Abstract
Human studies conducted more than half a century ago have suggested that superficial pain induces excitatory effects on the sympathetic nervous system, resulting in increases in blood pressure (BP) and heart rate (HR), whereas deep pain is believed to cause vasodepression. To date, no studies have addressed whether deep or superficial pain produces such differential effects on muscle sympathetic nerve activity (MSNA). Using microneurography we recorded spontaneous MSNA from the common peroneal nerve in 13 awake subjects. Continuous blood pressure was recorded by radial arterial tonometry. Deep pain was induced by intramuscular injection of 0.5 ml hypertonic saline (5%) into the tibialis anterior muscle, superficial pain by subcutaneous injection of 0.2 ml hypertonic saline into the overlying skin. Muscle pain, with a mean rating of 4.9 ± 0.8 (s.e.m.) on a 0–10 visual analog scale (VAS) and lasting on average 358 ± 32 s, caused significant increases in MSNA (43.9 ± 10.0%), BP (5.4 ± 1.1%) and HR (7.0 ± 2.0%) – not the expected decreases. Skin pain, rated at 4.9 ± 0.6 and lasting 464 ± 54 s, also caused significant increases in MSNA (38.2 ± 12.8%), BP (5.1 ± 2.1%) and HR (5.6 ± 2.0%). The high-frequency (HF) to low-frequency (LF) ratio of heart rate variability (HRV) increased from 1.54 ± 0.25 to 2.90 ± 0.45 for muscle pain and 2.80 ± 0.52 for skin pain. Despite the different qualities of deep (dull and diffuse) and superficial (burning and well-localized) pain, we conclude that pain originating in muscle and skin does not exert a differential effect on muscle sympathetic nerve activity, both causing an increase in MSNA and an increase in the LF : HF ratio of HRV. Whether this holds true for longer lasting experimental pain remains to be seen.
It has been suggested that pain is part of a homeostatic mechanism that signals the presence of tissue damage and encourages the animal to alter its behavioural state (Craig, 2002). The sensory attributes of pain depend on the tissue of origin: superficial pain, originating in skin, is perceived as sharp and/or burning and is limited to a small well-defined area, whereas deep pain, such as that originating in muscle, is dull and aching and difficult to localize (Henderson et al. 2006). In addition to these differences in the quality of superficial and deep pain, it has been observed that pain originating in deep structures evokes very different behavioural and cardiovascular responses to pain originating in superficial structures. Lewis (1942) observed that pain originating in skin evokes ‘a rise of pulse rate’ and a ‘sense of invigoration’ whereas pain originating in deep structures evokes quiescence, a ‘slowing of the pulse’ and ‘falling of the blood pressure’. Similar studies confirmed Lewis's findings that muscle pain was associated with a fall in blood pressure and bradycardia in awake human subjects (Feinstein et al. 1954).
Since the initial observations by Lewis and Feinstein, very few studies have examined the effects of pain on the cardiovascular system in awake human subjects. Intraneural recordings of muscle sympathetic nerve activity (MSNA), which is vasoconstrictor in function, have shown that increases in MSNA and blood pressure (BP) can be induced by various forms of noxious stimulation: instillation of soap solution in the eye (Nordin & Fagius, 1995), strong pressure on the nail (Nordin & Fagius, 1995), immersion of a hand in ice-water (Fagius & Karhuvaara, 1989) and mechanical pressure on the skin (Schobel et al. 1996). Increases in MSNA can also be induced by the pain associated with spontaneous or evoked cluster headaches (Nordin et al. 1997).
Painful cutaneous stimuli have been shown to cause an increase in MSNA in the cat. Horeyseck & Jänig (1974a) found that noxious mechanical and thermal cutaneous stimulation caused activity in most sympathetic postganglionic muscle fibres to increase (Horeyseck & Jänig, 1974a). This occurred in both anaesthetized (Horeyseck & Jänig, 1974a) and spinalized cats (Horeyseck & Jänig, 1974b), suggesting the involvement of a spinal reflex: ‘… the reflex pattern in muscular sympathetic fibres is the same on the spinal and on the brain stem level’ (Horeyseck & Jänig, 1972). Sato and colleagues found that whilst rotation of the knee joint within the normal physiological working range did not produce any autonomic effects in anaesthetized cats, the same movements caused increases in BP and heart rate (HR) when the knee joint was sensitized by inflammation (Sato et al. 1984). Furthermore, they found that noxious rotation of knee joints (deep pain) caused an increase in both control and inflamed knee joints – with greater increases in BP and HR being seen with rotation of the inflamed joint. It has also been shown that sciatic nerve stimulation, at intensities that activate Aδ (Group III) afferent fibres, is capable of causing increases in arterial BP and HR in spontaneously hypertensive rats (Yao et al. 1982).
Contrasting with these findings, Keay et al. (1994) have reported that in rats pain originating in deep structures such as joints, muscle and viscera evokes profound decreases in BP and HR. They hypothesize that these different cardiovascular responses are components of an integrated response aimed at dealing effectively with the noxious stimulus: activation of a discrete region within the midbrain, the lateral periqueductal grey matter (lPAG), evokes an integrated response similar to that evoked by cutaneous pain, i.e. flight/fight coupled with increased BP and HR (Depaulis et al. 1992), whereas activation of the ventrolateral periqueductal grey matter (vlPAG) evokes a response similar to that evoked by deep pain, i.e. conservation/withdrawal with decreased BP and HR (Hassan & Togawa, 2001). Furthermore, Keay and colleagues have shown that cutaneous pain preferentially activates the lPAG whereas deep pain preferentially activates the vlPAG (Keay & Bandler, 1993). These data strongly suggest that the tissue from which pain originates has a significant influence on both the behavioural and cardiovascular response.
Given the role that the cardiovascular system has in both chronic and acute pain states it is important to determine the precise nature of sympathetic neural responses during pain originating in different tissues. To date, the differential effects of acute muscle versus cutaneous pain in MSNA in awake healthy human subjects have not been investigated. The aim of this study was to use microneurography and standard cardiorespiratory recording techniques to determine the cardiovascular responses to deep and superficial acute pain in humans. We hypothesized that muscle pain will evoke decreases and cutaneous pain increases in BP, HR and MSNA.
Methods
Subjects
Twenty-six healthy subjects (13 males, 13 females) with a mean age of 28 years and no reported history of skeletal muscle pain participated in this study. All subjects had abstained from caffeine and/or smoking on the day of the experiment and were not on any medication. The study was approved by the Human Research Ethics committee of the University of New South Wales, and written informed consent was obtained prior to participation in the study. The project conformed to the Declaration of Helsinki.
Experimental procedures
Standard cardiorespiratory measurements
All subjects rested with their eyes closed, in a semireclined position at a comfortable ambient temperature. HR was measured via standard Ag–AgCl ECG chest electrodes (n= 26). Out of the 26 subjects included in the study, effector organ responses (respiration, skin blood flow, EMG, BP) were recorded in 21 subjects. Respiration was recorded with a strain gauge transducer attached to a strap around the chest (Pneumotrace, UFI, Morro Bay, CA, USA). Changes in pulsatile skin blood volume were monitored via infrared photo-plethysmography probes attached to the subjects’ big toe ipsilateral to nociceptive stimuli (MLT1020EC, ADInstruments, Australia). Surface EMG was recorded over the ipsilateral tibialis anterior, extensor digitorum longus and soleus muscles with standard Ag–AgCl electrodes to ensure that the subject was fully relaxed. Continuous BP was measured non-invasively using pulse plethysmography (Finometer Pro, Finapres Medical Systems, Amsterdam, the Netherlands) placed on an index finger of the subject's non-dominant hand and the calibrating cuff placed around the contralateral upper arm. All BP calibration was performed prior to recording. All sensors were connected to a computer-based data acquisition system (PowerLab 16 SP, ADInstruments, Sydney, Australia) and analysed using Chart v5.5.4 software (ADInstruments, Sydney, Australia).
Microneurography
In 13 of the 26 subjects, in addition to the standard cardiorespiratory measurements, muscle sympathetic nerve activity (MSNA) was also recorded. The common peroneal nerve was located by transcutaneous stimulation around the fibular head with a surface probe (2 mm diameter) attached to an isolated current stimulator (0.2 ms, 1 Hz; Stimulus Isolator, ADInstruments, Sydney, Australia). Once the nerve was isolated an insulated tungsten microelectrode (Frederick Haer & Co. Inc., Brunswick, USA) was inserted percutaneously and electrical stimulation was used to guide the electrode into a muscle fascicle of the nerve. The electrode was manipulated until spontaneous, pulse-synchronous, muscle sympathetic nerve activity was encountered. Neural activity was recorded with a low-noise headstage (Neuro Amp EX, ADInstruments), amplified (2 × 104), filtered (0.3–3.0 kHz), digitized at 10 kHz and stored on magnetic and optical media with ECG (digitized at 2 kHz), blood pressure (400 Hz) and respiration (100 Hz).
Noxious stimulation
MSNA and effector-organ activity was stabilized for approximately 5 min. Following this, two 23 gauge butterfly cannulae primed with sterile hypertonic (5%) saline (hNaCl) were inserted into the same leg from which microneurographic recordings were obtained, one approximately 1.5 cm deep into the belly of tibialis anterior (TA), the other inserted under the overlying skin. Subjects were instructed to keep their eyes closed. A quiet resting period of 3–5 min (baseline activity) was recorded before injection of 0.5 ml (muscle) or 0.2 ml (subdermal) was made in a quasi-random order. Following complete cessation of pain a further 5 min was recorded. The alternate injection was then administered and the results recorded. Alternate injections were delivered at least 20 min apart.
For 18 of the 26 subjects, the pain profile during the experiment was obtained in real time by instructing subjects to turn a potentiometer (100 Hz); the majority (14/18) of these recordings were obtained for both muscle and skin pain in the same subjects. Subjects were instructed on the use of the pain potentiometer, in which pain intensity was expressed on a 0–10 visual analog scale (VAS), prior to the commencement of the study: 0 indicated ‘no pain’ and 10 was described as ‘the most intense pain ever experienced’; subjects were instructed to use the potentiometer as soon as they felt the onset of pain, to report any relative changes in intensity, and to indicate ‘0’ at the cessation of pain. In addition, at the conclusion of the recording subjects were asked to choose the appropriate descriptors from the McGill pain questionnaire.
Data analysis
The onset of pain was defined as the time following injections where subjective pain levels rose above zero. The time points of individual peak pain levels were used to identify the correct time frame to assess effector organ and neural responses with reference to each individual's peak pain level. Instantaneous HR was calculated from the ECG. Mean HR, BP and pain (VAS) values were calculated for every 15 s block during the 2 min period immediately prior to each hNaCl injection and during the 6 min period immediately following each hNaCl injection, i.e. a continuous 8 min period. Effector-organ (n= 21) responses were normalized to individual baseline values and changes were expressed as percentage change from baseline (%), in order to allow comparisons between individuals.
Heart rate variability (HRV) was calculated over a 2 min period of stable ECG immediately prior to the injection, and over a 2 min period after the heart rate had stabilized following the injection, using an automatic R-wave detection and analysis program (Heart Rate Variability module; ADInstruments, Sydney, Australia). Ectopic beats were excluded and fast-Fourier transforms computed (FFT 1024 points, half-overlap, Welch window) for 22 of 26 subjects. Four subjects were excluded from HRV analysis as they did not satisfy the principle of stationarity (Camm et al. 1996). Low-frequency (LF) and high-frequency (HF) components were calculated and the LF/HF ratio calculated by the program. Low-frequency HRV is believed to reflect the level of sympathetic drive to the heart, while the high-frequency component is believed to represent parasympathetic drive (Camm et al. 1996).
MSNA activity was analysed using the Peak Analysis module (ADInstruments). The mean number of bursts min−1 and burst area (%) min−1 were calculated for 2 min prior to and 6 min immediately following each hNaCl injection. Mean MSNA burst area values were normalized to individual baseline values and expressed as percentage change compared with baseline (%). Correlations between change in burst counts (%) and burst area (%), pain intensity (peak pain) and MSNA changes at the time of peak pain (burst area (%), peak pain intensity and BP and were performed using Spearman's rank correlation coefficient (rs).
All statistical analysis was performed using SPSS v15 (SPSS Inc., Chicago, IL, USA) and Gnumeric v1.8.3 software (GNU General Public License). Student's t test for paired data was used for comparing baseline MSNA, mean BP and respiration values (%), with a 15 s sample block corresponding to each individual's peak pain response. Paired t tests were also used to compare the transient peak increases in HR (which occurred 15 s after the onset of both muscle and skin pain) with baseline values. Wilcoxon's signed rank test was used to analyse changes in heart rate variability (baseline versus muscle pain; baseline versus skin pain). For all statistical tests, a probability level of less than 5% was regarded as significant. All values are expressed as means and s.e.m.
Results
Muscle pain
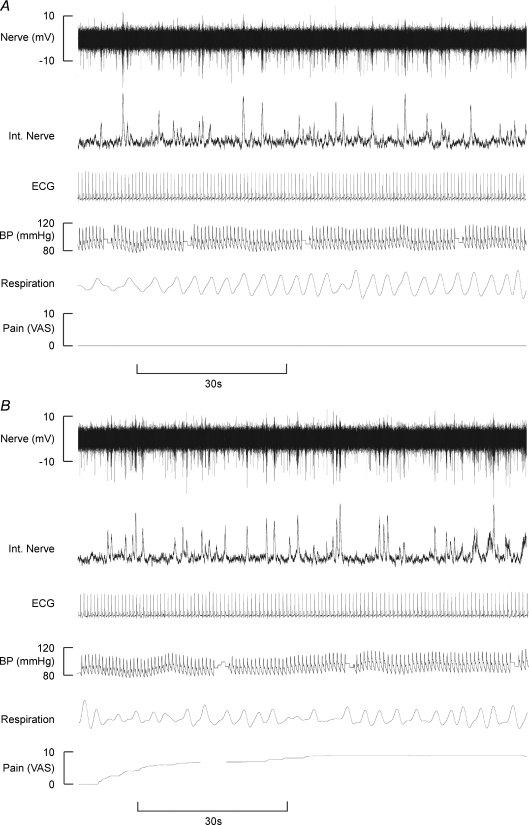
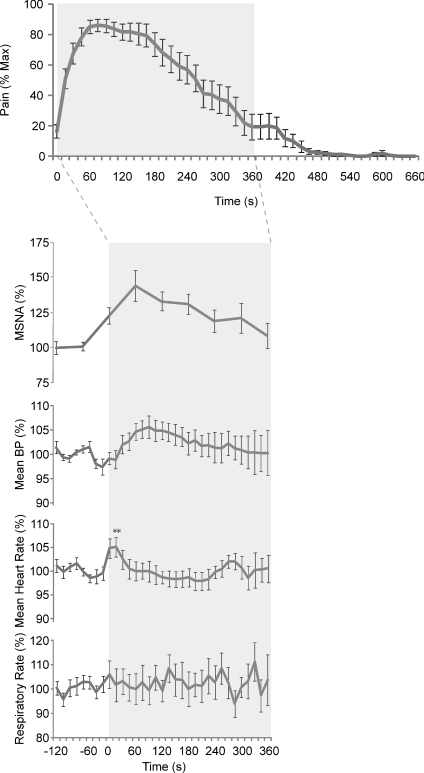
Real-time pain profiles (VAS) were obtained from 18 out of 26 subjects. In the majority of subjects (16/18) intramuscular injection of 0.5 ml hypertonic saline (hNaCl) induced pain within the first 15 s, with the remaining two subjects experiencing the onset of pain within the first 30 s following the intramuscular injection. Muscle pain, as calculated from individual subjects, peaked at 111 ± 17 s (4.9 ± 0.8, VAS), with the pain lasting 358 ± 32 s. The average time-trend, computed from all subjects, is shown in Fig. 1A. Intramuscular hNaCl injections evoked pain that was described as dull, diffuse and throbbing in all subjects and often spread from the belly of TA into the ankle and foot. Despite the pain subjects remained relaxed throughout, as evidenced by the absence of EMG from the ipsilateral leg.
Figure 1. Mean pain profiles for intramuscular (A) and subdermal (B) pain.
n= 18.
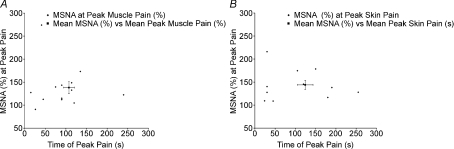
Muscle sympathetic nerve activity (MSNA) was recorded in 13 subjects. Muscle pain caused MSNA to increase above baseline levels in 12 subjects and decrease in one (Fig. 2A). Figure 3 shows raw data from one subject. Measured across all subjects, mean MSNA increased from 27 ± 2 to 29 ± 2 bursts min−1 at the peak of muscle pain, but this failed to reach statistical significance (P= 0.204, paired t test). However, MSNA total burst area (%) did show a significant increase at the time of peak muscle pain (38.2 ± 12.8%; P= 0.015, paired t test). There was no significant correlation between the intensity of peak muscle pain and the increase in MSNA burst area at the time of peak pain (P= 0.658).
Figure 2. The relationship between individual peak pain times and change in MSNA burst area following intramuscular (A) and subdermal (B) injections.
Mean MSNA (%) versus Mean Peak Pain values are shown with error bars (s.e.m.).
Figure 3. Raw data figures illustrating the increases in MSNA comparing baseline activity (A) with activity following intramuscular injection of hypertonic saline (B) in one subject.
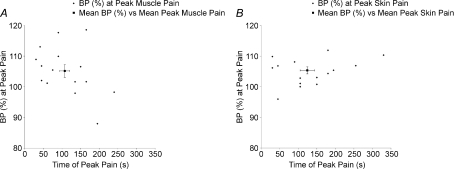
Blood pressure increased in 12 and decreased in 3 of 15 subjects. On average, there was a significant increase in BP at the time of peak muscle pain (5.1 ± 2.1%, P= 0.028; Fig. 5A), but there was no significant correlation between the increase in BP and peak pain (P= 0.284). Heart rate (HR) increased transiently following intramuscular injection of hNaCl in 18/21 subjects, with the peak response occurring 15 s after the injection (5.6 ± 2.0%; P= 0.011, paired t test; Fig. 4). HR returned to baseline within 60 s. The increase in HR was also reflected in a significant increase in the mean ratio of low-frequency to high-frequency (LF : HF) spectral power of heart rate variability (HRV): the mean ratio increased from 1.18 ± 0.26 at rest to 2.96 ± 0.49 during pain (P= 0.028 Wilcoxon). Representative data from one subject are shown in Fig. 6. Changes in respiratory rate were inconsistent and statistically insignificant at the time of peak muscle pain (P= 0.924; Fig. 4).
Figure 5. The relationship between individual peak pain times and BP following intramuscular (A) and subdermal (B) injections.
Figure 4. Neural and effector organ responses to intramuscular injections of hypertonic saline.
n= 13 for neural response; n= 21 for effector organ responses; n= 18 for pain data. Mean values; error bars depict s.e.m.
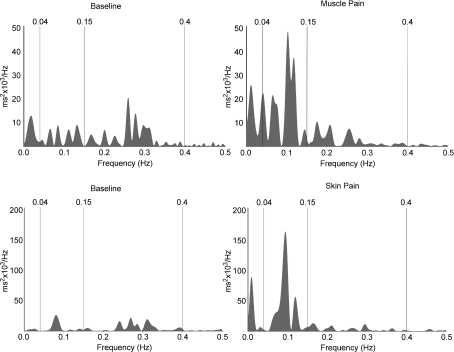
Figure 6. Representative data depicting heart rate variability spectra from two subjects.
Values between 0.04 and 0.15 Hz represent sympathetic drive; values between 0.15 and 0.4 Hz reflect parasympathetic drive to the heart.
Skin pain
Similar to muscle pain, skin pain commenced within the first 15 s in 16/18 subjects, with the remaining two subjects feeling pain within the first 30 s following subdermal injection of hNaCl. Skin pain peaked at 132 ± 22 s (4.9 ± 0.6 VAS (Mean Peak Pain)) with the pain lasting 464 ± 54 s (Fig. 1B). All subjects described the pain as hot, burning, localized to a small area surrounding the injection site, and qualitatively distinct from that of muscle pain. As with muscle pain, skin pain did not cause any detectable increases in EMG activity.
In all 11 subjects, MSNA increased from 27 ± 3 to 31 ± 3 bursts min−1 at the time of peak pain (P= 0.003, paired t test). Additionally, MSNA burst area (%) significantly increased at the time of peak muscle pain (43.9 ± 10.0%; P= 0.007, paired t test; Fig. 2B). There was no significant correlation between peak skin pain and MSNA (burst area,%) at the time of peak pain (P= 0.803).
Blood pressure increased in 15 and decreased in 1 of 16 subjects following subcutaneous injection, with significant increases in BP occurring at the time of peak muscle pain intensity (5.4 ± 1.1%; P= 0.001, paired t test; Fig. 5B). There was no significant correlation between the intensity of peak skin pain and BP at the time of peak skin pain (P= 0.370). Heart rate transiently increased by 7.0 ± 2.0% (P= 0.001, paired t test), the peak response occurring 15 s after the injection in 16/19 subjects (Fig. 7) and returning to baseline within 60 s of the injection. The mean LF : HF increased significantly from 1.18 ± 0.26 to 2.48 ± 0.59 (P= 0.014, Wilcoxon). Representative data from one subject are shown in Fig. 6. Changes in respiratory rate at the time of peak skin pain were not significant (P= 0.811; Fig. 7).
Figure 7. Neural and effector organ responses to subdermal injections of hypertonic saline were similar to those seen following intramuscular injections.
n= 11 for neural response; n= 21 for effector organ responses; n= 18 for pain data. Mean values; error bars depict s.e.m.
Discussion
Our results are in direct contrast to those of previous experimental human investigations, which found that acute cutaneous pain evokes large and sustained increases in BP and HR, whereas acute muscle pain evokes large and sustained decreases in BP and HR (Lewis, 1942; Feinstein et al. 1954). We did not find this differentiation between deep and superficial pain. Rather, we found that both acute deep and cutaneous pain evoked mild increases in BP and HR.
Both muscle and cutaneous pain evoked significant increases in MSNA. Whilst the MSNA increases were small in terms of absolute burst count number, MSNA burst area increased by some 30–50% during muscle and cutaneous pain. Similar to BP, these MSNA increases followed the pattern of perceived pain intensity changes. The modest increases in MSNA seen during peak muscle pain in our study are similar to those observed during mental stress, where burst amplitudes increased but not burst incidence (Hjemdahl et al. 1989). Since hypertonic saline injections in muscle and skin failed to elicit clinically significant effector organ responses in spinal cord injured subjects – who could not feel the noxious stimuli (Burton et al. 2008) – we believe the MSNA responses seen in our study most likely reflect cognitive processes associated with the affective components of the pain experience, rather than as the result of spinal or supraspinal reflexes.
Our observation that cutaneous pain causes an increase in MSNA reflects the findings of Horeyseck & Jänig, 1974), who reported that noxious mechanical and thermal cutaneous stimulation caused an increased activity in most sympathetic postganglionic muscle fibres (Horeyseck & Jänig, 1974a). Furthermore, Boczek-Funcke et al. (1992) found that most muscle vasoconstrictor neurones which displayed strong cardiac rhythmicity (indicative of strong baroreceptor control) were excited by noxious mechanical stimulation to the skin of the head of the anaesthetized cat (Boczek-Funcke et al. 1992).
Although the increases in BP and MSNA during muscle pain appear at odds with some previous animal and human studies (Keay et al. 1994), it has been suggested that the division of pain into superficial and deep may not reflect the true nature of pain. Keay and colleagues have suggested that pain should instead be divided into escapable (cutaneous pain is almost always escapable) and inescapable (deep pain is almost always inescapable; Keay & Bandler, 2002). Similarly, these authors speculate that pain may exert differential autonomic effects depending on whether the source of pain is transient or prolonged. It may be that all pain initially evokes an increase in BP and behavioural arousal to warn an animal that tissue damage has occurred. If this pain persists, BP falls and the animal adopts withdrawal in an effort to conserve energy and deal with tissue damage. Indeed, those animal investigations in which acute deep pain evokes profound falls in BP and HR have used stimuli of relatively long duration – such as intraperitoneal injections of acetic acid or injection of formalin into the triceps surae muscle (Clement et al. 1996). Because these studies are undertaken in animals, it is difficult to determine the pain profile (intensity and duration). It may be that intramuscular injections of formalin cause pain that is stronger and longer-lasting than that produced by intramuscular injection of hypertonic saline injections, which may explain why deep pain produced vasodepressive effects in the studies by Keay and colleagues but not in our study. It would be interesting to follow changes in BP and MSNA in humans resulting from a deep pain stimulus that lasts some 10 times longer – 1 h rather than ∼6 min.
Differential sympathetic responses to painful stimuli may be dependent not only on the tissue in which the pain originates, but also on the type of stimuli used to induce pain. Whilst the tissues were different (joint pain versus muscle pain) between the studies by Sato et al. (1984) and Keay et al. (1994), the stimuli were also different (joint manipulation versus formalin injections). It is interesting to note that deep joint pain (Sato et al. 1984) caused increases in BP and HR, as did intramuscular hypertonic saline in our study. Differences between the nature of the stimuli and their differential effects on the sympathetic nervous system may be an additional factor to consider with experimentally induced pain.
Since a small number of subjects (3 out of 15) showed decreases in BP in response to deep pain, it is possible that vasodepressive responses may indeed occur in human subjects; however, this type of response was the exception to the rule, with the predominant response to deep pain being an excitatory rather than depressive effect on BP.
It is also possible that the MSNA changes reported in our study reflect specific changes in regional perfusion (i.e. the leg) and do not accurately reflect the muscle vasoconstrictor drive to other muscle vascular beds. The finding that BP increased only slightly whereas MSNA increased by a greater amount following both deep and superficial pain supports this possibility, but it is important to recognize that sympathetic nerve traffic cannot simply be directly translated into sympathetic effector-organ function (Elam, 2001). It has been shown that activation of a region of the midbrain, the lateral periaqueductal grey matter (lPAG), evokes an integrated behavioural and cardiovascular response that is equivalent to that evoked by cutaneous pain (Depaulis et al. 1992). Furthermore, Depaulis et al. (1992) found that activation of the lateral PAG by cutaneous pain is regionally specific; for example, stimulation of the hindlimb of the cat evokes a flight response accompanied by increased BP and HR with blood flow being redirected specifically towards skeletal muscles in the legs. Again, stimulation of the pretentorial part of the PAG resulted in a confrontational behavioural response, with increases in BP and HR and blood flow being directed towards the face (Carrive & Bandler, 1991).
In contrast to the sustained increases in BP and MSNA, HR increased only transiently at the onset of pain, regardless of whether the pain originated in muscle or skin. HR had returned to baseline levels before the peak pain had been reached, where it remained for the rest of the period. These transient changes are likely to reflect brief arousal responses to both deep and superficial pain. It is interesting to note that despite pain originating in different tissues (muscle versus skin) similar profiles in HR increases were seen. This was reflected by spectral analysis of the R-R intervals, which showed similar increases in LF : HF ratio, indicative of an increase in sympathetic drive to the heart. These results suggest that the balance of neural outflow to the heart (sympathetic versus parasympathetic) may not be differentially affected by pain originating in different tissues (deep versus cutaneous); rather the same patterned response occurs regardless of the source of pain.
Methodological considerations
For technical reasons, adequate muscle sympathetic nerve data could not be obtained from all subjects, so the number of subjects from whom effector-organ data were obtained is larger. Nevertheless, we feel that the MSNA data are sufficiently robust to allow us to conclude that increases in muscle vasoconstrictor drive would also have occurred in those subjects in whom it was not directly recorded. With respect to heart rate variability, at least 2 min is required to adequately represent the low-frequency (sympathetic) components (Camm et al. 1996). Since the changes in HR were transient (∼30 s) and HR variability analysis encompassed 2 min, the spectral changes we observed may not only reflect the neural drive to the heart during the transient increase in HR following the onset of pain – there may be a longer-lasting increase in sympathetic drive to heart. Future studies in which the pain lasts longer (induced by infusion, rather than bolus injection, of hypertonic saline) will allow us to address this.
Unlike chronic pain (defined as pain lasting more than 3 months), the pain induced by intramuscular or subcutaneous injections of hypertonic saline last only 6–8 min. Furthermore, each subject was aware that the injections had no persistent side-effects beyond the experimental period. Therefore, coping mechanisms (both cognitive and emotional) probably differ between acute experimental pain and ‘real-world’ acute pain in relation to the concepts of escapable and inescapable pain. Feinstein et al. (1954) reported that vasodepressive effects associated with intramuscular injections of hypertonic saline most commonly occurred with injections to the thoracic region, whilst vasodepressive effects were comparatively rare when deep pain was induced in cervical or sacral regions. The nociceptive stimuli used in our study were confined to the tibialis anterior muscle and nearby skin. Despite hypertonic saline causing pain irrespective of where it is injected, it is possible that different autonomic effects may be seen in regard to deep pain versus cutaneous pain originating in other areas.
It is unclear in the studies by Lewis (1942) and Feinstein et al. (1954) as to the exact time frame over which blood pressure measurements were taken. Our studies focused on the acute effects (first 6 min) of deep and superficial pain, after which pain ceased and neural and effector organ responses appeared to return to baseline. It is conceivable that vasodepressive effects may occur after the cessation of deep or superficial pain; however, vasodepressive effects did not occur during either painful period in our study.
Conclusions
Acute deep and cutaneous pain originating in the tibialis anterior muscle and overlying skin is associated with moderate increases in MSNA. These increases were associated with small increases in BP which culminated around the time of peak pain. Changes in HR were transient and occurred irrespective of whether the pain originated in deep (muscle) or superficial (cutaneous) tissue. These data are in contrast to some animal investigations, which have shown that deep pain evokes decreases in BP and HR whilst cutaneous pain increases BP and HR. However, our results support other animal studies which found that deep pain, produced by different stimuli to those we used, is capable of causing increases in BP and HR. These findings suggest that differential sympathetic responses to painful stimuli may depend not only on the tissue in which the pain originates, but also on the type of stimulus used to induce pain. Whether this also holds during longer-lasting experimental pain remains to be seen.
Acknowledgments
This work was supported by grant 350889 to VGM and LAH from the National Health and Medical Research Council of Australia.
References
- Boczek-Funcke A, Dembowsky K, Häbler HJ, Jänig W, McAllen RM, Michaelis M. Classification of preganglionic neurones projecting into the cat cervical sympathetic trunk. J Physiol. 1992;453:319–339. doi: 10.1113/jphysiol.1992.sp019231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AR, Brown R, Macefield VG. Selective activation of muscle and skin nociceptors does not trigger exaggerated sympathetic responses in spinal-injured subjects. Spinal Cord. 2008;46:660–665. doi: 10.1038/sc.2008.33. [DOI] [PubMed] [Google Scholar]
- Camm A, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, Coumel P, Fallen EL, Kennedy HL, Kleiger RE, Lombardi F, Malliani A, Moss AJ, Rottman JN, Schmidt G, Schwartz PJ, Singer DH. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Carrive P, Bandler R. Control of extracranial and hindlimb blood flow by the midbrain periaqueductal grey of the cat. Exp Brain Res. 1991;84:599–606. doi: 10.1007/BF00230972. [DOI] [PubMed] [Google Scholar]
- Clement CI, Keay KA, Owler BK, Bandler R. Common patterns of increased and decreased fos expression in midbrain and pons evoked by noxious deep somatic and noxious visceral manipulations in the rat. J Comp Neurol. 1996;366:495–515. doi: 10.1002/(SICI)1096-9861(19960311)366:3<495::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90:307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Elam M. What lies above and beyond the concept of ‘sympathetically maintained pain’? Clin Auton Res. 2001;11:331–333. doi: 10.1007/BF02292762. [DOI] [PubMed] [Google Scholar]
- Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14:511–517. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- Feinstein B, Langton JN, Jameson RM, Schiller F. Experiments on pain referred from deep somatic tissues. J Bone Joint Surg. 1954;36:981–997. [PubMed] [Google Scholar]
- Hassan M, Togawa T. Observation of skin thermal inertia distribution during reactive hyperaemia using a single-hood measurement system. Physiol Meas. 2001;22:187–200. doi: 10.1088/0967-3334/22/1/322. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Bandler R, Gandevia SC, Macefield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2006;120:286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol Endocrinol Metab. 1989;257:E654–E664. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- Horeyseck G, Jänig W. Response patterns in vasoconstrictors to the skin and muscles upon stimulation of skin receptors in chronically spinalized cats. Pflugers Arch. 1972;332(Suppl.):R64. [PubMed] [Google Scholar]
- Horeyseck G, Jänig W. Reflexes in postganglionic fibres within skin and muscle nerves after noxious stimulation of skin. Exp Brain Res. 1974a;20:125–134. doi: 10.1007/BF00234007. [DOI] [PubMed] [Google Scholar]
- Horeyseck G, Jänig W. Reflex activity in postganglionic fibres within skin and muscle nerves elicited by somatic stimuli in chronic spinal cats. Exp Brain Res. 1974b;21:155–168. doi: 10.1007/BF00234387. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett. 1993;154:23–26. doi: 10.1016/0304-3940(93)90162-e. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: observations and speculation. Exp Physiol. 2002;87:275–279. doi: 10.1113/eph8702355. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Owler BK, Depaulis A, Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61:727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Lewis T. Pain. London: McMillan; 1942. [Google Scholar]
- Nordin M, Fagius J. Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol. 1995;489:885–894. doi: 10.1113/jphysiol.1995.sp021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Fagius J, Waldenlind E. Sympathetic vasoconstrictor outflow to extremity muscles in cluster headache. recordings during spontaneous and nitroglycerin-induced attacks. Headache. 1997;37:358–367. doi: 10.1046/j.1526-4610.1997.3706358.x. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Schmidt RF. Changes in blood pressure and heart rate induced by movements of normal and inflamed knee joints. Neurosci Lett. 1984;52:55–60. doi: 10.1016/0304-3940(84)90350-1. [DOI] [PubMed] [Google Scholar]
- Schobel HP, Ringkamp M, Behrmann A, Forster C, Schmieder RE, Handwerker HO. Hemodynamic and sympathetic nerve responses to painful stimuli in normotensive and borderline hypertensive subjects. Pain. 1996;66:117–124. doi: 10.1016/0304-3959(96)03079-5. [DOI] [PubMed] [Google Scholar]
- Yao T, Andersson S, Thorén P. Long-lasting cardiovascular depression induced by acupuncture-like stimulation of the sciatic nerve in unanaesthetized spontaneously hypertensive rats. Brain Res. 1982;240:77–85. doi: 10.1016/0006-8993(82)90645-x. [DOI] [PubMed] [Google Scholar]