Abstract
To examine the programming effects of maternal renal dysfunction (created by subtotal nephrectomy in ewes prior to mating; STNx), renal and cardiovascular function were studied in 6-month-old male and female offspring of STNx and control pregnancies. After studies were conducted on a low salt diet (LSD) some female offspring underwent salt loading (0.17 m NaCl in the drinking water for 5–7 days; HSD). On LSD both male and female offspring of STNx had similar mean arterial pressures (MAP), heart rates, cardiac outputs and renal function to those measured in offspring of control ewes. In female STNx offspring on a HSD, plasma sodium levels increased and haematocrits fell, indicating volume expansion (P < 0.05). Plasma renin levels were not suppressed despite the increases in plasma sodium concentrations, but aldosterone levels were reduced. In control animals plasma renin levels fell (P < 0.05) but there was no change in plasma aldosterone concentrations. There was a positive relationship between GFR and MAP which was present only in female STNx offspring. In conclusion, in STNx offspring there was an impaired ability to regulate glomerular filtration independent of arterial pressure, renin release was insensitive to a high salt intake and control of aldosterone secretion was abnormal. This study provides evidence of abnormal programming of the renin–angiotensin system and glomerular function in offspring of pregnancies in which there is impaired maternal renal function.
Developmental programming is a term used to describe the process whereby a stimulus or insult, at a crucial period of development, has lasting or lifelong significance (Barker, 1994). Epidemiological evidence has shown a link between an altered intrauterine environment and hypertension in adult life (Barker et al. 1989; Dabelea et al. 2000). Animal models of fetal programming have reinforced this hypothesis, showing that hypertension can present itself in young adulthood after development in an altered intrauterine environment (Dodic et al. 1998; Gilbert et al. 2005).
Very few animal studies have investigated whether or not maternal renal dysfunction programs those mechanisms critical to control of fluid and electrolyte balance. In previous studies from our laboratory we have shown that maternal renal dysfunction, produced by subtotal nephrectomy (STNx) of the non-pregnant ewe, has effects on fetal renal function and glomerular haemodynamics and alters renal development in the lamb. As fetuses, STNx offspring had high sodium excretion rates, low haematocrits, low plasma renin levels and abnormal glomerular haemodynamics (Gibson et al. 2007). After birth, STNx offspring had glomerular hypertrophy and there was a positive relationship between total glomerular volume and urinary protein excretion, indicating a predisposition for renal disease later in life (Brandon et al. 2008).
In human beings, a high salt intake is associated with hypertension (Campese, 1994; Meneton et al. 2005) and an increased salt intake can exacerbate existing hypertension (Payne et al. 2004). There are strains of rats in which blood pressure is salt sensitive (Zhao et al. 2000) and in some strains of spontaneously hypertensive rats blood pressure rises further when salt intake is increased (Huang et al. 2004). Non-pregnant sheep in which renal mass has been reduced also develop hypertension when salt intake is increased (Whitworth et al. 1979). When pregnant STNx ewes were given a high salt diet, their fetuses had altered renal blood flows compared with fetal sheep carried by ewes in which renal function was intact (Boyce et al. 2008). Furthermore, fetal glomerular filtration rate and urinary protein excretion correlated with plasma renin levels in these animals (Boyce et al. 2008).
Because they had glomerular hyperfiltration as fetuses (Turner, 2008) and glomerular hypertrophy in early life (Brandon et al. 2008) we hypothesized that by 6 months of age both male and female offspring of STNx ewes would have higher arterial pressures than offspring of ewes with normal renal function. We also postulated that a high salt intake would cause a rise in arterial pressure in offspring of STNx ewes, whether or not these offspring already had higher blood pressures on a normal low salt intake. To avoid the risk of infection from long-term indwelling bladder catheters and because bladder catheters can easily be inserted and removed as required in female sheep, the effects of high salt diet were only conducted in female offspring.
Methods
Surgical preparation
Non-pregnant ewes
Surgical and experimental protocols were approved by the Animal Care and Ethics Committee of the University of New South Wales. Prior to mating, 13 maiden Border Lester × Merino cross ewes underwent surgery for subtotal nephrectomy as previously described at ∼1 years of age (Gibson et al. 2006). Ewes were anaesthetized with an intravenous (i.v.) injection of 1 g thiopentone sodium (Pentothal, Abbott Australasia Pty Ltd, Australia) and intubated, and anaesthesia was maintained by 1–3% halothane (Fluothane, AstraZeneca Ltd) in oxygen.
Using sterile techniques, the kidneys were exposed via a paravertebral incision. One kidney was removed and weighed and at least one branch of the renal artery to the other kidney was ligated to produce a colour change over 30–50% of the kidney surface. After surgery, intramuscular (i.m.) injections of procaine penicillin (Ilium Propen, 600 mg, Troy Laboratories) and oxytetracycline (Alamycin, 288 mg, Norbrook Laboratories Ltd, UK) were given to the ewes. All incisions were infiltrated with bupivicaine (Marcain 0.5%, AstraZeneca, Australia) and ewes were given 300 μg of buprenorphine i.m. (Temgesic, Reckitt Benckiser, Australia).
After surgery, sheep were housed individually in metabolic cages in the same temperature controlled room (18–22°C) as other ewes. They had free access to food (1200 g chaff and 300 g oats daily) and water. Once ewes had recovered from surgery they were returned to the farm and housed outside on pasture, where they were mated at least 2 months after surgery. Since the STNx ewes had at least 2 months to recover before mating, no sham surgery was carried out in the control animals.
Offspring
Control and STNx ewes spontaneously delivered lambs and cared for their offspring. Lambs weaned spontaneously and remained with their mothers on pasture (low salt diet) until coming into the laboratory. Male offspring were castrated via normal husbandry measures before the age of 12 weeks, and brought into the laboratory at ∼6 months of age. Female offspring were brought into the laboratory at ∼4 months for bilateral oophorectomy.
There was a similar ratio of singletons to twins in the two male cohorts (control, 5 : 4; STNx, 3 : 3), while in the females there were fewer singleton and more twin STNx offspring (control, 5 : 5; STNx, 3 : 9). Offspring were similar in age at the first experiment (males: control 196 ± 2 versus STNx 194 ± 2 days; females: control 197 ± 2 versus STNx 193 ± 2 days).
Oophorectomy
At ∼4 months of age, female lambs were anaesthetized with an i.v. injection of thiopentone sodium (20 mg kg−1) and intubated, and anaesthesia was maintained by 2% halothane in oxygen. The ovaries were removed via a midline incision following ligation of the vessels supplying them. The wound was infiltrated with 0.5% bupivicaine (Marcain 0.5%, AstraZeneca, Australia), and 150 μg of buprenorphine (Temgesic, Reckitt Benckiser, Australia) was given i.m.
Insertion of catheters
From ∼6 months of age, both male and female offspring were housed individually in metabolic cages in the same temperature controlled room (18–22°C) as other offspring. They had free access to food (1200 g chaff and 300 g oats daily; low salt diet) and their food and water intake and urine output were measured daily. Polyvinyl catheters (2.7 mm o.d., 1.5 mm i.d.) were placed in a femoral artery and both femoral veins under the same anaesthetic regime as used for oophorectomy. A Swann-Ganz catheter was inserted into the pulmonary artery via the jugular vein as previously described (Burrell et al. 1999). In male offspring only, a suprapubic bladder catheter was inserted. After surgery, injections of procaine penicillin (10 mg kg−1) and oxytetracycline (5.8 mg kg−1) were given i.m. to the offspring. All wounds were infiltrated with 0.5% bupivicaine and the animal was given 150 μg of buprenorphine i.m. For the first three days post-surgery catheters were flushed with heparinized (100 IU ml−1) 0.15 m saline, and the offspring were given the same doses of antibiotics as above. Thereafter, catheters were flushed every second day.
Experimental procedure
Renal and cardiovascular function were measured in both male and female sheep at least 4 days after catheterization while animals were on their normal, low salt diet (LSD; 1200 g chaff (sodium content about 33 mmol kg−1; Gibson & Lumbers, 1992) and 300 g oats with 6 l water). In the females, a bladder catheter was inserted via the urethra the evening before the experiment. In the males, the indwelling bladder catheter was opened at least 40 min prior to the start of the experiment. Bolus doses of 150 μmol kg−1 lithium chloride and 4.8 mg kg−1 of para-amino hippurate (PAH, Sigma-Aldrich, USA) were given i.v., followed by a continuous infusion of 10 μmol kg−1 h−1 LiCl and 810 mg h−1 PAH in 0.15 m saline delivered using a Braun Perfusor VII pump at a rate of 20 ml h−1 throughout the experiment. After a 40 min equilibration period, the experiment began, consisting of 4 × 30 min periods. Blood samples (males 10 ml; females 6 ml) collected into heparinized (20 IU ml−1) tubes were taken at the midpoint of the 2nd and 4th periods and immediately centrifuged at 1560 g for 10 min at 4°C. Plasma samples were stored at −20°C until analysis. Arterial blood pressure and heart rates were continuously measured with the animal standing throughout the experiment using pressure transducers (MLT0670 Disposable BP Transducer, ADInstruments Pty Ltd, Australia) placed beside the cage at the level of the heart. Transducers were connected to a polygraph (Model 79D, Grass Instrument Co., Quincy, MA, USA) and data were collected using an IBM compatible PC and a National Instruments interface card (Model 371). Cardiac output was measured by thermodilution once during each collection period using a cardiac output computer (model COM-1, American Edwards Laboratories) as previously described (Burrell et al. 1999).
After finishing this experiment male sheep were killed by i.v. injection of 5 g sodium pentobarbitone (Lethabarb) and a postmortem was carried out. Approximately 2 weeks later, a subset of the female offspring were placed on a high salt diet (HSD; 0.17 m NaCl in place of drinking water with continued access to 1200 g chaff and 300 g oats per day). After 5–7 days on the HSD the renal and cardiovascular studies described above were repeated, after which the animals were killed as described above and a postmortem was carried out. Salt intake was determined from the amount the lambs ate and drank over the 24 h period preceding each experiment. There were five control and six STNx offspring with a similar ratio of singletons and twins (control 1 : 4; STNx 2 : 4).
Postmortem procedures
All offspring were weighed prior to killing. Both kidneys were weighed and the left kidney was photographed on a 1 cm grid for length and width measurements. Samples were snap frozen in liquid nitrogen and stored at −80°C until analysis of renal renin content. The lungs, liver and adrenals were weighed. STNx ewes were also weighed and killed via an i.v. injection of 5 g pentobarbitone sodium approximately 4–6 months after giving birth. Their remnant kidneys were weighed and compared with the weights of the kidney removed 1–2 years earlier to determine the degree of compensatory renal hypertrophy.
Biochemical analysis
Blood gases and pH were measured at 37°C using a ABL 715 blood gas analyser (Radiometer Pacific Pty Ltd) and corrected to 39.5°C. Plasma potassium and sodium concentrations were also measured using this analyser. Plasma and urinary osmolality were determined using a Fiske One-Ten Osmometer (Fiske Associates, Massachusetts). Sodium and potassium concentrations in urine were measured in duplicate using a FLM3 flame photometer (Radiometer Pacific Pty Ltd). Glomerular filtration rate (GFR) was determined by the clearance of endogenous creatinine and the clearance of PAH was used to determine effective renal plasma flow (ERPF). Creatinine concentrations were determined in duplicate using the method of Haeckel (1980) using a microplate reader (model 680XR, Bio-Rad Laboratories Pty Ltd Australia) spectrophotometer at 510 nm. The interassay coefficient of variation (CV) for this assay was 8.2% for plasma and 9% for urine. PAH concentrations were measured in duplicate using methods already described (Bauer et al. 1968; Gibson et al. 2006); the interassay CV was 8.9% for plasma and 10.2% for urine. Protein concentrations were determined in duplicate by a Lowry protein assay (Lowry et al. 1951); interassay CV was 7.0% for plasma and 4.6% for urine. Lithium clearance was used to determine proximal sodium reabsorption as lithium is reabsorbed with sodium and water in the proximal tubule, but it is not reabsorbed distally (Lumbers et al. 1988). Lithium concentrations were measured in duplicate using a Varian-Techtron AA5 atomic absorption spectrophotometer (Melbourne, Australia).
Plasma renin levels were measured in duplicate using methods previously described (Marsh et al. 2001), as the rate of formation of angiotensin I (Ang I) in ng ml−1 h−1 when 90 μl of plasma was incubated for 2 h at pH 7.5 and 37°C with excess substrate (nephrectomised sheep plasma; NSP); the interassay CV was 30%. Kidney renin levels were measured using methods previously described (Boyce et al. 2005). Briefly, 0.5 g of renal cortex was homogenized in 4 ml of 0.03 m phosphate buffer. The supernatant diluted 1 : 400 was incubated with NSP for 2 h at pH 7.5 and 37°C and the rate of formation of Ang I in ng ml−1 h−1 measured. Ang I was measured by radioimmunoassay (Lumbers & Lee Lewes, 1979). Kidney renin levels were expressed relative to protein levels (μg Ang I h−1 (mg protein)−1). Plasma aldosterone levels were measured in duplicate by ELISA (Aldosterone EIA kit-Monoclonal, Cayman Chemical Co., MI, USA): the interassay CV was 9.5%, although all samples were measured in one assay.
Data analysis and statistics
Data from the 4 × 30 min periods were averaged to obtain a single value. All data were expressed as means ± standard error of the mean (s.e.m). Salt intake was determined as the amount of sodium consumed in food and water over the 24 h period prior to each experiment.
There were nine male sheep in the control group and six in the STNx offspring group unless stated otherwise. There were ten female sheep in the control group and twelve female STNx offspring in the low salt diet (LSD) studies, and five female control offspring and six female STNx offspring in studies on the effects of a high salt diet unless stated otherwise. Differences between the control and STNx groups during baseline and at postmortem were determined separately for each sex using Student's unpaired t test and SPSS (SPSS/PC; SPSS Inc., Chicago, IL, USA). To determine the effects of salt intake, data within each experimental group were analysed using an ANOVA for repeated measures. In the STNx group urine flow rate and free water clearance were analysed using Wilcoxon's non-parametric test for paired samples. To test for differences between groups, a two-way ANOVA for repeated measures was used with the two factors being mother (STNx or Control) and diet (LSD or HSD). Regression equations were determined by the method of least squares and the slopes were compared via the methods of Zar (1984).
Results
STNx ewes had considerable hypertrophy of their remaining kidney. At postmortem, the remnant kidney weighed twice as much as the kidney that was removed at surgery (135.1 ± 4.5 versus 65.1 ± 2.1 g, P < 0.001).
Baseline comparisons
At birth both male and female control and STNx offspring had similar body weights (male: control 4.7 ± 0.3 versus STNx 4.2 ± 0.3 kg; female: control 4.6 ± 0.4 versus STNx 3.9 ± 0.3 kg). However by ∼6 months of age, male STNx offspring weighed less than control males (34.2 ± 1.6 versus 40.4 ± 1.5 kg, P < 0.01) and there was also a tendency for the STNx female offspring to have a lighter body weight (31.6 ± 1.5 versus 36.1 ± 1.8 kg, P= 0.06).
On a normal, low salt diet, there were no differences between the control and STNx offspring in blood pressure, heart rate or cardiac output in either males or females (Table 1). Haematocrit and plasma composition (osmolality and concentrations of protein and electrolytes) were similar between groups in both males and females, as were plasma renin and aldosterone levels (Table 2). Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were not different between groups in either the male or female cohort, nor were any other renal parameters (Table 3) including the tubular handling of sodium (data not shown).
Table 1.
Blood pressure, heart rate and cardiac output in 6-month-old offspring of control or subtotally nephrectomised (STNx) ewes on a low salt diet
| Males | Females | |||
|---|---|---|---|---|
| Control | STNx | Control | STNx | |
| Systolic pressure (mmHg) | 117 ± 4 | 116 ± 3 | 107 ± 3 | 102 ± 1 |
| Diastolic pressure (mmHg) | 79 ± 3 | 76 ± 2 | 72 ± 3 | 66 ± 1 |
| Mean pressure (mmHg) | 93 ± 3 | 92 ± 3 | 87 ± 3 | 82 ± 1 |
| Heart rate (beats min−1) | 116 ± 6 | 115 ± 10 | 122 ± 5 | 131 ± 5 |
| Cardiac output (l min−1) | 7.2 ± 0.5 (6) | 6.6 ± 1.0 (5) | 6.6 ± 0.6 (6) | 5.7 ± 0.2 |
Values are means ±s.e.m. Males: control n= 9, STNx n= 6; females: control n= 10; STNx n= 12, except where shown in parentheses.
Table 2.
Plasma composition in 6-month-old-offspring of control or subtotally nephrectomised (STNx) ewes on a low salt diet
| Males | Females | |||
|---|---|---|---|---|
| Control | STNx | Control | STNx | |
| Haematocrit (%) | 29.5 ± 0.8 | 30.0 ± 1.5 | 29.4 ± 0.8 | 27.6 ± 0.6 |
| Protein (mg ml−1) | 82.7 ± 2.9 | 80.0 ± 2.5 | 90.5 ± 2.1 | 84.6 ± 2.5 |
| Osmolality (mosmol (kg H2O)−1) | 304 ± 4 | 297 ± 5 | 302 ± 2 | 303 ± 1 |
| Na+ (mmol l−1) | 145 ± 0 | 144 ± 1 | 145 ± 0 | 145 ± 0 |
| K+ (mmol l−1) | 4.3 ± 0.2 | 4.1 ± 0.2 | 3.8 ± 0.1 | 4.0 ± 0.1 |
| Cl− (mmol l−1) | 109 ± 1 | 108 ± 2 | 110 ± 1 | 111 ± 1 |
| Renin (ng ml−1 h−1) | 0.13 ± 0.09 | 0.35 ± 0.17 | 0.38 ± 0.19 | 0.44 ± 0.32 |
| Aldosterone (pg ml−1) | 907 ± 294 | 1091 ± 352 | 407 ± 42 | 491 ± 50 |
Values are mean ±s.e.m. Males: control n= 9, STNx n= 6; females: control n= 10; STNx n= 12.
Table 3.
Renal function in 6-month-old-offspring of control or subtotally nephrectomised (STNx) ewes on a low salt diet
| Males | Females | |||
|---|---|---|---|---|
| Control | STNx | Control | STNx | |
| GFR (ml min−1 kg−1) | 4.4 ± 0.7 | 4.4 ± 0.7 | 5.6 ± 0.3 | 5.1 ± 0.4 |
| ERPF (ml min−1 kg−1) | 13.7 ± 1.9 | 18.8 ± 1.9 | 22.4 ± 1.4 | 21.7 ± 1.2 |
| Urine flow rate (ml min−1 kg−1) | 0.09 ± 0.02 | 0.09 ± 0.04 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| Urinary osmolality (mosmol (kg H2O)−1) | 912 ± 191 | 873 ± 213 | 1479 ± 116 | 1282 ± 82 |
| ENa (μmol min−1 kg−1) | 7.3 ± 1.8 | 4.7 ± 2.3 | 3.4 ± 0.7 | 2.4 ± 0.7 |
| EK (μmol min−1 kg−1) | 11.0 ± 1.1 | 10.1 ± 2.5 | 10.6 ± 1.0 | 11.0 ± 1.3 |
| EProtein (μg min−1 kg−1) | 415 ± 37 | 490 ± 90 | 466 ± 49 | 522 ± 56 |
| Na/K ratio | 0.67 ± 0.18 | 0.36 ± 0.16 | 0.35 ± 0.08 | 0.18 ± 0.03 |
Values are means ±s.e.m. GFR, glomerular filtration rate; ERPF, effective renal plasma flow; Ex, excretion of x. Males: control n= 9, STNx n= 6; females: control n= 10; STNx n= 12.
Response to salt load
Five control and six STNx female offspring were studied on both low salt diet (LSD) and high salt diet (HSD). On a HSD, salt intake increased to the same extent in both groups (control from 30 ± 6 to 857 ± 154 mmol day−1, P < 0.005; STNx from 28 ± 3 to 880 ± 151 mmol day−1, P < 0.005). The increase in salt intake relative to body weight was also similar between groups (Control 0.86 ± 0.17 to 22.26 ± 4.64 mmol salt day−1 (kg BW)−1, P < 0.05); STNx 0.87 ± 0.11 to 24.02 ± 3.76 mmol salt day−1 (kg BW)−1, P < 0.05).
Cardiovascular parameters
Although systolic, diastolic and mean arterial pressures appeared to increase slightly on the HSD, this was not significant in either group (Table 4). Heart rate did not change in either group. Cardiac output in the STNx offspring increased from 5.6 ± 0.4 l min−1 on the LSD to 6.3 ± 0.4 (P < 0.05) on HSD. In the control group there were insufficient measurements for comparison (Control LSD, n= 2, 5.1 and 5.0 l min−1, HSD, n= 1, 5.1 l min−1).
Table 4.
Blood pressure, heart rate and plasma composition of 6-month-old-female offspring of control (C) or subtotally nephrectomised (S) ewes studied under conditions of low (LSD) and high salt diet (HSD)
| Control | STNx | |||
|---|---|---|---|---|
| LSD | HSD | LSD | HSD | |
| Systolic pressure (mmHg) | 108 ± 4 | 113 ± 2 | 102 ± 2 | 108 ± 3 |
| Diastolic pressure (mmHg) | 72 ± 5 | 78 ± 2 | 66 ± 1 | 72 ± 2 |
| Mean pressure (mmHg) | 88 ± 5 | 93 ± 2 | 82 ± 1 | 88 ± 3 |
| Heart rate (beats min−1) | 119 ± 9 | 113 ± 2 | 126 ± 8 | 123 ± 6 |
| Protein (mg ml−1) | 91.7 ± 2.0 | 89.0 ± 5.0 | 86.8 ± 4.1 | 86.2 ± 4.8 |
| Osmolality (mosmol (kg H2O)−1) | 301 ± 2 | 305 ± 4 | 301 ± 4 | 303 ± 3 |
| Cl− (mmol l−1) | 110 ± 0.6 | 113 ± 2 | 111 ± 1 | 114 ± 1 |
| Na/K ratio | 38.5 ± 1.5 | 32.9 ± 1.7* | 36.3 ± 0.8 | 37.6 ± 1.5 |
Values are means ±s.e.m.
Different from LSD, P < 0.05. Control, n= 5; STNx, n= 6.
Haematocrit and plasma composition
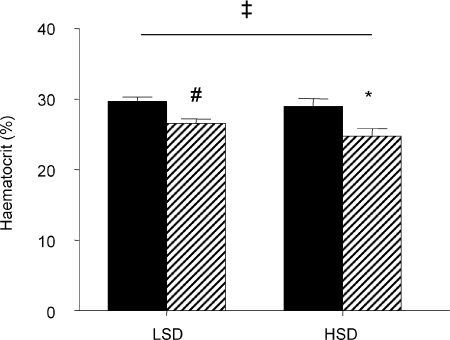
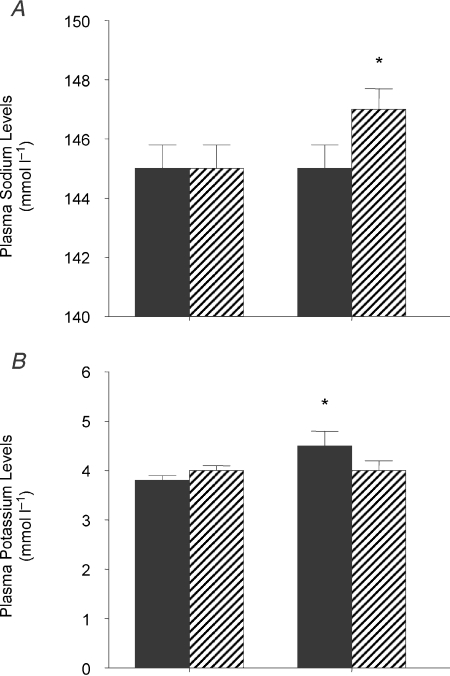
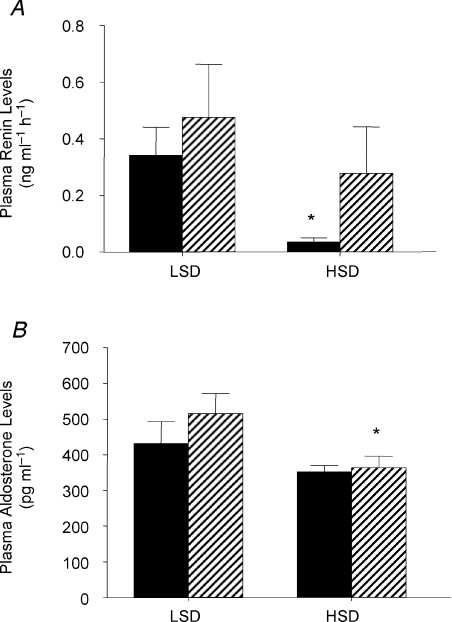
On a LSD haematocrit was lower in the STNx group than the control group (STNx 26.5 ± 0.7; control 29.7 ± 0.6%, P < 0.01). On the HSD, haematocrit decreased in STNx offspring only, so that there was a significant interaction between mother (control or STNx) and salt intake (Fig. 1). Plasma sodium levels increased only in STNx offspring (Fig. 2A), while there was an increase in plasma potassium (Fig. 2B) and consequent decrease in the Na/K ratio only in the control group (Table 4). There were no other changes in plasma electrolytes. Plasma renin levels fell in the control animals (P < 0.05) but remained unchanged in STNx offspring (Fig. 3A). By contrast, plasma aldosterone levels were unchanged in control offspring on a HSD but decreased in STNx group (P < 0.05; Fig. 3B).
Figure 1.
Haematocrit in 6-month-old female offspring of control (filled) or subtotally nephrectomised ewes (STNx, hatched) studied under conditions of low (LSD) and high salt (HSD) diets #Difference between groups on LSD, P < 0.05; *different from LSD, P < 0.05; ‡interaction between mother (control or STNx) and salt intake, P < 0.05. Values are means ±s.e.m.
Figure 2.
Plasma sodium levels (A) and plasma potassium levels (B) in 6-month-old female offspring of control (filled, n= 5) or subtotally nephrectomised ewes (STNx, hatched, n= 6) studied under conditions of low (LSD) and high salt (HSD) diets *Different from LSD, P < 0.05. Values are means ±s.e.m. Control, n= 5; STNx, n= 6.
Figure 3.
Plasma renin levels (A) and plasma aldosterone levels (B) in 6-month-old female offspring of control (filled, n= 5) or subtotally nephrectomised ewes (STNx, hatched, n= 6) studied under conditions of low (LSD) and high salt (HSD) diets *Different from LSD, P < 0.05. Values are means ±s.e.m. Control, n= 5; STNx, n= 6.
Renal parameters
There were no changes in glomerular filtration rate (GFR) or effective renal plasma flow (ERPF) on the HSD (Table 5). Urine flow rate increased in both groups (Control 0.03 ± 0.00 to 0.11 ± 0.03 ml min−1 kg−1, P < 0.05; STNx 0.03 ± 0.00 to 0.10 ± 0.03 ml min−1 kg−1, P < 0.05). On LSD, urinary osmolality was lower in STNx offspring (1125 ± 105 mosmol (kg H2O)−1) than controls (1440 ± 83, P < 0.05). Urinary osmolaltiy fell in controls on HSD (986 ± 163 mosmol (kg H2O)−1, P < 0.05) but did not change in STNx offspring (1066 ± 87). Free water clearance became more negative in both groups (Control −0.12 ± 0.01 to −0.18 ± 0.02 ml min−1 kg−1P < 0.05; STNx −0.13 ± 0.06 to −0.21 ± 0.04 ml min−1 kg−1, P < 0.05). While renal sodium excretion increased in both groups of animals on the HSD, the excretion rates of potassium did not change (Table 5).
Table 5.
Renal function in 6-month-old female offspring of control (C) or subtotally nephrectomised (S) ewes studied under conditions of low (LSD) and high salt diet (HSD)
| Control | STNx | |||
|---|---|---|---|---|
| LSD | HSD | LSD | HSD | |
| GFR (ml min−1 kg−1) | 5.1 ± 0.4 | 5.1 ± 0.5 | 5.2 ± 0.6 | 5.4 ± 0.5 |
| ERPF (ml min−1 kg−1) | 23.3 ± 2.5 | 22.4 ± 4.5 | 22.8 ± 1.7 | 22.9 ± 2.2 |
| ENa (μmol min−1 kg−1) | 3.7 ± 0.7 | 29.9 ± 7.1* | 2.3 ± 0.9 | 28.3 ± 8.8* |
| EK (μmol min−1 kg−1) | 10.2 ± 1.3 | 8.8 ± 1.1 | 10.2 ± 2.0 | 8.5 ± 1.5 |
| Na/K Ratio | 0.36 ± 0.08 | 3.35 ± 0.99* | 0.20 ± 0.04 | 3.26 ± 1.02* |
| FRNa (%) | 99.5 ± 0.1 | 95.9 ± 1.0* | 99.7 ± 0.1 | 96.4 ± 0.8* |
| FRNaP (%) | 84.5 ± 1.6 | 82.5 ± 2.2 | 87.8 ± 2.2 | 83.0 ± 1.7* |
| DDNa (mmol min−1 kg−1) | 0.12 ± 0.02 | 0.13 ± 0.2 | 0.09 ± 0.02 | 0.14 ± 0.03* |
| FRNaD (%) | 15.0 ± 1.5 | 13.4 ± 1.2 | 11.9 ± 2.1 | 13.4 ± 1.1 |
| DRNaDD (%) | 96.5 ± 0.7 | 77.9 ± 3.4* | 97.3 ± 0.8 | 79.7 ± 2.6* |
Values are means ±s.e.m. GFR, glomerular filtration rate; ERPF, effective renal plasma flow; Ex, excretion of x; FRNa fractional reabsorption of Na; FRNaP and FRNaD, fractional reabsorption of Na by the proximal and distal tubules; DDNa distal delivery of Na; DRNaDD, distal reabsorption of Na as a percentage of distal delivery.
Different from LSD, P < 0.05. Control, n= 5; STNx, n= 6.
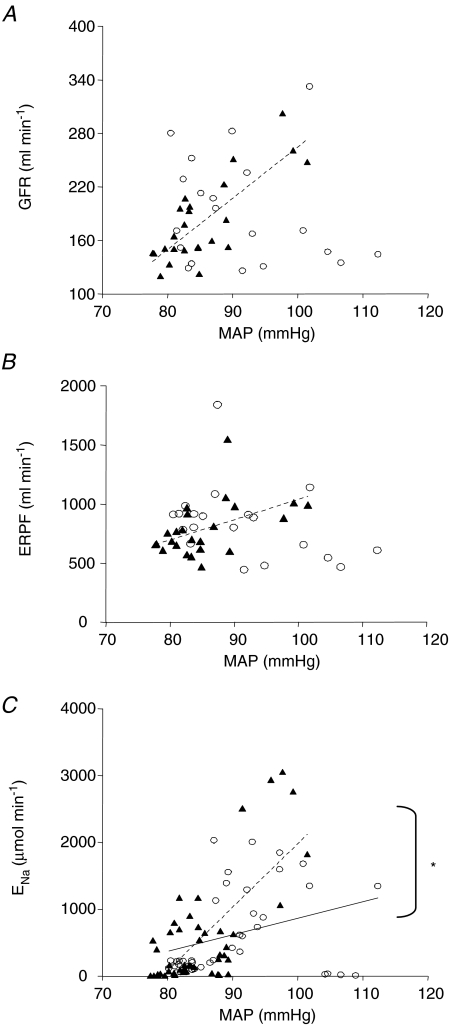
Figure 4A shows that there was a positive relationship between GFR (ml min−1) and MAP (mmHg) in the STNx animals (GFR = 5.7 × MAP−310; r2= 0.619, P < 0.01, n= 24 data points) which was not present in the controls (r2= 0.033, n= 20 data points). A similar positive relationship was found between ERPF (ml min−1) and MAP (mmHg) that was present only in the STNx offspring (ERPF = 17 × MAP − 678, r2= 0.235, P < 0.05, n= 24 data points; Fig. 4B). There was also a positive relationship between sodium excretion (ENa, μmol min−1) and MAP (mmHg) in the STNx animals, described by the equation ENa= 95.0 × MAP − 7524 (r2= 0.498, P < 0.001, n= 48 data points; Fig. 4C). This was stronger and steeper (P < 0.05) than the very weak relationship found in the control group (ENa= 24.8 × MAP − 1615; r2= 0.111, P < 0.05, n= 40 data points; Fig. 4C).
Figure 4.
The relationship between glomerular filtration rate (GFR) (A), effective renal plasma flow (B) and the excretion of sodium (ENa) and mean arterial pressure (MAP) (C) in 6-month-old female offspring born to either control (○) or STNx ewes (▴) on both a low and high salt diet *Difference between control and STNx, P < 0.05. A and B, control n= 20 data points and STNx n= 24 data points (2 points per animal per diet). C, control n= 40 data points and STNx n= 48 data points (4 points per animal per diet).
Table 5 also shows the tubular handling of sodium. Both groups of offspring had a decreased fractional reabsorption of sodium on the HSD (FRNa, P < 0.05). The fractional reabsorption of sodium by the proximal tubule (FRNaP) decreased in the STNx offspring and as a consequence more sodium was delivered to the distal tubule (DDNa) in this group (P < 0.05). The distal reabsorption of sodium as a percentage of distal delivery (DRNaDD) decreased in both groups (Table 5).
Organ weights and kidney renin levels
In males, absolute heart weight and right ventricular free wall weight were lower in the STNx offspring, although they were similar to controls when expressed per kg body weight (total heart weight: control 5.1 ± 0.1 vs. STNx 5.2 ± 0.1 g kg−1; right ventricular weight: control 1.0 ± 0.0 vs. STNx 0.9 ± 0.0 g kg−1). Weights of the kidneys, lungs, liver and adrenals and kidney dimensions were similar between the groups. In females, all organ weights and kidney dimensions were similar between groups. Renal renin levels were not different between groups in male (control 0.75 ± 0.20 vs. STNx 0.56 ± 0.13 μg Ang I h−1 mg protein−1) or female offspring (control 0.52 ± 0.11 vs. STNx 0.55 ± 0.26 μg Ang I h−1 (mg protein)−1).
Discussion
The major findings of this study are that at 6 months of age, and under baseline conditions on a low salt intake, male and female offspring of STNx ewes were not hypertensive and their renal function was similar to that of control sheep. The arterial pressures of female offspring of STNx ewes did not increase on a HSD, but their cardiac outputs were greater than those measured on a LSD. In contrast to control animals, haematocrits fell in STNx offspring on a HSD. This, coupled with the increase in plasma sodium levels in these offspring on a HSD, indicates that they were retaining salt and water whilst on HSD. Since their plasma renin levels were not suppressed on high salt, we further suggest that this salt and water retention was due to the inability of STNx offspring to respond appropriately to a high salt intake by reducing renin secretion, although there was a decrease in plasma aldosterone levels. Also, in female offspring of STNx ewes, we found that GFR and effective renal plasma flow (ERPF) were directly related to MAP suggesting that these ewes’ ability to autoregulate ERPF and GFR was impaired.
Despite high plasma sodium concentrations and probable volume expansion, STNx offspring were not able to suppress plasma renin levels on a HSD. Normally, as was the case in control offspring, a high salt intake causes marked suppression of renin release from the kidney (Carillo et al. 2007). The inability of STNx offspring to suppress renin release on a HSD probably accounts for the rise in plasma sodium levels and their lower haematocrits The failure of STNx offspring to reduce renin secretion when dietary salt intake increased is remarkable given that there was a strong signal at the macula densa for suppression of renin release. On the HSD, STNx offspring had a marked reduction in the fraction of filtered sodium reabsorbed by the proximal tubule and a consequent increase in the amount of sodium delivered to the distal nephron. This increase in distal sodium delivery, which did not occur in control offspring, should have led to macula densa mediated suppression of renin release.
Unexpectedly, plasma aldosterone levels did not decrease significantly after 5–7 days on a HSD in control animals (Fig. 3B). However, the aldosterone response to a high salt intake in each group must be viewed in light of the major regulators of aldosterone secretion, namely plasma potassium and Ang II concentrations, with plasma sodium levels exerting a weaker influence (Williams, 2005). Plasma potassium levels were increased in control offspring on high salt (Fig. 2B), and this would directly oppose the effects of lowered levels of circulating renin in these animals. Interestingly in STNx offspring there was a fall in plasma aldosterone levels which was independent of any changes in the renin–angiotensin system as plasma renin levels did not change. However unlike control animals, plasma potassium did not increase in STNx offspring (Fig. 2B) and it is possible that this unmasked a weaker, but still well described, direct inhibitory effect of a high salt intake. Thus not only do STNx offspring have altered regulation of the renin–angiotensin system (RAS) in response to salt loading and to haemorrhage (O’Connell, 2007), but the balance between key factors that control aldosterone secretion when salt intake varies is also different.
Despite our earlier observations that at 1–2 weeks of age STNx offspring had higher rates of protein excretion that correlate with glomerular volume (Brandon et al. 2008), there was no evidence of impaired renal function in STNx offspring at 6 months of age. However female STNx offspring were unable to autoregulate either ERPF or GFR effectively when salt intake was varied. This is in sharp contrast to the control animals in which there were no relationships between arterial pressure and GFR or ERPF. Given this finding, plus the inappropriately high levels of renin in the presence of a high salt intake, we postulate that their in utero exposure to a transplacental fluid load (Gibson et al. 2007) has programmed macula densa function.
Glomerular filtration is a major determinant of renal sodium excretion because of the high concentration of this cation in the extracellular fluid and the high rate of filtration of plasma in the adult mammal. The ability to autoregulate GFR is of prime importance in maintenance of the volume and composition of the extracellular fluid. The much steeper relationship between sodium excretion and MAP seen in STNx offspring (Fig. 4C) means that these animals are programmed so that they are susceptible to abnormal fluctuations in extracellular volume as arterial pressure fluctuates. We suggest that this puts these animals at risk of hypovolaemia as well as hypervolaemia (as demonstrated in the present study).
In some human beings and strains of rat, a high salt intake causes a rise in blood pressure (Campese, 1994; Meneton et al. 2005). Also, an increase in salt intake can exacerbate existing hypertension (Payne et al. 2004). Surprisingly the expanded extracellular volume did not translate into an increase in the arterial pressures of STNx offspring on a HSD (Table 4) even though their cardiac outputs were increased. These animals also did not have higher arterial pressures than control animals when they were on a low salt diet. Other models of fetal programming, including those in sheep, have shown that hypertension can present in young adult life. Maternal infusion of dexamethasone in early gestation caused hypertension in 4-month-old sheep (Dodic et al. 1998) whereas the fetuses and neonates were normotensive (Moritz et al. 2002; Roghair et al. 2005). In another study, maternal undernutrition resulted in offspring that were hypertensive at 9 months of age (Gilbert et al. 2005). Although STNx offspring did not have a reduced nephron complement (Brandon et al. 2008), which is a feature of other models of fetal programming associated with hypertension in the adult offspring (Wintour et al. 2003; Gilbert et al. 2005), they were hyperfiltering as fetuses in late gestation (Turner, 2008) and had glomerular hypertrophy by 1–2 weeks of age (Brandon et al. 2008). Therefore, we had hypothesized that over time this structural abnormality would result in the development of hypertension (Brenner et al. 1996).
By 6 months of age both male and female STNx offspring were lighter than control offspring, even though at birth they were not growth retarded. In females this may have been related to the fact that there were more twins in the STNx group than in the control group. However, body weights were also less in males, where twinning rates were similar between groups. Thus, while intrauterine growth does not appear to be affected in this model of maternal renal dysfunction, extrauterine growth is slowed. Most organs were appropriately grown for the body weight, so the reduction in growth was symmetrical, as was the case in another study carried out 10 days postpartum (Brandon et al. 2008).
In summary, male and female offspring of mothers with renal dysfunction have normal blood pressure and renal function under baseline conditions. Also, when female offspring of STNx mothers were placed on a HSD, they did not become hypertensive. However, their fluid and electrolyte homeostasis was altered, as their plasma sodium levels increased and their haematocrits fell on a HSD, indicating that they retained salt and water under these conditions. They were also unable to autoregulate GFR and ERPF in response to fluctuations in arterial pressure. Thus the offspring of ewes with maternal renal dysfunction cannot maintain fluid and electrolyte balance in the face of a high salt intake because they cannot regulate their plasma renin levels appropriately and because autoregulation of GFR, a key factor in control of salt balance, is also impaired. The difference in the aldosterone response of control and STNx animals to HSD is due to their differences in plasma sodium and potassium levels on a HSD. These findings complement our already established observations of glomerular dysfunction and glomerular hypertrophy in offspring of ewes with chronic renal dysfunction.
Acknowledgments
This work was supported by a grant to Dr K. Gibson and Prof E. Lumbers from Kidney Health Australia (PI040506). We would like to thank Ms Vasumathy Kumarasamy and Ms Carrie Chan for their technical assistance.
References
- Barker DJP. Mothers, Babies, and Disease in Later Life. London: BMJ. Medical Publishing Group; 1994. [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JD, Ackerman PG, Toro G. Bray's Clinical Laboratory Methods. St Louis: C. V. Mosby Co.; 1968. [Google Scholar]
- Boyce AC, Gibson KJ, Thomson CL, Lumbers ER. Interactions between maternal subtotal nephrectomy and salt: effects on renal function and the composition of plasma in the late gestation sheep fetus. Exp Physiol. 2008;93:262–270. doi: 10.1113/expphysiol.2007.039149. [DOI] [PubMed] [Google Scholar]
- Boyce AC, Gibson KJ, Wintour EM, Koukoulas I, Lumbers ER. Effects of 7-day amino acid infusion on renal growth, function, and renin-angiotensin system in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1099–R1106. doi: 10.1152/ajpregu.00055.2005. [DOI] [PubMed] [Google Scholar]
- Brandon AE, Boyce AC, Lumbers ER, Zimanyi MA, Bertram JF, Gibson KJ. Glomerular hypertrophy in offspring of subtotally nephrectomised ewes. Anat Rec (Hoboken) 2008;291:318–324. doi: 10.1002/ar.20651. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Lumbers ER, Bernasconi C. Effects of ACTH-induced hypertension in the pregnant ewe. J Cardiovasc Pharmacol. 1999;34:818–823. doi: 10.1097/00005344-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- Carillo BA, Beutel A, Mirandola DA, Vidonho AF, Jr, Furukawa LN, Casarini D, Campos RR, Dolnikoff MS, Heimann JC, Bergamaschi CT. Differential sympathetic and angiotensinergic responses in rats submitted to low- or high-salt diet. Regul Pept. 2007;140:5–11. doi: 10.1016/j.regpep.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Gibson KJ, Boyce AC, Karime BM, Lumbers ER. Maternal renal insufficiency alters plasma composition and renal function in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1204–R1211. doi: 10.1152/ajpregu.00188.2006. [DOI] [PubMed] [Google Scholar]
- Gibson KJ, Lumbers ER. Mechanisms by which the pregnant ewe can sustain increased salt and water supply to the fetus. J Physiol. 1992;445:569–579. doi: 10.1113/jphysiol.1992.sp018940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KJ, Thomson CL, Boyce AC, Karime BM, Lumbers ER. Effects of a reduction in maternal renal mass on pregnancy and cardiovascular and renal function of the pregnant ewe. Am J Physiol Renal Physiol. 2006;290:F1153–F1162. doi: 10.1152/ajprenal.00241.2005. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel R. Simplified determinations of the ‘true’ creatinine concentration in serum and urine. J Clin Chem Clin Biochem. 1980;18:385–394. doi: 10.1515/cclm.1980.18.7.385. [DOI] [PubMed] [Google Scholar]
- Huang BS, Van Vliet BN, Leenen FHH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr LA, Randall RJ. Protein Measurement with the Folin reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lumbers ER, Hill KJ, Bennett VJ. Proximal and distal tubular activity in chronically catheterized fetal sheep compared with the adult. Can J Physiol Pharmacol. 1988;66:697–702. doi: 10.1139/y88-111. [DOI] [PubMed] [Google Scholar]
- Lumbers E, Lewes JL. The actions of vasoactive drugs on fetal and maternal plasma renin activity. Biol Neonate. 1979;35:23–32. doi: 10.1159/000241150. [DOI] [PubMed] [Google Scholar]
- Marsh AC, Gibson KJ, Wu J, Owens PC, Owens JA, Lumbers ER. Insulin-like growth factor I alters renal function and stimulates renin secretion in late gestation fetal sheep. J Physiol. 2001;530:253–262. doi: 10.1111/j.1469-7793.2001.0253l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- O’Connell AE. Sydney: University of New South Wales; 2007. Consequences of an altered intrauterine environment on the offspring's renal, cardiovascular and renin angiotensin systems. PhD Thesis. [Google Scholar]
- Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension. 2004;43:420–427. doi: 10.1161/01.HYP.0000111832.47667.13. [DOI] [PubMed] [Google Scholar]
- Roghair RD, Segar JL, Sharma RV, Zimmerman MC, Jagadeesha DK, Segar EM, Scholz TD, Lamb FS. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1169–R1176. doi: 10.1152/ajpregu.00369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ. Sydney: University of New South Wales; 2008. Control of renal hemodynamics in the developing kidney: Implications for fetal programming. PhD Thesis. [Google Scholar]
- Whitworth JA, Coghlan JP, Denton DA, Hardy KJ, Scoggins BA. Effect of sodium loading and ACTH on blood pressure of sheep with reduced renal mass. Cardiovasc Res. 1979;13:9–15. doi: 10.1093/cvr/13.1.9. [DOI] [PubMed] [Google Scholar]
- Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev. 2005;10:7–13. doi: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. Englewood Cliffs, NJ, USA: Prentice Hall International; 1984. [Google Scholar]
- Zhao X, White R, Van Huysse J, Leenen FH. Cardiac hypertrophy and cardiac renin-angiotensin system in Dahl rats on high salt intake. J Hypertens. 2000;18:1319–1326. doi: 10.1097/00004872-200018090-00018. [DOI] [PubMed] [Google Scholar]