Despite the numerous efforts to produce new drugs for the treatment of neuropathic pain, our options are still rather limited. This is somewhat surprising if one takes into account the high prevalence of this condition, about 5–7% of the general population, and the resources invested in the last two decades trying to develop new drugs.
There are three reasons one may think have prevented real advances in this field in the last years. First, there is no clear surrogate marker for spontaneous pain, which is the most common symptom of neuropathic pain, in laboratory animals. Except for some recent efforts in trying to produce objective measures of spontaneous pain, virtually all animal models of neuropathic pain are based on the measure of withdrawal latencies in response to mechanical or thermal stimuli. This may represent an adequate model for the evoked symptoms of hyperalgesia and allodynia that some neuropathic pain patients express, but it is an inadequate surrogate model for spontaneous pain. Second, past science has heavily focused on the mechanisms of synaptic plasticity, and even structural changes, at the level of the CNS, particularly the dorsal horn of the spinal cord. Actually, there have even been strong claims that central mechanisms are more relevant to explain peripheral neuropathic pain symptoms and signs than abnormal spike generation in diseased peripheral neurons. Finally, and as a consequence of this last, there has been considerably less research efforts directed at understanding the mechanisms behind axonal membrane hyperexcitability.
This situation is somewhat surprising as hyperexcitability of diseased peripheral somatosensory neurons is an important phenomenon in the pathogenesis of neuropathic pain symptoms. It is important because these abnormally generated ectopic impulses contribute a direct afferent input into the central nervous system. Moreover, this abnormal afferent barrage may set up a state of altered central sensitization that could further amplify the effect of this abnormal input. Therefore, understanding the mechanisms responsible for spontaneous impulse generation in diseased peripheral nociceptors is of the utmost importance, and designing drugs that stop it seems a logical way of treating neuropathic pain.
How can we record abnormalities in single nociceptors in humans? Conventional nerve conduction studies and somatosensory evoked potentials have proved an invaluable method to study the function and dysfunction of the somatosensory system. However, these methods are only capable of recording compound nerve action potentials and do not discriminate individual action potentials travelling in single, identified peripheral afferent fibres. Therefore, they are unsuitable for exploring the bases for positive sensory phenomena mediated by both large calibre fibres, such as spontaneous tactile paresthesias and dysesthesias, and small fibres, such as the different spontaneous pains. Although there is direct evidence from several authors that ectopic impulse generation does occur both in the experimental and the human setting, research has not traditionally focused on its basic underlying mechanisms. Additionally, up to now, there has been little interest from the pharmaceutical industry to generate new membrane-stabilizing agents.
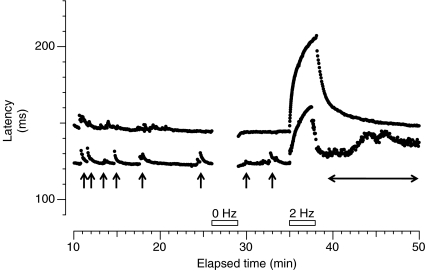
Microneurography is a minimally invasive technique, which allows single-fibre recordings from peripheral axons in conscious subjects (Hagbarth, 2002). It provides useful information on the physiology of nociceptors. Microneurography has traditionally been regarded as time-consuming and difficult, requiring both an expert investigator and a collaborative patient. Moreover, analysis of the data obtained during a recording session could sometimes take several days. For this reason, and despite its great potential, microneurography has been used on very few occasions to study neuropathic pain patients. It can be definitively said, however, that this situation has changed. For example, patterns of activity-dependent slowing of conduction velocity in response to repetitive stimulation permit classification of different functional types of peripheral C fibres (Serra et al. 1999), among them mechano-sensitive and mechano-insensitive C-nociceptors (Serra et al. 2004).There are now several investigators using microneurography, and recent developments in analysis software allow multiple simultaneous recordings of C fibres. For these reasons, new insights into the physiology and pathophysiology of peripheral nociceptor fibres have emerged. For example, there are clear reports of ongoing abnormal activity arising from the periphery in patients with peripheral small fibre neuropathies and neuropathic pain (Ochoa et al. 2005). An example from a personal unpublished observation is illustrated in Fig. 1.
Figure 1. Microneurographic raster plot of latencies in the C fibre range during baseline stimulation (0.25 Hz), pause (0 Hz, first open bar) and tetanus (2Hz, second open bar) from a patient with small fibre neuropathy and spontaneous burning pain.
Two different fibres with activity-dependent profiles characteristic of mechano-insensitive nociceptors can be observed (baseline latency 123 ms and 150 ms, respectively). Both units were spontaneously active, giving rise to some ‘saw-tooth’ markings (marked with arrows for the fibre at a shorter latency) before the pause. After the 2 Hz tetanus the fibre at a shorter latency engages in intense spontaneous activity with progressive increase of conduction latency (horizontal double-ended arrow).
In this issue of The Journal of Physiology, Namer et al. (2009) expand our knowledge by performing microneurography in aged patients. This is an extremely important previous step if we want to be able to interpret abnormal findings in patients with peripheral neuropathies, for example in diabetes. To evaluate these abnormalities, it is important to understand the physiological changes of C fibre function that occur as a result of ageing itself, and this is what has been accomplished in this interesting study. The authors found significant changes in aged, otherwise normal, subjects. However, these changes do not create a problem when interpreting abnormal behaviour of peripheral C nociceptors in neuropathic pain patients. In the words of the authors, ‘It is of major importance to quantify these age-dependent changes in order to separate them from neuropathy related processes: the number of pathologically changed C fibres was much lower than that found in patients with neuropathy and their occurrence therefore does not pose a major problem for our subjects’.
References
- Hagbarth KE. Muscle Nerve Suppl. 2002;11:S28–S35. doi: 10.1002/mus.10144. [DOI] [PubMed] [Google Scholar]
- Namer B, Barta B, Ørstavik K, Schmidt R, Carr RW, Schmelz M, Handwerker HO. J Physiol. 2009;587:419–428. doi: 10.1113/jphysiol.2008.162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JL, Campero M, Serra J, Bostock H. Muscle Nerve. 2005;32:459–472. doi: 10.1002/mus.20367. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. J Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. J Neurophysiol. 2004;91:2770–27781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]