Abstract
We investigated the role of somatosensory feedback from locomotor muscles on central motor drive (CMD) and the development of peripheral fatigue during high-intensity endurance exercise. In a double-blind, placebo-controlled design, eight cyclists randomly performed three 5 km time trials: control, interspinous ligament injection of saline (5KPlac, L3–L4) or intrathecal fentanyl (5KFent, L3–L4) to impair cortical projection of opioid-mediated muscle afferents. Peripheral quadriceps fatigue was assessed via changes in force output pre- versus postexercise in response to supramaximal magnetic femoral nerve stimulation (ΔQtw). The CMD during the time trials was estimated via quadriceps electromyogram (iEMG). Fentanyl had no effect on quadriceps strength. Impairment of neural feedback from the locomotor muscles increased iEMG during the first 2.5 km of 5KFentversus 5KPlac by 12 ± 3% (P < 0.05); during the second 2.5 km, iEMG was similar between trials. Power output was also 6 ± 2% higher during the first and 11 ± 2% lower during the second 2.5 km of 5KFentversus 5KPlac (both P < 0.05). Capillary blood lactate was higher (16.3 ± 0.5 versus 12.6 ± 1.0%) and arterial haemoglobin O2 saturation was lower (89 ± 1 versus 94 ± 1%) during 5KFentversus 5KPlac. Exercise-induced ΔQtw was greater following 5KFentversus 5KPlac (−46 ± 2 versus−33 ± 2%, P < 0.001). Our results emphasize the critical role of somatosensory feedback from working muscles on the centrally mediated determination of CMD. Attenuated afferent feedback from exercising locomotor muscles results in an overshoot in CMD and power output normally chosen by the athlete, thereby causing a greater rate of accumulation of muscle metabolites and excessive development of peripheral muscle fatigue.
Peripheral locomotor muscle fatigue develops only up to a threshold unique for each individual, and exercise is either voluntarily terminated once peripheral fatigue has reached this critical threshold (open-loop design) or the exercise intensity is drastically reduced once a critical rate of fatigue development is reached (closed-loop design; Hogan & Welch, 1984; Hogan et al. 1999; Duhamel et al. 2004; Sandiford et al. 2005; Amann et al. 2006a, 2007a,b; Katayama et al. 2007; Romer et al. 2007; Amann & Dempsey, 2008). We interpreted these observations to mean that the rate of development of peripheral fatigue is associated with increasing sensory feedback from locomotor muscles to the central nervous system (CNS). In turn, this feedback regulates central motor drive in order to limit the level of peripheral fatigue development and thereby avoid intolerable levels of effort/‘pain’ perception and/or excessive muscle dysfunction.
To evaluate the potential inhibitory influences of ascending sensory pathways on the determination of central motor drive, we recently blocked somatosensory feedback during cycling time trial exercise using lumbar epidural anaesthesia (0.5% lidocaine). We observed a substantially higher central motor drive with epidural anaesthesia, which confirmed our postulate. However, the local anaesthetic also affected peripheral motor nerves, leading to significant losses in locomotor muscle strength, which prevented the increased central neural drive to be reflected in an improved power output. Thus, time trial power output was significantly lower during the epidural versus control time trial (Amann et al. 2008b). These confounding effects of epidural lidocaine did not allow us to perform adequate testing of the role of afferent feedback effects, per se, on exercise performance and the development of peripheral muscle fatigue.
To circumvent the lidocaine-induced forfeit of locomotor muscle force-generating capacity and to make an adequate determination of the effect of neural feedback from exercising muscle on power output and the development of peripheral fatigue during high-intensity whole body endurance exercise, we now use fentanyl, an opioid analgesic, to selectively block the activity in ascending sensory pathways without affecting motor nerve activity or maximal force output (Grant et al. 1996; Standl et al. 2001). Lumbar intrathecal fentanyl increases the subject's tolerance for pain in the lower extremities by binding to spinal opiate receptors and attenuating the ascending activity of nociceptive and metaboreceptive Aδ (group III) and C fibres (group IV; Kalliomaki et al. 1998), which are thought to project to various brain regions, including the primary somatosensory cortex (Mark & Steiner, 1958; Handwerker & Zimmermann, 1972; Schouenborg et al. 1986; Kalliomaki et al. 1993; Almeida et al. 2004). The cortical projection of these nociceptors/metaboreceptors increases in proportion to noxious stimuli and metabolic byproducts of fatiguing muscular contractions (Mense, 1977; Kniffki et al. 1978; Rotto & Kaufman, 1988; Adreani et al. 1997).
Based on these considerations, we hypothesized that lumbar intrathecal fentanyl would attenuate the normally occurring inhibitory feedback influence from the working muscles on the process of determining the magnitude of central motor drive, as ‘chosen’ by the subject during time trial exercise (closed-loop design). We further expected the higher central motor drive to be reflected in a higher power output during the race and consequently to cause the locomotor muscles to fatigue beyond an individual's critical threshold of fatigue; a level of peripheral fatigue that is never exceeded under intact sensory feedback conditions (Hogan & Welch, 1984; Hogan et al. 1999; Gandevia, 2001; Duhamel et al. 2004; Sandiford et al. 2005; Amann et al. 2006a, 2007a,b; Katayama et al. 2007; Romer et al. 2007; Amann & Dempsey, 2008).
Methods
Subjects
Eight competitive male cyclists volunteered to participate in the study [age, 25.0 ± 2.4 years; body mass, 75.3 ± 2.1 kg; stature, 1.77 ± 0.11 m; maximal O2 consumption 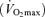 , 63.9 ± 3.4 ml kg−1 min−1]. Written informed consent was obtained from each participant. All procedures were approved by the University of Wisconsin's Health Science Institutional Review Board. The experimental protocols conformed to the Declaration of Helsinki.
, 63.9 ± 3.4 ml kg−1 min−1]. Written informed consent was obtained from each participant. All procedures were approved by the University of Wisconsin's Health Science Institutional Review Board. The experimental protocols conformed to the Declaration of Helsinki.
Protocol
At preliminary visits to the laboratory, subjects were thoroughly familiarized with the procedures used to assess neuromuscular functions. All participants performed a practice 5 km cycling time trial and a maximal incremental exercise test (20 W + 25 W min−1; Amann et al. 2004) on a computer-controlled electromagnetically braked cycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA, USA) for the determination of peak power output (Wpeak) and 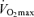 . On separate days, double blind, placebo controlled and in random order, all participants performed three time trials (Amann et al. 2008a): a control time trial (5KCtrl), a placebo time trial with a sham interspinous ligament injection of sterile normal saline at vertebral interspace L3–L4 (5KPlac) and an experimental time trial with intrathecal fentanyl applied at vertebral interspace L3–L4 (5KFent).
. On separate days, double blind, placebo controlled and in random order, all participants performed three time trials (Amann et al. 2008a): a control time trial (5KCtrl), a placebo time trial with a sham interspinous ligament injection of sterile normal saline at vertebral interspace L3–L4 (5KPlac) and an experimental time trial with intrathecal fentanyl applied at vertebral interspace L3–L4 (5KFent).
All time trials were preceded by a 10 min warm-up at 1.5 W (kg body mass)−1. The subjects remained seated throughout exercise. During various time trials, an antecubital intravenous 20-gauge cannula was inserted, and a bolus injection of ∼500 ml of normal saline was administered prior to the start of the race. The cannula was removed upon completion of the experiment. To avoid initial peak force outputs, subjects were instructed to pick up their pace slowly, and the recording period started after the starting power output and pedal cadence, adopted from the practice time trial, was reached (within 10–15 s). Neuromuscular functions were assessed before and 3 min after exercise. To test for the effects of the opioid analgesic on resting quadriceps strength, resting neuromuscular functions were assessed before and 5 min after the fentanyl administration (prior to any exercise). The subjects were naive to the purpose of the study and the expected outcomes. Each exercise session was separated by at least 72 h and was completed at the same time of day. Subjects were instructed to refrain from caffeine for 12 h and stressful exercise for 48 h before each exercise trial. Ambient temperature and relative humidity were not different between conditions.
Exercise responses
Pulmonary ventilation 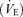 and pulmonary gas exchange were measured breath-by-breath at rest and throughout exercise using an open-circuit system including two pneumotachographs (model 3800, Hans Rudolph, Shawnee, KS, USA; for inspiration and expiration) and two Perkin-Elmer mass spectrometers (model 1100, Waltham, MA, USA) for the analysis of mixed expired and end-tidal gases (Harms et al. 1998). Arterial O2 saturation measured by pulse oxymetery
and pulmonary gas exchange were measured breath-by-breath at rest and throughout exercise using an open-circuit system including two pneumotachographs (model 3800, Hans Rudolph, Shawnee, KS, USA; for inspiration and expiration) and two Perkin-Elmer mass spectrometers (model 1100, Waltham, MA, USA) for the analysis of mixed expired and end-tidal gases (Harms et al. 1998). Arterial O2 saturation measured by pulse oxymetery  was estimated using a pulse oximeter (Nellcor N-595, Pleasanton, CA, USA) with adhesive forehead sensors. Heart rate (HR) was measured from the R–R interval of an electrocardiogram using a three lead arrangement. Ratings of perceived exertion (RPE) were obtained at rest and upon completion of various trials using Borg's modified CR10 scale (Borg, 1998). Arterialized (Finalgon, Boehringer Ingelheim, Germany) capillary blood samples were collected from an earlobe at rest and every kilometre during the time trials for determination of total whole blood lactate concentration ([La−]B) using an electrochemical analyser (YSI 1500 Sport, YSI Life Sciences, Yellow Springs, OH, USA).
was estimated using a pulse oximeter (Nellcor N-595, Pleasanton, CA, USA) with adhesive forehead sensors. Heart rate (HR) was measured from the R–R interval of an electrocardiogram using a three lead arrangement. Ratings of perceived exertion (RPE) were obtained at rest and upon completion of various trials using Borg's modified CR10 scale (Borg, 1998). Arterialized (Finalgon, Boehringer Ingelheim, Germany) capillary blood samples were collected from an earlobe at rest and every kilometre during the time trials for determination of total whole blood lactate concentration ([La−]B) using an electrochemical analyser (YSI 1500 Sport, YSI Life Sciences, Yellow Springs, OH, USA).
Neuromuscular function
Electromyography
Quadriceps electromyograms (EMG) were recorded from the right vastus lateralis (VL), vastus medialis (VM) and rectus femoris (RF) using monitoring electrodes with full-surface solid adhesive hydrogel (Kendall H59P, Mansfield, MA, USA), with on-site amplification. Electrodes were placed in a bipolar configuration over the middle of the respective muscle belly, and the active electrode was placed over the motor point of the muscle. The recording electrode was moved along the muscle until a good configuration, confirmed by a ‘maximal’ M-wave (muscle compound action potential) shape, was achieved. The reference electrode was placed over an electrically neutral site. The position of the EMG electrodes was marked with indelible ink to ensure that they were placed in the same location at subsequent visits. Proper electrode configuration was checked before the beginning of every experiment. To minimize movement artifacts, electrode cables were fastened to the subject's quadriceps using adhesive tape and wrapped in elastic bandage. The VL, VM and RF electrodes were used to record: (a) magnetically evoked compound muscle action potentials (M-waves) to evaluate changes in membrane excitability; and (b) EMG for VL throughout exercise to estimate central motor drive. The M-wave properties included conduction time, peak amplitude and area (Caquelard et al. 2000; Sandiford et al. 2005; Katayama et al. 2007). Membrane excitability was maintained from pre- to postexercise in all trials as indicated by unchanged M-wave characteristics. This suggests that the observed changes in potentiated quadriceps twitch force (Qtw,pot) are mainly due to changes within the quadriceps and that peripheral failure of electrical transmission might be excluded.
Raw EMG signals from VL, VM and RF corresponding to each muscle contraction during the exercise trials and the pre- and postexercise maximal voluntary contraction (MVC) manoeuvres were recorded for later analysis. The EMG signals were amplified and filtered by a Butterworth bandpass filter (BMA −830, CWE, Inc., Ardmore, PA, USA) with a low-pass cut-off frequency of 10 Hz and a high-pass cut-off frequency of 1 kHz. The slope of the filters was −6 dB per octave. The filtered EMG signals were sampled at 2 kHz by a 16 bit A/D converter (PCI-MIO-16XE-50, National Instruments, Austin, TX, USA) with custom software (Labview 6.0, National Instruments). A computer algorithm identified the onset of activity where the rectified EMG signals deviated by more than two standard deviations above the baselines for at least 100 ms. Each EMG burst was inspected to verify the timing identified by the computer. For data analysis, the integral of each burst [integrated EMG (iEMG)] was calculated as follows:
where m is the raw EMG signal. As an estimate of central neural drive, mean iEMG over each 500 m segment of the time trial was normalized to the iEMG obtained from pre-exercise MVC manoeuvres performed either without (control and placebo trial) or with blockade of locomotor muscle afferents (experimental trial).
Magnetic stimulation
For a detailed description we refer to previous studies from our laboratory (Amann et al. 2006a,b; Amann & Dempsey, 2008). Briefly, subjects lay semi-recumbent on a table with the right thigh resting in a preformed holder, the knee joint angle set at 1.57 rad (90 deg) of flexion and the arms folded across the chest. A magnetic stimulator (Magstim 200, The Magstim Company Ltd, Whitland, Wales, UK) connected to a double 70 mm coil was used to stimulate the femoral nerve. The evoked quadriceps twitch force was obtained from a calibrated load cell (Model SM 1000, Interface, Scottsdale, AZ, USA) connected to a non-compliant strap, which was placed around the subject's right leg just superior to the malleoli. To determine whether nerve stimulation was supramaximal, unpotentiated quadriceps single twitch forces (Qtw) were obtained every 30 s at 50, 60, 70, 80, 85, 90, 95 and 100% of maximal stimulator power output. A near plateau in baseline Qtw and M-wave amplitudes with increasing stimulus intensities was observed in every subject, indicating maximal depolarization of the femoral nerve.
For the evaluation of quadriceps strength, we used eight unpotentiated and six potentiated single twitch forces (Qtw and Qtw,pot, respectively). The Qtw,pot is more sensitive for detecting fatigue than the non-potentiated twitch, particularly when the degree of fatigue is small or when differences in exercise-induced potentiation effects exist (Kufel et al. 2002). Accordingly, we measured quadriceps twitch force 5 s after a 5 s MVC of the quadriceps and repeated this procedure six times such that six Qtw,pot values were obtained (Kufel et al. 2002). Like Kufel et al. (2002), we found that the degree of potentiation was slightly smaller after the first and, to a lesser extent, after the second MVC; therefore, we discarded the first two measurements. Activation of the quadriceps during the MVCs was assessed using a superimposed twitch technique (Merton, 1954; Strojnik & Komi, 1998). Briefly, the force produced during a superimposed single twitch on the MVC was compared with the force produced by the potentiated single twitch delivered 5 s afterwards. The assessment procedure was performed before exercise and 3 min after exercise, which represented the duration of the break between the prefatigue trials and the subsequent time trials. Peak force, contraction time (CT), maximal rate of force development (MRFD), one-half relaxation time (RT0.5) and maximal relaxation rate (MRR) were analysed for all Qtw,pot values (Lepers et al. 2002; Sandiford et al. 2005).
Intrathecal fentanyl
A 20 gauge antecubital intravenous cannula was inserted, and a bolus of ∼500 ml of normal saline was infused to prevent a potential drop in blood pressure (Shannon & Ramanathan, 1998). The subjects were placed in the flexed sitting position and the skin and subcutaneous tissue were anaesthesitized at the L3–L4 vertebral interspace using 2–4 ml of 1% (10 mg ml−1) lidocaine. In the experimental trial (5KFent), a 25 gauge, 9 cm Pecan (pencil-point) needle was advanced to the subarachnoid space. Free-flowing cerebrospinal fluid (CSF) confirmed subarachnoid positioning of the needle tip. A small amount of CSF was aspirated, and 1 ml of fentanyl (0.05 mg ml−1) injected. The needle was then removed, and the subjects remained in an upright sitting position to minimize the potential risk of cephalad movement within the CSF. The participants were observed for 5 min with repeated sampling of vital signs. In the placebo trial, the same needle was advanced (L3–L4), but just prior to entering the subarachnoid space 1 ml of preservative-free normal saline was injected. The needle was then removed and the subjects were observed for 5 min with repeated sampling of vital signs. To evaluate the effects of intrathecal fentanyl on resting neuromuscular functions, percentage voluntary muscle activation, MVC force and twitch forces (Qtw and Qtw,pot) were assessed before and after the application of the opioid analgesic. The time between the application of fentanyl and the start of the time trial was about 20 min.
Reliability, reproducibility and technical considerations
Magnetic femoral nerve stimulation
For between-day reliability, five of the eight subjects repeated the magnetic stimulation protocol at rest on separate visits to the laboratory about a month prior to the present study; those results have been published (Amann et al. 2008b). There was no systematic bias in the baseline measurements between days. Mean between-day, within-subject coefficients of variation were 4.9 ± 0.7% (range, 1.3–7.4) for Qtw,pot, 2.5 ± 1.4% (range 0.8–6.3) for MVC and 1.4 ± 0.6% (range 0.0–4.1) for voluntary muscle activation. Additional reliability measures regarding magnetic nerve stimulation, as well as technical considerations addressing the limitations of surface EMG and magnetic femoral nerve stimulation can be found elsewhere (Enoka & Stuart, 1992; Keenan et al. 2005; Amann et al. 2006a,b, 2008b).
Exercise trials
Previously, five of the eight subjects also repeated 5KCtrl twice on different days for reproducibility measures (Amann et al. 2008b). No systemic changes occurred in group mean values for performance time and mean power output. Between-day coefficient of variation was 0.8 ± 0.3% (range 0.1–1.8) for performance time and 1.6 ± 0.5% (range 0.2–3.4) for mean power output.
Statistical analyses
Student's paired t tests were conducted to compare the effect of sensory feedback blockade on various physiological parameters. A Pearson product–moment correlation was computed to evaluate the relationship between the two performance variables (i.e. time to completion and average power output). Results are expressed as the means ±s.e.m. Statistical significance was set at P < 0.05.
Results
Effects of intrathecal fentanyl on resting neuromuscular functions
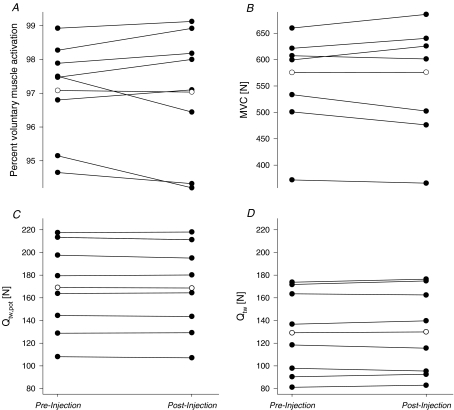
Intrathecal fentanyl resulted in cutaneous hypoaesthesia to pinprick below T1–T3. Figure 1 illustrates individual neuromuscular responses following the subarachnoid injection of fentanyl at rest. Intrathecal fentanyl had no effect on percentage voluntary muscle activation (97 ± 1 versus 97 ± 1%, P= 0.85), MVC force (575 ± 37 versus 576 ± 41 N, P= 0.95), Qtw,pot (169 ± 14 versus 169 ± 14 N, P= 0.58) and Qtw (129 ± 13 versus 130 ± 14 N, P= 0.49), indicating that efferent nerve activity and quadriceps strength were left intact by the opioid receptor agonist.
Figure 1. Individual (•) and group mean effects (○) of intrathecal fentanyl on resting neuromuscular functions.
Measures were taken before (pre-injection) and ∼10 min after (postinjection) the subarachnoid administration of fentanyl through the vertebral interspace L3–L4. A and B reflect the effects of fentanyl on voluntary muscle activation and maximal voluntary contraction force (MVC; P= 0.85 and P= 0.95, respectively). C and D illustrate the effects of fentanyl on potentiated (Qtw,pot; P= 0.38) and unpotentiated quadriceps twitch force (Qtw; P= 0.39), respectively.
Effects of intrathecal fentanyl on time trial performance, iEMG and locomotor muscle fatigue
Power output
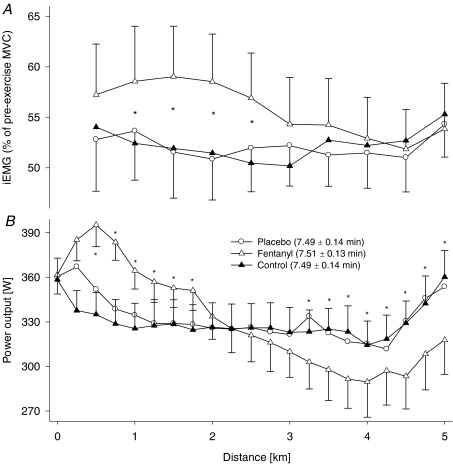
Mean power output during the first 2.5 km of 5KFentversus 5KPlac was higher in all eight subjects (6 ± 2%, P < 0.05; range, 1–20%), whereas during the second 2.5 km, power output was 4–18% (11 ± 2%) lower in seven of the eight subjects (Fig. 2 and Table 1). Mean power output during the first versus second half of 5KFent was significantly higher in all eight subjects (358 ± 12 versus 304 ± 20 W, P < 0.001). Mean power output during the first 2.5 versus the second 2.5 km was not significantly different during 5KPlac (334 ± 17 versus 328 ± 16 W, respectively; P= 0.10). Overall mean power output was not different between 5KCtrl and 5KPlac (328 ± 16 versus 330 ± 16 W, respectively; P= 0.59). Furthermore, overall mean power output was similar between 5KFent and 5KPlac (328 ± 16 and 330 ± 16 W, respectively; P= 0.81).
Figure 2. Effect of modified somatosensory feedback on neural drive and power output during a 5 km cycling time trial.
A, effects of opioid analgesic (intrathecal fentanyl) on group mean integrated EMG (iEMG) of vastus lateralis. Mean iEMG of the vastus lateralis was normalized to the iEMG obtained from pre-exercise MVC manoeuvres performed either without (5KPlac and 5KCtrl) or with intrathecal fentanyl (5KFent). Mean iEMG during the pre-exercise MVC manoeuvres on the fentanyl day was not different between pre- versus postfentanyl injection (0.23 ± 0.03 versus 0.24 ± 0.03 V s−1, respectively; P= 0.74). Each point represents the mean iEMG of the preceding 0.5 km section. Mean iEMG during the time trial was significantly increased from the trial with interspinous ligament injection of saline (5KPlac) to the intrathecal fentanyl trial (5KFent; P < 0.05). B, group mean power output during the 5 km time trial with and without impaired afferent feedback. The subjects were required to reach an individual target power output before the race was launched (361 ± 15 W). Group mean power output/time to completion were 330 ± 16 W/7.49 ± 0.14 min and 328 ± 16 W/7.51 ± 0.13 min for 5KPlac and 5KFent, respectively (P= 0.82 and P= 0.65, respectively). *P < 0.05, 5KFentversus 5KPlac; n= 8 subjects.
Table 1.
Mean physiological responses to the first and second 2.5 km and overall mean of 5 km of cycling at maximal self-selected effort
| Control | Placebo | Intrathecal fentanyl | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean over first 2.5 km | Mean over second 2.5 km | Mean over 5 km | Mean over first 2.5 km | Mean over second 2.5 km | Mean over 5 km | Mean over first 2.5 km | Mean over second 2.5 km | Mean over 5 km | |
| Power output (W) | 329 ± 16 | 328 ± 16† | 328 ± 16 | 334 ± 17 | 328 ± 16 | 330 ± 16 | 358 ± 12* | 304 ± 20*† | 328 ± 16 |
| Pedal frequency (r.p.m.) | 104 ± 2 | 100 ± 2† | 102 ± 2 | 103 ± 2 | 100 ± 2† | 102 ± 2 | 102 ± 2 | 96 ± 2*† | 99 ± 2* |
| Exercise time (min) | 3.74 ± 0.07 | 3.75 ± 0.07 | 7.49 ± 0.14 | 3.73 ± 0.07 | 3.76 ± 0.07 | 7.49 ± 0.14 | 3.63 ± 0.04* | 3.88 ± 0.09*† | 7.51 ± 0.13 |
| Heart rate (beats min−1) | 174 ± 2 | 187 ± 2† | 178 ± 1 | 175 ± 3 | 189 ± 3† | 180 ± 2 | 174 ± 3 | 184 ± 3*† | 179 ± 2 |
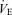 (l min−1) (l min−1) |
140 ± 7 | 171 ± 6† | 155 ± 6 | 142 ± 5 | 172 ± 5† | 158 ± 5 | 137 ± 4 | 165 ± 7† | 151 ± 5* |
| fR (breaths min−1) | 44.4 ± 2.0 | 56.6 ± 2† | 50.5 ± 1.7 | 46.1 ± 2.2 | 57.2 ± 2.2† | 51.8 ± 2.1 | 46.7 ± 2.2 | 58.4 ± 2.7† | 52.6 ± 2.3 |
| VT (l) | 2.91 ± 0.12 | 2.84 ± 0.12† | 2.89 ± 0.13 | 2.90 ± 0.12 | 2.80 ± 0.11† | 2.84 ± 0.12 | 2.84 ± 0.10 | 2.69 ± 0.09 | 2.77 ± 0.10* |
 (l min−1)a (l min−1)a
|
3.84 ± 0.16 | 4.40 ± 0.14† | 4.16 ± 0.14 | 3.86 ± 0.16 | 4.40 ± 0.16† | 4.20 ± 0.16 | 4.06 ± 0.12* | 4.27 ± 0.11† | 4.22 ± 0.12 |
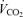 (l min−1) (l min−1) |
4.36 ± 0.16 | 4.63 ± 0.16† | 4.49 ± 0.15 | 4.43 ± 0.14 | 4.64 ± 0.17† | 4.54 ± 0.16 | 4.72 ± 0.12* | 4.49 ± 0.17 | 4.60 ± 0.14 |
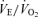 a a
|
36.4 ± 1.6 | 39.0 ± 1.7† | 37.4 ± 1.4 | 36.5 ± 2.1 | 38.9 ± 1.6† | 37.8 ± 1.7 | 34.0 ± 1.7* | 38.7 ± 1.2† | 36.0 ± 1.2 |
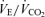 |
32.2 ± 0.8 | 37.2 ± 0.9† | 34.6 ± 1.1 | 31.9 ± 1.0 | 36.9 ± 0.6† | 34.5 ± 1.1 | 28.9 ± 1.0* | 36.6 ± 1.2† | 32.7 ± 1.0 |
 (mmHg) (mmHg) |
107.2 ± 1.5 | 111.7 ± 1.4† | 109.5 ± 1.4 | 108.4 ± 0.7 | 112.0 ± 0.7† | 111.1 ± 0.9 | 107.1 ± 1.5 | 111.8 ± 1.3† | 109.7 ± 1.3 |
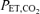 (mmHg) (mmHg) |
39.3 ± 0.8 | 33.4 ± 0.9† | 36.7 ± 0.8 | 38.3 ± 0.5 | 32.8 ± 0.6† | 35.7 ± 0.7 | 42.8 ± 0.9* | 34.5 ± 0.9*† | 38.7 ± 0.9* |
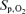 (%) (%) |
97.5 ± 0.5 | 95.0 ± 0.5† | 96.2 ± 0.5 | 97.2 ± 0.6 | 94.8 ± 0.8† | 96.0 ± 0.7 | 95.7 ± 0.5* | 91.1 ± 0.7*† | 93.4 ± 0.6* |
| RPE | — | — | 8.5 ± 0.3 | — | — | 8.7 ± 0.2 | — | — | 9.4 ± 0.3* |
| [La−]B (mmol l−1) | 6.6 ± 0.3 | 11.2 ± 0.5† | 7.9 ± 0.3 | 6.7 ± 0.4 | 11.5 ± 0.9† | 8.1 ± 0.6 | 8.9 ± 0.6* | 15.5 ± 0.6*† | 10.9 ± 0.4* |
Values are expressed as means ±s.e.m. Abbreviations: 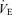 , minute ventilation; fR, breathing frequency; VT, tidal volume;
, minute ventilation; fR, breathing frequency; VT, tidal volume; 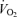 , O2 uptake;
, O2 uptake; 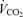 , CO2 elimination; PET,O2, end tidal O2; PET, CO2, end tidal CO2; SP,O2, arterial saturation; RPE, ratings of perceived exertion; LA−, capillary blood lactate. Note that all 2.5 and 5 km values (with the exemption of power output and lactate) are given as the means over the preceding 250 m (∼21 s).
, CO2 elimination; PET,O2, end tidal O2; PET, CO2, end tidal CO2; SP,O2, arterial saturation; RPE, ratings of perceived exertion; LA−, capillary blood lactate. Note that all 2.5 and 5 km values (with the exemption of power output and lactate) are given as the means over the preceding 250 m (∼21 s).
n = 6 subjects;
P < 0.05 versus placebo and control;
P < 0.05 versus mean over first 2.5 km; n= 8 subjects.
Integrated EMG
Vastus lateralis surface EMG was highly sensitive to the effects of intrathecal fentanyl. Group mean average iEMG over the entire time trial was not different between 5KPlacversus 5KCtrl (P= 0.65). However, average iEMG was 12 ± 3% (range, −2 to +25%) higher during the first 2.5 km of 5KFentversus 5KPlac (P < 0.01; Fig. 2). Average iEMG during the second 2.5 km was similar between 5KFentversus 5KPlac (P= 0.39). Over the entire time trial, group mean average iEMG was 8 ± 3% higher in 5KFentversus 5KPlac (P < 0.05; range, 1–21% in 7 of 8 subjects; Fig. 2).
Performance time
Time to complete the first 2.5 km was faster in 5KFentversus 5KPlac in all eight subjects (3 ± 1%, P < 0.05; range, 1–9%), whereas during the second 2.5 km, time to completion was 1–7% (4 ± 1%) slower in seven of the eight subjects (Fig. 2 and Table 1). Time to complete the first versus second half of 5KFent was significantly faster in all eight subjects (3.63 ± 0.04 versus 3.88 ± 0.09 min, P < 0.001). Performance time during the first 2.5 versus the second 2.5 km was not different during 5KPlac (3.73 ± 0.07 versus 3.76 ± 0.07 min, respectively; P= 0.21). Overall performance time was not different between 5KCtrl and 5KPlac (7.49 ± 0.14 versus 7.49 ± 0.14 min, respectively; P= 0.75). Furthermore, overall performance time was similar between 5KFent and 5KPlac (7.51 ± 0.13 and 7.49 ± 0.14 min, respectively; P= 0.65).
Contractile function
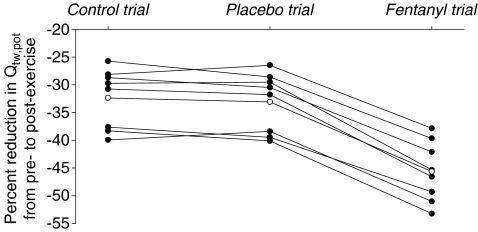
Immediately after each time trial, group mean Qtw,pot was reduced from pre-exercise baseline (P < 0.01). Exercise-induced changes in quadriceps muscle functions were similar between 5KPlac and 5KCtrl (Table 2 and Fig. 3). Time trial-induced reductions in Qtw,pot were significantly greater following 5KFent (−46 ± 2%; range, −37 to −53%) versus 5KPlac (−33 ± 2%; range, −26 to −40%), and this increase occurred in all eight subjects. The MVC force and all within-twitch measurements (MRFD, MRR, CT and RT0.5) were altered from baseline immediately after each time trial (P < 0.01); again, the exercise-induced changes were similar between 5KPlac and 5KCtrl and significantly altered after 5KFent. Percentage voluntary muscle activation was similar before versus after 5KPlac (96 ± 1 versus 95 ± 1%, P= 0.14) and 5KCtrl (94 ± 2 versus 94 ± 1%, P= 0.94); however, group mean percentage voluntary muscle activation was reduced by 1.6 ± 0.7% 3 min after 5KFent (97 ± 1 versus 96 ± 1%, P= 0.049). This exercise-induced reduction in percentage voluntary muscle activation was observed in seven of eight subjects (0.1–4.1%).
Table 2.
Effects of 5 km time trial exercise on quadriceps muscle functions
| Percentage change from pre- to 3 min postexercise |
|||
|---|---|---|---|
| Control | Placebo | Intrathecal fentanyl | |
| Qtw,pot (N) | −32.4 ± 1.9 | −33.1 ± 1.9 | −45.6 ± 1.9* |
| MRFD (N s−1) | −28.2 ± 1.6 | −29.3 ± 2.9 | −43.8 ± 2.7* |
| MRR (N s−1) | −30.1 ± 1.3 | −30.7 ± 2.4 | −43.4 ± 2.9* |
| CT (s) | −6.8 ± 0.6 | −6.9 ± 0.6 | −6.9 ± 0.6 |
| RT0.5 (s) | 7.3 ± 0.3 | 7.2 ± 0.3 | 9.4 ± 0.6* |
| MVC peak force (N) | −7.9 ± 0.6 | −8.5 ± 0.8 | −14.5 ± 2.0* |
| Percentage voluntary muscle activation | 0.0 ± 0.64† | −0.8 ± 0.52† | −1.6 ± 0.7 |
Peripheral fatigue was assessed via supramaximal magnetic stimulation of the femoral nerve before and 3 min after exercise. Changes in fatigue variables are expressed as a percentage change from pre-exercise baseline to 3 min after the completion of exercise. Values are expressed as means ±s.e.m. Abbreviations: Qtw,pot, potentiated single twitch; MRFD, maximal rate of force development; MRR, maximal rate of relaxation; CT, contraction time; RT0.5, one-half relaxation time; and MVC, maximal voluntary contraction. Percentage muscle activation is based on superimposed twitch technique. Most of the variables changed significantly compared with baseline 3 min after exercise (P < 0.01).
P < 0.01 versus control and placebo.
Not significantly different from pre-exercise baseline. Pre-exercise, resting mean values for potentiated single twitch, MRFD, MRR, CT and RT0.5 were 171 ± 3 N, 1499 ± 17 N s−1, 1011 ± 10 N s−1, 0.26 ± 0.00 s and 0.12 ± 0.00 s, respectively; n= 8 subjects.
Figure 3. Individual (•) and group mean effects (○) of 5 km time trial without (control trial and placebo trial) and with intrathecal fentanyl (fentanyl trial) on potentiated quadriceps twitch force (Qtw,pot).
Exercise performance was similar between 5KCtrl and 5KPlac (7.49 ± 0.14 and 7.49 ± 0.14 min, respectively; P= 0.75), which was also reflected in similar exercise-induced reductions in Qtw,pot from before to 3 min after the time trial (intraclass correlation coefficient = 0.95, P= 0.26). Despite a similar overall exercise performance (7.51 ± 0.13 min), end-exercise quadriceps fatigue was significantly exacerbated following 5KFentversus 5KPlac (P < 0.001).
Interrelation between iEMG, power output and peripheral fatigue
During the first 2.5 km, group mean iEMG was higher during 5KFentversus 5KPlac (58 ± 5 versus 52 ± 5% of pre-exercise MVC, respectively; P < 0.001), and this higher central motor drive was reflected in a higher mean power output (358 ± 12 versus 338 ± 16 W, respectively; P < 0.05) and a faster performance time (3.63 ± 0.04 versus 3.73 ± 0.07 min, respectively; P < 0.05) over the first half of the race. However, during the second 2.5 km mean iEMG was not different between 5KFentversus 5KPlac, and this similar central motor drive coincided with a lower mean power output (304 ± 20 versus 327 ± 16 W, respectively; P < 0.05) and a slower performance time (3.88 ± 0.09 versus 3.76 ± 0.07 min, respectively; P < 0.05) over the second half of 5KFentversus 5KPlac. The apparent inability of quadriceps force output to respond to equal central motor drive during the second half of the 5KFent was probably a consequence of the greater level of peripheral muscle fatigue when force output was high during the preceding 2.5 km.
Effects of intrathecal fentanyl on blood lactate, haemoglobin saturation and cardiorespiratory responses
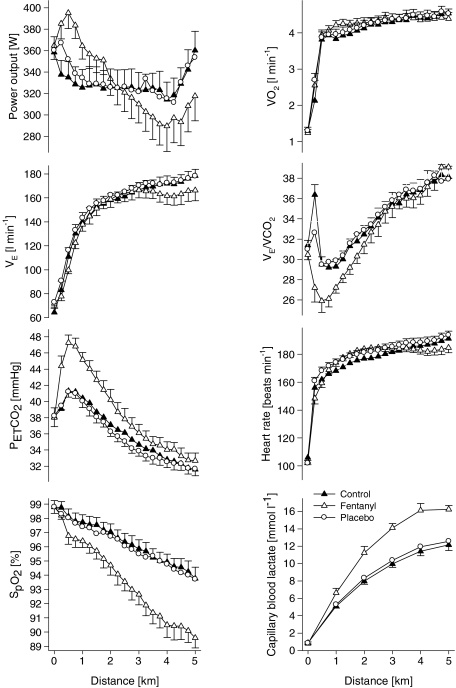
Arterialized blood lactate and haemoglobin saturation were nearly identical throughout 5KPlac and 5KCtrl (P > 0.4). The rate of lactate accumulation was increased during 5KFentversus 5KPlac, leading to a 35 ± 11% higher end-exercise capillary lactate concentration compared with 5KPlac (P < 0.01). The fentanyl trial was also characterized by a consistently steeper fall in 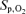 , leading to a 7 ± 1% lower haemoglobin saturation at the end of 5KFentversus 5KPlac (P < 0.001; Table 1 and Fig. 4).
, leading to a 7 ± 1% lower haemoglobin saturation at the end of 5KFentversus 5KPlac (P < 0.001; Table 1 and Fig. 4).
Figure 4. Physiological responses to a 5 km cycling time trial without (5KCtrl and 5KPlac) and with (5KFent) partly blocked somatosensory neural feedback from the fatiguing locomotor muscles.
Group mean performance (average power output/time to completion) was similar in all trials (329 ± 16 W/7.49 ± 0.14 min, 330 ± 16 W/7.49 ± 0.14 min and 328 ± 16 W/7.51 ± 0.13 min for 5KCtrl, 5KPlac and 5KFent, respectively; n= 8 subjects).
Various physiological responses to 5 km time trial exercise with and without intrathecal fentanyl are shown in Table 1 and Fig. 4. The cardiorespiratory responses to exercise were similar between 5KPlac and 5KCtrl. The group mean average 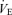 over the time trial was 5 ± 2% lower during 5KFentversus 5KPlac (P < 0.05), attributable to a 3 ± 1% lower tidal volume (VT; P < 0.05). Since power output and therefore CO2 output
over the time trial was 5 ± 2% lower during 5KFentversus 5KPlac (P < 0.05), attributable to a 3 ± 1% lower tidal volume (VT; P < 0.05). Since power output and therefore CO2 output 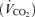 were not identical, it is important to note that the ratio of pulmonary ventilation to CO2 output
were not identical, it is important to note that the ratio of pulmonary ventilation to CO2 output 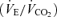 was 7 ± 2% lower over 5KFentversus 5KPlac (P < 0.05), and this hypoventilation was reflected in an elevated end-tidal partial pressure of CO2
was 7 ± 2% lower over 5KFentversus 5KPlac (P < 0.05), and this hypoventilation was reflected in an elevated end-tidal partial pressure of CO2 during 5KFent. Overall oxygen uptake
during 5KFent. Overall oxygen uptake  was similar between 5KFentversus 5KPlac (P= 0.6). However, mean
was similar between 5KFentversus 5KPlac (P= 0.6). However, mean 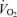 during the first 2.5 km was higher during 5KFentversus 5KPlac (P < 0.05);
during the first 2.5 km was higher during 5KFentversus 5KPlac (P < 0.05); 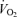 during the second half of 5KFentversus 5KPlac was smaller, but the difference was not significant (P= 0.09).
during the second half of 5KFentversus 5KPlac was smaller, but the difference was not significant (P= 0.09).
Discussion
We wished to determine the role of feedback from working locomotor muscles on central motor drive, power output and peripheral muscle fatigue developed during the performance of closed-loop exercise, in which the subject is free to choose their central motor drive voluntarily on a second-by-second basis. To this end, we pharmacologically modulated opioid-mediated muscle afferents originating in the working limbs and attenuated their cortical projection via the subarachnoid injection of fentanyl into the cerebrospinal fluid bathing the lumbosacral spinal cord. Our experiments were the first to attenuate sensory feedback from the working limbs without affecting their neuromuscular function. Blockade of opioid-mediated muscle afferents resulted in a substantially higher iEMG and presumably central motor drive, as well as higher power output and faster cycling performance time during the first half of 5KFentversus 5KPlac. Over the second half of 5KFent, iEMG was reduced to levels similar to those seen during 5KPlac, but remained significantly higher over the entire time course of 5KFentversus 5KPlac. Peripheral locomotor muscle fatigue following 5KFent developed substantially beyond the individual critical threshold for each subject as established reproducibly in the control and placebo trials. We interpret these observations, namely, the initial substantially higher central motor drive and power output and the excessive peripheral fatigue developed during 5KFent, as direct support for our hypothesis claiming that ascending sensory pathways from the working locomotor musculature exert inhibitory influences to supraspinal areas of the CNS, thereby contributing to the conscious and/or subconscious determination of the magnitude of central motor drive during high-intensity endurance exercise. These findings confirm our experiments during which we used a local anaesthetic to block muscle afferent feedback during constant-load cycling to show that peripheral feedback inhibits central motor output (Amann et al. 2008b).
Did lumbar intrathecal fentanyl migrate within the cerebrospinal fluid to affect cerebral opioid receptors?
Controversy exists over whether appreciable amounts of lipid-soluble opioids can migrate within the CSF from the lumbar injection site to cervical and medullary regions (Gourlay et al. 1989; Hill & Kaufman, 1990; Ummenhofer et al. 2000; Swenson et al. 2001). A potential direct effect of fentanyl on brain opioid receptors might not only attenuate the respiratory response to exercise by blunting the CO2-dependent respiratory drive (i.e. CO2 sensitivity; Lalley, 2008), but might also affect the subject's perception of discomfort and/or effort sensation independent of the reduced locomotor muscle afferent feedback achieved by the local effect of lumbar intrathecal fentanyl. To minimize the possibility of cephalad movement of lumbar intrathecal fentanyl, we administered the drug in the flexed sitting position and the subjects were kept upright throughout the remainder of the experiment. We have excluded a significant systemic absorption or migration of appreciable amounts of the drug beyond the cervical CSF based on three observations. Firstly, immediately prior to the time trial (about 25 min postinjection) hypoalgesia to pinprick in all eight subjects only occurred below T1–T3. Secondly, in a separate study including the same subjects (M. Amann, L. Proctor, J. Sebranek, D. Pegelow & J. Dempsey, unpublished findings) we observed no detectable level of fentanyl in systemic venous blood, sampled before and after about 10 and 50 min following intrathecal fentanyl. Thirdly, in this same separate study we evaluated the effects of lumbar intrathecal fentanyl on resting ventilation and on the ventilatory response to a range of increased arterial partial pressures of CO2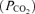 achieved via a modified hyperoxic rebreathing technique. These data showed that neither resting eupnoeic ventilation and end-tidal
achieved via a modified hyperoxic rebreathing technique. These data showed that neither resting eupnoeic ventilation and end-tidal 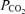 nor ventilatory sensitivity to CO2 were affected by lumbar interthecal fentanyl, indicating that fentanyl probably remained below medullary neuronal sites concerned with the chemical control of breathing.
nor ventilatory sensitivity to CO2 were affected by lumbar interthecal fentanyl, indicating that fentanyl probably remained below medullary neuronal sites concerned with the chemical control of breathing.
Why was fatigue significantly exacerbated following 5KFentversus 5KPlac despite similar time trial performances?
The fentanyl-induced increase in locomotor muscle power output during the first half of 5KFent substantially exacerbated metabolic acidosis over control/placebo conditions, and this acidosis was accomplished by a relative hypoventilation and concomitant respiratory acidosis (Fig. 4). The resultant rightward shift in the oxyhaemoglobin dissociation curve, together with the hypoventilation-induced reduction in alveolar partial pressure of O2, resulted in greater oxyhaemoglobin desaturation throughout the 5KFent. Together, these factors, namely, the high locomotor muscle power output during the first half of 5KFent and the exaggerated arterial hypoxaemia, are likely to have resulted in a faster rate of accumulation of inorganic phosphate (Pi; Hogan et al. 1999). Combined, the presumed increased rate of H+ (due to metabolic and respiratory acidosis) and Pi (due to power output and arterial hypoxaemia) accumulation in locomotor muscles during 5KFent accelerated the rate of peripheral fatigue (Hogan et al. 1999; Allen et al. 2008a,b; Amann & Calbet, 2008; Fitts, 2008). Since the accumulation of muscle metabolites was ‘unseen’ by the brain owing to the diminished afferent feedback, the subjects ‘pushed’ harder and power output was higher during 5KFentversus 5KPlac, and the associated H+/Pi-induced exacerbated rate of peripheral fatigue development during exercise resulted in substantially more end-exercise peripheral locomotor muscle fatigue.
We also observed subjective evidence of severely impaired locomotor muscle function following 5KFent. For example, upon completion of the time trials subjects were required to walk about 3 m from the bike ergometer to the station where the fatigue assessment was performed. Following 5KFent, all subjects needed assistance in disembarking the ergometer, standing upright and walking to the bed. Furthermore, in the evening, several hours following 5KFent, subjects reported continued problems with ambulation and muscle soreness. In our laboratory, these ambulatory problems have never been observed in intact subjects following various types of high-intensity whole body exercise performed during the present study or in prior investigations, even following those trials conducted in severe hypoxic environments or those requiring exercise-induced fatigue prior to a subsequent time trial (Amann et al. 2006a, 2007a,b; Katayama et al. 2007; Romer et al. 2007; Amann & Dempsey, 2008). We believe this markedly impaired motor function following 5KFent reflects the effect of the extra high levels of peripheral fatigue presently incurred (−37 to −53%ΔQtw) in the absence of inhibitory feedback versus the lesser level of fatigue (−26 to −40%ΔQtw) experienced uniformly when subjects were intact.
Mean power output and time to completion were unchanged in 5KPlacversus 5KFent. Although our present results demonstrate the inhibitory influences of muscle afferents on central motor drive during high-intensity whole body endurance exercise, the data are not suitable for addressing neural feedback effects, by themselves, on exercise performance, because of the multiple negative secondary effects of this reduced feedback. That is, the increase in central motor drive (iEMG) and therefore power output during the first half of 5KFentversus 5KPlac occurred at the expense of severe acidosis and arterial oxyhaemoglobin desaturation and presumably greater Pi accumulation in locomotor muscles. Significant hypoventilation also contributed to the arterial hypoxaemia and acidosis (Fig. 4). These substantial changes in the metabolic milieu of the working locomotor muscles during 5KFent is likely to have contributed during the second half of the race to the significant reduction in power output below 5KPlac despite only small attenuation of the central motor drive (iEMG). Thus, the enhanced time trial performance in the first half of the 5KFent (6 ± 2 s faster) was offset by a reduced performance during the second half (7 ± 2 s slower), with a net result of nearly identical overall time trial performances in 5KFent and 5KPlac. The results of the present study emphasize the critical role of locomotor muscle afferents in determining the subject's choice of the ‘optimal’ exercise intensity that will allow for maximal performance while preserving a certain level of locomotor muscle ‘functional reserve’ at end-exercise.
The use of surface EMG to reflect neural control of movement
We and others have used surface EMG as an indirect estimate of central motor drive. However, many factors can influence the EMG signal, which potentially limits and compromises its validity (Yang & Winter, 1983; Arendt-Nielsen & Mills, 1985; Enoka & Stuart, 1992; Petersen et al. 2003; Farina et al. 2004; Keenan et al. 2005; Martin et al. 2008). For example, significant filtering effects on the EMG will occur, which are related to the thickness of the subcutaneous tissues and over-represent motor units located near the recording electrodes. Additionally, amplitude cancellation can attenuate increases in motor unit activity measured by surface EMG. This insensitivity of the surface EMG technique might underestimate increases in neural drive (Keenan et al. 2005). Furthermore, voluntary muscle contractions might alter the efficacy of corticospinal synapses on motor neurons (Petersen et al. 2003, Martin et al. 2008); consequently, measures of muscle EMG may under- or overestimate the central motor drive. Despite these shortcomings, we found in the present study in the initial 2.5 km of 5KFent that power output was also increased coincident with the increase in iEMG (Fig. 2). This tight correspondence between changes in EMG and power output prior to the onset of substantial muscle fatigue gives credence to our present use of iEMG as an estimate of the relative changes in the motor output to locomotor muscle.
The final rise in central motor drive and power output
We observed a rise in central motor drive and power output towards the very end of the time trial (Amann et al. 2006a, 2008b; Amann & Dempsey, 2008). This consistent observation confirms that inhibitory somatosensory feedback from the working limbs to the CNS is only one of several potential influences determining the magnitude of central motor drive (Nybo & Secher, 2004). Apparently, this inhibitory feedback can voluntarily be ‘overridden’ for a short duration (i.e. the final ∼30 s of a 5 km time trial) as suggested (Calbet et al. 2003; Amann & Dempsey, 2008). We also need to point out that the rising power output at the end of 5KFent did not reach, even for a brief period, the levels achieved over the initial 2.5 km. Furthermore, power output over the final kilometre of 5KFent was also significantly lower compared with 5KPlac, despite similar iEMG values, revealing the effects of peripheral fatigue per se on locomotor muscle force output.
Attenuated exercise hyperpnoea via fentanyl
We observed a substantial hypoventilation during the initial 2.5 km of the 5KFentversus 5KPlac (Fig. 4). This hypoventilation may have occurred due to the absence of feedback from muscle metaboreceptors and/or mechanoreceptors, which have been shown to contribute to exercise hyperpnoea induced by muscle contractions in animal models (Pomeroy et al. 1986; Hill & Kaufman, 1990). This hypothesis needs to be addressed via further studies in which the effects of peripheral feedback blockade on cardiorespiratory responses are evaluated over a wide range of fixed exercise intensities carried out at comparable levels of metabolic demand and chemoreceptor stimuli.
Conclusion
These experiments were the first ever to selectively block lower limb afferent feedback during whole body exercise in humans without affecting the force and power-generating capacity of the locomotor muscles. By blocking the central projection of opioid-mediated muscle afferents, a centrally mediated ‘brake’ on central motor drive was released, and the CNS ‘allowed’ or ‘tolerated’ the exercise-induced development of peripheral locomotor muscle fatigue substantially beyond levels as observed following the same exercise but with an intact neural feedback system. The outcome of this study further confirms our hypothesis claiming that locomotor muscle afferent feedback exerts an inhibitory influence on the determination of central motor drive during high-intensity whole body endurance exercise and restricts the development of peripheral locomotor fatigue to an individual critical threshold.
Acknowledgments
This research was supported by a National Heart, Lung, and Blood Institute (NHLBI) RO1 grant (HL-15469). Markus Amann is the recipient of a Postdoctoral Fellowship from the American Heart Association. We thank Mrs Cynthia E. Bird and Mrs Rose E. Voelker for valuable assistance with lactate assessment and EMG data analyses.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008a;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008b;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000:40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue. J Physiol. 2006a;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Hopkins WG, Marcora SM. Similar sensitivity of time to exhaustion and time trial to changes in endurance. Med Sci Sports Exerc. 2008a;40:574–578. doi: 10.1249/MSS.0b013e31815e728f. [DOI] [PubMed] [Google Scholar]
- Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R2036–R2045. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008b doi: 10.1152/japplphysiol.90456.2008. doi 10.1152/Japplphysiol 90456 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol. 2006b;101:119–127. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol. 2007b;581:389–403. doi: 10.1113/jphysiol.2007.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc. 2004;36:613–622. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Mills KR. The relationship between mean power frequency of the EMG spectrum and muscle fibre conduction velocity. Electroencephalogr Clin Neurophysiol. 1985;60:130–134. doi: 10.1016/0013-4694(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL, USA: Human Kinetics; 1998. [Google Scholar]
- Calbet JA, De Paz JA, Garatachea N, Cabeza De Vaca S, Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol. 2003;94:668–676. doi: 10.1152/japplphysiol.00128.2002. [DOI] [PubMed] [Google Scholar]
- Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP, Jammes Y. Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci (Lond) 2000;98:329–337. [PubMed] [Google Scholar]
- Duhamel TA, Green HJ, Sandiford SD, Perco JG, Ouyang J. Effects of progressive exercise and hypoxia on human muscle sarcoplasmic reticulum function. J Appl Physiol. 2004;97:188–196. doi: 10.1152/japplphysiol.00958.2003. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gourlay GK, Murphy TM, Plummer JL, Kowalski SR, Cherry DA, Cousins MJ. Pharmacokinetics of fentanyl in lumbar and cervical CSF following lumbar epidural and intravenous administration. Pain. 1989;38:253–259. doi: 10.1016/0304-3959(89)90210-8. [DOI] [PubMed] [Google Scholar]
- Grant GJ, Susser L, Cascio M, Moses M, Zakowski MI. Hemodynamic effects of intrathecal fentanyl in nonlaboring term parturients. J Clin Anesth. 1996;8:99–103. doi: 10.1016/0952-8180(95)00174-3. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Zimmermann M. Cortical evoked responses upon selective stimulations of cutaneous group 3 fibers and the mediating spinal pathways. Brain Res. 1972;36:437–440. doi: 10.1016/0006-8993(72)90751-2. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507:619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol. 1990;68:2466–2472. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Welch HG. Effect of varied lactate levels on bicycle ergometer performance. J Appl Physiol. 1984;57:507–513. doi: 10.1152/jappl.1984.57.2.507. [DOI] [PubMed] [Google Scholar]
- Kalliomaki J, Luo XL, Yu YB, Schouenborg J. Intrathecally applied morphine inhibits nociceptive C fiber input to the primary somatosensory cortex (SI) of the rat. Pain. 1998;77:323–329. doi: 10.1016/S0304-3959(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Kalliomaki J, Weng HR, Nilsson HJ, Yu YB, Schouenborg J. Multiple spinal pathways mediate cutaneous nociceptive C fibre input to the primary somatosensory cortex (SI) in the rat. Brain Res. 1993;622:271–279. doi: 10.1016/0006-8993(93)90828-b. [DOI] [PubMed] [Google Scholar]
- Katayama K, Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Effect of arterial oxygenation on quadriceps fatigability during isolated muscle exercise. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1279–R1286. doi: 10.1152/ajpregu.00554.2006. [DOI] [PubMed] [Google Scholar]
- Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol. 2005;98:120–131. doi: 10.1152/japplphysiol.00894.2004. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol. 2002;92:1487–1493. doi: 10.1152/japplphysiol.00880.2001. [DOI] [PubMed] [Google Scholar]
- Mark RF, Steiner J. Cortical projection of impulses in myelinated cutaneous afferent nerve fibres of the cat. J Physiol. 1958;142:544–562. doi: 10.1113/jphysiol.1958.sp006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Secher NH. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol. 2004;72:223–261. doi: 10.1016/j.pneurobio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci. 2003;23:7974–7980. doi: 10.1523/JNEUROSCI.23-22-07974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res. 1986;381:385–389. doi: 10.1016/0006-8993(86)90095-8. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R598–R606. doi: 10.1152/ajpregu.00269.2006. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Sandiford SD, Green HJ, Duhamel TA, Schertzer JD, Perco JD, Ouyang J. Muscle Na-K-pump and fatigue responses to progressive exercise in normoxia and hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;289:R441–R449. doi: 10.1152/ajpregu.00652.2004. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Kalliomaki J, Gustavsson P, Rosen I. Field potentials evoked in rat primary somatosensory cortex (SI) by impulses in cutaneous Aβ- and C-fibres. Brain Res. 1986;397:86–92. doi: 10.1016/0006-8993(86)91371-5. [DOI] [PubMed] [Google Scholar]
- Shannon MT, Ramanathan S. An intravenous fluid bolus is not necessary before administration of intrathecal fentanyl for labor analgesia. J Clin Anesth. 1998;10:452–456. doi: 10.1016/s0952-8180(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Standl TG, Horn E, Luckmann M, Burmeister M, Wilhelm S, Schulte am Esch J. Subarachnoid sufentanil for early postoperative pain management in orthopedic patients: a placebo-controlled, double-blind study using spinal microcatheters. Anesthesiology. 2001;94:230–238. doi: 10.1097/00000542-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Strojnik V, Komi PV. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol. 1998;84:344–350. doi: 10.1152/jappl.1998.84.1.344. [DOI] [PubMed] [Google Scholar]
- Swenson JD, Owen J, Lamoreaux W, Viscomi C, McJames S, Cluff M. The effect of distance from injection site to the brainstem using spinal sufentanil. Reg Anesth Pain Med. 2001;26:306–309. doi: 10.1053/rapm.2001.25069. [DOI] [PubMed] [Google Scholar]
- Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- Yang JF, Winter DA. Electromyography reliability in maximal and submaximal isometric contractions. Arch Phys Med Rehabil. 1983;64:417–420. [PubMed] [Google Scholar]