Abstract
Acute exercise performance represents a major metabolic challenge for the skeletal muscle, but also for the liver as the most important source of energy. However the molecular adaptation of the liver to one single bout of exercise is largely unknown. C57BL/6 mice performed a 60 min treadmill run at high aerobic intensity. Liver, soleus and white gastrocnemius muscle were removed immediately after exercise. The single bout of exercise resulted in a very rapid and pronounced induction of hepatic metabolic enzymes and regulators of metabolism or transcription: glucose-6-phosphatase (G6Pase; 3-fold), pyruvate dehydrogenase kinase-4 (PDK4; 4.8-fold), angiopoietin-like 4 (2.1-fold), insulin receptor substrate (IRS)-2 (5.1-fold), peroxisome proliferator activated receptor-γ coactivator 1α (PGC-1α; 3-fold). In soleus and white gastrocnemius muscle the up-regulation of IRS-2 and PDK4 was less pronounced compared with the liver and no significant induction of PGC-1α could be detected at this early time point. Activation of AMPK was found in both liver and white gastrocnemius muscle as phosphorylation of Thr-172. The induction of endogenous insulin secretion by a glucose load directly after the exercise bout resulted in a significantly higher PKB/Akt phosphorylation in the liver of exercised mice. The markedly enhanced IRS-2 protein amount, and presumably reduced serine/threonine phosphorylation of the IRS proteins induced by the acute exercise could be responsible for this enhanced action of insulin. In conclusion, acute exercise induced a rapid and pronounced transcriptional adaptation in the liver, and regulated hepatic IRS proteins leading to improved cellular insulin signal transduction.
Exercise performance represents in addition to fasting the major challenge for the organism to supply fuels for the energy spending tissues, i.e. during physical activity for the working skeletal muscle (Wasserman & Cherrington, 1991; Kjaer, 1998). In both conditions, during exercise and during fasting, the liver is the central organ for the control of glucose homeostasis and for providing glucose (Fritsche et al. 2008). The effects of fasting on hepatic gene expression have been studied in detail and the regulation of major metabolic key enzymes has been demonstrated: the adaptation of the liver to fasting involves enhanced expression of the gluconeogenic enzymes phosphoenolpyruvate carboxylase (PEPCK) and glucose-6-phosphatase (G6Pase) (Cimbala et al. 1982; Herzig et al. 2001), up-regulation of pyruvate dehydrogenase kinase (PDK)4 (Wu et al. 2000) and induction of insulin receptor substrate (IRS)-2 (Canettieri et al. 2005). Activation and up-regulation of peroxisome proliferator activated receptor-γ coactivator (PGC)-1α has been elucidated as an important transcriptional amplifier for these adaptations (Herzig et al. 2001; Yoon et al. 2001). In contrast, limited information is available on the effects of exercise on metabolic regulators in the liver. Earlier studies showed increased activities of the gluconeogenic enzymes as well as decreased lipogenic enzyme activities (Dohm et al. 1985; Nizielski et al. 1996). A few studies have investigated the effects of long-term exercise training over several weeks on hepatic gene expression in rodents (Wilson & Johnson, 2000; Aoi et al. 2004; Colombo et al. 2005; Lee et al. 2006), but the acute transcriptional response of hepatic genes to one single bout of exercise remains largely unknown.
Most studies in humans and with rodents have focused on the working skeletal muscle, and acute exercise has been shown to induce the transcription of genes involved in glucose and fatty acid metabolism (Neufer & Dohm, 1993; Seip et al. 1995; O'Doherty et al. 1996; Kraniou et al. 2000; Jones et al. 2003), myogenic genes (Yang et al. 2005), expression of transcriptional regulators (Puntschart et al. 1998; Baar et al. 2002; Russell et al. 2005) and stress-response genes (Essig et al. 1997). The induction of some of these genes is very rapid and could be detected immediately after cessation of the exercise bout, e.g. the expression of PDK4 after 45 min of high intensive treadmill exercise in rats (Hildebrandt et al. 2003), but the up-regulation of most metabolic genes peaked in the first hours of the recovery phase, both in rodents (Leick et al. 2008) and in humans (Yang et al. 2005). From the first instance it appears obvious that the contracting muscle is the predominantly affected organ during exercise, which has to cope with an acutely increased energy demand and has the need to respond to this challenge with adaptive responses. Interestingly, a recent study investigating the energy charge in the liver of mice after one single bout of exercise found a clear increase of AMP and a strong decrease of ATP in the liver (Camacho et al. 2006). Of note, the concentration of ATP in the muscle was found to be unaffected by the intensity of the exercise protocol (Camacho et al. 2006; Leick et al. 2008). This prompted us to speculate that the liver although not directly consuming energy due to enhanced work load has to adapt to an enormous metabolic challenge during acute exercise. This could result in similar rapid and significant gene activation as in the working muscle. To test this hypothesis, we submitted C57BL/6 mice to 60 min of acute exercise at high aerobic intensity on a treadmill.
Immediately after cessation of the physical activity we found a very rapid and pronounced expression of metabolic enzymes and of regulators of metabolism and transcription in the liver. The hepatic response to glucose-induced insulin action shown as tyrosine phosphorylation of IRS-1 and phosphorylation of Akt/PKB was improved in exercised compared with sedentary mice.
Methods
Materials
A mouse Accupacer treadmill with motorized grade adjust was from Hugo Sachs Elektronik (March – Hugstetten, Germany); a LightCycler system was from Roche (Mannheim, Germany). Oligonucleotides were synthesized by Invitrogen (Karlsruhe, Germany). Antibodies against phospho-AMPK α Thr-172, β-actin, phospho-Akt Ser-473, and phospho-tyrosine were from Cell Signalling (Frankfurt, Germany); antibodies against AMPK α1 and α2, and IRS-1 and IRS-2 were from Upstate Biotechnology (Lake Placid NY, USA); the antibody against Akt was from BD Biosciences (San Diego CA, USA). λ-Protein phosphatase (PPase) was purchased from New England BioLabs (Beverly, MA, USA), protease inhibitor mixture was from Roche, and 20%d-glucose was from B. Braun (Melsungen, Germany).
Animal care and exercise protocol
All animal experiments were conducted in accordance with the guidelines of laboratory animal care and were approved by the local governmental commission for animal research (Regierungspraesidium). Male C57BL/6 mice were purchased from Charles River Wiga GmbH (Sulzfeld, Germany) and kept under an inverted light–dark cycle (dark period 09.30 h–21.30 h, light period 21.30 h–09.30 h) with free access to standard chow (Sniff, Soest, Germany) and tap water. All experiments were performed between 10.00 h and 13.00 h in non-fasted animals. Mice were habituated to treadmill running for 10 min at 5 m min−1 and 5 deg inclination twice 1 and 2 weeks prior to the experiment. For acute exercise, 12-week-old mice (n= 8) ran 60 min at 14 m min−1 and 14 deg inclination after 5 min warm-up (5 m min−1 and 5 deg inclination). Mice attempting to rest were encouraged to continue running by gently tapping on their back. Directly after running animals were anaesthetized with an intraperitoneal injection of ketamine (150 mg (kg body weight)−1) and xylazine (10 mg (kg body weight)−1) and killed by decapitation. Tissues were immediately removed and frozen in liquid nitrogen or directly homogenized.
In a second acute exercise protocol, immediately after an acute bout of exercise as described above, mice (n= 6) were intraperitoneally injected with a single bolus of d-glucose (2 g (kg body weight)−1). Either directly after running or following 30 min of glucose stimulation without access to food, animals were anaesthetized and killed by decapitation as described above.
Blood parameters
Capillary blood samples were taken from the tail vein for determination of glucose and lactate concentrations. FFA and hormones were measured in the EDTA–plasma collected after decapitation. Glucose was quantified using an Accu-Chek Aviva glucometer (Roche, Mannheim, Germany), lactate concentration was determined with an Ebio plus 6668 analyser (Eppendorf AG, Hamburg, Germany) using lactate oxidase containing electrodes, FFA concentrations were detected by an enzymatic method (ADVIA 1650®, Siemens Medical Solutions, Fernwald, Germany), and insulin and glucagon levels were determined by radio-immunoassay (Linco Research, St Charles, MO, USA).
RNA isolation, RT-PCR and real-time quantitative PCR analysis
Frozen tissue was homogenized in a TissueLyser (Qiagen, Hilden, Germany) and RNA was extracted with the RNeasy Fibrous Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Reverse transcription of total RNA (1 μg) was performed in a volume of 20 μl using random hexamers with the First strand cDNA synthesis kit for RT-PCR (Roche, Mannheim, Germany). Aliquots of 2 μl of the reverse transcription reactions were then submitted to online quantitative PCR with the Light Cycler system (Roche, Mannheim, Germany) with SYBR® green using FastStart DNA-MasterSYBR Green I (Roche, Mannheim, Germany) or the QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany). The following primer pairs were used: COX-1 sense: GCC TTT CGA GAA TAC CAC GA, antisense: AGG TTG GTT CCT CAG ATG TG, product of 233 bp; PGC-1α sense: ATG TGT CGC CTT CTT GCT CT, antisense: ATC TAC TGC CTG GGG ACC TT, product of 179 bp; G6Pase sense: GTC GAC TCG CTA TCT CCA AG, antisense: GCA ATG CCT GAC AAG ACT CC, product of 528 bp; PEPCK sense: CAC CTC CTG GAA GAA CAA GG, antisense: CTA CGG CCA CCA AAG ATG AT, product of 161 bp; Angptl-4 sense: CAA AAC AGC AAG ATC CAG CA, antisense: TTG GAA GAG TTC CTG GCA GT, product of 246 bp; IRS-1 sense: GAT AGC GAG GCT GAG CAA GA, antisense: CAC CAC GGA GTC ATC CAC TT, product of 429 bp; β-actin sense: AGC CAT GTA CGT AGC CAT CC, antisense: CTC TCA GCT GTG GTG GTG AA, product of 227 bp; 28S-RNA sense: CCA GTA CTT CAC TCC TGT CT, antisense: TCT AAG AGT GAG CAA CGA CG, product of 194 bp; PDK4: Mm_Pdk4–1_SG QuantiTect Primer Assay, Fasn: Mm_Fasn_1_SG QuantiTect Primer Assay, CTP1a: Mm_CPT1a_1_SG QuantiTect Primer Assay, CTP1b: Mm_CPT1b_1_SG QuantiTect Primer Assay, IRS-2: Mm_LOC384783–1_SG QuantiTect Primer Assay (Qiagen, Hilden, Germany). The quantitative PCR was performed in a total volume of 20 μl: 2 μl FastStart DNA-MasterSYBR Green I, MgCl2 4 mmol l−1, and primers at a concentration of 1 μmol l−1 or according to the instructions of the QuantiTect Primer Assay.
The instrument settings were as follows. After denaturing at 95°C for 10 min, cycling was performed by denaturing at 95°C for 15 s, annealing at 66°C for 10 s, elongation for 8 s for PGC-1α; annealing at 66°C for 10 s, elongation for 21 s for G6Pase; annealing at 61°C for 10 s, elongation for 15 s for PEPCK; annealing at 66°C for 10 s, elongation for 16 s for Angptl4; annealing at 68°C for 10 s, elongation for 17 s for IRS-1; annealing at 69°C for 10 s, elongation for 10 s for β-actin; annealing at 61°C for 10 s, elongation for 8 s for 28S-RNA; the number of cycles was 50; or the instrument settings were according to the QuantiTect Primer Assay.
The mRNA content is given in arbitrary units. Normalization to β-actin mRNA content (liver) or 28S mRNA content (muscle) gave results comparable to non-normalized values. β-Actin mRNA was slightly up-regulated by acute exercise in muscle.
Tissue lysates and Western blotting
Liver and muscles were removed as described and immediately homogenized at 4°C in lysis buffer (50 mm Tris pH 7.6, 150 mm NaCl, 1% Triton X-100 containing protease and phosphatase inhibitors). After solubilization on ice for 30 min, the homogenates were clarified by three rounds of centrifugation (10 min at 12 000 g). To visualize tyrosine phosphorylation, 1 mg of protein was used for immunoprecipitation with 2.5 μg antibody against IRS-1 or IRS-2. For dephosphorylation, 150 μg of protein was incubated with λ-PPase at 30°C for 30 min according to instruction of the manufacturer. SDS-PAGE (7.5%) and Western blotting were performed as previously described (Weigert et al. 2006).
Statistical analysis
Means ±s.e.m. were calculated and groups of data were compared using Student's t test. Statistical significance was set at P < 0.05.
Results
Parameters of metabolism in the plasma
Plasma glucose levels were not different between sedentary mice and mice before the exercise bout (150 ± 13 versus 144 ± 6, respectively, data not shown) and dropped significantly by the exercise performance (Fig. 1A). The insulin levels showed a clear tendency to be lower after the exercise (0.19 ± 0.1 versus 0.39 ± 0.25 ng ml−1, P= 0.06, Fig. 1B), while the glucagon levels did not change (64 ± 23 versus 52 ± 32 pg ml−1, Fig. 1C). Concomitantly, the insulin/glucagon ratio was reduced (Fig. 1D). Free fatty acid levels increased in the exercise group (880 ± 188 versus 580 ± 212 μmol l−1, Fig. 1E). Moreover, the physical activity led to a slight but significant increase in plasma lactate concentrations (Fig. 1F), which indicates that the animals were exercising at a high aerobic intensity (Ferreira et al. 2007).
Figure 1. Parameters of metabolism in the plasma.
Plasma concentrations are shown of glucose (A), insulin (B), glucagon (C), FFA (E) and lactate (F), as well as the insulin/glucagon ratio (D) of sedentary (sed) and exercised (run) mice (n= 8, mean ±s.e.m.). In A and F individual values of the same animals before and after the exercise bout are given.
Hepatic mRNA expression
To evaluate the effect of the single bout of exercise on the regulation of genes in the liver, we studied the expression of genes known to be up-regulated by acute exercise in the muscle or shown to be involved in the transcriptional adaptation of the liver to fasting.
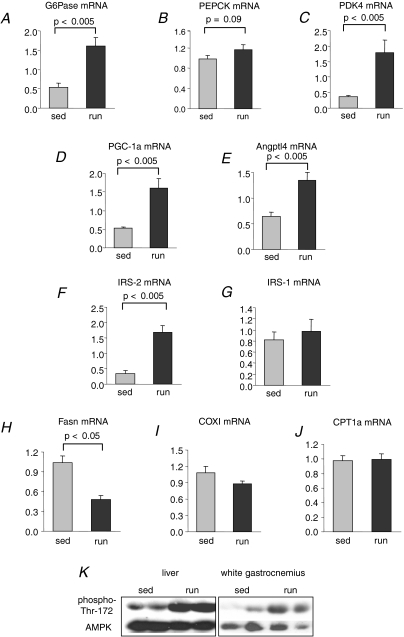
The mRNA expression of the gluconeogenic enzyme G6Pase was up-regulated 3-fold, and the expression of PEPCK tended to be increased (Fig. 2A and B). The mRNA content of PDK4 was 4.8-fold increased (Fig. 2C). The mRNA expression of the transcriptional coactivator PGC-1α was 3-fold up-regulated (Fig. 2D). The mRNA content of angiopoietin-like 4 (Angptl-4), also known as fasting-induced adipose factor and previously shown to be induced by fasting in liver and muscle (Dutton & Trayhurn, 2008), was 2.1-fold increased (Fig. 2E). IRS-2 mRNA expression was 5.1-fold up-regulated, while the mRNA content of IRS-1 was not influenced by acute exercise (Figs 2F and G). Moreover, the exercise bout induced an acute decrease in fatty acid synthase (Fasn) mRNA expression (Fig. 2H). The mRNA content of the mitochondrial-encoded cytochrome c oxidase subunit (COX)I and of the hepatic carnitine palmitoyltransferase (CPT) isoform 1a was unchanged (Fig. 2I and J).
Figure 2. Hepatic mRNA expression.
G6Pase (A), PEPCK (B), PDK4 (C), PGC-1α (D), Angptl-4 (E), IRS-2 (F), IRS-1 (G), Fasn (H), COXI (I), and CPT1a (J) mRNA content in the liver of sedentary (sed) or exercised (run) mice. Values are shown as arbitrary units (n= 8, mean ±s.e.m.). K, 150 μg protein of liver and white gastrocnemius muscle extracts were separated by 7.5% SDS-PAGE and immunoblotted with antiphospho-Thr-172 antibodies and reprobed with mixed anti-AMPK α1 +α2 antibodies. Shown are representative blots with two animals of each group.
The question arises which signalling pathways are activated in the liver to mediate the detected transcriptional adaptation of the metabolic genes. Acute exercise results in rapid decrease in hepatic ATP levels and concomitantly a pronounced increase in hepatic AMP levels (Camacho et al. 2006), and therefore activation of the metabolic master switch enzyme AMP-activated kinase (AMPK) could be one mechanism. We studied the phosphorylation of Thr-172 of AMPK, which indicates the activation of this kinase. The exercise performance resulted in a clear increase in this phosphorylation in the liver and also the white gastrocnemius muscle (Fig. 2K). Thus, AMPK could be involved in the transcriptional response in the liver.
Muscle mRNA expression
In the next step, we studied the effect of the single exercise bout on the expression of the genes shown to be regulated in the liver, in the white gastrocnemius (fast twitch glycolytic muscle fibres) and soleus muscle (slow oxidative fibres). We found significant increases in the mRNA content of IRS-2 in soleus muscle (3.4-fold) and in the white gastrocnemius (2.4-fold) (Fig. 3A and B). Expression of IRS-1 was not increased in both muscles (data not shown). Expression of Angptl-4 was also up-regulated (2.0-fold in soleus, 2.2-fold in white gastrocnemius muscle, Fig. 3C and D). Moreover, expression of PDK4 was slightly elevated in both muscle tissues (Fig. 3E and F). Due to the lack of gluconeogenic properties of skeletal muscle the expression level of G6Pase and PEPCK was not determined. The expression of PGC-1α was not significantly enhanced in soleus and in white gastrocnemius muscle (Fig. 3G and H). Previous data show that the transcription of PGC-1α is rapidly increased after acute exercise in skeletal muscle and peaked in the first hours of the recovery phase with (Pilegaard et al. 2003; Mathai et al. 2008) or without significant up-regulation directly after the exercise bout (Hildebrandt et al. 2003; Leick et al. 2008). Expression of Fasn, COXI and the CPT1 muscle isoform b was not influenced in both muscle tissues (Fig. 3I–N).
Figure 3. Muscle mRNA expression.
IRS-2 (A and B), Angptl-4 (C and D), PDK4 (E and F), PGC-1α (G and H), Fasn (I and J), COXI (K and L) and CPT1b (M and N) mRNA content in soleus (A, C, E, G, I, K and M) and white gastrocnemius muscle (B, D, F, H, J, L and N) of sedentary (sed) and exercised (run) mice. Values are shown as arbitrary units (n= 8, mean ±s.e.m.).
Regulation of IRS proteins and insulin signalling in the liver by acute exercise
The beneficial effects of one single bout of exercise on whole body insulin sensitivity have been extensively demonstrated both in humans and in rodents, but studies on the underlying cellular signalling mechanisms have focused on the skeletal muscle (Zierath, 2002; Holloszy, 2005; Wojtaszewski & Richter, 2006). Since we had found markedly enhanced transcription of genes in the liver by the acute exercise protocol, we also investigated the effect on the hepatic insulin signalling cascade.
First we verified if the change in IRS mRNA expression was reflected on the protein level. Similar to the data obtained for mRNA expression we found no change in IRS-1 protein expression while IRS-2 protein expression was strongly enhanced in exercised mice (Fig. 4A). Furthermore we observed a reduced electrophoretic mobility of both IRS-1 and -2 in sedentary mice. This finding was studied further as described below.
Figure 4. Regulation of IRS proteins and insulin signalling in the liver by acute exercise.
A, detection of IRS proteins in liver extracts of sedentary (sed) and exercised (run) mice. Liver protein (150 μg) was separated by 7.5% SDS-PAGE and immunoblotted with anti-IRS-2, -IRS-1 and -β-actin antibodies. B, C and D, plasma concentration of insulin (B), FFA (C) and glucose (D) of sedentary mice (sed), exercised mice immediately after the exercise bout (run), sedentary and exercised mice 30 min after the glucose load (+ glucose i.p.). (n= 6, mean ±s.e.m., *P < 0.05 versus mice of the same group without glucose, #P < 0.05 versus sedentary mice with glucose). E. 150 μg protein of liver extracts was separated by 7.5% SDS-PAGE and immunoblotted with anti-phospho-Ser-473 antibodies and membranes were reprobed with anti-Akt/PKB antibodies. Shown is one representative immunoblot and the densitometric quantification, phosphorylation of Akt/PKB in extracts obtained from sendentary, glucose-treated mice was set as 1 (n= 6, mean ±s.e.m., *P < 0.05 versus mice of the same group without glucose, #P < 0.05 versus sedentary mice with glucose). F and G, tyrosine phosphorylation of IRS-1 (F) and IRS-2 (G). Protein of liver extracts (1 mg) was immunoprecipitated with anti-IRS-1 or anti-IRS-2 antibodies, and immunoprecipitates were separated by 7.5% SDS-PAGE and immunoblotted with anti-phospho-tyrosine antibodies. Membranes were reprobed with anti-IRS-1 or anti-IRS-2 anitbodies. Shown are representative immunoblots and the densitometric quantification (n= 6, mean ±s.e.m., *P < 0.05 versus mice of the same group without glucose). H, 150 μg protein of liver extracts were left untreated, incubated with buffer, or incubated with buffer and λ protein phosphatase (λPPase) for 30 min at 30°C, separated by 7.5% SDS-PAGE and immunoblotted with anti-IRS-2-antibodies.
To evaluate if the increased expression of genes involved in metabolism and proximal insulin signalling has functional consequences on insulin signal transduction we performed a second exercise study where we induced endogenous insulin secretion by an intraperitoneal glucose load (2 g kg−1) directly after the exercise bout and studied the effect on plasma parameters and insulin signalling molecules 30 min later. The insulin concentration was reduced by the treadmill run (0.26 ± 0.06 versus 0.44 ± 0.05 ng ml−1, P= 0.04, Fig. 4B), and the glucose treatment resulted in increased insulin levels in the exercised and sedentary group compared to the groups without the glucose load (Fig. 4B). The insulin concentration in the sedentary group after the glucose load appeared to be higher, but this did not reach statistical significance. The increased plasma free fatty acid levels in the exercised group declined to the concentrations found in the sedentary groups, while plasma glucose levels were still increased in both the exercised and the sedentary group 30 min after the glucose load (Fig. 4C and D). The phosphorylation of the kinase Akt/PKB, an important target in the insulin signalling pathway, was induced in both groups of glucose-treated mice, and this effect was clearly enhanced in the exercised mice (Fig. 4E). This result suggests that the liver could be more sensitive to the action of insulin directly after an acute exercise bout. The tyrosine phosphorylation of IRS-1 appeared slightly enhanced only in the exercised group after glucose treatment (Fig. 4F), while the relative tyrosine phosphorylation of IRS-2 was not different in the four groups, although the signal intensity was clearly intensified after the exercise performance due to the strong up-regulation of IRS-2 protein (Fig. 4G).
Another striking finding is the clearly reduced mobility of IRS-1 and IRS-2 in liver extracts of sedentary mice in the SDS-PAGE (Fig. 4A and H). Thus, acute exercise alters the post-translational modification of IRS proteins, leading to an increased electrophoretic mobility. Alteration in the phosphorylation of tyrosine residues appeared not to be responsible for this phenomenon (Fig. 4F and G), but treatment of the liver extracts obtained from sedentary mice with protein phosphatase resulted in a similar increase in the mobility and density of IRS-2 in the SDS-PAGE compared with exercise (Fig. 4H). The more diffuse IRS-2 band detected in liver extract of sedentary mice could be due to the existence of differently phosphorylated IRS-2 proteins. Together, these data suggest that acute exercise could regulate serine/threonine phosphorylation of IRS-2, presumably leading to enhanced insulin signal transduction.
Discussion
The main findings of the present study could be summarized as follows. Firstly, the liver responds to the single bout of treadmill exercise with a very rapid and pronounced induction of metabolic enzymes, and regulators of metabolism or transcription; secondly, the up-regulation of genes well known as acute-exercise-regulated genes in the working muscle is more pronounced in liver compared to soleus and white gastrocnemius muscle at this time point; thirdly, acute exercise regulates IRS proteins on a transcriptional and post-transcriptional level and might improve insulin signalling in the liver post-exercise.
We focused the study on genes known as acute exercise-responsive genes in the muscle which could also be relevant for the adaptation of hepatic metabolism to exercise. In the studied group of genes, the hepatic expression of PDK4 and PGC-1α was markedly up-regulated. Both proteins are important for the regulation of hepatic gluconeogenesis. PDK4 phosphorylates and inactivates the pyruvate dehydrogenase complex, thus inhibiting glucose oxidation and promoting gluconeogenesis (Holness et al. 2003; Watt et al. 2004). PGC-1α serves as transcriptional coactivator for the expression of the gluconeogenic enzymes G6Pase and PEPCK and is also involved in PDK4 gene activation (Yoon et al. 2001; Wende et al. 2005). Accordingly, we found a strong up-regulation of G6Pase mRNA content in the liver directly after the acute exercise. G6Pase is necessary for the delivery of glucose, including glucose derived from glycogenolysis, which serves as a glucose pool before the onset of gluconeogenesis (Kjaer, 1998; Wahren & Ekberg, 2007). In contrast, the increase in hepatic PEPCK mRNA was very weak and not significant directly after the exercise and may peak in recovery or after prolonged exercise, when the glycogen stores are depleted and gluconeogenesis is the only source of glucose.
In addition to PGC-1α, G6Pase and PDK4, acute exercise increased the expression of Angptl-4 and IRS-2 and decreased the expression of Fasn in the liver. Since all these genes have also been demonstrated to be up-regulated by long-term fasting in rodents (Wu et al. 2000; Kersten et al. 2000; Yoon et al. 2001; Kubota et al. 2008) it could be speculated that 60 min of acute exercise induces similar transcriptional responses in the liver of mice to prolonged starvation. This is supported by previous data showing that after 35 min of moderate treadmill exercise of mice (20 m min−1, 5 deg) liver glycogen is clearly reduced and after approximately 80 min of running till exhaustion almost depleted (Camacho et al. 2006). The present exercise protocol reduced liver glycogen to less than 10% compared with sedentary controls (data not shown). To almost completely deplete hepatic glycogen by starvation, mice have to be fasted for 12 h (Chen et al. 1992; Pederson et al. 2005).
The similarities of transcriptional adaptations of the liver to acute exercise and to fasting are of course not unexpected, since in both conditions the liver has to supply glucose, to produce glucose from gluconeogenic precursors and to maintain glucohomeostasis. In the present study, we provided for the first time data showing that for certain genes the transcriptional response to exercise is unexpectedly rapid and pronounced in the liver when compared with the response of the working muscle. With the exception of Angptl-4, which was similarly up-regulated in liver and muscle, the mRNA expression of the other genes tested was more increased in the liver than in both soleus and white gastrocnemius muscle immediately after cessation of exercise. We did not study the time course of hepatic gene expression after the exercise bout, but it is possible that the kinetics of hepatic and muscle transcriptional response to exercise are different, since the expression of several exercise-regulated genes in the muscle has been shown to peak in the recovery phase (Hildebrandt et al. 2003; Yang et al. 2005).
Important questions which arise from the present study are what is the hepatic sensor and what are the signalling molecules during acute exercise that mediate the crosstalk between the working muscle and the liver? As mentioned above, acute exercise strongly reduces liver glycogen content, and this is accompanied by a significant decrease in hepatic energy charge (Camacho et al. 2006). This clearly is an important activator of AMPK, and exercise-induced activation of hepatic AMPK was demonstrated in several studies including the present one (Park et al. 2002; Kelly et al. 2004). Since alterations in the energy charge of the exercising muscle depend on the exercise protocol and were not detected even after exhaustive treadmill running (Camacho et al. 2006), it could be argued that the activation of AMPK may be less pronounced in the working muscle compared with liver and that may explain the stronger hepatic transcriptional response. However contraction-induced activation of calcium-dependent pathways as it occurs in the working muscle potentiates AMPK activation even in the absence of altered energy charge (Hurley et al. 2005; Birnbaum, 2005). Thus the mechanisms leading to AMPK activation appear to be different in the liver and the working muscle, but it is unclear whether this could explain the different transcriptional response of, for example, the AMPK-regulated expression of PGC-1α.
An often discussed mechanism and intensively studied hypothesis is the existence of an exercise factor which is released from the working muscle into circulation and signals to the liver. Most reports focused on interleukin (IL)-6 (Febbraio & Pedersen, 2002). However, the local concentration of IL-6 in the muscle fibres is much higher than in the circulation (Rosendal et al. 2004). Therefore the activation of IL-6-dependent pathways as an explanation for the strong hepatic response to acute exercise appears unlikely. In accordance with this, we detected increased phosphorylation of the IL-6 signal transducer STAT-3 in all tissues directly after the exercise bout, in liver, soleus and white gastrocnemius muscle (C. Weigert, unpublished data). Nitric oxide is another signalling molecule which is produced during exercise, but the reported increase might be restricted to the working muscle (Steensberg et al. 2007). Moreover, exercise has been shown to reduce nitric oxide-dependent nitrosation of insulin signalling proteins (Pauli et al. 2008).
Altered substrate availability, namely the detected increase in plasma free fatty acids and the decline in glucose, the increased glucagon/insulin ratio and the reported rapid decline in ATP (Camacho et al. 2006) certainly contribute to the regulation of hepatic metabolism during exercise. Indirect central effects on the liver and the action of catecholamines should also be considered. However, the exact molecular mechanisms responsible for the rapid and pronounced transcriptional response of the liver to exercise need to be identified.
A further important finding of the present study is the striking regulation of IRS proteins by acute exercise. The up-regulation of IRS-2 mRNA expression and protein content in the liver is well in accordance with the prominent role of IRS-2 during fasting and immediately after refeeding (Kubota et al. 2008). Of note, while the first increase in IRS-2 mRNA levels were detected after 12 h of starvation (Kubota et al. 2008), only 60 min of exercise were sufficient to induce a pronounced up-regulation of IRS-2 mRNA and protein levels. Acute exercise also induced a marked mobility shift of hepatic IRS-1 and IRS-2 which was not due to reduced tyrosine phosphorylation. Based on the results obtained with protein phosphatase treatment, we suggest that reduced serine/threonine phosphorylation is the most likely explanation for the higher electrophoretic mobility of the IRS proteins. Since increased serine phosphorylation of IRS-1 is an important negative feedback mechanism to reduce insulin action (Tanti et al. 1994), this could explain the slightly improved tyrosine phosphorylation of IRS-1 in the exercised mice after the glucose load. A previous study reported on reduced Ser-307 phosphorylation of IRS-1 after one single bout of exercise in the muscle of rats, which exhibited reduced muscle insulin signalling and increased serine phosphorylation of IRS-1 due to a high fat diet (Ropelle et al. 2006). We studied the phosphorylation of Ser-307 and Ser-318, but we could only detect a weak phosphorylation of Ser-318 without any difference in the signal intensity between sedentary and exercised mice (data not shown). Information about serine/threonine phosphorylation sites of IRS-2 is very limited and no phospho-site specific antibodies are commercially available.
Well in accordance with the increased IRS-2 protein content and presumably reduced serine/threonine phosphorylation of IRS proteins, we could demonstrate improved hepatic insulin signalling on the cellular level. The induction of endogenous insulin secretion by glucose resulted in a slightly enhanced tyrosine phosphorylation of IRS-1 and significantly higher PKB/Akt phosphorylation in the liver of the exercised mice. This was not reflected by lower plasma glucose levels 30 min after the glucose load in the exercised mice, but previous reports suggest that insulin action on glucose uptake could be even impaired immediately after exercise due to the increased free fatty acid and catecholamine levels (Wojtaszewski et al. 2002).
We conclude that the liver has to be considered as a working organ during exercise and that some of the beneficial effects of physical activity on insulin sensitivity and metabolism occur in the liver. Moreover, acute exercise appears to be a very efficient stimulus similar to starvation with pronounced effects on hepatic gene expression after only 60 min of running.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft to C.W. (WE 4176/1-1) and to E.S. (GRK 1302/1) and by a grant from the Landesstiftung Baden-Wuerttemberg to R.L. (P-LS-Prot/29). We gratefully acknowledge the technical assistance of Isolde Riedlinger and Elisabeth Metzinger.
References
- Aoi W, Ichiishi E, Sakamoto N, Tsujimoto A, Tokuda H, Yoshikawa T. Effect of exercise on hepatic gene expression in rats: a microarray analysis. Life Sci. 2004;75:3117–3128. doi: 10.1016/j.lfs.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell. 2005;19:289–290. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Camacho RC, Donahue EP, James FD, Berglund ED, Wasserman DH. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2006;290:E405–E408. doi: 10.1152/ajpendo.00385.2005. [DOI] [PubMed] [Google Scholar]
- Canettieri G, Koo SH, Berdeaux R, Heredia J, Hedrick S, Zhang X, Montminy M. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005;2:331–338. doi: 10.1016/j.cmet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Chen C, Williams PF, Cooney GJ, Caterson ID, Turtle JR. The effects of fasting and refeeding on liver glycogen synthase and phosphorylase in obese and lean mice. Horm Metab Res. 1992;24:161–166. doi: 10.1055/s-2007-1003285. [DOI] [PubMed] [Google Scholar]
- Cimbala MA, Lamers WH, Nelson K, Monahan JE, Yoo-Warren H, Hanson RW. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982;257:7629–7636. [PubMed] [Google Scholar]
- Colombo M, Gregersen S, Kruhoeffer M, Agger A, Xiao J, Jeppesen PB, Orntoft T, Ploug T, Galbo H, Hermansen K. Prevention of hyperglycemia in Zucker diabetic fatty rats by exercise training: effects on gene expression in insulin-sensitive tissues determined by high-density oligonucleotide microarray analysis. Metabolism. 2005;54:1571–1581. doi: 10.1016/j.metabol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Kasperek GJ, Barakat HA. Time course of changes in gluconeogenic enzyme activities during exercise and recovery. Am J Physiol Endocrinol Metab. 1985;249:E6–E11. doi: 10.1152/ajpendo.1985.249.1.E6. [DOI] [PubMed] [Google Scholar]
- Dutton S, Trayhurn P. Regulation of angiopoietin-like protein 4/fasting-induced adipose factor (Angptl4/FIAF) expression in mouse white adipose tissue and 3T3-L1 adipocytes. Br J Nutr. 2008;100:18–26. doi: 10.1017/S0007114507882961. [DOI] [PubMed] [Google Scholar]
- Essig DA, Borger DR, Jackson DA. Induction of heme oxygenase-1 (HSP32) mRNA in skeletal muscle following contractions. Am J Physiol Cell Physiol. 1997;272:C59–C67. doi: 10.1152/ajpcell.1997.272.1.C59. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol. 2007;34:760–765. doi: 10.1111/j.1440-1681.2007.04635.x. [DOI] [PubMed] [Google Scholar]
- Fritsche L, Weigert C, Haring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver – implications for health and disease. Curr Med. 2008;15:1316–1329. doi: 10.2174/092986708784534956. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Physiol Endocrinol Metab. 2003;285:E1021–E1027. doi: 10.1152/ajpendo.00234.2003. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003;369:687–695. doi: 10.1042/BJ20021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Jones TE, Baar K, Ojuka E, Chen M, Holloszy JO. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2003;284:E96–E101. doi: 10.1152/ajpendo.00316.2002. [DOI] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Hepatic glucose production during exercise. Adv Exp Med. 1998;441:117–127. [PubMed] [Google Scholar]
- Kraniou Y, Cameron-Smith D, Misso M, Collier G, Hargreaves M. Effects of exercise on GLUT-4 and glycogenin gene expression in human skeletal muscle. J Appl Physiol. 2000;88:794–796. doi: 10.1152/jappl.2000.88.2.794. [DOI] [PubMed] [Google Scholar]
- Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, Sasako T, Moroi M, Sugi K, Kakuta S, Iwakura Y, Noda T, Ohnishi S, Nagai R, Tobe K, Terauchi Y, Ueki K, Kadowaki T. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. doi: 10.1016/j.cmet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Lee KY, Kim SJ, Cha YS, So JR, Park JS, Kang KS, Chon TW. Effect of exercise on hepatic gene expression in an obese mouse model using cDNA microarrays. Obesity (Silver Spring) 2006;14:1294–1302. doi: 10.1038/oby.2006.147. [DOI] [PubMed] [Google Scholar]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1a is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1a mRNA and protein in human skeletal muscle. J Appl Physiol. 2008;105:1098–1105. doi: 10.1152/japplphysiol.00847.2007. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Dohm GL. Exercise induces a transient increase in transcription of the GLUT-4 gene in skeletal muscle. Am J Physiol Cell Physiol. 1993;265:C1597–C1603. doi: 10.1152/ajpcell.1993.265.6.C1597. [DOI] [PubMed] [Google Scholar]
- Nizielski SE, Arizmendi C, Shteyngarts AR, Farrell CJ, Friedman JE. Involvement of transcription factor C/EBP-b in stimulation of PEPCK gene expression during exercise. Am J Physiol Regul Integr Comp Physiol. 1996;270:R1005–R1012. doi: 10.1152/ajpregu.1996.270.5.R1005. [DOI] [PubMed] [Google Scholar]
- O'Doherty RM, Bracy DP, Granner DK, Wasserman DH. Transcription of the rat skeletal muscle hexokinase II gene is increased by acute exercise. J Appl Physiol. 1996;81:789–793. doi: 10.1152/jappl.1996.81.2.789. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Pauli JR, Ropelle ER, Cintra DE, Carvalho-Filho MA, Moraes JC, De Souza CT, Velloso LA, Carvalheira JB, Saad MJ. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL, DePaoli-Roach AA, Roach PJ. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem. 2005;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1a gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntschart A, Wey E, Jostarndt K, Vogt M, Wittwer M, Widmer HR, Hoppeler H, Billeter R. Expression of fos and jun genes in human skeletal muscle after exercise. Am J Physiol Cell Physiol. 1998;274:C129–C137. doi: 10.1152/ajpcell.1998.274.1.C129. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Prada PO, De Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol. 2006;577:997–1007. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2004;182:379–388. doi: 10.1152/japplphysiol.00130.2004. [DOI] [PubMed] [Google Scholar]
- Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988. doi: 10.1096/fj.04-3168fje. [DOI] [PubMed] [Google Scholar]
- Seip RL, Angelopoulos TJ, Semenkovich CF. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am J Physiol Endocrinol Metab. 1995;268:E229–E236. doi: 10.1152/ajpendo.1995.268.2.E229. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Keller C, Hillig T, Frosig C, Wojtaszewski JF, Pedersen BK, Pilegaard H, Sander M. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007;21:2683–2694. doi: 10.1096/fj.06-7477com. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Gremeaux T, Van Obberghen E, Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- Wahren J, Ekberg K. Splanchnic regulation of glucose production. Annu Rev Nutr. 2007;27:329–345. doi: 10.1146/annurev.nutr.27.061406.093806. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Cherrington AD. Hepatic fuel metabolism during muscular work: role and regulation. Am J Physiol Endocrinol Metab. 1991;260:E811–E824. doi: 10.1152/ajpendo.1991.260.6.E811. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJ, LeBlanc PJ, Inglis JG, Spriet LL, Peters SJ. Rapid upregulation of pyruvate dehydrogenase kinase activity in human skeletal muscle during prolonged exercise. J Appl Physiol. 2004;97:1261–1267. doi: 10.1152/japplphysiol.00132.2004. [DOI] [PubMed] [Google Scholar]
- Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schauble M, Haring HU, Schleicher ED. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem. 2006;281:7060–7067. doi: 10.1074/jbc.M509782200. [DOI] [PubMed] [Google Scholar]
- Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1a coactivates PDK4 gene expression via the orphan nuclear receptor ERRa: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DO, Johnson P. Exercise modulates antioxidant enzyme gene expression in rat myocardium and liver. J Appl Physiol. 2000;88:1791–1796. doi: 10.1152/jappl.2000.88.5.1791. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 2006;42:31–46. doi: 10.1042/bse0420031. [DOI] [PubMed] [Google Scholar]
- Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98:1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zierath JR. Invited review: Exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93:773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]