Abstract
GABA is expressed in carotid body (CB) chemoreceptor type I cells and has previously been reported to modulate sensory transmission via presynaptic GABAB receptors. Because low doses of clinically important GABAA receptor (GABAAR) agonists, e.g. benzodiazepines, have been reported to depress afferent CB responses to hypoxia, we investigated the potential contribution of GABAAR in co-cultures of rat type I cells and sensory petrosal neurones (PNs). During gramicidin perforated-patch recordings (to preserve intracellular Cl−), GABA and/or the GABAA agonist muscimol (50 μm) induced a bicuculline-sensitive membrane depolarization in isolated PNs. GABA-induced whole-cell currents reversed at ∼−38 mV and had an EC50 of ∼10 μm (Hill coefficient =∼1) at −60 mV. During simultaneous PN and type I cell recordings at functional chemosensory units in co-culture, bicuculline reversibly potentiated the PN, but not type I cell, depolarizing response to hypoxia. Application of the CB excitatory neurotransmitter ATP (1 μm) over the soma of functional PN induced a spike discharge that was markedly suppressed during co-application with GABA (2 μm), even though GABA alone was excitatory. RT-PCR analysis detected expression of GABAergic markers including mRNA for α1, α2, β2, γ2S, γ2L and γ3 GABAAR subunits in petrosal ganglia extracts. Also, CB extracts contained mRNAs for GABA biosynthetic markers, i.e. glutamate decarboxylase (GAD) isoforms GAD 67A,E, and GABA transporter isoforms GAT 2,3 and BGT-1. In CB sections, sensory nerve endings apposed to type I cells were immunopositive for the GABAAR β subunit. These data suggest that GABA, released from the CB during hypoxia, inhibits sensory discharge postsynaptically via a shunting mechanism involving GABAA receptors.
The mammalian carotid body (CB) is a polymodal chemosensor that is excited by a variety of stimuli in arterial blood including low O2 (hypoxia), high CO2/H+ (acid hypercapnia), and low glucose (hypoglycaemia), and initiates compensatory reflex responses via an increase in sensory discharge in the carotid sinus nerve (CSN) (Kumar & Bin-Jaliah, 2007). Though ATP appears to be a key excitatory neurotransmitter that mediates these responses following its release from CB chemoreceptor (type I) cells, other neurotransmitters and neuromodulators including ACh, dopamine, adenosine and 5-HT, may contribute to or help fine-tune the final afferent signal (Iturriaga & Alcayaga, 2004; Nurse, 2005). In fact, some of these chemicals may have a dual role as fast-acting neurotransmitters that mediate their effects via ionotropic receptors, and as slow-acting neuromodulators that utilize metabotropic G-protein-coupled receptors. For example, ATP may act postsynaptically on ionotropic P2X receptors located on CSN afferent terminals (Prasad et al. 2001; Gourine, 2005; Nurse, 2005), as well as presynaptically on G-protein-coupled P2Y receptors on type I or glial-like type II cells (Xu et al. 2003, 2005). Also, co-released ACh may act postsynaptically on ionotropic nicotinic ACh receptors (nAChRs) located on CSN afferent terminals, as well as presynaptically on both nAChR and G-protein-coupled muscarinic ACh receptors (mAChRs) on type I cells (Fitzgerald, 2000; Zhang et al. 2000; Nurse, 2005). Serotonin (5-HT) and adenosine may also contribute to positive feedback autocrine/paracrine regulation of transmitter output from type I cells via presynaptic G-protein-coupled 5-HT2a and adenosine A2a receptors, respectively (Zhang et al. 2003; Xu et al. 2006). Thus, several chemical mediators present in the CB may function as both neurotransmitters and/or neuromodulators during sensory transmission, and in some cases these roles may even be antagonist.
Another classical neurotransmitter that is expressed in CB type I cells is γ-aminobutyric acid (GABA) (Oomori et al. 1994; Pokorski & Ohtani, 1999; Fearon et al. 2003), though its role in the control of respiration is not completely understood. In a previous study from this laboratory using a functional co-culture model of rat CB type I cells and sensory (petrosal) neurones, GABA was found to inhibit the afferent response via a presynaptic autoregulatory mechanism involving GABAB receptors on type I cells (Fearon et al. 2003). In spite of these findings, there are compelling reasons to consider the possibility that GABA might have other actions in the CB, resembling those of the classical inhibitory transmitter. Of particular interest is the observation that benzodiazepines, a clinically important class of ionotropic GABAA receptor agonists, have been reported to depress the hypoxic ventilatory response in part by inhibiting CB chemoreceptor afferent activity. Thus, perfusion of the cat and rabbit CB with low doses of midazolam (and/or diazepam) reduced afferent chemoreceptor responses to hypoxia (Shirahata, 2002; Kim et al. 2006), and reversal of the effect by bicuculline confirmed that GABAA receptors were involved (Shirahata, 2002; see also, Alexander & Gross, 1988). These data suggested an inhibitory role of GABA at sensory nerve endings during hypoxic chemotransmission. In contrast, however, an excitatory role of GABAA receptors in the CB has been implied by observations that exogenous GABA increased CSN discharge in the isolated adult rabbit petrosal ganglion in vitro, and depolarized isolated petrosal neurones during sharp electrode intracellular recordings (Iturriaga et al. 2007; see also Koga & Bradley, 2000). These findings, together with earlier evidence questioning an inhibitory role of the endogenous benzodiazepine system in the control of respiration (Pokorski et al. 1994), point to a need to elucidate the role of GABAA receptors in CB function.
The goal of the present study was to clarify the role of GABA and GABAA receptors (GABAARs) in hypoxic chemotransmission in the CB using an established co-culture model of the rat type I cell clusters and dissociated petrosal neurones (Nurse, 2005). We confirm the presence of functional GABAAR on these neurones and show that, although the action of GABA is mainly depolarizing, GABAAR activation during hypoxic chemotransmission causes inhibition of the sensory response that is probably due to a shunting mechanism. Additionally, we used RT-PCR techniques to probe for an array of GABAergic markers in the rat chemoafferent pathway in order to validate a synaptic role of GABA in the rat CB. In particular, because GABAARs are pentamers derived from potentially multiple combinations of some 19 different subunits including α1–6, β1–3, γ1–3 and δ (Möhler, 2006; Michels & Moss, 2007), we probed for tissue expression patterns of selected GABAAR subunits. Also, we probed for the expression of several isoforms of GABA biosynthetic enzymes (glutamic acid decarboxylases, GADs) and GABA transporters (GATs). Finally, confocal immunofluorescence labelling was used to show that chemosensory nerve endings in contact with type I cells of the rat CB in situ expressed the β subunit of GABAARs.
Methods
Cell culture
The procedures for culture of dissociated rat petrosal neurones, with or without co-cultured carotid body (CB) type 1 cell clusters, were identical to those described in detail elsewhere (Stea & Nurse, 1992; Zhong et al. 1997; Zhang et al. 2000). Rat pups, 9–14 days old (Wistar, Charles River, Quebec), were first rendered unconscious by a blow to the back of the head, and then killed immediately by decapitation. All procedures for animal handling and tissue dissections were carried out according to the guidelines of the Canadian Council on Animal Care (CCAC). Cultures were grown at 37°C in a humidified atmosphere of 95% air–5% CO2. Electrophysiological, patch-clamp recordings from co-cultures were usually carried out 3–6 days after the neurones were plated.
Perforated-patch whole-cell recording
In the majority of electrophysiological experiments reported in the text, the gramacidin perforated-patch, whole-cell recording technique was used to preserve intracellular Cl− levels (Akaike, 1996). Nystatin perforated-patch recordings were obtained in a few cases where indicated, and procedures were similar to those described in previous studies from this laboratory (Zhong et al. 1997; Zhang et al. 2000; Fearon et al. 2003). The series resistance was typically less than 30 MΩ for gramicidin recordings and was compensated ∼80%. Membrane potential and ionic currents were recorded with the aid of an Axopatch 1D or a dual headstage MultiClamp 700B patch clamp amplifier and a Digidata 1200 or 1322A analog-to-digital converter (Axon Instruments Inc., Union City, CA, USA), and stored on a personal computer. Data acquisition and analysis were performed using pCLAMP software (versions 6.0.2 and 9.0; Axon Instruments Inc.). Results are represented in the text as mean ± standard error of the mean (s.e.m.). For statistical analysis, a paired non-parametric test (Mann–Whitney) was used for ratio comparisons (Fig. 4C), and ANOVA was used for multiple comparisons (Fig. 5D) as appropriate; the level of significance was set at P < 0.05.
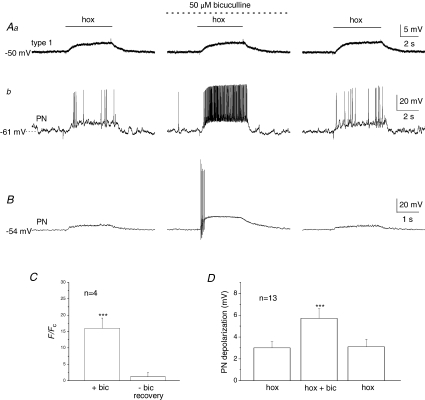
Figure 4. Bicuculline selectively potentiates the postsynaptic hypoxia-evoked chemosensory response in carotid body co-cultures.
Simultaneous gramicidin perforated-patch recordings were obtained from a presynaptic type I cell (that was part of a cluster) (Aa), and an adjacent petrosal neurone (PN) (Ab). Note depolarizing responses to hypoxia (hox; PO2∼ 5 mmHg) were recorded in both the type I cell (Aa, left trace) and the functional PN (Ab, left trace), where suprathreshold responses were evoked. Interestingly, in the presence of the GABAA receptor blocker, bicuculline (50 μm), there was a marked potentiation of the PN response resulting in a robust increase in spike discharge (Ab, middle trace), whereas the type I cell response was unaltered (Aa, middle trace). Reversibility of these responses following wash-out of the drug is shown in Aa and b (right traces). Summary data from 4 similar PNs are shown in C, where hypoxia-induced PN spike frequency (F) during bicuculline (bic) and after wash-out (recovery) is plotted as a ratio relative to initial control frequency (Fc); note the ratio F/Fc was significantly augmented (∼15×) during bicuculline, compared to after bicuculline (∼1), i.e. recovery (***P < 0.001; Mann–Whitney U test). B, another representative example from a different co-cultured PN, where only the PN postsynaptic response was recorded and was initially subthreshold; note the marked enhancement of the response in the presence of bicuculline (middle trace). D, summary data of the mean (±s.e.m.) postsynaptic depolarization before, during and after bicuculline from 13 similar PNs; hypoxia-evoked (hox) responses in the presence of bicuculline were significantly different from control hypoxic response (***P < 0.01; Student's t test).
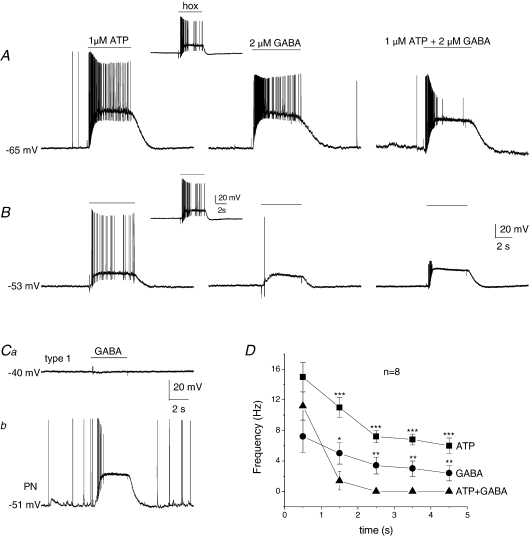
Figure 5. Interaction between ATP- and GABA-evoked responses in functional petrosal neurones in co-culture.
A and B, two functional petrosal neurones (PNs) in co-culture were first identified by the presence of an excitatory response to hypoxia (insets) during gramicidin perforated-patch membrane potential recordings. In each case, separate application of the excitatory carotid body neurotransmitter ATP (1 μm; left traces) and GABA (2 μm; middle traces) over the soma, during period indicated by upper horizontal bars, produced excitatory responses of different intensities. Interestingly, simultaneous application of the two transmitters at the same concentrations produced inhibition, resulting in a marked suppression of firing especially noticeable after ∼1 s (right traces). Combined data of firing frequency versus time (s) are shown in D for 8 similarly treated functional PNs; note the marked decrease in firing frequency after ∼1 s when both transmitters were applied together, compared to separate application (***P < 0.001; **P < 0.01; *P < 0.05; one-way ANOVA). During simultaneous recordings from a functional PN and a type I cell from an adjacent cluster, it was found that the concentration of exogenous GABA (2 μm) that produced a depolarizing response in the PN (Cb) in these experiments had a negligible effect on the membrane potential of the adjacent type I cell (Ca); example representative of 18 similar cases. Similar results have previously been reported for effects of ATP (Zhang et al. 2000; Prasad et al. 2001; Campanucci et al. 2006), suggesting that at these concentrations the interaction between the transmitters was predominantly postsynaptic, and not due to secondary effects resulting from changes in membrane potential of adjacent type I cells.
Solutions and drugs
Most electrophysiological experiments were performed using extracellular fluid (ECF) of the following composition (mm): NaCl, 110; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 10; sucrose, 10; NaHCO3 24; at pH ∼7.4 maintained by bubbling with 5% CO2. In a few experiments (indicated in the text), a Hepes-buffered ECF with the following composition was used (mm): NaCl, 135; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 10; Hepes, 10; at pH ∼7.4. Pipette solutions for gramicidin (or nystatin) perforated-patch, whole-cell recording contained (mm): potassium glutamate or gluconate, 115; KCl, 25; NaCl, 5; CaCl2, 1; Hepes, 10 and gramicidin 30 μg ml−1 (or nystatin 300 μg ml−1), at pH 7.2. A correction for liquid junction potentials of 9 mV between pipette and bath solutions was applied to membrane potential measurements as indicated. The pore-antibiotics (nystatin or gramicidin) were dissolved in dimethylsulfoxide (DMSO, 30 mg ml−1), vortexed for ∼30 s, sonicated for 5 min and kept as a stock solution at −20°C for 1 week. During recordings the culture was continuously perfused under gravity flow and removal of excess fluid by suction ensured that the fluid level in the recording chamber remained relatively constant. To prepare hypoxic solutions, the PO2 of the extracellular solution was lowered by bubbling N2 through it and the hypoxic stimulus was applied to the cells with the aid of a rapid perfusion system utilizing a double-barrelled pipette as previously described (Zhong et al. 1997; Zhang et al. 2000). In all experiments, the temperature in the recording chamber was ∼35°C.
The drugs bicuculline, muscimol, baclofen, and phaclofen were obtained from Research Biochemicals Inc. (RBI; Natick, MA, USA). Gramicidin, nystatin, tetrodotoxin (TTX), tetraethylammonium (TEA), GABA and ATP were obtained from Sigma-Aldrich (Oakville, ON, Canada).
Application of GABA agonists and antagonists
GABA was freshly prepared in a 10 mm stock solution of ECF and then diluted to its final concentration just before use. GABA and agonists were applied to petrosal cell bodies in one of three ways. In some experiments GABA (0.8–100 μm) was delivered by pressure ejection from a ‘puffer’ pipette (diameter ∼30 μm), positioned 20–40 μm from the neuronal surface. This procedure permitted fast application of the agonist at a low pressure (4–8 p.s.i.; 50–200 ms) under the control of a solenoid valve (General Valve, NJ, USA). In experiments designed to vary GABA concentrations and maximize speed of application, double-barrelled pipettes were used as previously described (Zhong et al. 1997). One barrel contained GABA at variable concentrations and the other contained ECF. Fast exchange between pipettes, aided by an electromechanical switch (Piezo Systems Inc., MA, USA), allowed the dose–response relation to be studied under voltage clamp conditions. In these experiments (see below), the peak current response elicited by the test GABA concentration was normalized to that elicited by 100 μm GABA at a holding potential of −60 mV. The dose–response curve for GABA-evoked responses was fitted with the Hill equation using pCLAMP software (version 9.0; Axon Instruments Inc.).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Whole carotid bodies (CBs) and petrosal ganglia (PG) were excised from P14 Wistar rats as described above and immediately frozen in lysis solution (Bio-Rad Laboratories, Hercules, USA) for a minimum of 24 h at −80°C. Samples were homogenized upon thawing and total RNA was extracted using the Aurum Total RNA Mini Kit in spin-column format (Bio-Rad). Yields were quantified by OD measurement at 260 nm with a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Reverse transcription was carried out on 100 ng of total RNA using SuperScript III reverse transcriptase and random primers (Invitrogen, Carlsbad, USA). Amplification was performed with a PTC-200 DNA Engine PCR machine (MJ Research, Braintree, UK) using Platinum Taq polymerase (Invitrogen) and primers specific for several GABAergic markers (Table 1). Control reactions were performed where RT was omitted from the reaction mix. Products were visualized on 2% agarose gels stained with ethidium bromide and viewed under UV illumination.
Table 1.
Forward (F) and reverse (R) primers used for RT-PCR analysis of GABAergic marker expressiona
| Marker | Accession number | Primer sequences | Annealing position | Product size (bp) | Reference |
|---|---|---|---|---|---|
| Receptor subunits | |||||
| α1 | NM_183326 | F 5′-AGCTATACCCCTAACTTAGCCAGG-3′ | 1341–1364 | 304 | Hayasaki et al. 2006 |
| R 5′-AGAAAGCGATTCTCAGTGCAGAGG-3′ | 1645–1622 | ||||
| α2 | L08491 | F 5′-ACAACGGGAAAAAGTCAGTG-3′ | 329–348 | 282 | Stewart et al. 2002 |
| R 5′-TGGCCCAGCAAATCATAC-3′ | 661–594 | ||||
| β2 | X15467 | F 5′-GAGAAGATGCGCCTGGATGTCA-3′ | 1127–1148 | 389 | Stewart et al. 2002 |
| R 5′-ATGGGAGGCTGGAGTTTAGTTCAC-3′ | 1516–1453 | ||||
| γ2S | L08497 | F 5′-AAGAAAAACCCTGCCCCTACCATT-3′ | 1158–1181 | 336 | Stewart et al. 2002 |
| R 5′-TTCGTGAGATTCAGCGAATAAGAC-3′ | 1494–1471 | ||||
| γ2L | BC092617 | F 5′-CTTCTTCGGATGTTTTCCTTCAAG-3′ | 389–412 | 390 | Stewart et al. 2002 |
| R 5′-CATAGGGTATTAGATCGTTGGACT-3′ | 779–756 | ||||
| γ3 | NM_024370 | F 5′-CATCCAGATTCAACAAGATG-3′ | 1189–1208 | 253 | Hayasaki et al. 2006 |
| R 5′-AGCTCAGAGACGTCAATG-3′ | 1442–1425 | ||||
| δ | NM_017289 | F 5′-TGAGGAACGCCATTGTCCTCTTCT-3′ | 1116–1139 | 333 | Hayasaki et al. 2006 |
| R 5′-ACCACCGCACGTGGTACATGTAAA-3′ | 1449–1426 | ||||
| Transporter isoforms | |||||
| GAT 1 | NM_024371 | F 5′-ACGCTTCGACTTCCTCATGTCCTGT-3′ | 296–320 | 698 | Fujimori et al. 2006 |
| R 5′-GAATCAGACAGCTTTCGGAAGTTGG-3′ | 994–970 | ||||
| GAT 2 | NM_133623 | F 5′-CCGGGTGTTCCGTAAGAAGAACCG-3′ | 1346–1369 | 541 | Howd et al. 1997 |
| R 5′-GTGTCGCTGGAGAAGTCAGCTCTG-3′ | 1887–1864 | ||||
| GAT 3 | NM_024372 | F 5′-ACCCCAAGGCTGTCACTATG-3′ | 1142–1161 | 479 | Fujimori et al. 2006 |
| R 5′-TGTTGTACTTGAGCGGCTTG-3′ | 1621–1602 | ||||
| BGT-1 | NM_017335 | F 5′-AGGGAGGCGTTCACCTCAGG-3′ | 2092–2111 | 388 | Fujimori et al. 2006 |
| R 5′-TTGGGCTCTGCAAGGCTGC-3′ | 2480–2462 | ||||
| Biosynthesis enzyme isoforms | |||||
| GAD 65 | NM_012563 | F 5′-GGCTCTGGCTTTTGGTCCTTC-3′ | 87–107 | 437 | Hayasaki et al. 2006 |
| R 5′-TGCCAATTCCCAATTATACTCTTGA-3′ | 524–500 | ||||
| GAD 67A | NM_017007 | F 5′-GCTGGAAGGCATGGAAGGTTTTAA-3′ | 621–644 | 302 | Hayasaki et al. 2006 |
| R 5′-AATATCCCATCACCATCTTTATTTGACC-3′ | 923–896 | ||||
| GAD 67E | M383510 | F 5′-GCTGGAAGGCATGGAAGGTTTTA-3′ | 538–560 | 243 | Szabo et al. 2000 |
| R 5′-TGAGCCCCATCACCGTAGCA-3′ | 781–762 | ||||
| Control housekeeping | |||||
| Lamin | NM_001002016 | F 5′-GCAGTACAAGAAGGAGCTA-3′ | 933–951 | 318 | Brown & Nurse, 2008 |
| R 5′-CAGCAATTCCTGGTACTCA-3′ | 1251–1233 | ||||
All primers were synthesized by MOBIX (McMaster University, Hamilton, Canada). Annealing temperature during amplification was 55°C, except for GAT 1 (60°C), GAT 3 (60°C) and BGT-1 (65°C).
Immunofluorescence localization of GABAergic markers
GABAA receptor immunostaining in tissue sections
Rat pups (∼3 weeks old) were anaesthetized by intraperitoneal administration of Somnotol (65 mg kg−1) and perfused via the aorta with phosphate-buffered saline (PBS) followed by PBS containing 2% paraformaldehyde and 0.1% glutaraldehyde. The carotid bifurcation was excised bilaterally, cleaned of surrounding tissue, and immersed in the same fixative for 4 h at room temperature. The tissue was washed 3× with PBS over a period of 30 min with constant stirring, followed by overnight incubation in 30% sucrose at 4°C. Sections (thickness, 18–20 μm) were cut in a cryostat and collected on glass slides coated with 2% silane (Sigma) and stored at −20°C. For immunostaining, sections were rehydrated with PBS, blocked with 5% fetal bovine serum, and then incubated overnight with a mouse bd17 monoclonal anti-GABAA receptor β chain antibody (MAB341; Chemicon, Temecula, CA, USA) at 1/20 dilution. This bd17 monoclonal antibody recognizes an extracellular epitope on the β subunit of the GABAA receptor (Chen et al. 2000). This was followed by washing the sections 3× with PBS over 30 min, and incubating in the dark for 1 h at room temperature with FITC-conjugated goat anti-mouse antibody (Jackson Laboratories, 115-095-166) at 1/50 dilution. Sections were washed 3× with PBS over 15 min, followed by incubation with rabbit anti-tyrosine hydroxylase (TH) antibody (AB151, Chemicon, USA) overnight at 4°C (dilution 1/1500). After washing 3× with PBS over 30 min, sections were incubated in Texas Red-conjugated goat anti-rabbit antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA) at 1/50 dilution for 1 h in the dark. Finally, sections were washed 3× with PBS, mounted in Vectashield Mounting Medium (Vector Laboratories, Burlington, Ontario, Canada) and viewed with a Zeiss LSM 510 confocal microscope equipped with argon and helium/neon lasers. In control experiments, omission of the primary antibodies produced negligible background staining of the tissue.
GABA immunostaining in cell culture
Three-day-old cultures of ∼2-week-old rat carotid bodies were rinsed in warm PBS and fixed for 1 h at −20°C in 95% methanol–5% acetic acid. After washing 3× in PBS over 10 min, cultures were incubated overnight at 4°C in polyclonal guinea pig anti-GABA antibody (AB175, Chemicon; 1/500 dilution). Following a 3× wash in PBS over 30 min, cultures were incubated with FITC-conjugated goat anti-guinea pig IgG (1/10 dilution; Jackson Laboratories) for 1 h at room temperature in the dark. Samples were covered with Vectashield Mounting Medium before viewing under epi-fluorescence with a Zeiss IM35 microscope equipped with fluorescein and rhodamine filter sets.
Results
Because GABAA receptors are Cl−-selective ion channels, we routinely used gramicidin perforated-patch recordings in order to preserve intracellular [Cl−] levels and therefore prevent artifactual changes in the chloride equilibrium potential, ECl (Akaike, 1996; Macdonald & Olsen, 1994). In a few experiments the nystatin perforated-patch technique was used, but unlike gramicidin pores which are impermeable to Cl−, nystatin pores are relatively Cl− permeable and allow equilibration of Cl− between pipette and cell interior (Akaike, 1996). Unless stated otherwise the data represented in the figures below were obtained using the gramicidin perforated-patch technique.
Expression of functional GABAA receptors on dissociated petrosal neurones in culture
Isolated petrosal neurones (PNs) were tested for the presence of GABAA receptors in dissociated cell cultures of 9- to 14-day-old rat petrosal ganglia cultured alone, or in the presence of co-cultured type I cells. During rapid application of GABA from a ‘puffer’ pipette or by fast perfusion, ∼85% of PNs (n= 196) responded with a rapid depolarization from a resting potential of ∼−60 mV under current clamp (Fig. 1A and C), and/or activation of an inward current at a holding potential of −60 mV under voltage clamp (Figs 1D and 2A). The depolarization was associated with a conductance increase as indicated during application of constant hyperpolarizing current pulses (Fig. 1C), and was observed with both gramicidin and nystatin perforated-patch recordings. The GABA-induced depolarization was reversibly blocked by the GABAA receptor antagonist bicuculline (50–100 μm) (Fig. 1A; n= 12) and could be mimicked by the GABAA receptor agonist, muscimol (50 μm; Fig. 1B, n= 5). The muscimol-induced depolarization was also reversibly blocked by bicuculline (Fig. 1B). In this study we did not encounter PNs that produced hyperpolarizing responses to GABA or muscimol, as previously observed in some PNs from older (20- to 40-day-old) rats (Koga & Bradley, 2000). Under voltage clamp, the GABA-induced inward current (IGABA) at −60 mV showed rapid activation followed by a slower desensitization (Figs 1D, and 2A and D). The desensitization phase of IGABA could be fitted with a single exponential with mean time constant τ= 2.2 ± 0.3 s (n= 6; Fig. 2D).
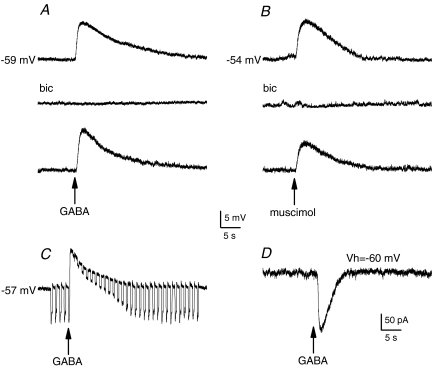
Figure 1. Effects of GABA on membrane potential and ionic currents in isolated petrosal neurones.
A, application of a ‘puff’ of GABA (at arrow; 0.1 mm in pipette) elicited membrane depolarization (upper trace) that was blocked in the presence of the GABAA receptor antagonist (bicuculline (bic); 0.1 mm) in the bath (middle trace), and the effect was reversible (lower trace). B, using the same procedure, muscimol (50 μm), a GABAA receptor agonist, mimicked the effects of GABA (upper and lower traces), and the response was similarly inhibited by bicuculline (middle trace). The depolarization induced by puffer-applied GABA was associated with a conductance increase, as revealed by application of constant hyperpolarizing current pulses (C). Under voltage clamp, a puff of GABA produced an inward current at a holding potential Vh of −60 mV. All recordings were obtained using the gramicidin perforated-patch technique.
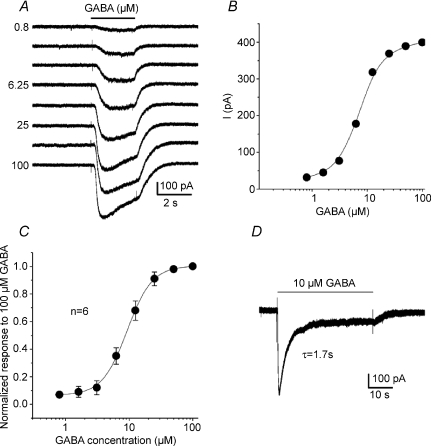
Figure 2. Dose–response curve and kinetics of IGABA in identified chemosensory petrosal neurones.
Under voltage clamp at a holding potential of −60 mV, rapid perfusion of GABA over the soma of a ‘functional’ petrosal neurone (PN) caused dose-dependent inward current (A and B); in A, GABA was applied during the period indicated by upper horizontal bar and GABA concentrations are indicated on the left of the traces. The functional PN was first identified in co-culture by the presence of a depolarizing response to a hypoxic stimulus. The sigmoidal dose–response curve for 6 functional PNs is shown in C, where the data are normalized to the maximum current evoked at 100 μm GABA; data represent mean (±s.e.m.) and the curve was fitted by the Hill equation with EC50= 9.1 μm and Hill coefficient = 1. In D, prolonged application of GABA (10 μm) during the period indicated by the upper bar reveals slow desensitization of IGABA at −60 mV. The desensitization phase was fitted by a single exponential with time constant τ= 1.7 s.
Baclofen, a GABAB receptor agonist, had no detectable effect on the membrane potential of isolated PNs (n= 6; e.g. Fig. 2D) when applied from a ‘puffer’ pipette (1 mm), and phaclofen (100 μm), a GABAB receptor antagonist, had no effect on the GABA-induced depolarization (n= 8; data not shown). These results indicate that the GABA-induced depolarization in PNs was mediated by activation of ionotropic GABAA receptors (MacDonald & Olsen, 1994), and that metabotropic GABAB receptors were not involved.
Dose–response relation and reversal potential of IGABA in functional chemosensory neurones
In these experiments functional petrosal neurones (PNs) were first identified in co-culture by the presence of a depolarizing response to hypoxia, as previously described (Zhong et al. 1997; Zhang et al. 2000). In such PNs, the magnitude of IGABA at a holding potential of −60 mV increased with GABA concentration in a sigmoidal manner over the concentration range 0.8–100 μm as exemplified in Fig. 2A and B. The response evoked by 100 μm GABA activated faster than that by lower GABA concentrations (Fig. 2A). To obtain the average dose–response curve for IGABA, the peak response at each GABA concentration was normalized to that elicited by 100 μm GABA and the pooled data from six functional PNs were fitted by the Hill equation with EC50= 9.1 μm and Hill coefficient of 1.0 (Fig. 2C). Similar results were obtained from a random sample of PNs cultured alone, i.e. without type 1 cells (EC50= 11.5 μm; Hill coefficient = 1.1; n= 6).
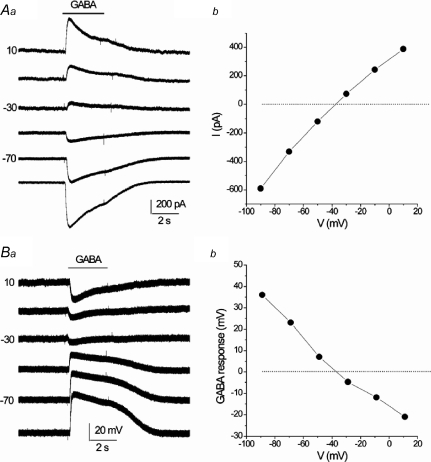
The reversal potential of GABA-evoked responses in functional PNs was determined under voltage clamp from plots of IGABAversus voltage as illustrated in Fig. 3Aa and b. After correcting for liquid junction potentials, the mean reversal potential EGABA was −38.1 ± 1.7 mV (n= 4) and −37 ± 2.9 mV (n= 2) for recordings in Hepes- and CO2/bicarbonate-buffered solutions, respectively. In two cases studied with Hepes-buffered solutions, the reversal potential of IGABA was similar when the same cell was studied first with gramicidin perforated-patch and then with conventional whole-cell recording, and EGABA was close to the Cl− equilibrium potential (ECl=–40 mV). An estimate of the reversal potential of GABA-evoked responses was also obtained under current clamp, after blockade of voltage-dependent Na+ and K+ currents with tetrodotoxin (1 μm) and tetraethylammmonium (5 mm), respectively (Fig. 3Ba and b); the mean apparent EGABA under these conditions was −38.4 ± 3.2 mV (n= 5) and −38.9 ± 3.5 mV (n= 3) in Hepes- and CO2/bicarbonate-buffered solutions, respectively. These data, combined with those in Figs 1 and 5, are consistent with an excitatory effect of GABA when acting alone, as previously reported for subpopulations of adult rat and rabbit petrosal neurones (Koga & Bradley, 2000; Iturriaga et al. 2007).
Figure 3. Reversal potential of GABA-evoked responses.
Aa, under voltage clamp, currents evoked by rapid application of GABA (2 μm) over the soma of a functional petrosal neurone in co-culture (IGABA) at different holding potentials. The functional neurone was first identified by the presence of a depolarizing response to hypoxia (not shown). Ab, corresponding I–V plot shows a reversal potential of approximately −38 mV for IGABA. In Ba and b, GABA-evoked responses for the same functional neurone were examined under current clamp conditions in the presence of TTX (1 μm) and TEA (5 mm) to block voltage-gated Na+ and K+ channels, respectively. Changes in membrane potential due to GABA application were measured at different holding potentials (Ba). Note similar reversal potential under these conditions. Both recordings were obtained using gramicidin perforated-patch technique and Hepes-buffered extracellular medium. Membrane potentials have been corrected for liquid junction potential of 9 mV between patch pipette and bath solution.
GABAA receptor blockers selectively potentiate the hypoxia-induced postsynaptic sensory response in co-culture
In previous studies from this laboratory a co-culture model of rat type I cell clusters and juxtaposed PNs was used to investigate the role of presynaptic GABAB receptors in sensory transmission (Fearon et al. 2003). In the present study we used similar co-cultures to elucidate the role of endogenous GABA, which is expressed in cultured type I cells (Fig. 9A), and GABAA receptors in the hypoxia-induced chemosensory response. Dual gramicidin-perforated patch recordings from a type I cell within a cluster and an adjacent PN allowed simultaneous monitoring of both presynaptic and postsynaptic responses during chemosensory stimulation. As illustrated in Fig. 4Aa and b, hypoxia (PO2∼5 mmHg) induced a depolarizing receptor potential in the recorded type 1 cell (Fig. 4Aa; left trace) and simultaneously, a depolarizing excitatory response in the adjacent PN (Fig. 4Ab; left trace). Interestingly, perfusion of the GABAA receptor blocker bicuculline (50 μm) caused a robust potentiation of the PN sensory discharge (Fig. 4Ab; middle trace) without affecting the type I cell response (Fig. 4Aa; middle trace), and the effect was reversible (Fig. 4Aa and b; right traces). Data from four similar cases are summarized in Fig. 4C, where the PN spike frequency (F) during and after bicuculline is expressed as a ratio relative to initial control (Fc). In these experiments, bicuculline caused a dramatic ∼13× increase in PN firing frequency; the mean frequency in the presence of bicuculline was 16 ± 3.1 Hz versus 1.2 ± 1.3 Hz in control solution (n= 4; P < 0.001). In other cases where the initial hypoxia-induced PN response in co-culture was subthreshold, bicuculline still caused potentiation of the response, sometimes leading to a burst of action potentials (Fig. 4B). Data showing an approximately 2-fold potentiation of the hypoxia-induced PN depolarizing response by bicuculline are summarized in Fig. 4D for a group of 13 such cells. Because the hypoxia-induced receptor potential in the adjacent type I cell in control conditions (ΔVc) was similar to that during perfusion of bicuculline (ΔVbic) in all cases tested (ratio ΔVbic/ΔVc= 0.97 ± 0.11; n= 9), it appears that the effect of bicuculline was predominantly on postsynaptic GABAA receptors expressed on petrosal neurones and/or their terminals.
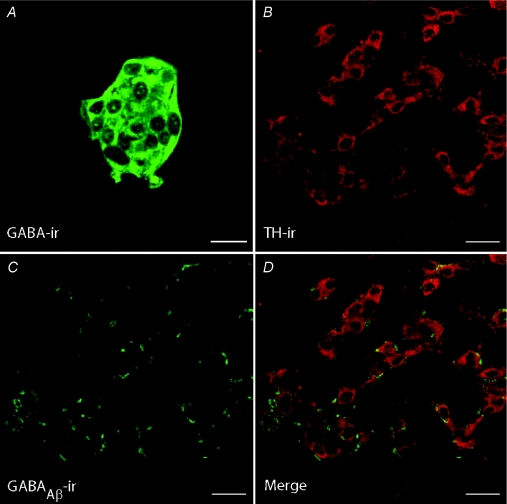
Figure 9. Immunofluorescence staining GABA in cultured cells and of GABAAR β subunit in tissue sections of whole rat carotid body.
A, a type 1 cell cluster from a carotid body culture showed positive GABA-immunoreactivity. B–D, the same section of rat carotid body was immunostained with a polyclonal antibody against tyrosine hydroxylase (TH; red fluorescence) and a monoclonal antibody (bd17) against the β subunit of the GABAAR (green fluorescence). Note positive TH- and bd17-immunofluorescence in type I cells (B) and nerve endings (C), respectively. In D (merge), the bd17-positive nerve endings are closely apposed to TH-positive type 1 cells, suggesting that in situ type 1 cells are contacted by afferent terminals expressing postsynaptic GABAARs. Scale bar = 10 μm in A and 20 μm in B–D.
Evidence that activation of GABAA receptors produces shunting of excitatory stimuli in chemosensory petrosal neurones
The data in Figs 1 and 3 indicated that the predominant effect of exogenous GABA on these petrosal neurones was excitatory, yet release of endogenous GABA during hypoxia chemotransduction appeared to inhibit the sensory discharge in co-culture (Fig. 4). We hypothesized that a GABAA-mediated ‘shunting’ mechanism that results in the attenuation or blunting of a coincident excitatory input might explain this discrepancy (Monsivais & Rubel, 2001). To test this possibility in co-cultured PNs, we compared the separate and combined effects of GABA and ATP, a key excitatory neurotransmitter in carotid body chemotransmission that mediates its effects via postsynaptic P2X2/3 receptors (Zhang et al. 2000; Prasad et al. 2001; Gourine, 2005; Nurse, 2005). Functional PNs that were excited by a hypoxic stimulus in co-culture were first identified (Fig. 5A and B; insets), and then ATP and/or GABA applied by rapid perfusion over the soma.
As exemplified in Fig. 5A and B, separate application of 1 μm ATP, a value less than the EC50 for purinergic receptors on these neurones (EC50= 2.7 μm; Zhang et al. 2000), and 2 μm GABA produced an excitatory discharge of varying intensities in functional PNs, but in each case the response was significantly blunted when ATP and GABA were applied together. The relationship between spike frequency, averaged over 1 s intervals, versus time is summarized for a group of eight similarly treated functional PNs (Fig. 5D). To rule out secondary effects of exogenous GABA (at these low concentrations) on neighbouring type I cells in the co-culture, dual gramicidin perforated-patch recordings were obtained simultaneously from a PN and an adjacent type I cell. In each case (n= 18), the membrane potential of the PN was strongly depolarized by GABA (2 μm) whereas that of the type I cell was unaffected (Fig. 5Ca and b). It was previously shown that ATP also had negligible effects on the membrane potential of type I cells at the concentrations used here (Zhang et al. 2000; Campanucci et al. 2006). Taken together, these data indicate that co-activation of GABAA and P2X receptors on these neurones suppresses excitability that is especially pronounced at later times (> 1 s). This inhibition probably occurs via a shunting mechanism.
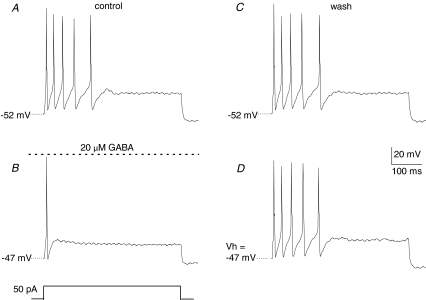
As previously reported in dorsal root ganglion neurones (Sokolova et al. 2001; Toulméet al. 2007), inhibition of afferent excitation may also arise from functional cross-talk between adjacent activated GABAA and P2X2/3 receptors, though usually at higher ligand concentrations than those used in Fig. 5. Though not studied in detail, in preliminary voltage clamp studies we found no convincing evidence for such cross-inhibition, at least at the level of the petrosal soma (data not shown). To obtain additional evidence for the proposed GABAA-mediated shunting inhibition, we injected suprathreshold depolarizing current pulses into cultured PNs before, during, and after bath application of GABA. As exemplified in Fig. 6A, a long-duration depolarizing current pulse (50 pA) triggered multiple spikes in an isolated PN. However, after bath application of 20 μm GABA the membrane potential depolarized by ∼5 mV and only a single spike could be elicited with the same depolarizing stimulus (Fig. 6B), and the effect was reversible (Fig. 6C). To eliminate the confounding effect of the GABA-induced depolarization on membrane excitability, current was continuously injected to depolarize the cell artificially by 5 mV, and then the depolarizing stimulus re-applied. This artificial depolarization did not mimic the inhibitory effect of GABA on PN excitability (Fig. 6D). Similar results were observed in 8 out of 10 neurones tested in this way, supporting the idea that GABA mediates its effects mainly via a shunting mechanism.
Figure 6. Exogenous GABA reduces excitability of isolated petrosal neurones.
A, under current clamp, a long depolarizing current pulse (50 pA; 50 ms) triggered multiple action potentials in a petrosal neurone. B, bath application of 20 μm GABA depolarized the membrane potential by 5 mV and only a single action potential could be elicited by the same depolarizing stimulus. C, on washing out GABA, the control response and initial membrane potential recovered. D, the magnitude of the GABA-induced depolarization was mimicked by artificially injecting a continuous current to depolarize the cell by ∼5 mV. However, this artificial depolarization did not mimic the inhibitory effect of GABA, as the depolarizing current pulse still triggered multiple action potentials similar to A. These data suggest that the effects of GABA were not simply due to secondary effects of depolarization on membrane excitability. Data are representative of 8/10 cells that behaved in this way.
Expression of GABAergic markers in the carotid body and petrosal ganglion
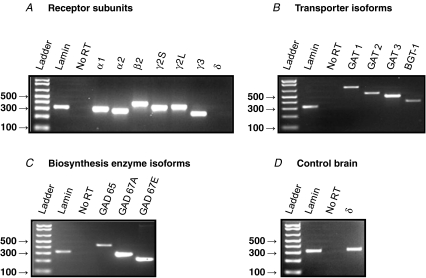
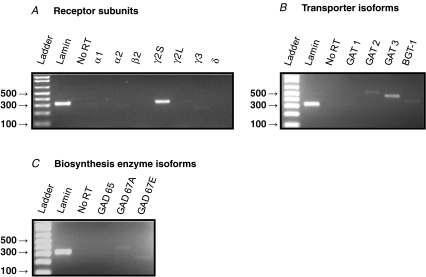
If GABA acts as an afferent carotid body (CB) neurotransmitter then it is expected that GABAergic markers should be expressed in both the CB and petrosal ganglion (PG). While there is evidence for the presence of GABA and GABAB receptor subunits in chemoreceptor type I cells of the CB in situ (Oomori et al. 1994; Pokorski & Ohtani, 1999; Fearon et al. 2003), as well as cultured rat type I cells (Fig. 9A), little is known about the expression of GABAergic markers in the CB chemoafferent pathway. To address this, we used RT-PCR to probe for a variety of GABAergic markers in tissue extracts of both the PG and CB. The forward and reverse primer sets for the various markers tested are listed in Table 1 and include GABAA receptor (GABAAR) subunits, GABA transporter (GAT) isoforms, and isoforms of the biosynthetic enzyme, glutamic acid decarboxylase (GAD). A summary of the results of the GABAergic expression pattern in the PG and CB is shown in Table 2. Consistent with the electrophysiological results reported above, mRNA for a variety of GABAAR subunits was detected in PG extracts including α1, α2, β2, γ2S, γ2L and γ3 (Fig. 7A). Interestingly, the δ subunit, that is usually associated with extrasynaptic GABAAR (Michels & Moss, 2007), was not detectable in PG (Fig. 7A), but was present in control brain tissue (Fig. 7D). Additionally, mRNA for GABA transporter isoforms GAT 1,2,3 and BGT-1 (Fig. 7B), as well as the biosynthetic markers GAD 65, GAD 67A and GAD 67E (Fig. 7C) were detected in PG extracts. In contrast, in the CB, mRNAs for GAT 2,3 and BGT-1 (Fig. 8B), and GAD 67A,E (Fig. 8C) were detectable, though, of the GABAAR subunits only γ2S was detectable (Fig. 8A). Taken together, these data indicate that an array of GABAergic markers is expressed in the rat CB chemoafferent pathway.
Table 2.
Summary of GABAergic marker expression in whole postnatal day 14 carotid bodies (CBs) and petrosal ganglia (PGs) as determined by RT-PCR analysis
| Marker | CB (Y/N)a | PG (Y/N) |
|---|---|---|
| Receptor subunits | ||
| α1 | N | Y |
| α2 | N | Y |
| β2 | N | Y |
| γ2S | Y | Y |
| γ2L | N | Y |
| γ3 | N | Y |
| δ | N | N |
| Biosynthesis enzyme isoforms | ||
| GAD 65 | N | Y |
| GAD 67A | Y | Y |
| GAD 67E | Y | Y |
| Transporter isoforms | ||
| GAT 1 | N | Y |
| GAT 2 | Y | Y |
| GAT 3 | Y | Y |
| BGT-1 | Y | Y |
Y refers to yes for expression and N to no expression.
Figure 7. RT-PCR analysis of GABAergic marker expression in extracts of postnatal day (P) 14 rat petrosal ganglia.
Products were run on 2% agarose gel stained with ethidium bromide and viewed under UV illumination. A, expression of GABAA receptor subunits; note lack of expression of the δ subunit. B, expression of the indicated GABA transporter (GAT) isoforms, including BGT-1. C, expression of the indicated GABA biosynthetic enzyme, glutamic acid decarboxylase (GAD) isoforms. D, positive expression of GABAAR δ subunit in rat brain tissue verifies primer function. Lamin (A/C) housekeeping gene was used as a positive control. ‘No RT’ lane refers to negative control reactions in which water replaced Superscript III reverse transcriptase. All band identities were verified by sequencing (MOBIX, McMaster University, Canada).
Figure 8. RT-PCR analysis of GABAergic marker expression in whole P14 rat carotid bodies.
Products were run on 2% agarose gel stained with ethidium bromide and viewed under UV illumination. A, expression of GABAA receptor subunits. B, expression of GABA transporter (GAT) isoforms. C, expression of GABA biosynthetic enzyme isoforms. Ladder band sizes are given in base pairs. Lamin (A/C) housekeeping gene was used as a positive control. No RT lane refers to negative control reactions in which water replaced Superscript III reverse transcriptase. All band identities were verified by sequencing (MOBIX, McMaster University, Canada).
Immunofluorescence localization of GABAAR β subunit on petrosal chemoafferent terminals in the rat carotid body in situ
Though the co-culture model provided strong evidence for a postsynaptic role of GABA acting via GABAAR in carotid body function, the operation of a similar mechanism in vivo requires that GABAAR subunits be expressed on afferent nerve terminals in situ. We therefore used confocal immunofluorescence to localize the GABAAR β subunit in tissue sections of ∼3-week-old rat CB. As illustrated in Fig. 9B–D, the monoclonal bd17 antibody, which recognizes the β subunit of the GABAAR (Chen et al. 2000), labels sensory nerve endings (red fluorescence) apposed to tyrosine hydroxylase (TH)-positive type I cells (green fluorescence) in CB tissue sections (n= 2 animals; 10 sections stained). In control experiments, omission of the primary antibodies alone resulted in the abolition of all immunostaining (not shown).
Discussion
In this study we present evidence for a postsynaptic inhibitory role of GABA, acting via ionotropic GABAA receptors (GABAARs), in modulating chemosensory transmission in the rat carotid body (CB). Accordingly, it is proposed that during hypoxia chemotransduction, CB chemoreceptor (type I) cells release several neurotransmitters including GABA, which acts on postsynaptic GABAA receptors on petrosal terminals and contributes to rapid inhibition via a shunting mechanism. These data complement previous studies from this laboratory, based on a similar co-culture model, demonstrating a slower-acting inhibitory role of GABA mediated via a presynaptic mechanism (Fearon et al. 2003). This latter mechanism involved autocrine–paracrine stimulation of G-protein-coupled GABAB receptors, linked to inhibition of protein kinase A (PKA) and activation of background (TASK-1-like) K+ channels on chemoreceptor type I cells. A neurotransmitter role of GABA in the CB is supported by previous studies demonstrating expression of GABA immunoreactivity in type I cells in situ (Oomori et al. 1994; Pokorski & Ohtani, 1999; Fearon et al. 2003). In the present study, we confirmed GABA expression in isolated rat type I cells in culture, and further showed that isoforms of GABA biosynthetic enzymes (GAD 67A and GAD 67E), and of GABA transporters (GAT 2,3 and BGT-1) were expressed in CB extracts using RT-PCR. Taken together, these data are consistent with GABA playing a central role in synaptic transmission in the CB.
The majority of petrosal neurones encountered in this study expressed functional GABAARs that were activated by GABA and/or the GABAA agonist muscimol, but not GABAB agonists, during gramicidin perforated-patch recordings. Under current clamp, the predominant effect of GABA agonists was depolarizing, sometimes leading to action potentials. Under voltage clamp, GABA activated fast, desensitizing, inward currents at −60 mV and IGABA reversed direction at depolarized membrane potentials (approximately −38 mV). Because GABAARs are highly Cl− permeable (Macdonald & Olsen, 1994), the use of gramicidin perforated-patch recording in this study ensured that intracellular Cl− concentration was maintained at physiological levels (Akaike, 1996), and therefore the Cl− equilibrium potential (ECl) was not artefactually biased by the recording conditions. In addition to Cl−, we considered possible contributions from HCO3− ions; however, these appeared minor because the reversal potential of IGABA was similar in Hepes- and bicarbonate-buffered extracellular media. Indeed, HCO3− fluxes were expected to be small given the low HCO3−/Cl− permeability (∼0.18) of the GABAAR channels and an estimated EHCO3− of ∼−10 mV (Howard et al. 2007). Based on these data, as well as recordings from the same neurone with gramicidin perforated-patch followed by conventional whole-cell recording, we estimate that the physiological intracellular Cl− concentration in these 12- to 15-day-old petrosal neurones is ∼30 mm. Thus, the effect of GABA alone on these neurones was depolarizing and excitatory, with the reversal potential of IGABA above the action potential threshold. In studies on older (20–40 days old) rats, Koga & Bradley (2000) found subpopulations of petrosal neurones that hyperpolarized or depolarized in response to both GABA and muscimol.
These authors also concluded that GABAB receptors did not play a major role in GABA-mediated responses in these neurones. Our failure to observe hyperpolarizing responses to GABA is unlikely to be due to the recording conditions as explained above, but might be attributed to developmental age or the possibility that our dissection procedure and/or culture conditions favoured the survival of certain subtypes of petrosal neurones (Zhong et al. 1997). There is abundant evidence that central neurones expressing GABAAR may undergo a shift from depolarizing GABA-mediated responses in the fetus to hyperpolarizing responses in the adult as intracellular Cl− is lowered, coincident with developmental up-regulation of K+–Cl− cotransporter (KCC2) activity (Ben-Ari, 2002; Banke & McBain, 2006). However, depolarizing GABA-evoked responses are not limited to fetal or early postnatal neurones because they have also been observed in the adult (Howard et al. 2007). Also, in the isolated adult rabbit petrosal ganglion (PG) in vitro, exogenous GABA caused a dose-dependent increase in sinus nerve discharge that was blocked by the non-competitive GABAA antagonist picrotoxin (Iturriaga et al. 2007). Further, following dissociation of these adult ganglia, isolated PG neurones were depolarized by GABA during sharp electrode recordings (Iturriaga et al. 2007).
Physiological role of postsynaptic GABAA receptors in carotid body function: clinical significance
The results of the present study provide a satisfactory explanation of several reported observations on the effects of GABA agonists and antagonists on the hypoxic ventilatory response and chemosensory discharge in the isolated CB–sinus nerve preparation in vitro. Sedative doses of benzodiazepines (e.g. midazolam and diazepam), which act as positive allosteric GABAAR modulators, have long been known to depress the hypoxic ventilatory response in cats (Shirahata, 2002; see, however, Pokorski et al. 1994), rabbits (Kim et al. 2006) and humans (Alexander & Gross, 1988). This effect was associated with an inhibition of CB chemoreceptor discharge in isolated CB–sinus nerve preparations, and could be blocked by GABAAR antagonists (Shirahata, 2002; Kim et al. 2006). In the present study, we showed immunofluorescence localization of GABAAR β subunits on sensory nerve terminals apposed to CB chemoreceptor type I cells in situ, and the expression of mRNA for several GABAAR subunits in tissue extracts of the parent petrosal ganglia. Thus GABA, released from receptor cells during CB chemoexcitation, could act postsynaptically on adjacent terminal GABAAR to inhibit parallel excitatory inputs, and this effect would be potentiated in the presence of exogenous benzodiazepines. We further demonstrated in functional petrosal neurones in co-culture that, even though the action of GABA alone may be depolarizing and excitatory (see also, Iturriaga et al. 2007), it produced inhibition when combined with a key CB excitatory neurotransmitter, i.e. ATP (Nurse, 2005). This inhibition was probably mediated by a shunting mechanism due mainly to the increased permeability of GABAAR to Cl− and concomitant decrease in the cell's input resistance. Thus GABAAR activation could effectively short circuit ATP-induced excitatory currents and may occur even in conditions where the effects of GABA alone are depolarizing (Banke & McBain, 2006; Howard et al. 2007). However, we cannot exclude a possible contribution due to negative cross-talk between GABAA and purinergic P2X2/3 receptors on petrosal terminals (Prasad et al. 2001). Such cross-talk, which appears to involve close, receptor-based interactions between these anionic and cationic channels, has been observed in sensory dorsal root ganglion neurones (Sokolova et al. 2001; Toulméet al. 2007), though at much higher ligand concentrations than used in the present study. Though our preliminary voltage clamp studies did not reveal evidence for such cross-talk at the petrosal soma, they did not address whether it could occur at the corresponding afferent terminals.
Substrates for GABAergic neurotransmission in the rat carotid body chemoafferent pathway
Though we did not probe for all possible GABAAR subunits, our RT-PCR data identified several subunits known to contribute to the broad heterogeneity in GABAAR in the central nervous system. In the petrosal ganglia, mRNAs for the α1, α2, β2, γ2L and γ2S subunits were detected; however, these data do not allow firm conclusions to be drawn about the actual pentameric GABAAR subunit composition in petrosal terminals. Most receptors are thought to contain 2α, 2β and 1γ/1δ subunits, and our detection of the γ2 subunit, known to be required for the benzodiazepine sensitivity discussed above (Michels & Moss, 2007), suggests it probably contributes to the functional receptor in petrosal afferents. GABAA receptors containing the γ2 subunit are expressed preferentially at synaptic sites where they contribute to phasic or transient effects due to high GABA concentrations (Michels & Moss, 2007). Additionally, in conjunction with the N-terminal tubulin-binding motif of GABAA receptor-associated protein (GABARAP), the γ2 subunit is required for clustering of GABAARs at synapses (Chen et al. 2000). Our confocal fluorescence images of anti-β-chain immunostaining of petrosal afferents in situ suggest that GABAARs may occur in patches or clusters, and this could well be due to the presence of the γ2 subunit. In this regard, it was of interest that of all the subunits tested only γ2 mRNA was detected by PCR in CB extracts. Though the significance of this finding is uncertain, it should be noted that the γ2 subunit cannot form a functional receptor on its own (Michels & Moss, 2007). One possible explanation is that the γ2 signal was amplified from mRNA in ‘contaminating’ petrosal terminals present in extracts of the CB, where it directed synthesis of the protein subunit required for targeting GABAAR at synaptic sites. On the other hand, the δ subunit, thought to be a substitute partner for γ2, and normally associated with extrasynaptic sites and tonic or persistent inhibitory effects at low GABA concentrations (Michels & Moss, 2007), was not detected in petrosal or CB extracts though it was present in brain.
In summary these data, taken together, are consistent with a fast, inhibitory, postsynaptic action of GABA at synaptic contacts between petrosal chemoafferents and CB receptor cells. This pathway appears to attenuate coincident excitatory effects of a key CB neurotransmitter, i.e. ATP (Nurse, 2005), via a shunting (and/or cross-talk) mechanism involving ionotropic GABAA and P2X2/3 receptors on petrosal terminals. Because GABAA receptors are known to cycle constantly between the cell surface and endosomal compartments (Michels & Moss, 2007), they provide a potential mechanism for rapidly altering the sensitivity of the CB chemoreflex. Therefore, in conjunction with the previously described autocrine–paracrine pathway involving presynaptic G-protein-coupled GABAB receptors on type I cells (Fearon et al. 2003), the chemoafferent GABAergic system is well positioned to contribute to CB plasticity during patterned stimulation in both the developing and mature animal.
Acknowledgments
This work was supported by a grant from the Canadian Institutes for Health Research (MOP 12037) to C.A.N.
References
- Akaike N. Gramacidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Molec Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Gross TB. Sedative doses of midazolam depress hypoxic ventilatory responses in humans. Anesth Analg. 1988;67:377–382. [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26:11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Brown ST, Nurse CA. Induction of HIF-2α is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line. Am J Physiol Cell Physiol. 2008;294:C1305–C1312. doi: 10.1152/ajpcell.00007.2008. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C, Nurse CA. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: Role of nitric oxide-mediated efferent inhibition. J Neurosci. 2006;26:9482–9493. doi: 10.1523/JNEUROSCI.1672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang H, Vicini S, Olsen RW. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc Natl Acad Sci U S A. 2000;97:11557–11562. doi: 10.1073/pnas.190133497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon IM, Zhang M, Vollmer C, Nurse CA. GABA mediates autoreceptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1. J Physiol. 2003;553:83–94. doi: 10.1113/jphysiol.2003.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS. Oxygen and carotid body chemotransduction: the cholinergic hypothesis – a brief history and new evaluation. Respir Physiol. 2000;120:89–104. doi: 10.1016/s0034-5687(00)00091-8. [DOI] [PubMed] [Google Scholar]
- Fujimori S, Hinoi E, Takarada T, Iemata M, Takahata Y, Yoneda Y. Possible expression of a particular gamma-aminobutyric acid transporter isoform responsive to upregulation by hyperosmolarity in rat calvarial osteoblasts. Eur J Pharmacol. 2006;550:24–32. doi: 10.1016/j.ejphar.2006.08.088. [DOI] [PubMed] [Google Scholar]
- Gourine A. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaki H, Sohma Y, Kanbara K, Maemura K, Kubota T, Wantanabe M. A local GABAergic system within rat trigeminal ganglion cells. Eur J Neurosci. 2006;23:745–757. doi: 10.1111/j.1460-9568.2006.04602.x. [DOI] [PubMed] [Google Scholar]
- Howard MA, Burger RM, Rubel EW. A developmental switch to the GABAergic inhibition dependent on increases in KvL-type K+ currents. J Neurosci. 2007;27:2112–2123. doi: 10.1523/JNEUROSCI.5266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howd AG, Rattray M, Butt AM. Expression of GABA transporter mRNAs in the developing and adult rat optic nerve. Neurosci Lett. 1997;235:98–100. doi: 10.1016/s0304-3940(97)00699-x. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev. 2004;47:46–53. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Varas R, Alcayaga J. Electrical and pharmacological properties of petrosal ganglion neurons that innervate the carotid body. Resp Physiol Neurobiol. 2007;157:130–139. doi: 10.1016/j.resp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Kim C, Shvarev Y, Takeda S, Sakamoto A, Lindahl SG, Eriksson LI. Midazolam depresses carotid body chemoreceptor activity. Acta Anaesthesiol Scand. 2006;50:144–149. doi: 10.1111/j.1399-6576.2005.00896.x. [DOI] [PubMed] [Google Scholar]
- Koga T, Bradley RM. Biophysiological properties and responses to neurotransmitters of petrosal and geniculate ganglion neurons innervating the tongue. J Neurophysiol. 2000;84:1404–1413. doi: 10.1152/jn.2000.84.3.1404. [DOI] [PubMed] [Google Scholar]
- Kumar P, Bin-Jaliah I. Adequate stimuli for the carotid body: More than an oxygen sensor. Resp Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Ann Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: Properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABAA receptor diversity and pharmacology. Cell Tiss Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Monsivais P, Rubel EW. Accomodation enhances depolarizing inhibition in central neurons. J Neurosci. 2001;21:7823–7830. doi: 10.1523/JNEUROSCI.21-19-07823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci. 2005;120:1–9. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Nakaya K, Tanaka H, Iuchi H, Ishikawa K, Sato Y, Ono K. Immunohistochemical and histochemical evidence for the presence of noradrenaline, serotonin and g-aminobutyric acid in chief cells of the mouse carotid body. Cell Tissue Res. 1994;278:249–254. doi: 10.1007/BF00414167. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Ohtani S. GABA immunoreactivity in chemoreceptor cells of the cat carotid body. Acta Histochem Cytochem. 1999;32:179–182. [Google Scholar]
- Pokorski M, Paulev P-E, Szereda-Przestaszewska M. Endogenous benzodiazepine system and regulation of respiration in the cat. Respir Physiol. 1994;97:33–45. doi: 10.1016/0034-5687(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol. 2001;537:667–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M. Neurotransmission in the carotid body and anesthesia. J Anesth. 2002;16:298–309. doi: 10.1007/s005400200047. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Nistri A, Giniatullin R. Negative cross talk between anionic GABAA and cationic P2X ionotropic receptors of rat dorsal root ganglion neurons. J Neurosci. 2001;21:4958–4968. doi: 10.1523/JNEUROSCI.21-14-04958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Nurse CA. Whole cell currents in two subpopulations of cultured rat petrosal neurons with different tetrodotoxin sensitivities. Neurosci. 1992;17:727–736. doi: 10.1016/0306-4522(92)90180-a. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neuroprogenitor cells of the neonatal rat subventricular zone express functional GABAA receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Szabo G, Katarova Z, Hoertnagl B, Somogyi R, Sperk G. Differential regulation of adult and embryonic glutamate decarboxylases in the rat dentate granule cells after kainate-induced limbic seizures. Neuroscience. 2000;100:287–295. doi: 10.1016/s0306-4522(00)00275-x. [DOI] [PubMed] [Google Scholar]
- Toulmé E, Blais D, Léger C, Landry M, Garret M, Séguéla P, Boué-Grabot E. An intracellular motif of P2 X 3 receptors is required for functional cross-talk with GABAA receptors in nociceptive DRG neurons. J Neurochem. 2007;102:1357–1368. doi: 10.1111/j.1471-4159.2007.04640.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Tse FW, Tse A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J Physiol. 2003;549:739–747. doi: 10.1113/jphysiol.2003.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu F, Tse FW, Tse A. ATP inhibits the hypoxia response in type I cells of rat carotid body. J Neurochem. 2005;92:1419–1430. doi: 10.1111/j.1471-4159.2004.02978.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytosolic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol. 2006;290:C1592–C1598. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- Zhang M, Fearon IM, Zhong H, Nurse CA. Preynaptic modulation of rat arterial chemoreceptor function by 5-HT: role of K+ channel inhibition via protein kinase C. J Physiol. 2003;551:825–842. doi: 10.1113/jphysiol.2002.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol. 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]