor ligand-binding pocket. J Biol Chem. 2006;281:24455–24461. doi: 10.1074/jbc.M601775200. [DOI] [PubMed] [Google Scholar]
- Holden J, Czajkowski C. α1G124-α1L132: a novel binding site region on the GABAA receptor that undergoes distinct conformational rearrangements during ligand binding and allosteric modulation. Soc Neurosci Abs. 2004;34:50.51. [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas M. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- Kash T, Dizon M, Trudell J, Harrison N. Charged residues in the β2 subunit involved in GABAA receptor activation. J Biol Chem. 2004;279:4887–4893. doi: 10.1074/jbc.M311441200. [DOI] [PubMed] [Google Scholar]
- Kash T, Jenkins A, Kelley J, Trudell J, Harrison N. Coupling of agonist binding to channel gating in the GABAA receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- Lester H, Dibas M, Dahan D, Leite J, Dougherty D. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lummis S, Beene D, Harrison N, Lester H, Dougherty D. A cation–π binding interaction with a tyrosine in the binding site of the GABAC receptor. Chem Biol. 2005;12:993–997. doi: 10.1016/j.chembiol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Mannuzzu L, Moronne M, Isacoff E. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Mourot A, Bamberg E, Rettinger J. Agonist- and competitive antagonist-induced movement of loop 5 on the a subunit of the neuronal α4β4 nicotinic acetylcholine receptor. J Neurochem. 2008;105:413–424. doi: 10.1111/j.1471-4159.2007.05151.x. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Czajkowski C, Jackson M. Local and global ligand-induced changes in the structure of the GABAA receptor. Biochemistry. 2006;45:7013–7022. doi: 10.1021/bi060222v. [DOI] [PubMed] [Google Scholar]
- Newell J, Czajkowski C. The GABAA receptor α1 subunit Pro174–Asp191 segment is involved in GABA binding and channel gating. J Biol Chem. 2003;278:13166–13172. doi: 10.1074/jbc.M211905200. [DOI] [PubMed] [Google Scholar]
- Olsen R. Picrotoxin-like channel blockers of GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:6081–6082. doi: 10.1073/pnas.0601121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless S, Dibas M, Lester H, Lynch J. Conformational variability of the glycine receptor M2 domain in response to activation by different agonists. J Biol Chem. 2007;282:36057–36067. doi: 10.1074/jbc.M706468200. [DOI] [PubMed] [Google Scholar]
- Sedelnikova A, Smith C, Zakharkin S, Davis D, Weiss D, Chang Y. Mapping ρ1 GABAC receptor agonist binding pocket: constructing a complete model. J Biol Chem. 2005;280:1535–1542. doi: 10.1074/jbc.M409908200. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Kellenberger S, Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992;11:2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Olsen R. Identification of a [3H]muscimol photoaffinity substrate in the bovine γ-aminobutyric acidA receptor α subunit. J Biol Chem. 1994;269:20380–20387. [PubMed] [Google Scholar]
- Venkatachalan S, Czajkowski C. A conserved salt bridge critical for GABAA receptor function and loop C dynamics. Proc Natl Acad Sci U S A. 2008;105:13604–13609. doi: 10.1073/pnas.0801854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Czajkowski C. Structure and dynamics of the GABA binding pocket: a narrowing cleft that constricts during activation. J Neurosci. 2001;21:67–74. doi: 10.1523/JNEUROSCI.21-01-00067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lester H, Dougherty D. Establishing an ion pair interaction in the homomeric ρ1 γ-aminobutyric acid type A receptor that contributes to the gating pathway. J Biol Chem. 2007;282:26210–26216. doi: 10.1074/jbc.M702314200. [DOI] [PubMed] [Google Scholar]
- Westh-Hansen S, Rasmussen P, Hastrup S, Nabekura J, Noguchi K, Akaike N, Witt M, Nielsen M. Decreased agonist sensitivity of human GABAA receptors by an amino acid variant, isoleucine to valine, in the α1 subunit. Eur J Pharmacol. 1997;329:253–257. [PubMed] [Google Scholar]
- Westh-Hansen S, Witt M, Dekermendjian K, Liljefors T, Rasmussen P, Nielsen M. Arginine residue 120 of the human GABAA receptor α1 subunit is essential for GABA binding and chloride ion current gating. Neuroreport. 1999;10:2417–2421. doi: 10.1097/00001756-199908020-00036. [DOI] [PubMed] [Google Scholar]
- Xu M, Covey D, Akabas M. Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.