Abstract
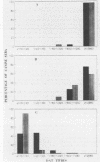
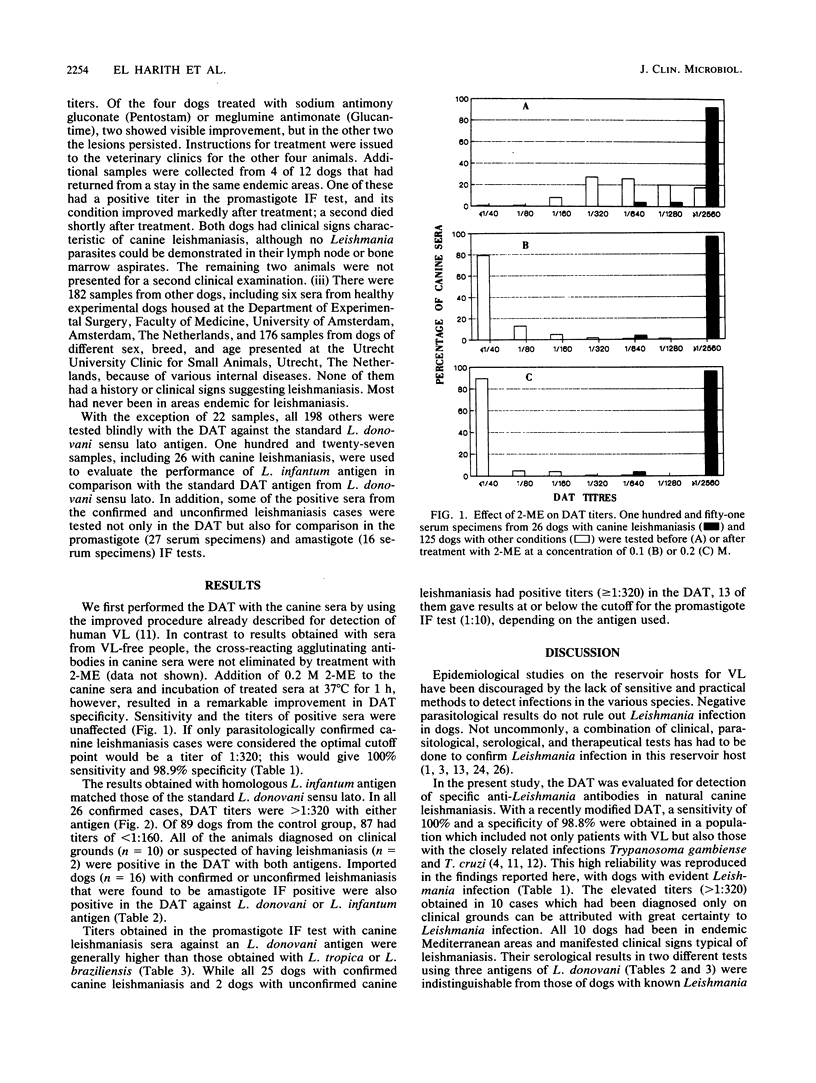
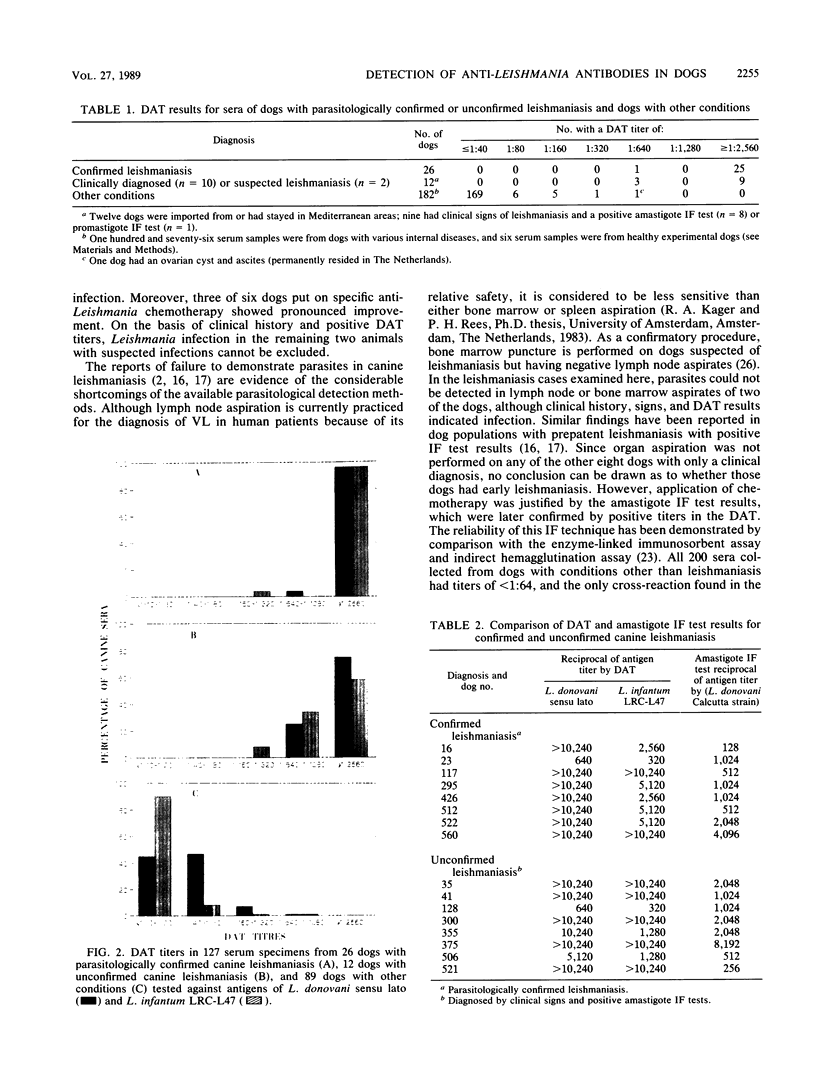
A direct agglutination test (DAT) for detection of visceral leishmaniasis in humans has been developed. In this study, it was evaluated for applicability to detection of infections in dogs, a reservoir species. The reliability of the test was improved by treating the test sera with 0.2 M 2-mercaptoethanol and incubating them at 37 degrees C. Sensitivity was 100% and specificity was 98.9% when the test was used on serum samples from 220 dogs, including 26 with parasitologically confirmed canine leishmaniasis, 12 with suspected but unconfirmed leishmaniasis, and 182 with other conditions. The DAT detected specific antibodies in 10 dogs with canine leishmaniasis diagnosed by case history, clinical signs of leishmaniasis, and seropositivity in an immunofluorescence test using either promastigotes or amastigotes, as well as in 2 dogs suspected of having leishmaniasis. The performance of an antigen prepared from a homologous isolate of Leishmania infantum in the DAT was compared with that of an antigen from a laboratory-adapted strain of L. donovani (sensu lato). The homologous antigen compared favorably with the standard antigen, and the results provided further evidence of the potential of the DAT for detection of Leishmania infection in the canine reservoir host. The results of this study, together with those of our previous studies in human visceral leishmaniasis, demonstrate that the DAT is highly suitable for wide-scale epidemiological and ecological field work. This technique could also facilitate diagnosis of leishmaniasis in dogs in veterinary health services.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abranches P., Lopes F. J., Fernandes P. S., Gomes L. T. Kala-azar in Portugal. I. Attempts to find a wild reservoir. J Trop Med Hyg. 1982 Jun;85(3):123–126. [PubMed] [Google Scholar]
- Andrade C. R., Silva O. A., Andrade P. P., Kolk A. H., Harith A. E. A direct agglutination test discriminative toward Chagas' disease for the diagnosis of visceral leishmaniasis in Brazil: preliminary results. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):457–459. doi: 10.1016/s0769-2625(87)80056-9. [DOI] [PubMed] [Google Scholar]
- Azab M. E., Rifaat M. A., Schnur L. F., Makhlouf S. A., El-Sherif E., Salem A. M. Canine and rodent leishmanial isolates from Egypt. Trans R Soc Trop Med Hyg. 1984;78(2):263–264. doi: 10.1016/0035-9203(84)90295-5. [DOI] [PubMed] [Google Scholar]
- Badaró R., Jones T. C., Lorenço R., Cerf B. J., Sampaio D., Carvalho E. M., Rocha H., Teixeira R., Johnson W. D., Jr A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986 Oct;154(4):639–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- Belazzoug S., Addadi K., Mokrani T., Hafirassou N., Hamrioui B., Belkaid M. La leishmaniose viscérale en Algérie: étude des cas hospitalisés entre 1975 et 1984. Ann Soc Belg Med Trop. 1985;65(4):329–335. [PubMed] [Google Scholar]
- Bray R. S. Leishmaniasis in the Old World. Br Med Bull. 1972 Jan;28(1):39–43. doi: 10.1093/oxfordjournals.bmb.a070891. [DOI] [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Faber F. J., Muigai R., Kiugu S., Laarman J. J. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans R Soc Trop Med Hyg. 1987;81(4):603–606. doi: 10.1016/0035-9203(87)90423-8. [DOI] [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Muigai R., Kiugu S., Kiugu S., Laarman J. J. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(4):583–536. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- Kammermann-Lüscher B. Leishmaniose beim Hund. Schweiz Arch Tierheilkd. 1980 Oct;122(10):585–603. [PubMed] [Google Scholar]
- Lainson R., Shaw J. J., Silveira F. T., Fraiha H. Leishmaniasis in Brazil. XIX: visceral leishmaniasis in the Amazon Region, and the presence of Lutzomyia longipalpis on the Island of Marajó, Pará State. Trans R Soc Trop Med Hyg. 1983;77(3):323–330. doi: 10.1016/0035-9203(83)90154-2. [DOI] [PubMed] [Google Scholar]
- Lanotte G., Rioux J. A., Perieres J., Vollhardt Y. Ecologie des leishmanioses dans le sud de la France. 10. Les formes évolutives de la leishmaniose viscérale canine. Elaboration d'une typologie bio-clinique à finalité épidémiologique. Ann Parasitol Hum Comp. 1979 May-Jun;54(3):277–295. [PubMed] [Google Scholar]
- Reiter I., Kretzschmar A., Boch J., Krampitz H. Zur Leishmaniose des Hundes. Infektionsverlauf, Diagnose und Therapieversuche nach exp. Infektion von Beagles mit Leishmania donovani (St. Kalkutta). Berl Munch Tierarztl Wochenschr. 1985 Feb 1;98(2):40–44. [PubMed] [Google Scholar]
- Rioux J. A., Lanotte G., Maazoun R., Perello R., Pratlong F. Leishmania infantum Nicolle, 1908, agent du bouton d'Orient autochtone. A propos de l'identification biochimique de deus souches isolées dans les Pyrénées-Orientales. C R Seances Acad Sci D. 1980 Oct 27;291(8):701–703. [PubMed] [Google Scholar]
- SOUTHGATE B. A., ORIEDO B. V. Studies in the epidemiology of East African leishmaniasis. 1. The circumstantial epidemiology of kala-azar in the Kitui District of Kenya. Trans R Soc Trop Med Hyg. 1962 Jan;56:30–47. doi: 10.1016/0035-9203(62)90087-1. [DOI] [PubMed] [Google Scholar]
- Silveira F. T., Lainson R., Shaw J. J., Póvoa M. M. Leishmaniasis in Brazil: XVIII. Further evidence incriminating the fox Cerdocyon thous (L) as a reservoir of Amazonian visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1982;76(6):830–832. doi: 10.1016/0035-9203(82)90119-5. [DOI] [PubMed] [Google Scholar]
- Walton B. C., Brooks W. H., Arjona I. Serodiagnosis of American leishmaniasis by indirect fluorescent antibody test. Am J Trop Med Hyg. 1972 May;21(3):296–299. doi: 10.4269/ajtmh.1972.21.296. [DOI] [PubMed] [Google Scholar]
- el Harith A., Kolk A. H., Leeuwenburg J., Muigai R., Huigen E., Jelsma T., Kager P. A. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J Clin Microbiol. 1988 Jul;26(7):1321–1325. doi: 10.1128/jcm.26.7.1321-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Harith A., Laarman J. J., Minter-Goedbloed E., Kager P. A., Kolk A. H. Trypsin-treated and coomassie blue-stained epimastigote antigen in a microagglutination test for Chagas' disease. Am J Trop Med Hyg. 1987 Jul;37(1):66–71. doi: 10.4269/ajtmh.1987.37.66. [DOI] [PubMed] [Google Scholar]