Abstract
We investigated how myofibrillar protein synthesis (MPS) and muscle anabolic signalling were affected by resistance exercise at 20–90% of 1 repetition maximum (1 RM) in two groups (25 each) of post-absorptive, healthy, young (24 ± 6 years) and old (70 ± 5 years) men with identical body mass indices (24 ± 2 kg m−2). We hypothesized that, in response to exercise, anabolic signalling molecule phosphorylation and MPS would be modified in a dose-dependant fashion, but to a lesser extent in older men. Vastus lateralis muscle was sampled before, immediately after, and 1, 2 and 4 h post-exercise. MPS was measured by incorporation of [1,2-13C] leucine (gas chromatography–combustion–mass spectrometry using plasma [1,2-13C]α-ketoisocaparoate as surrogate precursor); the phosphorylation of p70 ribosomal S6 kinase (p70s6K) and eukaryotic initiation factor 4E binding protein 1 (4EBP1) was measured using Western analysis with anti-phosphoantibodies. In each group, there was a sigmoidal dose–response relationship between MPS at 1–2 h post-exercise and exercise intensity, which was blunted (P < 0.05) in the older men. At all intensities, MPS fell in both groups to near-basal values by 2–4 h post-exercise. The phosphorylation of p70s6K and 4EBP1 at 60–90% 1 RM was blunted in older men. At 1 h post-exercise at 60–90% 1 RM, p70s6K phosphorylation predicted the rate of MPS at 1–2 h post-exercise in the young but not in the old. The results suggest that in the post-absorptive state: (i) MPS is dose dependant on intensity rising to a plateau at 60–90% 1 RM; (ii) older men show anabolic resistance of signalling and MPS to resistance exercise.
Exercise is known to stimulate the rate of post-exercise myofibrillar protein synthesis (MPS) in healthy young people, the extent of which probably depends upon several factors including nutritional state, mode, intensity and duration of exercise. (Chesley et al. 1992; Biolo et al. 1995; Phillips et al. 1997; Miller et al. 2005; Dreyer et al. 2006; Drummond et al. 2008; Wilkinson et al. 2008). However, little or no information is available in respect of the dose–response regarding exercise intensity. Muscles of older people are also capable of increases in MPS after resistance exercise, and feeding increases this (Sheffield-Moore et al. 2005; Drummond et al. 2008) but again dose–response information is lacking.
Normal ageing is associated with a loss of skeletal muscle mass at 0.5–2% per annum, causing sarcopenia with an incidence rate of 13–24% in those aged 50–70 years and up to 50% for those in their eighties (Baumgartner et al. 1998). However, maintaining physical activity appears to preserve muscle mass (Raguso et al. 2006) and even non-agenarians can benefit from resistance training (Fiatarone et al. 1990). Nevertheless, the dose–response relationship between increases in muscle synthetic rates and exercise intensity for older people is unknown. Thus, a comparison of the effects of exercise between young and old across a full spectrum of exercise intensity on MPS and associated changes in signalling activity is unavailable. Here we aim to fill this gap.
On the basis of a limited pilot study at exercise intensities of 60–90% (Bowtell et al. 2003), we hypothesized that the dose–response relationship between exercise intensity and increases in MPS would be hyperbolic with linear increases at intensities up to 60% of 1 RM and no further increase above this value, when all motor units would probably be activated; on the basis of that work, we also hypothesized that there would be a latent period of ∼1 h before any rise in MPS occurred. We have previously shown (Cuthbertson et al. 2005) that in older men (∼70 years) there is a decreased sensitivity and capacity of increases of MPS (and associated anabolic signalling) across a wide range of essential amino acid availability, a phenomenon we named anabolic resistance. We therefore hypothesized that decreased sensitivity and capacity to increase myofibrillar protein synthesis would occur in older men in response to resistance exercise.
Methods
Ethics
The study was approved by the University of Nottingham Ethics Committee and complied with the Declaration of Helsinki. Written informed consent was obtained from subjects after explaining the procedures and risks.
Study design
Twenty-five young (24 ± 6 years) and 25 older (70 ± 5 years) men were recruited for the study. They were recreationally active, physically independent and healthy overall, with no sign of insulin resistance (fasting blood glucose, 4.5 ± 1.0 versus 5.1 ± 0.9 mm, in young and old). Body mass indices were identical in the two groups (23 ± 4 versus 24 ± 2 kg m2) as were lean body masses (64 ± 17 versus 57 ± 14 kg) and right (11.9 ± 2.7 versus 10.7 ± 4 kg) and left (10.5 ± 2.5 versus 9.3 ± 2.3 kg) lean leg masses (all young versus older). The only major difference was the 1 repetition maximum (1 RM) weight lifted by unilateral leg extension which was significantly reduced in the older men (75 ± 14 kg versus 41 ± 11 kg, P < 0.05).
Participant screening by an experienced doctor included a clinical history, a physical examination, an electrocardiogram and routine blood tests. Subjects were excluded if they had a history of cardiac, pulmonary, liver, kidney, vascular or autoimmune disease, clotting disorders, uncontrolled hypertension, diabetes, thyroid disorders, obesity, anaemia, cancer, alcohol abuse, visually obvious muscle wasting, corticosteroid use or joint pain that restricted movement. Older subjects with mild controlled hypertension (< 140/90 mmHg without medication) were not excluded but they did not take medication on the study day. At least 1 week before the study each subject's dominant leg maximal strength was measured on a leg extension machine (ISO leg extension, Leisure Lines (GB) Ltd) and the subjects were familiarized with the study protocols. Body composition was measured using dual-energy X-ray absorptiometry (DEXA; GE Lunar Prodigy II, GE Healthcare) using the manufacturer's software.
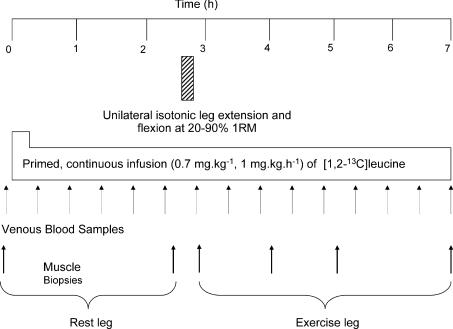
The participants were studied after an overnight fast after normal daily activity. On the morning of the study (∼09.00 h), they had an 18-g cannulae inserted into the antecubital vein of each arm for tracer infusion and venous blood sampling. Blood samples and muscle biopsies were taken according to the protocol (Fig. 1). Muscle biopsies were taken from the vastus lateralis using the conchotome biopsy technique under sterile conditions with subcutaneous 1% lignocaine as local anaesthetic. Muscle tissue was washed in ice-cold saline, blotted dry and frozen in liquid nitrogen, and stored at −80°C until analysis. A primed, continuous (0.7 mg kg−1, 1 mg kg−1 h−1) infusion of [1,2-13C2] leucine (99 Atoms%, Cambridge Isotopes, Cambridge, MA, USA) was started immediately after the first biopsy and maintained for ∼7 h. After 2.5 h of infusion, the subjects exercised with their dominant legs at intensities, randomly assigned, from 20% to 90% of 1 RM, with five subjects per group per intensity. The seated subjects performed unilateral leg extensions and flexions (1–2 s each) with 2 min rest between sets. The schedule of contractions was designed to equalize, as closely as possible, the volume of exercise, i.e. the force × time-under-tension product (often described as ‘work’). Thus, at an exercise intensity of 20% of 1 RM, the subjects completed 3 sets × 27 repetitions (reps); at 40%, 3 sets × 14 reps; at 60%, 3 sets × 9 reps; at 75%, 3 sets × 8 reps and those at 90%, 6 sets × 3 reps. Total work output (i.e.% 1 RM × number of repetitions × number of sets) was 1620–1800 units at different exercise intensities, and total time-under-tension was obtained by multiplying by 4 s.
Figure 1. Protocol for the measurement of the relationships of myofibrillar protein synthesis and muscle anabolic signal molecule phosphorylation.
Each subject was studied over the period shown in the fasted state with 5 young and 5 older subjects carrying out exercise with their dominant leg randomly assigned to 20–90% 1 RM.
Myofibrillar protein synthesis and muscle anabolic signalling
Myofibrillar protein was isolated as previously described (Wilkinson et al. 2008). The fractional synthesis rate (FSR) of myofibrillar protein was determined from the incorporation of [1,2-13C2] leucine between muscle biopsies using the labelling of venous plasma α-KIC as a surrogate for true precursor labelling (Watt et al. 1991) as described in our very recent work (Greenhaff et al. 2008). The methods for determination of the extent of phosphorylation of p70s6K1 on Thr389, 4EBP1 on Thr37/46 and the elongation factor eEF2 on Thr56, were as described in recent work (Smith et al. 2008) using antibodies from Cell Signalling Technology Inc. (Beverly, MA, USA).
Statistical analysis
All data are reported as means ±s.e.m. Between and within-group differences were tested by two-way ANOVA with a Bonferroni post hoc procedure to identify pair-wise differences. Significance was accepted as P < 0.05. Where appropriate, correlations were tested by assessing the existence of a linear fit between variables. All analyses were made using GraphPad Prism version 5.0 (Graph Pad Software, La Jolla, CA, USA).
Results
The only distinguishing features between the groups were age and a 51% smaller 1 RM leg extension force in the older men. Thus, the absolute total force–time integral was less in older men even though the relative work done was the same.
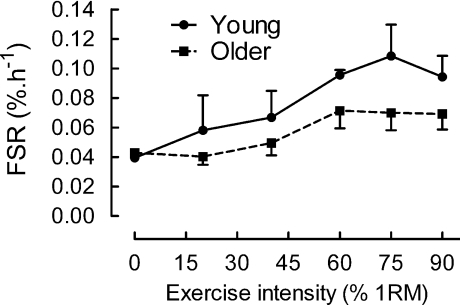
The basal rates of myofibrillar protein synthesis were identical in the two groups of subjects (0.039 ± 0.002 versus 0.043 ± 0.003% h−1, young versus older, respectively). In both groups there was a dose-related effect of resistance exercise on myofibrillar protein synthesis at 1–2 h post-exercise which was sigmoidal (Fig. 2); thus, there were small increases after exercise at 20 and 40% of 1 RM but a bigger rise to the values at 60, 75 and 90% of 1 RM, that were effectively at a maximal plateau (i.e. with no significant differences between them). There was a significant difference between the overall responses of MPS to exercise in the young and older subjects, with the area under the curves (rate of muscle protein synthesis × time, i.e.% of total protein synthesized) being 30 ± 6% higher (P < 0.04) in the younger men.
Figure 2. Dose–response relationship of myofibrillar protein synthesis (FSR, fractional synthetic rate,% h−1) measured at 1–2 h post-exercise for 5 young men and 5 older men at each intensity.
The responses of the young men overall were greater than those of the older men (P < 0.04). The responses between 60 and 90% of 1 RM in young and old were indistinguishable from each other but those in the young were together significantly higher than in the older men (P < 0.01) for 15 subjects in each group
No values of synthetic rates are presented for the exercise period as the exercise period was variable, and in most cases too short to obtain reliable results. The shapes of the time courses of the changes in protein synthesis thereafter were similar in both groups of subjects, with a lag over the first hour after exercise, a rise whose extent depended on intensity and the group studied, to a peak at 1–2 h and a fall thereafter to near basal values.
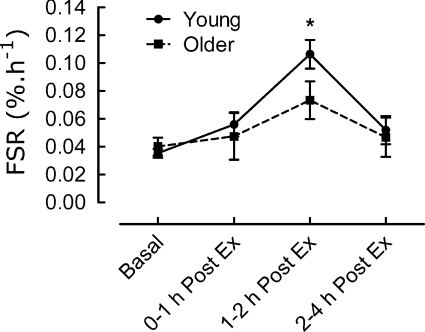
The values at 60–90% in each group were not different from each other, and so we averaged the data at these intensities (Fig. 3).
Figure 3. Time course of the averaged responses to exercise at 60–90% 1 RM of myofibrillar protein synthesis (FSR, fractional synthetic rate,% h−1) at 60–90% 1 RM for 15 subjects in each group of young and older subjects.
*P < 0.05. Note that protein synthesis is measured over 2.5 h in the basal pre-exercise state and then over the periods shown post-exercise.
The values in the young and older men were only different at 1–2 h. Inspection of the data for the extent of protein phosphorylation of p70s6K and 4EBP1 revealed a much greater degree of variation around mean values than that observed for myofibrillar protein synthesis and it was therefore difficult to discern more than a broad positive relationship between the size of the changes and exercise intensity (data not shown).
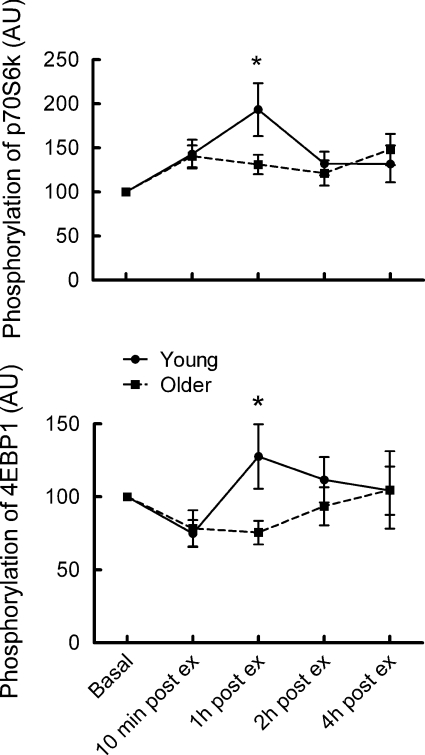
Nevertheless, as for myofibrillar protein synthesis, there were no significant differences between the phosphorylation responses of p70s6K and 4EBP1 at 60, 75 and 90% of 1 RM for young and old subjects, and combining these data produced more coherent images of the time courses and differences between the two groups of subjects (Fig. 4).
Figure 4. Time courses of the responses of phosphorylation of p70s6K and 4EBP1 (arbitrary units as percentage basal for each subject) averaged for intensities of 60–90% 1 RM.
n= 15 in each group. *P < 0.05.
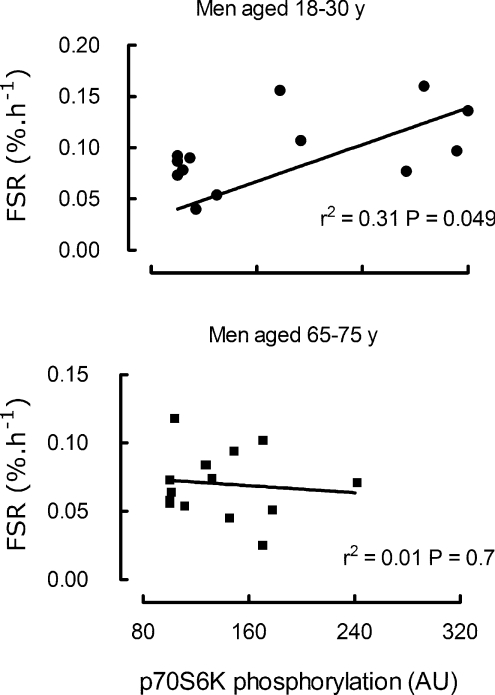
When the extent of phosphorylation of p70s6K at 1 h was related to the extent of MPS at 1–2 h there was a positive correlation for the young but not the old (Fig. 5).
Figure 5. Relationship between myofibrillar synthetic rate and extent of phosphorylation of p70s6K averaged for responses at 60–90% in young subjects (above) and older subjects (below).
There was a significant relationship (P= 0.049) between degree of phosphorylation (arbitrary units and protein synthetic rate (FSR,% h−1) only in the young. Note: some points overlaid.
For eEF2, phosphorylation showed a statistically non-significant fall of about 20% immediately after exercise then a rebound to about 120% of basal values by 1 h after exercise with no significant differences between the two age groups (data not shown).
Discussion
The present results provide new information concerning the responses of myofibrillar protein synthesis and muscle anabolic signalling in young and older men to exercise at a range of intensities, all in the post-absorptive state. In particular, rather than the hyperbolic relationship we postulated, they show a sigmoidal dose–response relationship of myofibrillar protein synthesis to exercise intensity, with little increase from 20 to 40% 1 RM, then a bigger rise at 60% of 1 RM, with no significant further increase up to 90% 1 RM. Older men showed a smaller response than the young subjects.
We previously showed in a pilot study that isometric exercise at 60, 75 and 90% of 1 RM increased myofibrillar protein synthesis at 90–150 min post-exercise to the same extent (Bowtell et al. 2003); the present results confirm that above 60% of 1 RM of isotonic exercise, the stimulatory effect is maximized. We ensured that the force × time integral (i.e. total external work) was equalized so far as possible so the results suggest that the total energy expenditure was constant and not a factor affecting the responses.
Might a change in muscle fibre type composition explain the age-related differences?
It is known that as contraction intensity increases an increasing proportion of type 2 fibres are recruited. Although in the basal or amino acid-stimulated conditions there is little difference in rates of protein synthesis in human muscle of markedly different fibre type compositions (Mittendorfer et al. 2005), it is possible that at high contraction intensities, type 2 fibres would show a greater response than type 1 fibres. Indeed, in type 2 fibres, phosphorylation of sarcoplasmic p70S6K1 occurs to a greater extent (∼25–30% more) than in type 1 fibres after resistance exercise at 75% of 1 RM (Koopman et al. 2006). This difference was suggested to be due to greater recruitment of type 2 fibres than type 1 fibres, which is feasible. Cross-sectional studies comparing individuals aged 60–70 years to those in their twenties (Larsson, 1983) suggested a slight increase in the proportion of type 1 fibres with age. However, other later cross-sectional studies did not confirm this (see Porter et al. 1995 for review). Furthermore, when fibre type proportions of muscles from the same individuals at 65 years and then at 75 years were assessed, there was a decrease from ∼40–60% of type 1 content (Frontera et al. 2000). Thus, evidence for a selective loss of type 2 fibres with age is poor and provides no explanation for our results.
A loss of total muscle mass would not explain the results given that relative intensity was equalized between groups; in any case we found little difference in the values of muscle mass in the young and the older men (although we may have overestimated this in the older subjects due to the inability of dual-energy X-ray absorptiometry to detect interfasicular fat and oedema in muscle). However, there was a marked difference in strength between the groups which is most commonly explained as being due to differences in tendon properties and efficiency of excitation–contraction coupling in older subjects (Narici & Maganaris, 2006). Nevertheless, these variations should not affect muscle protein synthesis per se.
Despite the existence of a blunted response of the exercise-stimulated rate of myofibrillar protein synthesis in the elderly, there were no differences in the shape of its post-exercise time course, or that of anabolic signalling, between the two groups. Therefore, there was no indication of any lag in the responses of the older subjects, as has been reported in a comparison of the post-exercise changes in young and old subjects fed after exercise (Drummond et al. 2008). However, it is noteworthy that we observed a fall in myofibrillar protein synthesis between 2 and 4 h after the peak at 1–2 h, which we believe has not been reported before in a full paper. The reason for this fall is puzzling but our exercise stimulus (presumably volume rather than intensity) might not have been sufficient to cause a long-lasting effect; after exercise of a greater volume (8 sets of 8 repetitions at 80% 1 RM) in the post-absorptive state by young subjects, the stimulatory effects lasted for up to 24 h (Phillips et al. 1997). The effects of volume of work and total time under tension remain to be investigated. Alternatively the fall might have been due to the lack of amino acid availability as occurs after feeding when the increase in MPS can be sustained for at least 24 h (Cuthbertson et al. 2006).
The precise molecular mechanisms by which resistance exercise stimulates myofibrillar protein synthesis remain to be determined but it is highly likely that enhanced phosphorylation of mammalian target of rapamycin (mTOR) and its downstream effectors, 4E binding protein 1 (4EBP1) and p70 ribosomal S6 kinase (p70s6K) are involved. (Deldicque et al. 2005; Cuthbertson et al. 2005; Terzis et al. 2008; Spiering et al. 2008; Wilkinson et al. 2008). Accordingly, we observed quantitatively similar increases in phosphorylation of both p70s6K and 4BP1, which were maximal for exercise at 60–90% 1 RM at 1 h post-exercise, i.e. just before the period of maximal increase in myofibrillar protein synthesis, and which were blunted in the older participants. Furthermore, we found for the first time in human muscle a positive correlation between extent of phosphorylation of p70s6K and MPS, albeit only in the young subjects. The extent of p70s6K phosphorylation reportedly predicts the extent of accretion of muscle in rats (Baar & Esser, 1999) and in weight lifters (Terzis et al. 2008), but no direct correlation between p70s6K phosphorylation and increases in muscle synthesis have been reported and certainly not in human muscle. This strengthens the support for a major role for p70s6K in stimulating MPS after exercise, and short-term changes in both predict the longer term changes. The lack of such a correlation in the older subjects is consonant with a blunted response of MPS to exercise, and reports that muscle hypertrophy after resistance exercise training is less in older men (Kosek et al. 2006). Nevertheless, the fact that both myofibrillar protein synthesis and p70S6 phosphorylation showed identical changes at 60–90% of 1 RM suggests that muscle adaptation may occur with exercise at less than the high intensities commonly assumed to be solely efficacious (Spiering et al. 2008).
The changes in 4EBP1 phosphorylation were more complex than those for p70s6K, in particular showing a marked fall in the biopsy taken immediately after exercise, which presumably mostly reflected the state of the molecule during exercise, as thereafter the change was reversed. This fall in 4EBP1 phosphorylation has been observed before (Dreyer et al. 2006; Koopman et al. 2006) and is likely to be associated with a fall in human muscle protein synthesis during exercise (Dreyer et al. 2006; Fujita et al. 2008). Although, like that of p70s6K, 4EBP1 phosphorylation showed a peak at 1 h post-exercise (although with much greater variability), and with blunted responses in the older subjects, no significant correlations with myofibrillar protein synthesis could be observed in either group.
We were unable to detect any effects of age or exercise intensity on the extent of phosphorylation of the elongation factor eEF2, which suggests that modulation of elongation at least by phosphorylation of eEF2 at Thr56 in the post-exercise period is of little biological relevance.
In summary, we have shown that acute bouts of resistance exercise at different intensities stimulate myofibrillar protein synthesis and anabolic signalling in a dose-dependent manner in both young and old men in the post-absorptive state. The stimulatory effect of exercise peaks at 1–2 h post-exercise is suppressed, but not delayed in older men. Although the extent of p70s6 kinase phosphorylation predicts the stimulation of myofibrillar protein synthesis in young men, older men appear not to match changes in anabolic signalling and myofibrillar protein synthesis, possibly explaining the deficiency in the muscle protein anabolic response.
Acknowledgments
We thank BBSRC (BB/X510697/1 and BB/C516779/1), the EC EXEGENESIS program Unilever plc and the University of Nottingham for support. Margaret Baker and Amanda Gates provided expert clinical assistance for which we are grateful.
References
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Bowtell JL, Park DM, Smith K, Cuthbertson DJR, Waddell T, Rennie MJ. Stimulation of human quadriceps protein synthesis after strenous exercise: no effects of varying intensity between 60 and 90% of one repetition maximum (1RM) J Physiol. 2003;547.P:P16. [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj JA, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie MJ. Anabolic signalling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Theisen D, Francaux M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol. 2005;94:1–10. doi: 10.1007/s00421-004-1255-6. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion prior to resistance exercise does not enhance post-exercise muscle protein synthesis. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.90395.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290:E1245–E1252. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117:469–471. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Raguso CA, Kyle U, Kossovsky MP, Roynette C, Paoloni-Giacobino A, Hans D, Genton L, Pichard C. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr. 2006;25:573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288:E922–E929. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE. 2008;3:e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiering BA, Kraemer WJ, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Maresh CM. Resistance exercise biology: manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008;38:527–540. doi: 10.2165/00007256-200838070-00001. [DOI] [PubMed] [Google Scholar]
- Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2008;102:145–152. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labelling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]