Abstract
Statins are used clinically for cholesterol reduction, but statin therapy is associated with myopathic changes through a poorly defined mechanism. We used an in vivo model of statin myopathy to determine whether statins up-regulate genes associated with proteasomal- and lysosomal-mediated proteolysis and whether PDK gene expression is simultaneously up-regulated leading to the impairment of muscle carbohydrate oxidation. Animals were dosed daily with 80 mg kg−1 day−1 simvastatin for 4 (n= 6) and 12 days (n= 5), 88 mg kg−1 day−1 simvastatin for 12 days (n= 4), or vehicle (0.5% w/v hydroxypropyl-methylcellulose and 0.1% w/v polysorbate 80; Control, n= 6) for 12 days by oral gavage. We found, in biceps femoris muscle, decreased AktSer473, FOXO1Ser253 and FOXO3aSer253 phosphorylation in the cytosol (P < 0.05, P < 0.05, P < 0.001, respectively) and decreased phosphorylation of FOXO1 in the nucleus after 12 days simvastatin when compared to Control (P < 0.05). This was paralleled by a marked increase in the transcription of downstream targets of FOXO, i.e. MAFbx (P < 0.001), MuRF-1 (P < 0.001), cathepsin-L (P < 0.05), PDK2 (P < 0.05) and PDK4 (P < 0.05). These changes were accompanied by increased PPARα (P < 0.05), TNFα (P < 0.01), IL6 (P < 0.01), Mt1A (P < 0.01) mRNA and increased muscle glycogen (P < 0.05) compared to Control. RhoA activity decreased after 4 days simvastatin (P < 0.05); however, activity was no different from Control after 12 days. Simvastatin down-regulated PI3k/Akt signalling, independently of RhoA, and up-regulated FOXO transcription factors and downstream gene targets known to be implicated in proteasomal- and lysosomal-mediated muscle proteolysis, carbohydrate oxidation, oxidative stress and inflammation in an in vivo model of statin-induced myopathy. These changes occurred in the main before evidence of extensive myopathy or a decline in the muscle protein to DNA ratio.
Statins inhibit synthesis of mevalonate by inhibiting 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting step in hepatic cholesterol and isoprenoid biosynthesis. Statins are used clinically for cholesterol reduction in hypercholesterolaemia, and their therapy has been associated with a 30% reduction in cardiovascular events in patients with vascular disease (Vaughan & Gotto, 2004).
Statins are generally well tolerated, but can have severe myopathic effects, albeit relatively infrequently (Thompson et al. 2003). Statin-related myopathy has varying degrees of severity, ranging from muscle myositis and myalgia (muscle aches or weaknesses with and without increased serum creatine kinase (CK) concentration, respectively) and, in the severest case, rhabdomyolysis (>10times the upper limit of normal serum CK concentration). Rhabdomyolysis is a rare event, with 0.15 deaths per million prescriptions; however, myalgia and myositis are more common, with an estimated occurrence of 1–5% in patients prescribed statins (Thompson et al. 2003). In a rat model of statin-induced muscle myopathy, a pattern of myopathy can be observed with raised serum CK and muscle necrosis occurring after 10–14 days of statin administration (Westwood et al. 2005) with type IIB (glycolytic) muscle fibres the most susceptible (Westwood et al. 2005).
The molecular mechanisms regulating statin-induced myopathy are poorly defined. However, up-regulation of gene expression in the ubiquitin–proteasome pathway has been shown to accompany statin administration in healthy volunteers (Urso et al. 2005). This pathway is reported to prevail in animal models of muscle wasting, which could account for up to 70% of muscle mass loss in rats during hepatoma-related cachexia (Baracos et al. 1995). In this pathway, ubiquitin conjugation to a protein substrate initiates its degradation by the 26S proteasome complex. In skeletal muscle, protein ubiquitination and degradation is reported to be regulated by two muscle-specific ubiquitin (E3) ligases; muscle atrophy F-box (MAFbx) and muscle RING finger-1 (MuRF-1) (Glass, 2003). The transcription of MAFbx and MuRF-1 is regulated by the forkhead box gene group O (FOXO) transcription factors, which are activated upon decreased signalling through the PI3k/Akt pathway (Sandri et al. 2004; Stitt et al. 2004). It has been demonstrated that statins decrease PI3k/Akt signalling in mouse C2C12 cells (Ogura et al. 2007), and that patients presenting with statin myopathy show increased MAFbx mRNA expression (Hanai et al. 2007). Furthermore, adult mouse muscle fibres with constitutively active FOXO3a also exhibit increased autophagy and lysosomal proteolysis (Mammucari et al. 2007). The lysosomal system is reported to account for ∼30% of muscle wasting observed in rodents during hepatoma-related cachexia (Baracos et al. 1995). Indeed, the transcription of the lysosomal protease cathepsin-L is up-regulated in many models of muscle atrophy (Lecker et al. 2004). A principal aim of the present study was to address whether statin administration impairs PI3k/Akt signalling in an in vivo model of statin myopathy, and if so, whether this activates genes involved in proteasome and lysosomal proteolysis by activating FOXO transcription factors. Furthermore, by performing a time course study we hoped to determine whether any signalling changes observed preceded associated downstream transcriptional and myopathic events. FOXO also activates the transcription of pyruvate dehydrogenase kinase (PDK) isoforms (1–4) by directly binding to the promoter region of the gene in C2C12 cells (Furuyama et al. 2003). Up-regulation of PDK phosphorylates and inactivates muscle pyruvate dehydrogenase complex (PDC) (Wieland, 1983), limiting oxidation of carbohydrate and promoting fat oxidation. Accordingly, increased PDK4 mRNA expression has been shown to parallel increased FOXO transcription in mouse muscle during starvation (Furuyama et al. 2003). Furthermore, statins and fibrates are known to increase PDK4 mRNA in rodent muscle (Motojima & Seto, 2003). Indeed, fibrate-mediated inhibition of muscle carbohydrate oxidation is achieved in part by increased PDK transcription and PDC inhibition in vivo, and appears to be elicited by altered Akt/FOXO signalling (Constantin et al. 2007). It is plausible therefore that if statins do activate FOXO transcription factors in vivo, this will result in the simultaneous up-regulation of muscle protein degradation and impairment of carbohydrate oxidation.
The direct mechanism by which statins decrease PI3k/Akt signalling is unclear; however, it has been suggested that inhibition of GTPase activity, through protein prenylation inhibition, may be involved (Flint et al. 1997; Johnson et al. 2004; Sakamoto et al. 2007). Prenylated proteins, or GTPases, are downstream intermediary metabolites of cholesterol synthesis and inhibition of HMG-CoA reductase by statins reduces the prenylation, and hence activation, of many GTPases (Goldstein & Brown, 1990). Indeed concomitant administration of statins with either mevalonate (i.e. the immediate product of HMG-CoA reductase necessary for the synthesis of isoprenoids) in a rat model of statin-induced myopathy (Westwood et al. 2005), or geranylgeraniol (the first isoprenoid metabolite of mevalonate responsible for prenylation of GTPases) in an in vitro model of statin-induced myotoxicity (Flint et al. 1997) reversed the myotoxic changes induced by statins. RhoA is a small GTPase activated by protein prenylation and is required for expression of MyoD, a key regulator of skeletal muscle differentiation (Carnac et al. 1998). Interestingly, inhibition of RhoA activity in differentiating myoblasts decreased signalling through the PI3k/Akt pathway specifically by dephosphorylation of Akt (Reuveny et al. 2004). Therefore, it may be suggested that inhibition of GTPase activity by statins could impair PI3k/Akt signalling and subsequently up-regulate ubiquitin- and lysosomal-mediated proteolysis.
To summarize, we measured Akt/FOXO signalling, MAFbx, MuRF-1, cathepsin-L, PDK2 and PDK4 mRNA expression, glycogen availability and RhoA activity in fast-twitch muscle in an in vivo rat model of statin myopathy to determine whether statins (i) blunt Akt/FOXO signalling and whether this occurs via decreased RhoA activity, (ii) up-regulate genes associated with proteasome and lysosomal proteolysis, and (iii) up-regulate PDK2 and 4 gene expression and impair muscle carbohydrate oxidation. We also aimed to determine whether Akt/FOXO signalling changes occurred before any associated downstream transcriptional or myopathic events. Using this approach we hoped to provide novel insight pertaining to the molecular aetiology of statin-induced myopathy.
Methods
Ethical approval
This study was planned in accordance with the standards of animal care and ethics described in Guidance on the Operations of the Animal (Scientific Procedures) Act 1986 issued by the UK Home Office and was conducted so that any clinical expression of toxicity remained within a moderate severity limit as described in guidelines agreed with the UK Home Office Inspector.
Animals and treatments
Simvastatin lactone (purity 98.4%) was obtained from Wuhan S&M Biochemie (Wuhan Hubei, China). The statin was formulated for dosing as a suspension in water containing 0.5% w/v hydroxypropyl methylcellulose and 0.1% w/v polysorbate 80.
Female Wistar Hanover rats, substrain Crl:WI (Glx/BRL/Han) BR (Charles River, UK), aged 8 weeks, were multiple-housed appropriate to each study and were acclimatized for 6 days. The animal rooms were illuminated in a 12 h light/dark cycle and temperature and humidity were controlled (limits 21 ± 2°C and 55 ± 15% RH). Pelleted RM1(E) SQC rodent diet and drinking water were freely available. Animals were dosed daily with 80 mg (kg body weight)−1 per day (mg kg−1 day−1) simvastatin for 4 (n= 6) and 12 days (n= 5), 88 mg kg−1 day−1 simvastatin for 12 days (n= 4), or vehicle (0.5% w/v hydroxypropyl methylcellulose and 0.1% w/v polysorbate 80; Control, n= 6) for 12 days by oral gavage. The time points in this study were chosen to determine if changes in Akt/FOXO signalling were early events that preceded transcriptional and/or histopathological changes. The latter normally occurring after 10–14 days of daily statin administration in this in vivo myopathy model (Westwood et al. 2005).
The work of Westwood et al. (2005) utilized a dose of 80 mg kg−1 day−1 simvastatin to induce necrosis. However, in this present study the incidence of necrosis was lower after 12 days simvastatin administration at this dose (Table 3), and therefore necrosis was induced in all animals by administering 88 mg kg−1 day−1 simvastatin for 12 days. Statin administration is known to predominantly affect type IIB fibres (Westwood et al. 2005), and therefore biceps femoris muscle, composed of approximately 70% type IIB fibres (Armstrong & Phelps, 1984), was investigated in this study. Muscles were harvested under terminal anaesthesia with halothane, immediately snap frozen and stored in liquid nitrogen.
Table 3.
Plasma creatine kinase (CK) activity and the incidence of necrosis in Control and simvastatin-treated animals
| Animal | Treatment | CK (IU l−1) | Necrosisa |
|---|---|---|---|
| 1 | Control | 170 | — |
| 2 | Control | 127 | — |
| 3 | Control | 149 | — |
| 4 | Control | 91 | * |
| 5 | Control | 211 | * |
| 6 | Control | 169 | — |
| 7 | Day 4, 80 mg | 168 | — |
| 8 | Day 4, 80 mg | 340 | — |
| 9 | Day 4, 80 mg | 497 | — |
| 10 | Day 4, 80 mg | 151 | — |
| 11 | Day 4, 80 mg | 504 | — |
| 12 | Day 4, 80 mg | 136 | — |
| 13 | Day 12, 80 mg | 189 | — |
| 14 | Day 12, 80 mg | 194 | — |
| 15 | Day 12, 80 mg | 161 | — |
| 16 | Day 12, 80 mg | 11 756 | ** |
| 17 | Day 12, 80 mg | 540 | ** |
| 18 | Day 12, 88 mg | 32 140 | ** |
| 19 | Day 12, 88 mg | 358 | ** |
| 20 | Day 12, 88 mg | 128 010 | * |
| 21 | Day 12, 88 mg | 32 500 | ** |
Incidence of necrosis: —, none;
minimal (up to 10 single fibres affected);
mild (up to 20% of fibres in section affected).
Glycogen and protein : DNA analysis
Snap-frozen muscle was divided into aliquots under liquid nitrogen; one part was freeze-dried and powdered. Glycogen was extracted from approximately 2 mg of powdered muscle using a modified protocol from Harris et al. (1974). The muscle glycogen was hydrolysed to glucosyl units with α-1,4-amyloglucosidase and measured using an enzymatic assay. Muscle alkaline-soluble protein and DNA were measured using the method described by Forsberg et al. (1991). The alkaline-soluble protein content was measured using the Lowry assay and DNA was measured by the diphenylamine reaction.
Quantitative RT-PCR
RNA was extracted from approximately 30 mg snap-frozen tissue using RNA Plus (Qbiogene). First strand cDNA was synthesized from 1 μg of total RNA using Powerscript reverse transcriptase (BD Biosciences) and random primers (Promega). A reaction was also carried out with the reverse transcriptase omitted to assess genomic DNA contamination.
All reactions were performed in the ABI Prism 7000 Sequence Detection System (Applied Biosystems, USA). Each well contained 2 μl cDNA, 12.5 μl Universal Taqman 2x PCR master mix (Eurogentec), 18 μm primer, 5 μm probe and 9.25 μl RNase-free water to make a volume of 25 μl. Each sample was measured in duplicate. The housekeeping gene used was hydroxymethylbilane synthase (HMBS), which was selected because it was not affected by the statin treatment. The thermal cycling conditions used were 2 min at 50°C, 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Genes analysed were metallothionein 1A (Mt1A), cathepsin-L, muscle-specific ubiquitin E3 ligases muscle atrophy F-box (MAFbx) and muscle RING finger-1 (MuRF-1), pyruvate dehydrogenase kinase (PDK) 2 and 4, peroxisome-proliferator-activated receptor-α (PPARα), interleukin-6 (IL6) and tumour necrosis factor-α (TNFα) (Applied Biosystems, Foster City, CA, USA). Relative quantification of the genes of interest was measured using the ΔΔCt method. The Ct values for the gene of interest were normalized with the Ct values of HMBS. The control group was used as calibrator with a value of 1.
Western blotting
Cytosolic and nuclear protein fractions were extracted from approximately 30 mg snap-frozen tissue using a method modified from Blough et al. (1999). Samples were homogenized in 50 mm Tris buffer pH 7.5 with the addition of protease and phosphatase inhibitors (Sigma-Aldrich, UK), centrifuged and the supernatant containing the cytosolic protein fraction was collected. The remaining pellet was resuspended in 20 mm Hepes buffer with the addition of protease and phosphatase inhibitors (Sigma-Aldrich, UK) to give the nuclear fraction. Protein content was measured using the Bradford assay. Protein samples were run on a 4–12% Bis-tris acrylamide gel (Invitrogen, UK) at 200 V for 1 h and transferred onto a polyvinylidene difluoride membrane (PVDF) overnight. Protein transfer was checked with Ponceau S staining before being blocked in 5% BSA–TBS–Tween. Membranes were probed with the primary antibody (1 : 500) overnight at 4°C. Membranes were washed with TBS–Tween and incubated with the secondary antibody horseradish (HRP)-linked anti-rabbit IgG (GE Healthcare) 1 : 2000 in 1% BSA–TBS–Tween. Membranes were incubated with ECL chemiluminescence detection reagent (Pierce, UK) and exposed to X-ray film.
Blots were scanned using Agfa Duoscan T1200. Bands were identified with Genetools from Syngene and the density volume was adjusted by subtracting the local background. Total Akt (Akt1, Akt2 and Akt3; tAkt) and phosphorylated Akt (Ser473; pAkt), total and phosphorylated FOXO1 (Ser253; tFOXO1 and pFOXO1) and FOXO3a (Ser253; tFOXO3a and pFOXO3a) proteins were measured and were normalized with actin (1 : 1000) or lamin (1 : 1000). All primary antibodies were purchased from Cell Signaling Technologies (USA).
Quantification of muscle necrosis
Muscle necrosis was quantified in muscle sections using the method described in Westwood et al. (2005). The magnitude of necrosis was graded subjectively: minimal, up to 10 muscle fibres affected in whole section; mild, up to 20% of fibres in section affected; moderate, up to 50% of fibres in section affected; and severe, more than 50% of fibres in section affected. Detailed histological images depicting muscle necrosis in the model used in the present study are presented in Westwood et al. (2005). Plasma creatine kinase activity was also determined using a spectrophotometric assay (Szasz et al. 1976).
RhoA GTPase activation assay
RhoA activation was measured using an absorbance-based G-LISA RhoA activation assay kit (Cytoskeleton, Denver, USA) according to the manufacturer's instructions.
Statistics
All data are expressed as mean ±s.e.m. Treatment effects were investigated using one-way ANOVA. When a significant F ratio was found, a least significant difference post hoc test was performed to locate specific differences. Any data that was not normally distributed was analysed with a Mann–Whitney U test. A significance level of P < 0.05 was used.
Results
Simvastatin reduces muscle Akt and FOXO phosphorylation and up-regulates gene expression consistent with the induction of ubiquitin proteasome- and lysosomal-mediated proteolysis
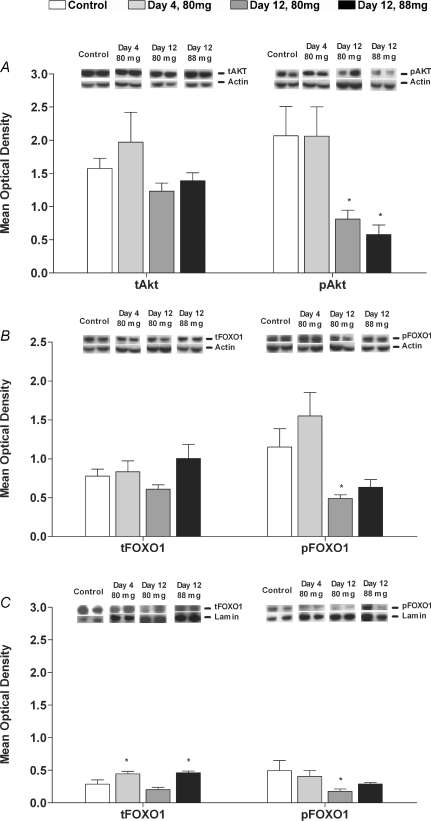
The phosphorylation of Akt (pAkt) was unchanged after 4 days simvastatin administration, but decreased after 12 days in the 80 mg kg−1 day−1 (P < 0.05) and 88 mg kg−1 day−1 (P < 0.05) groups compared to the corresponding time-matched control (Fig. 1A). There was also a trend for the phosphorylated Akt to total Akt ratio to decrease after 12 days administration of 88 mg kg−1 day−1 simvastatin (3.5 ± 0.2 fold, P= 0.064).
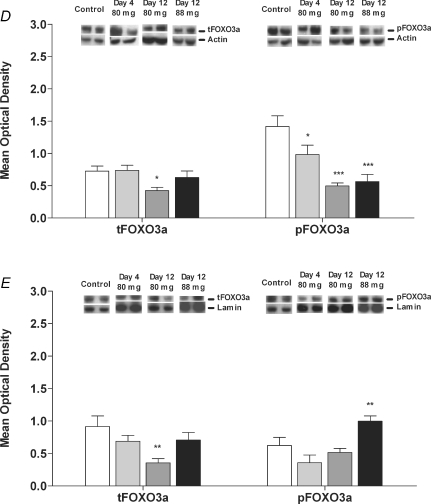
Figure 1. Protein expression of Akt, FOXO1 and FOXO3a in biceps femoris muscle of Control animals and animals dosed daily with 80 mg kg−1 day−1 simvastatin for 4 and 12 days and 88 mg kg−1 day−1 simvastatin for 12 days.
A, total Akt (tAkt) and phosphorylated Akt (pAkt) in the cytosolic protein fraction; B, total FOXO1 (tFOXO1) and phosphorylated FOXO1 (pFOXO1) in the cytosolic protein fraction; C, total FOXO1 (tFOXO1) and phosphorylated FOXO1 (pFOXO1) in the nuclear protein fraction; D, total FOXO3a (tFOXO3a) and phosphorylated FOXO3a (pFOXO3a) in the cytosolic protein fraction; E, total FOXO3a (tFOXO3a) and phosphorylated FOXO3a (pFOXO3a) in the nuclear protein fraction. Phosphorylated to total protein ratios are described in Results. Values expressed as mean optical density ±s.e.m.*P < 0.05, **P < 0.01, ***P < 0.001 when compared to Control. Images show a representative Western blot of the protein of interest and the corresponding actin or lamin blot.
Total FOXO1 in the cytosolic fraction was unchanged with simvastatin administration; however, this protein increased in the nuclear fraction after 4 days simvastatin at 80 mg kg−1 day−1 and 12 days simvastatin at 88 mg kg−1 day−1 (Fig. 1B and C). The phosphorylation of FOXO1 (pFOXO1) was unchanged at 4 days simvastatin administration, but significantly decreased after 12 days at 80 mg kg−1 day−1 (P < 0.05) in both the cytosolic and nuclear fractions (Fig. 1B and C). Accordingly, the phosphorylated FOXO1 to total FOXO1 ratio was lower in the cytosolic fraction at day 12 in the 80 mg kg−1 day−1 (1.9 ± 0.3 fold, P < 0.05) and the 88 mg kg−1 day−1 (2.4 ± 0.1 fold, P < 0.05) simvastatin-treated groups and was lower in the nuclear fraction of each simvastatin-treated group when compared to Control (day 4 (80 mg kg−1 day−1) 2.5 ± 0.4 fold, P < 0.05; day 12 (80 mg kg−1 day−1) 2.1 ± 0.2 fold, P < 0.05 and day 12 (88 mg kg−1 day−1) 3.0 ± 0.0 fold, P < 0.05). Total FOXO3a (tFOXO3a) decreased in both the cytosolic and nuclear fractions after 12 days of simvastatin administration at 80 mg kg−1 day−1 compared to Control (P < 0.05 and P < 0.01, respectively; Fig. 1D and E). Phosphorylated FOXO3a (pFOXO3a) in the cytosolic fraction decreased at all time points (Fig. 1D) as well as the phosphorylated FOXO3a to total FOXO3a ratio (day 4 (80 mg kg−1 day−1) 1.6 ± 0.3 fold, P < 0.05; day 12 (80 mg kg−1 day−1) 1.7 ± 0.5 fold, P < 0.05 and day 12 (88 mg kg−1 day−1) 2.3 ± 0.3 fold, P < 0.01). In the nuclear fraction, phosphorylated FOXO3a increased above Control with 12 days simvastatin at 88 mg kg−1 day−1 (Fig. 1E) and the phosphorylated FOXO3a to total FOXO3a ratio increased after 12 days simvastatin at 80 mg kg−1 day−1 (2.7 ± 0.1 fold, P < 0.05).
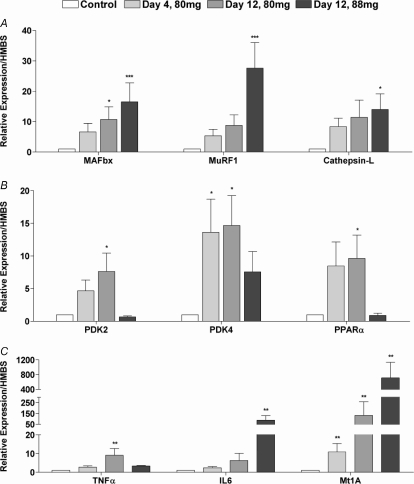
MAFbx expression was markedly greater than Control after 12 days in the 80 mg kg−1 day−1 group (P < 0.05) and the 88 mg kg−1 day−1 group (P < 0.001; Fig. 2A). MuRF-1 mRNA expression was also greater in the 88 mg kg−1 day−1 simvastatin group after 12 days (P < 0.001; Fig. 2A).
Figure 2. mRNA expression (fold changes relative to Control) determined in biceps femoris muscle of Control animals and animals dosed daily with 80 mg kg−1 day−1 simvastatin for 4 and 12 days and 88 mg kg−1 day−1 simvastatin for 12 days.
A, MAFbx, MuRF-1 and cathepsin-L expression; B, PDK2, PDK4 and PPARα expression; C, TNFα, IL6 and Mt1A expression. Values expressed as mean fold changes relative to Control ±s.e.m.*P < 0.05, **P < 0.01, ***P < 0.001 when compared to Control (which is set at 1).
Cathepsin-L mRNA expression became significantly different from Control after 12 days administration at 88 mg kg−1 day−1 (P < 0.05; Fig. 2A).
Simvastatin alters muscle glycogen content and increases muscle PDK2, PDK4 and PPARα gene expression
Muscle glycogen content was no different from Control after 4 days simvastatin administration at 80 mg kg−1 day−1, but was 30% greater after 12 days (P < 0.05, Table 1). Conversely, 12 days of simvastatin administration at 88 mg kg−1 day−1 reduced muscle glycogen by 46% compared with Control (P < 0.01).
Table 1.
Muscle glycogen content (absolute values) and the alkaline-soluble protein to DNA ratio in Control and simvastatin-treated animals
| Control | Day 4, 80 mg | Day 12, 80 mg | Day 12, 88 mg | |
|---|---|---|---|---|
| Glycogena | 137.2 ± 9.8 | 152.6 ± 9.1 | 178.1 ± 11.4* | 73.6 ± 17.4** |
| Protein : DNAb | 330.4 ± 32.2 | 269.4 ± 46.5 | 271.7 ± 20.0 | 237.5 ± 54.7* |
Values expressed as mean ±s.e.m.
mmol (kg dry matter (DM))−1,
g (kg DM) –1. Significantly different from Control:
P < 0.05,
P < 0.01.
PDK2 mRNA expression became significantly greater after 12 days simvastatin administration at 80 mg kg−1 day−1 (P < 0.05; Fig. 2B). Furthermore, PDK4 mRNA expression was markedly increased above Control after 4 and 12 days of simvastatin administration at 80 mg kg−1 day−1 (P < 0.05; Fig. 2B). PPARα mRNA expression was significantly greater than Control after 12 days simvastatin administration at 80 mg kg−1 day−1 (P < 0.05). PDK2, PDK4 and PPARα mRNA expression were no different from Control after 12 days of simvastatin administration at 88 mg kg−1 day−1.
Simvastatin up-regulates gene expression consistent with increased oxidative stress and inflammation in skeletal muscle
Metallothionein 1A (Mt1A) is a gene associated with oxidative stress that has been shown to be highly up-regulated in atrophying muscle (Lecker et al. 2004). In the current study, Mt1A mRNA expression was increased markedly compared with Control at all time points (P < 0.01; Fig. 2C). We also measured muscle TNFα and IL6 mRNA expression since inflammatory cytokines are known to induce oxidative stress (Matthys & Billiau, 1997), TNFα mRNA expression was significantly greater than Control after 12 days simvastatin administration at 80 mg kg−1 day−1 (P < 0.01; Fig. 2C), and IL6 mRNA expression was markedly greater than Control after 12 days administration at 88 mg kg−1 day−1 (P < 0.01; Fig. 2C).
Simvastatin does not impair RhoA activity in skeletal muscle
RhoA activity decreased significantly from Control after administration of simvastatin at 80 mg kg−1 day−1 for 4 days (P < 0.05, Table 2). However, RhoA activity was similar to Control after administration of simvastatin at 80 mg kg−1 day−1 and 88 mg kg−1 day−1 for 12 days.
Table 2.
Muscle RhoA activity (arbitrary units) in Control and simvastatin-treated animals
| Control | Day 4, 80 mg | Day 12, 80 mg | Day 12, 88 mg | |
|---|---|---|---|---|
| RhoA activity | 0.78 ± 2.71 | 0.56 ± 0.06* | 0.72 ± 0.06 | 0.76 ± 0.08 |
Values expressed as mean ± S.E.M. Significantly different from Control:
P < 0.05.
Simvastatin induces measurable myopathic changes
In the present study, administration of simvastatin at 88 mg kg−1 day−1 for 12 days increased plasma creatine kinase levels 315-fold above Control (P < 0.01, Table 3). Histological analysis revealed no evidence of muscle damage (necrosis) after 4 days simvastatin administration at 80 mg kg−1 day−1 (Table 3), but 2 out of 5 animals presented with mild necrosis at day 12 (which was also accompanied by an elevation in plasma creatine kinase). All animals in the group administered simvastatin at 88 mg kg−1 day−1 for 12 days presented with necrosis (Table 3). In keeping with these observations, animals in the 88 mg kg−1 day−1 treatment group also showed a 29% decrease in the alkaline-soluble protein to DNA ratio compared with Control (P < 0.05, Table 1), suggesting muscle protein degradation accompanied the necrosis evident in this group.
Discussion
The present study provides novel insight regarding the molecular basis of statin-induced myopathy by demonstrating that simvastatin administration resulted in the down-regulation of PI3k/Akt signalling, the activation of FOXO transcription factors, and the up-regulation of genes known to set in motion proteasomal- and lysosomal-mediated protein degradation and the impairment of carbohydrate oxidation in an in vivo model of statin-induced myopathy (Westwood et al. 2005). Furthermore, given that these changes occurred in the main concomitantly, and preceded a decline in the muscle protein to DNA ratio and evidence of wholesale myopathy, it is possible that statin-induced dampening of PI3k/Akt and activation of FOXO signalling was directly responsible for associated downstream transcriptional and myopathic events.
The molecular mechanisms responsible for statin myopathy are poorly defined, particularly in vivo. However, increased mRNA expression of the muscle-specific ubiquitin ligase, MAFbx, has been shown in patients with statin myopathy following chronic statin administration (which was not evident in non-myopathic patients treated with statins) (Hanai et al. 2007). In agreement with this observation, we were able to show markedly increased MAFbx mRNA expression in an in vivo model of statin myopathy and, for the first time, dramatically increased MuRF-1 mRNA expression (Fig. 2A). Furthermore, these changes were paralleled by a similarly large increase in muscle cathepsin-L mRNA expression. Cathepsin-L is an accepted marker of lysosomal-mediated proteolysis (Deval et al. 2001), and its transcription is up-regulated in several muscle atrophy models (Lecker et al. 2004), and by inflammatory cytokines (Ebisui et al. 1995). The present findings therefore suggest induction of both ubiquitin-proteasomal- and lysosomal-mediated proteolysis in this model; however, in the absence of cathepsin-L activity the mRNA data are equivocal.
MAFbx and MuRF-1 transcription is reported to be regulated by PI3k/Akt/FOXO signalling (Sandri et al. 2004; Stitt et al. 2004). When Akt is phosphorylated, it in turn phosphorylates and confines FOXO to the cytosol, inhibiting transcription of MAFbx and MuRF-1 and limiting protein degradation. Conversely, Akt dephosphorylation leads to dephosphorylation of FOXO, bringing about the translocation of these transcription factors to the nucleus, thereby increasing MAFbx and MuRF-1 transcription and increasing ubiquitin-proteasome-mediated proteolysis. In accordance with this, we have been able to show decreased Akt, FOXO1 and FOXO3a phosphorylation in the cytosol following 12 days simvastatin administration at 80 mg kg−1 day−1 in parallel with decreased phosphorylation of FOXO1, but not FOXO3a, in the nucleus. This suggests Akt dephosphorylation activates FOXO1, but not FOXO3a in statin myopathy. Indeed, these results are supported by evidence from McClung et al. (2007) where cytosolic dephosphorylation of FOXO3a did not lead to its nuclear localization in oxidative stress-induced atrophy and it may be suggested that there is a differential response of FOXO protein isoforms to different modes of muscle atrophy. The changes seen in Akt and FOXO1 coincided with increased MAFbx and MuRF-1 mRNA expression, but preceded a decline in the muscle protein to DNA ratio (Table 1) and extensive CK elevations and necrosis seen only after 12 days of simvastatin at 88 mg kg−1 day−1 (Table 3). Based on these data, we propose that the impairment of Akt/FOXO signalling is responsible for increased ubiquitin-proteasome-mediated proteolysis in statin myopathy. However, the data do not demonstrate whether the decline in the muscle protein to DNA ratio is solely attributable to an increase in protein degradation or whether a fall in muscle protein synthesis could also be involved. Indeed, dephosphorylation of Akt will also affect the mTOR protein translation initiation pathway and may lead to a reduction in protein synthesis, as well as an increase in ubiquitin-proteasome-mediated proteolysis. Future work should address whether the impairment of Akt signalling by simvastatin impacts on both muscle protein synthesis and degradation pathways.
It is not possible to distinguish from the present findings the mechanism by which simvastatin decreased Akt phosphorylation; however, statins have been shown to reduce Akt phosphorylation in differentiating myoblast cells by inhibiting RhoA activity (Reuveny et al. 2004). In the present study, RhoA activity declined significantly after 4 days of simvastatin at 80 mg kg−1 day−1 (Table 2); however, this was not paralleled by decreased Akt phosphorylation (Fig. 1A). When Akt phosphorylation was impaired (i.e. after 12 days of 80 mg kg−1 day−1 and 88 mg kg−1 day−1 simvastatin, Fig. 1A), RhoA activity was no different from Control suggesting RhoA inhibition was not responsible for simvastatin-mediated blunting of Akt signalling. This finding was surprising, as many studies have shown statin-induced RhoA inactivation (Laufs et al. 1999, 2002; Takeda et al. 2006). However, these studies were cell-based experiments and to our knowledge no in vivo study has shown statin-induced RhoA inhibition in skeletal muscle. Further investigation is required to determine the mechanism by which statins impair Akt/FOXO signalling in vivo.
Cytokines are also known to inhibit PI3k and Akt phosphorylation by reducing the ability of insulin receptor substrate-1 (IRS-1) to bind to the insulin receptor (Rui et al. 2001). Furthermore, it is known TNFα and IL6 can increase cathepsin-L (Ebisui et al. 1995) and MAFbx (Li et al. 2005) transcription directly, i.e. independent of PI3k/Akt signalling. Given that TNFα mRNA expression increased in parallel with the changes in Akt and FOXO phosphorylation and cathepsin-L and MAFbx mRNA expression, it is possible it played a regulatory role in the aetiology of events. However, the approximately 90-fold increase in IL6 mRNA expression after 12 days simvastatin at 88 mg kg−1 day−1 is in keeping with inflammatory cell infiltration occurring postnecrosis in statin-induced myopathy (Westwood et al. 2005).
Pyruvate dehydrogenase kinase phosphorylates and inactivates the pyruvate dehydrogenase complex (PDC) (Wieland, 1983), thereby limiting muscle carbohydrate oxidation and promoting fatty acid oxidation. Two PDK isoforms (2 and 4) predominate in skeletal muscle (Gudi et al. 1995; Rowles et al. 1996), and it has been demonstrated that an increase in PDK4 mRNA in rodent muscle during food deprivation is tightly coupled to increased FOXO transcription, and may be responsible for the decline in carbohydrate oxidation during starvation (Furuyama et al. 2003). We therefore hypothesized that if statins do activate FOXO transcription factors in vivo, along with the activation of muscle atrophy this would also result in the impairment of muscle carbohydrate oxidation by up-regulating muscle PDK gene expression. In keeping with this hypothesis, we have shown in the present study that PDK2 and PDK4 mRNA expression was markedly elevated (Fig. 2B) in parallel with the decline in Akt and FOXO1 phosphorylation after 12 days simvastatin administration at 80 mg kg−1 day−1 (Fig. 1). Furthermore, muscle glycogen content increased by 30% above Control following 12 days simvastatin administration at 80 mg kg−1 day−1 (Table 1), clearly indicating muscle glycogen oxidation was suppressed. To our knowledge, this is the first study to demonstrate impaired carbohydrate utilization in parallel with increased FOXO activity and PDK gene expression in an in vivo model of statin myopathy. In addition, this study provides novel evidence suggesting a dual role of FOXO signalling in the impairment of carbohydrate oxidation and up-regulation of ubiquitin-mediated proteolysis, a process which seems to be distinctive of statin myopathy. We were also able to demonstrate a 10-fold increase in PPARα mRNA expression (Fig. 2B). PPARα is considered to be an important regulator of fatty acid metabolism (Lefebvre et al. 2006), and it has been shown in mouse skeletal muscle that starvation-induced increases in PDK4 mRNA expression are mediated by PPARα (Wu et al. 2001). In accordance with this, we showed increased PDK4 and PPARα mRNA expression after 4 days simvastatin administration at 80 mg kg−1 day−1, events which were not paralleled by changes in Akt or FOXO phosphorylation. These data suggest PPARα also regulates muscle fuel metabolism in statin myopathy. Animals treated with the highest dose of simvastatin (88 mg kg−1 day−1) did not show increased PDK2, PDK4 or PPARα mRNA levels above Control, and there was a marked decline in muscle glycogen (Table 1). This collection of observations may have occurred as a consequence of the extensive necrosis observed in this group (Table 3). One of the core events in cell necrosis is bioenergetic failure (Zong & Thompson, 2006), and as a consequence regulators of energy metabolism, i.e. PDK and PPARα, would decline.
The gene most markedly up-regulated in the present study was metallothionein 1A (Mt1A, Fig. 2C). Mt1A is a gene associated with oxidative stress (Nath et al. 2000), and has been shown to be up-regulated in several models of muscle atrophy (Lecker et al. 2004), particularly in the presence of elevated TNFα levels (Matthys & Billiau, 1997). Furthermore, it has been proposed that components of the ubiquitin–proteasome pathway are transcriptional targets of reactive oxygen species (ROS) in cultured myotubes (Gomes-Marcondes & Tisdale, 2002), and that ROS can induce activation of FOXO (Nakamura & Sakamoto, 2008). The present data show early up-regulation of Mt1A mRNA prior to increases in TNFα and IL6 mRNA expression, with further profound changes occurring by day 12 (Fig. 2C). It is possible therefore that oxidative stress may have been a trigger for the signalling and gene expression changes observed in the present study and may play an important role in the aetiology of statin myopathy.
The plasma concentration of simvastatin in rats undergoing myopathy in this study is two to three orders of magnitude greater than that obtained in healthy volunteers under non-myopathic conditions (Lilja et al. 1998; Backman et al. 2002; Schneck et al. 2004). Compared with therapeutic use, the dose of statin required to induce myopathy in rodents and other species is high, being at or near a dose associated with general morbidity and body weight loss. However, given that preclinical studies are generally carried out in young, healthy, genetically ‘normal’ animals, it is perhaps not surprising that high doses of statins are required to induce myopathy. The doses of simvastatin used in the present study were, however, lower than those used in other preclinical animal models of statin myopathy, e.g. 100–150 mg kg−1 day−1 simvastatin (Smith et al. 1991), 200–1000 mg kg−1 day−1 lovastatin (Smith et al. 1991; Waclawik et al. 1993), 200–600 mg kg−1 day−1 pravastatin (Smith et al. 1991; Nakahara et al. 1992) and 120–160 mg kg−1 day−1 rosuvastatin (Westwood et al. 2008). Furthermore, clinical pathology assessment of animals in the present study showed elevation of plasma creatine kinase which is associated specifically with skeletal muscle damage. Finally, histology of the major organs demonstrates muscle necrosis to be a principal pathology of the model we have utilized (Westwood et al. 2005). It is apparent therefore that the present experiment has used a model of statin-induced myopathy and not statin-induced toxicity. Furthermore, the few reports of histological/biochemical analysis of skeletal muscle biopsies in patients undergoing myopathy suggest a very similar pattern of skeletal muscle damage to that observed in animal models; namely ragged red fibres and mitochondrial abnormalities. These experiments are frequently cited in reviews of statin-induced myopathy by clinical experts (Phillips et al. 2002; Thompson et al. 2003; Rosenson, 2004; Phillips & Haas, 2008) and would appear to be accepted as valid preclinical models. Indeed, in keeping with our own observations, Hanai et al. (2007) have recently shown changes in MAFbx gene expression in patients prescribed statins at clinical doses, therefore we believe the changes evident in the rodent model utilized here do reflect those occurring in patients with statin myopathy.
In summary, we have presented novel insight concerning the molecular basis of statin-induced myopathy by showing that simvastatin administration impaired PI3k/Akt signalling, and up-regulated FOXO transcription factors and downstream gene targets known to be implicated in proteasomal- and lysosomal-mediated protein breakdown, muscle carbohydrate oxidation, oxidative stress and inflammation in an in vivo model of statin-induced myopathy. We have also shown that a distinctive characteristic of statin myopathy is the concomitant regulation of protein and fuel metabolism via the FOXO signalling pathway. Additionally, given that the changes seen occurred before evidence of extensive myopathy, or a decline in the muscle protein to DNA ratio, it is possible that this statin-induced blunting of PI3k/Akt and activation of FOXO signalling was directly responsible for the myopathic events observed.
Acknowledgments
This study was supported by a grant from AstraZeneca Pharmaceuticals (UK).
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–691. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- Baracos V, DeVivo C, Hoyle D, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol Endocrinol Metab. 1995;268:E996–E1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- Blough E, Dineen B, Esser K. Extraction of nuclear proteins from striated skeletal muscle. Biotechniques. 1999;26:202–206. doi: 10.2144/99262bm05. [DOI] [PubMed] [Google Scholar]
- Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett AJ, Greenhaff PL. PPARd agonism induces a change in muscle fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function. J Physiol. 2007;583:381–390. doi: 10.1113/jphysiol.2007.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J. 2001;360:143–150. doi: 10.1042/0264-6021:3600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci. 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- Flint OP, Masters BA, Gregg RE, Durham SK. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol Appl Pharmacol. 1997;145:91–98. doi: 10.1006/taap.1997.8131. [DOI] [PubMed] [Google Scholar]
- Forsberg AM, Nilsson E, Werneman J, Berström J, Hultman E. Muscle composition in relation to age and sex. Clin Sci. 1991;81:249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;275:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gomes-Marcondes MCC, Tisdale MJ. Induction of protein catabolism and the ubiquitin proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180:69–74. doi: 10.1016/s0304-3835(02)00006-x. [DOI] [PubMed] [Google Scholar]
- Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme VP, Lecker SH. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Hultman E, Nordesjo LO. Glycogen, glycogen intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, Umbenhauer DR. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol. 2004;200:237–250. doi: 10.1016/j.taap.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27 (Kip1) J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPARa in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja JJ, Kivisto KT, Neuvonen PJ. Grapefruit juice–simvastatin interaction: Effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther. 1998;64:477–483. doi: 10.1016/S0009-9236(98)90130-8. [DOI] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997;13:763–770. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Motojima K, Seto K. Fibrates and statins rapidly and synergistically induce pyruvate dehydrogenase kinase 4 mRNA in the liver and muscles of mice. Biol Pharm Bull. 2003;26:954–958. doi: 10.1248/bpb.26.954. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Kuriyama M, Yoshidome H, Nagata K, Nagado T, Nakagawa M, Arimura K, Higuchi I, Osame M. Experimental simvastatin-induced myopathy in rabbits. J Neurol Sci. 1992;113:114–117. doi: 10.1016/0022-510x(92)90273-n. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2008;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Nath R, Kumar D, Li T, Singal PK. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology. 2000;155:17–26. doi: 10.1016/s0300-483x(00)00273-0. [DOI] [PubMed] [Google Scholar]
- Ogura T, Tanaka Y, Nakata T, Namikawa T, Kataoka H, Ohtsubo Y. Simvastatin reduces insulin-like growth factor-1 signalling in differentiating C2C12 mouse myoblast cells in an HMG-CoA reductase inhibition-independent manner. J Toxicol Sci. 2007;32:57–67. doi: 10.2131/jts.32.57. [DOI] [PubMed] [Google Scholar]
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther. 2008;6:971–978. doi: 10.1586/14779072.6.7.971. [DOI] [PubMed] [Google Scholar]
- Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- Reuveny M, Heller H, Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS Lett. 2004;569:129–134. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Rosenson R. Current overview of statin-induced myopathy. Am J Med. 2004;116:408–416. doi: 10.1016/j.amjmed.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Rowles J, Scherer SW, Xi T, Majer M, Nickle DC, Rommens JM, Popov KM, Harris RA, Riebow NL, Xia J, Tsui LC, Bogardus C, Prochazka M. Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J Biol Chem. 1996;271:22376–22382. doi: 10.1074/jbc.271.37.22376. [DOI] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim J, Shulman G, Lee A, Corbould A, Dunaif A, White M. Insulin/IGF-1 and TNFα stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Honda T, Yokoya S, Waguri S, Kimura J. Rab-small GTPases are involved in fluvastatin and pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J. 2007;21:1–8. doi: 10.1096/fj.07-8713com. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck DW, Birmingham BK, Zalikowski JA, Mitchell PD, Wang Y, Martin PD, Lasseter KC, Brown CD, Windass AS, Raza A. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004;75:455–463. doi: 10.1016/j.clpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Smith PF, Eydelloth RS, Grossman R, Stubbs J, Schwartz MS, Germershausen JI, Vyas KP, Kari PH, Macdonald JS. HMG-CoA reductase inhibitor-induced myopathy in the rat: Cyclosporine A interaction and mechanism studies. J Pharmacol Ther. 1991;257:1225–1235. [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Szasz G, Gruber W, Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem. 1976;22:650–656. [PubMed] [Google Scholar]
- Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol. 2006;35:722–729. doi: 10.1165/rcmb.2006-0034OC. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Clarkson P, Karas RH. Statin associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Urso ML, Clarkson P, Hittel D, Hoffman EP, Thompson PD. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler Thromb Vasc Biol. 2005;25:2560–2566. doi: 10.1161/01.ATV.0000190608.28704.71. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Gotto AM. Update on statins 2003. Circulation. 2004;110:886–892. doi: 10.1161/01.CIR.0000139312.10076.BA. [DOI] [PubMed] [Google Scholar]
- Waclawik AJ, Lindal S, Engel AG. Experimental lovastatin myopathy. J Neuropath Exp Neurol. 1993;52:542–549. doi: 10.1097/00005072-199309000-00012. [DOI] [PubMed] [Google Scholar]
- Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development and fibre selectivity. Toxicol Pathol. 2005;33:246–257. doi: 10.1080/01926230590908213. [DOI] [PubMed] [Google Scholar]
- Westwood FR, Scott RC, Marsden AM, Bigley A, Randall K. Rosuvastatin: Characterization of induced myopathy in the rat. Toxicol Pathol. 2008;36:345–352. doi: 10.1177/0192623307311412. [DOI] [PubMed] [Google Scholar]
- Wieland OH. The mammalian pyruvate dehydrogenase complex: Structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Wu P, Peters JM, Harris RA. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferato-activated receptor α. Biochem Biophys Res Commun. 2001;287:391–396. doi: 10.1006/bbrc.2001.5608. [DOI] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]