Abstract
Caffeine, a prototypic bitter stimulus, produces several physiological actions on taste receptor cells that include inhibition of KIR and KV potassium currents and elevations of intracellular calcium. These responses display adaptation, i.e. their magnitude diminishes in the sustained presence of the stimulus. Levels of the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) are well known to modulate many potassium channels, activating the channel by stabilizing its open state. Here we investigate a putative relationship of KIR and KV with PIP2 levels hypothesizing that inhibition of these currents by caffeine might be allayed by PIP2 resynthesis. Using standard patch-clamp techniques, recordings of either potassium current from rat posterior taste receptor cells produced essentially parallel results when PIP2 levels were manipulated pharmacologically. Increasing PIP2 levels by blocking phosphoinositide-3 kinase with wortmannin or LY294002, or by blocking phospholipase C with U73122 all significantly increased the incidence of adaptation for both KIR and KV. Conversely, lowering PIP2 synthesis by blocking PI4K or using the PIP2 scavengers polylysine or bovine serum albumin reduced the incidence of adaptation. Adaptation could be modulated by activation of protein kinase C but not calcium calmodulin kinase. Collectively, these data support two highly novel conclusions: potassium currents in taste receptor cells are significantly modulated by PIP2 levels and PIP2 resynthesis may play a central role in the gustatory adaptation process at the primary receptor cell level.
Adaptation, the reduced neurophysiological or psychophysical response in the presence of a constant stimulus, occurs in all sensory modalities. In gustation, it has been documented neurophysiologically at every level of the neuraxis, from receptor cells to central neurons, as well as in conscious perception. Underlying mechanisms for adaptation are not well understood, but are likely to include an amalgam of processes occurring at different sites (peripheral and central) as well as different taste qualities. Recent advances in the study of taste receptor cells (TRCs, e.g. Chandrashekar et al. 2006) have dramatically advanced our understanding of cellular transduction mechanisms underlying responses to tastant stimuli. Once canonical ideas of the role of cyclic nucleotides (Kinnamon & Margolskee, 1996; Gilbertson et al. 2000; Smith & Margolskee, 2001; Gilbertson & Boughter, 2003) have been displaced by a pathway that utilizes the trp channel TRPM5 (Perez et al. 2002; Zhang et al. 2003) activated downstream of the enzyme phospholipase C-β2 (PLCβ2, Rossler 1998; Zhang, 2003). This pathway, initiated by stimulation of members of the seven-transmembrane receptor families T1R or T2R, adequately explains how these receptor-expressing cells depolarize in response to an appropriate gustatory stimulus. These early, or primary, transduction events are subsequently followed by activation of later, or secondary, pathways involving cell-to-cell communication among the individual cells within the bud. At present, these secondary pathways are less well understood but they involve a number of neurotransmitters and neuropeptides (Herness et al. 2005; Roper, 2006). Quite probably, these primary pathways act in conjunction with the secondary pathways to ultimately excite the afferent nerve fibre and relay the presence of oral stimuli to the central nervous system.
The combination of the discovery of these new key signalling molecules in TRCs with advances in the knowledge of lipid regulation of ion channels has provided a new basis for investigation of adaptation at the primary receptor cell level. In particular, the understanding of lipid regulation by the phosphatidylinositiol 4,5-bisphosphate (PIP2) of ion channels has advanced greatly in the last several years (Hilgemann et al. 2001; Suh & Hille, 2005, 2008). It is now known that many members of the inwardly rectifying (KIR) and the delay-rectifying (KV) potassium channels are activated by PIP2, which interacts directly with the channel stabilizing its open state. Our previous work (Zhao et al. 2002) demonstrated that caffeine, a prototypic bitter stimulus, has multiple actions on TRCs that include inhibition of KIR, inhibition of KV and increases of intracellular calcium. Interestingly, all these responses display adaptation, i.e. the inhibition of the current or the elevation of calcium declines in magnitude during the continued present of caffeine. We hypothesize that PIP2 resynthesis plays a critical role in relieving the inhibition of both KIR and KV produced by caffeine. This paper provides the first evidence that potassium channels in TRCs are modulated by changing levels of PIP2 and suggests that the dynamic regulation of this lipid plays an important role in adaptation of the bitter stimulus caffeine.
Methods
Ethical approval
All procedures were approved by the University's Laboratory Animal Care and Use Committee and adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Anaesthesia and tissue/cell preparation
Experiments were performed on adult male Sprague–Dawley rats. Animals were brought to a surgical level of anaesthesia by intraperitoneal injection of 0.09 ml (100 g BW)−1 ketamine (91 mg ml−1; Hospira Inc. Lake Forest, IL) acepromazine (0.09 mg ml−1; Boehringer Ingelheim Vetmedica, Inc, St. Joseph, MO) mixture prior to kill by decapitation and excision of foliate and circumvallate papillae. Isolated taste receptor cells were dissociated from excised tissue by incubation in cysteine-activated (1 mg ml−1) papain (14 U ml−1) divalent-free bicarbonate-buffered solution (in mm: 80 NaCl, 5 KCl, 26 NaHCO3, 2.5 NaH2PO4H2O, 20 d-glucose, 1 EDTA) as previously described (Herness, 1989). This solution maintains a pH of 7.2–7.4 in a 5% CO2 incubator. After incubation, tissue blocks were transferred to a pseudo-extracellular fluid (ECF; in mm: 126 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 5 Na2Hepes (4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid, disodium salt), 1.25 NaH2PO4.H2O, 10 d-glucose, adjusted with HCl to pH 7.3) and the epithelium removed with fine forceps and subjected to mild agitation. Some papillae were maintained in an ice-cold ECF solution for later dissociation. Taste receptor cells were identified from other lingual cells by their emblematic morphology.
Reagents
Pharmacological reagents were obtained from commercial vendors. Bovine serum albumin (BSA), caffeine, heparin, 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride (LY294002), 4α-phorbol-12,13- didecanoate (4α-PDD), phosphatidylinositol (4,5) bisphosphate (PIP2), phosphatidylinositol (3,4,5) trisphosphate (PIP3), phorbol 12-myristate 13-acetate (PMA), polylysine, and wortmannin were purchased from Sigma (St Louis, MO, USA). KN-93 (N-[2-[[[3-(4′-chlorophenyl)-2- propenyl] methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4′-methoxybenzenesulpho-namide phosphate salt) and U73122 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione) were purchased from Calbiochem (San Diego, CA, USA) and ionomycin was obtained from Fluka (St Louis, MO, USA). Some agents (ionomycin, KN-93, LY294002, 4α-PDD, PMA, U73122, and wortmannin) required solubilization in dimethyl sulfoxide (DMSO). These reagents were dissolved in stock solution aliquots of 20 mg ml−1 and stored frozen. The highest tested concentration of these drugs was the equivalent of 1 : 1000 dilution of DMSO in ECF. We have previously tested ECF of standard composition and 1 : 1000 DMSO and noted no discernible effects on either sodium or potassium currents in whole cell configuration (Herness et al. 1997). PIP3 was solubilized in 10 mg ml−1 DMSO.
Electrophysiology
Potassium currents were recorded from voltage-clamped taste receptor cells isolated from rat foliate and circumvallate papillae using standard patch clamp recording techniques in the whole cell configuration as previously described (Herness, 2002). Microelectrode pipettes were pulled on a gas-cooled multistage puller from 1.5 mm (o.d.) borosilicate glass (World Precision Instruments, Sarasota, FL, USA). The composition of pseudo-intracellular fluid (ICF) used for recording KIR and KV consisted of (in mm) 130 potassium gluconate, 10 KCl, 2 MgCl2, 1 CaCl2, 11 EGTA (ethylenebis (oxonitrilo)tetraacetate), 10 Hepes (sodium salt), and 4 ATP (disodium salt). Recording electrode resistances were typically 5–7 MΩ when filled with ICF and measured in ECF. The extracellular solution for recording KIR consisted of the standard ECF recipe with the replacement of 25 mm NaCl by an equivalent amount of KCl (final extracellular potassium concentration of 30 mm). KV currents were recorded using the standard ICF and ECF compositions. A few experiments were performed using the perforated patch configuration with amphotericin B as the ionophore (400 μg ml−1 in the ICF). The composition of the perforated patch ICF was (in mm): 55 KCl, 75 K2SO4, 8 MgCl2, and 10 Hepes. A period of approximately 30 min was required to reach a stable level of recording after gigaseal formation.
To achieve the whole cell recording configuration, the pipette tip was positioned to contact the cell membrane and negative pressure was applied to its interior to facilitate gigaseal formation. Junction potentials were corrected before the electrode contacted the cell. Seal resistances were on the order of several decades of gigaohms. Further negative pressure was applied to enter whole-cell recording mode. Fast and slow capacitance compensation was employed as necessary with amplifier controls. Cell membrane capacitance and uncompensated series resistance were adjusted to produce optimal transient balancing. Membrane capacitance was 3–6 pF; series resistance averaged 10 MΩ in conventional whole-cell mode and 20–50 MΩ in most amphotericin B-perforated patch-clamp recordings. Low-pass filtering due to resistance–capacitance coupling was considered minimal. The product of these factors produces a time constant of 30–300 μs or a cutoff frequency (1/2πRC, where R is resistance and C is capacitance) of 1.6–16.6 kHz.
A test concentration of 20 mm was chosen for the caffeine stimulus. We have previously examined the effects of both 10 and 20 mm caffeine as test concentrations (Zhao et al. 2002). There were no significant differences in their electrophysiological responses. These concentrations were chosen based on their effectiveness in producing both neural and psychophysical responses in the rat. Twenty millimolar caffeine is about a mid-range concentration for the glossopharyngeal neural response and is a test concentration that is highly avoided in a two-bottle choice assay (Iwasaki & Sato, 1981). It also compares well to the human psychophysical range for caffeine, which similarly is the millimolar range (Drewnowski, 2001; Keast & Roper, 2007).
Caffeine was focally applied through a pipette positioned approximately five hundred micrometres from the recorded taste receptor cell. Flow was directed towards the apical portion of the cell against a backflow of ECF that prevented further diffusion of the stimulus. The flow rate of ECF, from a gravity-based perfusion system, was approximately 1 ml min−1. The dissociated cell preparation used in these studies potentially allows stimulation of apical and basolateral surfaces of the dissociated cell, a stimulation situation that for many tastant stimuli would be unlike that encountered under in situ conditions. In these studies we employed a stimulating technique that mostly restricts the stimulus application to the apical end of the taste receptor cell, thus mitigating the concern of basolateral exposure. Perhaps more importantly, it should be noted that basolateral exposure of the tastant stimulus caffeine most likely does occur in situ. Caffeine is membrane permeant and under in situ situations enters the taste receptor cell from the apical membrane and can reach intracellular and basolateral sites of the receptor cell. Peri et al. (2000) have demonstrated that a number of amphipathic bitter stimuli directly enter the taste receptor cell. Therefore since caffeine exposure to intracellular and basolateral sites occurs normally in situ, similar exposure in vitro is not considered problematic. Other assurances that specific transduction mechanisms are being studied include the observation that not all cells respond to a particular tastant, including caffeine, which agrees well with the heterogeneous distribution of transduction mechanisms across TRCs. Non-specific effects, such as the direct block of an ion channel or metabolic disturbances, would be expected to be common to all cells. As well, non-specific effects would not be blocked by specific pharmacological intervention. We have demonstrated, using this method, the PKC effects on caffeine as well as other studies including PKA mediation of potassium inhibition, the PKC effects on cycloheximide, β-blockers with noradrenaline stimulation, and CCK-A antagonists blocking the CCK effect. Finally, complimentary data, obtained with different techniques (patch clamp recordings or calcium imaging), are commonly impaired by pharmacological intervention of the transduction pathway. For example, tastants that produce measureable responses with both patch and fura-2 analysis, such as caffeine, are commonly blocked by agents that interfere with the IP3 pathway, suggesting a common mechanistic origin to the response as opposed to a coincident artifact. Collectively, these observations further underscore that physiological responses to caffeine stimulation are being measured.
It should be emphasized the percentage of cells responding to caffeine in our assay is not, on a scientific basis, directly comparable to the percentage of T2R expression within the taste bud as observed by other techniques, such as immunocytochemistry or in situ hybridization. Although there are several reasons that preclude this direct comparison, the most important involves sampling differences. Cells chosen for electrophysiological analysis do not represent a random sample from taste buds and hence cannot be quantitatively compared to expression patterns determined with other techniques. First, sampling for electrophysiological analysis involves an unknown bias imposed by the dissociation procedure on isolated cells. Second, isolated cells selected for electrophysiological analysis are purposefully chosen by morphological criteria; specifically, cells with large round nuclei which facilitates proper placement of the patch clamp electrode are chosen (as well as other criteria such as the absence of blebs, smooth membrane, and cytoplasmic contrast). These imposed biases could result in over selection of a particular cell type (e.g. type II cells, which are known to express T1R or T2R receptor members, have large round nuclei) that make quantitative comparison to other techniques invalid. Other considerations, such as that not all T2R expressing taste receptor cells might be responsive to caffeine or that all caffeine responsive cells necessarily require T2R expression, are further examined in the Discussion.
Data were acquired with a high-impedance amplifier (Axopatch 200A; Axon Instruments, Union City, CA, USA), a Pentium based computer, a 12-bit 330 kHz A/D converter (Digidata 1200; Axon Instruments), and a commercial software program (pCLAMP, v. 8.01; Axon Instruments). Recordings were made at room temperature. Membrane currents were acquired after low-pass filtering with a cut-off frequency of 5 kHz (at −3 dB). A software-driven digital-to-analog converter generated the voltage protocols. Data were elicited using ramp protocols. For both KIR and KV currents, ramps were driven from a holding potential of −50 mV. Ramps were generated from −140 mV to +10 mV for KIR currents and from −100 mV to +100 mV for KV currents. Command potential ramps were generated at 0.166 V s−1 and 0.125 V s−1 for measuring KIR and KV currents, respectively. Data were analysed off-line with a combination of software programs that included a software acquisition suite (pCLAMP, Axon Instruments) and a technical graphics/analysis program (Origin 7.5, OriginLab Corp., Northampton, MA, USA). KIR currents were analysed at the peak inward current, which typically occurred around −120 mV or for outward current at +10 mV. KV currents were analysed at peak outward current magnitudes, typically at +100 mV. A response was defined as adapting if its magnitude began to decline towards baseline (i.e. a reduction in the caffeine-induced inhibition) within the first 90 s during the sustained application of the stimulus and the decline continues within the next 4 min of stimulus presentation. This time period compares well with the decline of the neurophysiological response observed in the rat and psychophysical measures of adaptation in humans. Data are presented as means ± standard error of the mean. The value of current before drug application was normalized as 100%.
Statistics were performed using a one-tailed Chi-squared analysis used to evaluate the significance of the difference between control and experimental groups. Since there were no differences in the manner in which any of the control values were obtained (e.g. cell sampling, testing protocol, caffeine concentration), responses of control cells to caffeine were combined for all treatments since pooling control data in this fashion facilitates comparison as it yields a larger sample size for statistical comparison. Similarly, data obtained from foliate or circumvallate taste receptor cells were combined since our previous studies detailing ion currents in these cells have never demonstrated any significant differences between these groups. Values of P < 0.05 were considered to indicate statistical significance.
Results
Caffeine responses display adaptation
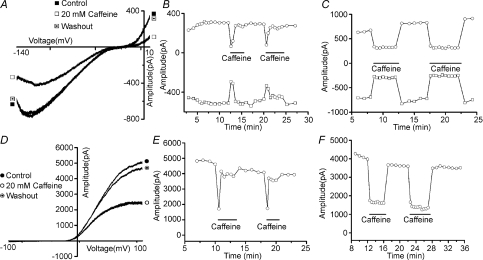
Focal stimulation of taste receptor cells with the bitter tastant caffeine results in two types of electrophysiological responses: inhibition of an inwardly rectifying potassium current (KIR; Fig. 1A) and inhibition of a delayed-rectifier potassium current (KV; Fig. 1D; Zhao et al. 2002). As expected for tastant stimulation, these inhibitions were easily reversible with washout of the stimulus. Also as expected, not all TRCs responded to caffeine. Over the varied number of control and test groups for KIR and KV, the percentage of cells unresponsive to caffeine ranged from 7 to 25%. When measuring KIR, both inward (Fig. 1A, open squares) and outward (Fig. 1A, open circles) components of the current were inhibited by caffeine exposure. Stimulation resulted in an average of 62 ± 1.2% and 40 ± 1.0% remaining current magnitudes for inward and outward components, respectively. For KV current, caffeine produced an average of 49 ± 2.4% remaining current. Many of these KIR and KV responses displayed adaptation. During the continued presence of caffeine, response magnitudes were maximal upon initial application of the stimulus and within the next few minutes declined back to prestimulus levels (Fig. 1B and E; KIR and KV, respectively). Some responses recorded from other cells did not display adaptation, i.e. the response magnitude was constant during protracted stimulus application (Fig. 1C and F; KIR and KV, respectively). Under control conditions, 58% of cells (100 of 171 cells) displayed adaptation when measuring KIR current and 31% of cells (39 of 128 cells) displayed adaptation when measuring KV.
Figure 1. Sample recordings of both KIR and KV currents from dissociated rat taste receptor cells before, during and after application of 20 mm caffeine.
Both KIR (A) and KV (D) currents, isolated for study, are inhibited by caffeine stimulation. When these responses are recorded during a prolonged continuous exposure to caffeine, some displayed adaptation, i.e. their response magnitude diminished over time. B and E illustrate adapting responses from KIR and KV, respectively. Note that the magnitude of the response, which is an inhibition of either current, diminishes over time and typically returns to baseline within 30 s although the stimulus remains present for 3 min. On the other hand, some electrophysiological responses to caffeine did not display adaptation. C and F illustrate non-adapting responses to KIR and KV, respectively, from two different cells. In these recordings the inhibition of either current was sustained throughout the stimulus presentation. In graphs representing data for KIR (B and C), open squares represent the maximum magnitude of the inward portion of the potassium current and open circles represent the maximum magnitude of the outward portion of the KIR current. In graphs representing data for KV (E and F), open circles represent the magnitude of the maximal outward KV current.
There were no differences in the averaged magnitude of the caffeine inhibition when comparing adapting and non-adapting responses (data not shown). The similarity of the observations for these two types of potassium current suggests that a similar mechanism may underlie this phenomenon. A role for PIP2, the precursor lipid for the second messenger IP3 and DAG, in this adaptation process was investigated since it has been shown to be an important regulator of ion channels, such as potassium channels, and since it is thought to play a central role in gustatory transduction mechanisms. Our results, during sustained taste stimulation, suggest that dynamic changes in PIP2 levels influence both KIR and KV potassium currents. The effects of a wide variety of pharmacological agents used in this study emerged only after the challenge of caffeine which implies study of a stimulus-dependent event. Specifically, after stimulus-evoked decline of PIP2 levels, its resynthesis in TRCs can transform non-adapting responses into those displaying adaptation.
Increasing PIP2 levels in TRCs enhances adaptation
In other cell types, PIP2 is well known to modulate both KIR and, to a lesser extent, KV channels, where its resynthesis typically activates channel activity (or in some cases removes inactivation). KIR channels typically display greater affinity for this phospholipid. Based on these observations, we hypothesized that increasing PIP2 levels should enhance adaptation of the caffeine response by relieving the inhibition of the channel produced by caffeine stimulation. To test this hypothesis, two regulatory enzymes of PIP2 were targeted: phosphatidylinositol 3-kinase (PI3K), which acts to decrease PIP2 levels by converting it to PIP3, and PLCβ2, which similarly acts to decrease PIP2 levels by using it as a substrate for IP3 and DAG production.
PI3K actions were tested with wortmannin, a cell-permeant fungal metabolite that acts as a potent and selective inhibitor of this enzyme. Wortmannin acts to block the catalytic activity of this kinase without affecting upstream signalling events. It also inhibits other kinases such as phosphoinositide 4-kinase (PI4K) at concentrations about 100-fold higher than that required for inhibition of PI3K (e.g. Suh & Hille, 2005; Etkovitz et al. 2007).
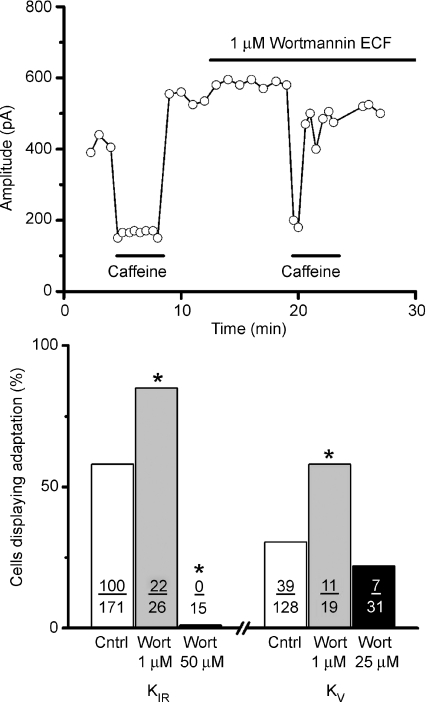
For both KIR and KV, treatment with wortmannin (1 μm) significantly increased the number of cells that responded to caffeine with adapting responses. In addition, for both currents, wortmannin treatment was able to transform a non-adapting response to an adapting response within a single cell, consistent with the notion that adaptation is promoted by wortmannin-induced PIP2 accumulation. A sample recording from a single cell is presented in Fig. 2 (top) for KIR current which illustrates a non-adapting response to 20 mm caffeine altered to an adapting response after the addition of 1 μm wortmannin. For KIR about 85% (22 of 26 cells) of TRC responses to caffeine displayed adaptation after 1 μm wortmannin exposure compared to 56% of cells in the ECF control (100 of 171 cells; Fig. 2B). This increase was statistically significant (P < 0.005) when compared with a one-tailed chi-squared analysis. Wortmannin treatment did not influence the magnitude of the inhibition produced by caffeine (38 ± 0.95%versus 35 ± 2.36%, control versus treated cells, P < 0.32; t test). These observations are consistent with the notion of PI3K blockage by wortmannin resulting in increased PIP2 levels. On the other hand, when the wortmannin concentration was increased to 50 μm, none of the tested cells displayed adaptation (0 of 15 cells), also statistically significant when compared to control cells (100 of 171 cells) using a one-tailed chi-squared analysis (P < 0.0008). These data suggest, in agreement with published data in other cell types, that in TRCs 1 μm wortmannin mainly inhibits PI3K activity, increasing PIP2 and promoting adaptation, whereas at higher concentration (50 μm) it strongly blocks PI4K, decreasing PIP2 and eliminating adaptation.
Figure 2. Adaptation of the caffeine response is strongly influenced by manipulations of endogenous levels of PIP2.
The application of 1 μm Wortmannin, an inhibitor of PI3K that acts to increase PIP2 levels by blocking its conversion to PIP3, significantly increased the number of caffeine responses displaying adaptation. Data from a representative cell are presented in the top panel. Wortmannin successfully transformed a non-adapting response to caffeine into an adapting response. In this cell, stimulation with 20 mm caffeine produced a sustained inhibition of KV yet during the application of wortmannin the response to caffeine displayed adaptation, i.e. the magnitude of the inhibition declined though the caffeine stimulus was still present. Summarized data are presented in bar graph form in the bottom panel for both KIR and KV. Wortmannin, at 1 μm (grey bars), which acts to increase PIP2 levels, significantly increased the number of cells displaying adaptation for both KIR and for KV when compared to their respective control groups. At higher concentration (black bars), where wortmannin is known to block PI4K, an enzyme in the biosynthetic pathway for PIP2, and would hence decrease PIP2 levels, wortmannin produced a significant decrease in the number of cells displaying adaptation for both KIR and KV. The number of cells displaying adaptation over the number of total cells tested is presented for each condition. Asterisks indicate statistical significance when compared to the control group (P < 0.05).
Similar effects of wortmannin were observed when recording KV currents. A low concentration of wortmannin (1 μm) statistically increased the percentage of cells displaying adaptation from a control value of 31% (39 of 128 tested cells) to 58% (11 of 19 tested cells; P < 0.0093). As with KIR, the magnitude of the inhibition of KV current by caffeine (49 ± 2.4%) was unaltered by wortmannin treatment (50 ± 2.4%). Using a high concentration of wortmannin (25 μm), expected to inhibit both PI3K and PI4K, the percentage of cells displaying responses with adaptation to caffeine dropped to 22% (7 of 31 tested cells). Although the effect of wortmannin at high concentration was in the same direction as its effect on KIR currents, this dosage failed to achieve statistical significance (P < 0.1924). Collectively, these data suggest that interfering with PIP2 production either by blocking its conversion to PIP3 or by limiting its production by PI4K produce results consistent with the hypothesis that PIP2 modulates these potassium channels.
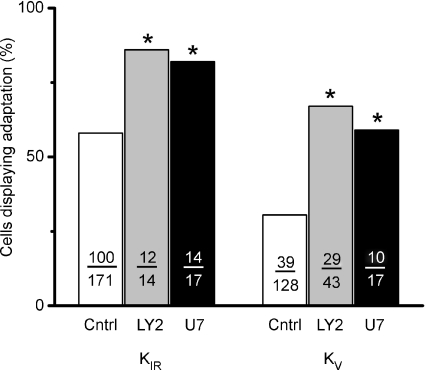
These effects of wortmannin were confirmed using additional pharmacological agents employed to manipulate endogenous PIP2 levels. Two were tested: LY294002, a potent inhibitor of PI3K, and U73122, an inhibitor of PLCβ2. Treatment with either agent would be expected to increase PIP2 levels since both enzymes use PIP2 as a substrate and therefore either agent would be predicted to increase the incidence of adaptation of the caffeine response. Consistent with previous data, both agents acted to increase the number of cells producing adapting responses to caffeine. While recording KIR, treatment with 50 μm LY294002, applied in the ICF, increased the number of caffeine responses displaying adaptation from 58% (100 of 171 cells) under control condition to 86% (12 of 14 cells; Fig. 3). Similarly, for caffeine mediated inhibition of KV, adapting responses increased from 31% to 67% (29 of 43 cells). Both effects were statistically significant when compared to control cells using a one-tailed chi-squared analysis (P < 0.0225, P < 0.0001, respectively). Similar to wortmannin treatment, the magnitude of the caffeine-induced inhibition of either KIR or KV was unaffected by LY294002 treatment. Remaining current for inward KIR current was 63 ± 3.3% after LY294002 application compared to 62 ± 1.0% for control cells. For KV measurements, the remaining current was 53 ± 2.6% compared to a control value of 49 ± 2.4%. Thus inhibition of PI3K with either low concentrations of wortmannin or LY294002 produced essentially parallel results in increasing the incidence of adaptation to caffeine stimulation.
Figure 3. Adaptation is facilitated by elevations of endogenous levels of PIP2.
Summary data for two pharmacological treatments, LY294002 (LY2), a potent inhibitor of phosphatidylinositol 3-kinase, and U73122 (U7), an inhibitor of phospholipase C, are presented as a bar graph. Both antagonists act to increase endogenous levels of PIP2, by blocking its phosphorylation to PIP3 or its hydrolysis to IP3 and DAG, respectively. Both treatments significantly increased the number of cells producing responses to caffeine with adaptation when compared to untreated control cells. These observations are consistent with the hypothesis that elevated levels of endogenous PIP2 facilitate the process of adaptation. The number of cells contributing to each point is indicated. Asterisks indicate statistical significance (P < 0.05).
Inhibition of PLCβ2 produced results qualitatively similar to that produced by LY294002 thus providing additional confirmatory evidence that elevating membrane PIP2 levels by blocking its hydrolysis facilitates the process of adaptation. While recording KIR, treatment with 125 μm U73122, applied through the pipette, increased the number of adapting cells from 58% (100 of 171 cells) to 82% (14 of 17 cells). Similarly, while recording KV, the number of adapting responses increased from 31% to 51% (10 of 17 tested cells). Both treatments were statistically significant (P < 0.0027; P < 0.0101, respectively). The magnitude of the inhibition produced by caffeine was unaffected by U73122 treatment. Remaining current for inward KIR current was 62 ± 2.6% after U73122 application compared to 62 ± 1.0% for control cells. For KV measurements, the remaining current was 51 ± 3.4% compared to a control value of 49 ± 2.4%.
Reducing PIP2 levels diminishes the incidence of caffeine adaptation
The previously described experiments produced data suggesting that pharmacological manipulations designed to increase endogenous levels of PIP2 facilitate the mechanism of adaptation of the caffeine response. Here we test additional manipulations of PIP2 designed to reduce rather than augment PIP2 levels.
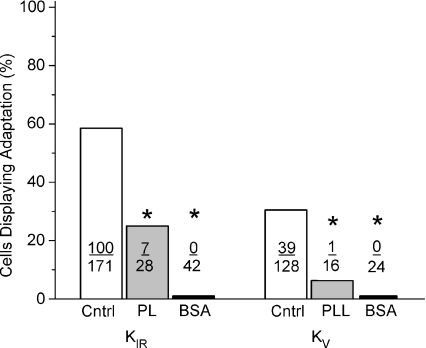
Polycations, such as neomycin, polylysine or BSA, are commonly employed as a practical treatment used for lowering PIP2 levels. They act as scavengers to block the anionic head-groups of PIP2 and consequently disrupt its ability to bind positive charges within target proteins. Here we tested polylysine and BSA on taste receptor cells hypothesizing it should effectively prevent elevations of PIP2 levels during resynthesis thus preventing PIP2 interactions with both KIR or KV channels and hence impeding the adaptation process. In preliminary experiments, different concentrations of polylysine applied via the ICF were tested. It was noted that stable recordings were difficult using high concentrations to the ICF (100 μg ml−1) and that low concentrations (5 μg ml−1) did not produce any functional changes. A mid-range concentration of 25 μg ml−1 polylysine was employed, which produced a strong and significant inhibitory effect on adaptation of the caffeine response, reducing the number of responses that displayed adaptation. For KIR, the number of responses displaying adaptation decreased from 58% to 25% (7 of 28 cells, P < 0.0005, Fig. 4). For KV, the number of adapting responses was reduced from 31% to 6% (1 of 16 cells; P < 0.0001). Polylysine treatment had minimal effects on the magnitude of the caffeine inhibition. For KIR currents, the remaining current (treatment versus control) was 78 ± 2.4%versus 62 ± 1.2% and for KV currents 47 ± 4.3%versus 49 ± 4.2%. Overall, these data thus nicely counterbalance the previously described pharmacological manipulations of PIP2 levels that demonstrated that elevations of PIP2 in TRCs facilitated the process of adaptation to the caffeine.
Figure 4. Reduction of PIP2 levels reduces the incidence of adaptation of potassium current response to caffeine.
The use of scavenger polycationic molecules, such as polylysine (PL) or bovine serum albumin (BSA), is known to decrease the effective concentration of PIP2 by binding to its anionic head-group and consequently disrupting its ability to bind positive charges within target proteins, such as potassium channels. Treatment with polylysine (PL; grey bars) produced a strong inhibitory action of the adaptation of the caffeine response. For KIR the incidence of adaptation was reduced from a control value of 58% to 25%. When recording KV currents, the incidence of adaptation was reduced from 31% to 6%. Both effects were statistically significant, as indicated by asterisks. The effect produced by BSA treatment proved to be more robust than that of polylysine. BSA acted to eliminate adaptation of the caffeine response for both tested potassium currents. No cells were observed to elicit adapting responses to caffeine in the presence of BSA when measuring either KIR or KV currents. The number of cells contributing to each bar of the graph is indicated. Asterisks indicate statistical significance (P < 0.05).
Moreover, these data are corroborated by similar treatment with BSA. BSA virtually eliminated adaptation without any significant effect on the magnitude of the inhibition produced by caffeine. When recording KIR, the number of caffeine responses displaying adaptation decreased from 58% to zero (0 of 42 cells, P < 0.0001; Fig. 4) after treatment with 0.5% BSA. For KV, the number of adapting responses was reduced from 31% to zero (0 of 24 cells; P < 0.0001). The magnitude of inhibition of the KIR by caffeine was unaffected for either KIR (63 ± 2.9% treated versus 62 ± 1.2% control) or KV (40.3 ± 2.2 treated versus 49 ± 4.2% control) currents.
Applying PIP2 or PIP3 reduced responsiveness to caffeine
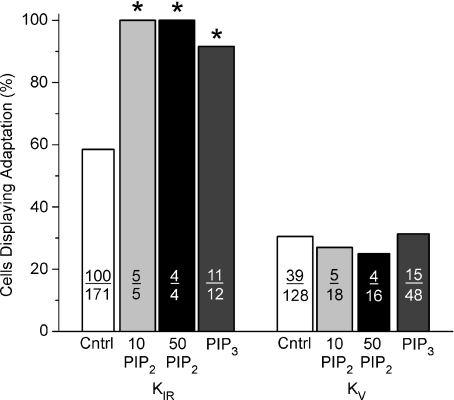
In separate experiments, the effect of direct application of exogenous PIP2 or PIP3 was tested on KIR and KV currents. PIP2 was applied to cells via pipette administration during whole-cell patch recording. It is important to note that PIP2 diffusion allows only steady increase of PIP2 as it diffuses into the cytoplasm and subsequently incorporates into the plasma membrane. This technique, though practical, does not permit precise temporal control of PIP2 concentration so that the decline and subsequent elevation occurring with resynthesis during tastant stimulation cannot be mimicked. Instead, pipette administration permits caffeine stimulation against a sustained elevated background level of PIP2. Under these conditions the most striking result when recording either KIR or KV currents was a substantial decrease in the number of cells which responded to caffeine. When measuring KIR, only 17% of TRCs (n= 9 of 52 tested cells) responded to caffeine in the presence of sustained PIP2 levels (tested at either 10 or 50 μm) compared to about 75% of tested cells under control conditions. Of those cells that responded to caffeine, all displayed responses with adaptation compared to 58% adaptation under control conditions (Fig. 5, P < 0.0311 10 μm; P < 0.047 50 μm). Thus, sustained PIP2 levels may act to stabilize KIR channels, preventing their subsequent inhibition by caffeine.
Figure 5. Exogenous application of PIP2 or PIP3 differentially affected caffeine inhibitions of KIR or KV.
Sustained application of PIP2, at either 10 μm or 50 μm, substantially increased the number of responses displaying adaptation when compared to control responses for KIR currents. At these same concentrations, exogenous PIP2 application had little effect on the incidence of adaptation when measuring KV currents. Similarly, PIP3, another phosphoinositide signalling molecule, produced an unexpected increase in adaptation of KIR but was without effect on KV. PIP3 increased the value for KIR to 92% whereas that for KV was the same as the control value (31%). The number of cells contributing to each bar is indicated. Asterisks indicate statistical significance (P < 0.05).
On the other hand, caffeine stimulation during the sustained presence of PIP2 while recording KV was more complex. Sustained PIP2 also reduced the number of cells that responded to caffeine to about 56% (10 and 50 μm; 34 of 60 tested cells). However, unlike KIR, those cells that responded to caffeine did so either with or without adaptation. The number of adapting responses was not significantly different from control values. PIP2, tested at 10 μm, produced 27% of adapting responses (n= 5/18 cells; P < 0.41) and, at 50 μm, 25% of responses displayed adaptation (n= 4/16 cells; P < 0.33).
In additional experiments, the potential role of PIP3 was tested. PIP3 was chosen for investigation since, after the initial discovery that PIP2 produced profound influence over a variety of ion channels, reports appeared that additional phosphoinositides, such as PIP3, could produce similar actions (cf. Czech, 2000). These effects, though, can be quite diverse on different classes of channels. Exogenous PIP3 was added to the patch recording pipette using the whole cell recording configuration. A test concentration of 1 μm was chosen as preliminary experiments using 5 μm failed to produce stable recordings. PIP3 had a strong effect on the adaptation of the caffeine response on KIR but was essentially without effect when recording KV currents. For KIR currents, the addition of PIP3 in the pipette increased the incidence of adaptation in the caffeine response to 92% (11 of 12), a significant increase when compared to control cells (58%; P < 0.0115). On the other hand, application of PIP3 was without effect on KV currents; 31% (15 of 48) of tested cells displayed adaptation compared to 31% for control cells (Fig. 5). These data represent one of the few examples where analogous results for KIR and KV were not observed and might be due to the stronger influence that phosphoinositides exert over inwardly rectifying potassium channels versus outward potassium channels.
Adaptation of the caffeine response can be modulated by protein kinase C
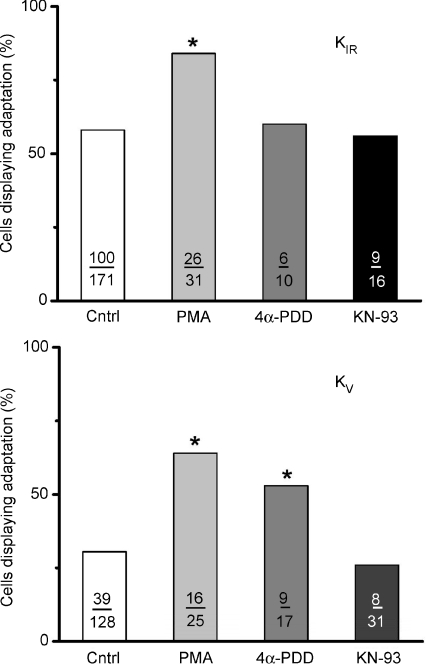
Many of the enzymes influential in the regulation of PIP2 levels are themselves subject to modulation. For example, since our present data demonstrated that PLCβ2 activity may modulate adaptation, potential regulatory actions of targets downstream of PLCβ2 were investigated. These included Ca2+/calmodulin-dependent kinase II (CaMK-II) and PKC. To test for modulatory actions of PKC, phorbol 12-myristate 13-acetate (PMA), a well-known PKC activator, was employed. PMA is a phorbol ester that affects PKC by mimicking diacylglycerol and activates both group A (α, βI, βII, γ) and group B (δ, ɛ, η, θ) protein kinases in the 1–100 nm range. Application of PMA significantly enhanced adaptation when added either to the pipette ICF solution (1 μm) or applied extracellularly (1 μm) while recording KIR. After PMA application, 84% of tested cells (26 of 31 cells) displayed adaptation compared to the control value of 58% (Fig. 8, P < 0.0036). Similar to all other pharmacological treatments, PMA had only modest effects on the magnitude of the inhibition produced by caffeine stimulation (67 ± 3.3% treated cells versus 62 ± 1.2% for untreated cells). In addition to PMA, its inactive analogue 4α-phorbol-12,13-didecanoate (4α-PDD) was tested under the same conditions. It was without effect on adaptation, yielding a value of 60% (6 of 10 cells, P > 0.4622). It was also without effect on the magnitude of inhibition (61 ± 3.7% treated cells versus 62 ± 1.2% untreated cells).
When measuring KV, similar phorbol ester effects were observed. PMA, added either to the pipette (1 μm) or to the ECF (1 μm), produced adaptation of 64% (16 of 25) of tested cells compared to 31% (39 of 128) for control cells. A modest decrease in the magnitude of the inhibition was noted (42 ± 3.5% inhibition versus 49 ± 4.2% in control cells). However, the inactive phorbol ester 4α-PDD, added to the ICF (1 μm), produced an unexpected 53% of responses with adaptation (9 of 17 tested cells) though no real effect was noted on the magnitude of inhibition (45 ± 4.2% in treated cells versus 49 ± 4.2% in control cells). The unexpectedly large effect of 4α-PDD on the incidence of adaptation suggests that PKC modulation on these potassium channels cannot be convincingly concluded.
To test for CaMK-II activity, N-[2-[[[3-(4′- chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]- N-(2-hydroxyethyl)-4′-methoxybenzenesulphonamide phosphate salt (KN-93) a selective and cell permeant CaMK-II inhibitor, and its inactive analogue, 2-[N-(4′-methoxybenzenesulphonyl)]amino-N-(4′- chlorophenyl)-2-propenyl-N-methylbenzylamine phosphate (KN-92), were tested. Inhibition of CaMK-II by KN-93 was without effect on adaptation. For KIR currents, 56% of tested cells displayed adaptation (9 of 16 cells; Fig. 6) compared to 58% (100 of 171) for control cells. The magnitude of inhibition was slightly depressed at 71 ± 5.2% remaining current compared to 62 ± 1.2% for controls cells. Similarly when recording KV current, 26% of tested cells (8 of 31 cells) displayed adaptation compared to 31% (39 of 128 cells) for control cells. The magnitude of inhibition was unaffected (50 ± 2.1% tested cells versus 49 ± 4.2% control cells). Thus neither current magnitude of inhibition was significantly affected by pharmacological manipulation of CaMK-II. Additionally, calmodulin itself appears to be vital to cell function, since 50 and 100 μmN-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide hydrochloride (W-7) added into the pipette (25, 50, or 100 μm) quickly produced irreversible damage to the cell (n= 7; data not shown). KN-92, which served as a negative control, produced results similar to KN-93 (28%; 8 of 29 cells). Collectively, these data suggest that whereas calmodulin has important functions in taste receptor cells, its participation through CaMK-II does not occur in the adaptation process to caffeine.
Figure 6. The activation of PKC could effectively increase the number of taste receptor cell responses to caffeine that displayed adaptation whereas activation of calcium–calmodulin was ineffective in modulating adaptation.
When recordings KIR current, the phorbol ester PMA, an activator of PKC, increased the number of cells displaying adaptation to caffeine from a control value of 58% to 84%. 4α-PDD, an inactive analogue of PMA, did not significantly differ from control values. KN-93, an activator of calcium–calmodulin, gave a value of 61%, which was not significantly different from control values. When recording KV currents, both PMA and its inactive analogue 4α-PDD produced significant increases in the percentage of cells displaying adaptation responses to caffeine when compared to untreated control cells (64% or 53%versus 31% for PMA, 4α-PDD, or control cells, respectively). Hence we are unable to distinguish between the possibilities that the PMA effect is artifactual (since the inactive analogue is also effective) or that the PMA is effective (as for KIR) but the inactive compound produces some artifactual response with KV channels. The calcium calmodulin activator KN-93 gave a value of 36%, which was not different from control values, suggesting it to be inactive. The number of cells contributing to each treatment value are indicated. Asterisks indicate statistical significance (P < 0.05).
Discussion
Collectively, these data strongly suggest that the response of TRCs to a gustatory stimulus is influenced by changing levels of phosphatidylinositol 4,5-bisphosphate, the first such demonstration to our knowledge. They further document modulation of both KIR and KV currents by PIP2 as an underlying mechanism for this phenomenon. PIP2 has long been hypothesized to be important in taste transduction mechanisms as a substrate for the hydrolytic production of the second messengers IP3 and DAG. As well, in other cell types PIP2 is well known to influence the gating of potassium channels, both inwardly and delayed-rectifying types (e.g. Hilgemann et al. 2001; Suh & Hille, 2005, 2008). In this study data suggest a similar phenomenon occurs in TRCs. To date, most studies of taste transduction mechanisms have focused on events leading to the decline of PIP2 while consequences of its resynthesis have remained essentially unstudied. Levels of PIP2 can be changed by multiple enzymes involved in phosphoinositide metabolism, such as phosphoinositide kinases, phosphoinositide phosphatases, and phospholipase C. Since PIP2 levels are low in most cells, estimated at only 1% of total plasma membrane phospholipid (e.g. Gamper & Shapiro, 2007), membrane proteins that are responsive to PIP2 are highly sensitive to small changes in its levels, such as receptor-induced depletions and subsequent resynthesis even during prolonged stimulation. The data of the present communication suggest that potassium channels in TRCs are strongly influenced by PIP2 levels and these events may influence later events occurring after the initial transduction mechanisms, namely adaptation.
Actions of caffeine on TRCs
Our previous study on the effects of the bitter stimulus caffeine on TRCs (Zhao et al. 2002) suggested multiple effects occur including inhibition of potassium channels and elevation of intracellular calcium. To date the transduction steps for caffeine remain unresolved. In the canonical view of bitter taste transduction, PIP2 levels would be expected to be initially reduced via a caffeine-initiated stimulation of the T2R–gustducin–PLCβ2 cascade. Alterations in membrane potential of the TRC subsequently result from depolarization via TRPM5 activation followed by subsequent activation of voltage-activated ion channels, such as sodium and potassium channels. Activation of PLCβ2, the PLC isoform expressed in TRCs (Rossler et al. 1998), and subsequent PIP2 hydrolysis, is widely acknowledged as essential for the transduction of stimuli such as sweet and bitter, due, for example, to the inability of PLCβ2 knockout mice to respond, either neurophysiologically or behaviourally, to several compounds representing these qualities (Zhang et al. 2003). Steps leading to activation of the TRPM5 ion channel in this cascade at present remain obscure but are hypothesized to involve calcium. The release of calcium from intracellular stores by IP3 production may be necessary to gate the TRPM5 channel (Zhang et al. 2007). An influx of a cation current through the TRPM5 channel fully depolarizes the TRC, which in turn activates voltage-gated ion channels within the cell to potentially trigger an action potential. The large majority of the caffeine-responsive TRCs in our study (Zhao et al. 2002) expressed sodium currents, suggesting these TRCs are electrically excitable and capable of firing action potentials. Further caffeine application did not inhibit these sodium currents, thus not interfering with the cell's ability to fire action potentials.
Others have suggested that T2R-independent mechanisms may exist for bitter stimuli (e.g. Rosenzweig et al. 1999; Peri et al. 2000; Caicedo et al. 2003; Dotson et al. 2005; Nelson et al. 2005; Zubare-Samuelov et al. 2005). Support for this notion comes from observations that elimination of transduction elements such as α-gustducin or PLCβ2 did not completely eliminate bitter responses or that other pathways, such as cGMP may be involved. Our data do not directly address whether caffeine may operate via T2R-dependent or T2R-independent mechanisms. Although it is likely that caffeine operates through a receptor in the T2R family, to date such a receptor in the T2R family with caffeine as its ligand has yet to be identified. However, whereas T2R-dependent mechanisms may exist, T2R-indepdnent mechanisms for caffeine are also likely. Caffeine is known to enter cells directly and may interact with a variety of intracellular enzymes and/or ion channels. Further, caffeine was not tested on PLCβ2 knock-out mice so that a complete dependence of the caffeine response on PLCβ2 has not yet been established. As well, the caffeine response was not eliminated in an IP3R knockout mouse (Hisatsune et al. 2007), suggesting that the PLCβ2-IP3 canonical pathway may not be essential to caffeine transduction. Finally, in our data the inhibition of potassium current by caffeine was not eliminated by inhibition of PLCβ2, though adaption was effected. Caffeine may well have more than one transduction mechanism. As the avoidance of bitter stimuli is essential to survival, multiple bitter detection mechanisms would be evolutionarily advantageous.
The inhibition of potassium currents in TRCs by bitter stimuli has been observed in several organisms (e.g. Avenet & Lindemann, 1987; Cummings & Kinnamon, 1992; Chen & Herness, 1997; Seto et al. 1999; Zhao et al. 2002; Straub et al. 2003). Bitter stimuli inhibit potassium channels during both active (KV) and resting (KIR) states. The mechanistic steps connecting bitter stimulation and potassium channel inhibition is not known with certainty. KV channels in TRCs are known to be inhibited by cAMP (Herness et al. 1997) and bitter stimuli may influence cyclic nucleotide levels. For example, quench-flow analysis has shown that caffeine stimulation produced not only measurable increases in IP3 production (Spielman et al. 1994) but also more robust production of cGMP (Rosenzweig et al. 1999). Some KIR channels may be inhibited by the βγ subunit of G-proteins, which could occur after receptor-mediated G-protein stimulation. Regardless of the mechanism, inhibition of potassium currents by a tastant stimulus such as caffeine has several potential actions on the TRC. Like other cell types, KIR contributes substantially to the resting potential of TRCs (Sun & Herness, 1996) and its inhibition would act to depolarize the cell, potentially stimulating voltage-gated channels, such as the sodium channel, and action potential production. Inhibition of KV by either the potassium channel blocker TEA or by caffeine acts to broaden the action potential (Chen et al. 1996; Zhao et al. 2002) keeping the cell in a depolarized state for a longer period of time. The relaxation of caffeine's inhibition on these inhibitory potassium currents that we propose occurs by PIP2 resynthesis thus acts to reduce the cell's excitability, as would occur during adaptation of a tastant response.
PIP2 regulation of potassium channels
Our data demonstrate that two types of potassium currents, KIR and KV, are influenced by dynamic changes in PIP2 levels in TRCs. A variety of pharmacological manipulations, all targeted to alter PIP2 levels during stimulus-induced resynthesis, produced consistent results on the incidence of adaptation: increasing PIP2 levels increased adaptation while decreasing PIP2 levels produced the converse effect. These events are summarized in Fig. 7. Pharmacological manipulation of enzymes at a number of points in this synthetic/hydrolytic pathway of PIP2 produced similar results. Three manipulations that would elevate PIP2 levels were performed: blocking PI3K with either wortmannin or LY294002 or blocking PLCβ2 using U73122. All significantly enhanced adaptation, as would be predicted with elevated PIP2 levels in the membrane. Conversely, blocking its synthetic enzyme PI4K with higher concentrations of wortmannin and thus reducing PIP2 levels produced the expected results of decreasing adaption observed with KIR and KV. Similarly, reducing PIP2 levels using a complimentary method of polylysine or BSA scavengers, which prevent the phospholipid from interacting with the channel, also diminished the incidence of adaptation of the caffeine response.
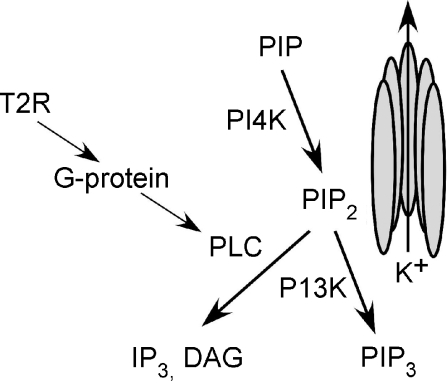
Figure 7. Summary of the pharmacologically targeted enzymes that influence PIP2 interactions with potassium channels.
The phospholipid PIP2 is hypothesized to interact directly with either KIR or KV potassium channels where dynamic fluctuations in its level influence channel activity. Pharmacological inhibition of the enzymes PLC or PI3K, either of which acts to increase PIP2 levels, resulted in an increase in the occurrence of adaptation of either potassium current to caffeine stimulation. On the other hand, treatments acting to decrease PIP2 levels, such as pharmacologically blocking its synthetic enzyme, PI4K, or BSA or polylysine application, which interferes with PIP2 interactions with the channels, resulted in a decrease in the occurrence of adaptation to caffeine stimulation. These findings suggest that potassium channels expressed in taste receptor cells are sensitive to fluctuations of PIP2 and that PIP2 resynthesis, which would occur after tastant stimulation, may act to restore channel activity.
Two additional observations support the notion of relief of the caffeine inhibition by PIP2 resynthesis. First, these effects emerged only after TRCs were challenged with a caffeine stimulus, suggesting stimulus-dependent resynthesis is required for the relaxation of the caffeine-induced inhibition. Pharmacological alterations of the potassium currents prior to caffeine stimulation were not noted. Second, none of these manipulations altered the initial magnitude of the caffeine-induced inhibition of the potassium current. Hence, their effects were noted only after stimulation, when resynthesis would occur, but not during the start of the caffeine-initiated inhibition. Collectively, they suggest that the inhibition of the potassium current induced by caffeine is mitigated by elevating levels of PIP2 as would occur during its resynthesis subsequent to a stimulus-induced decline.
Direct application of phospholipid also affected the incidence of adaptation though these data represented one of the few examples where analogous results for KIR and KV were not observed. Both PIP2 and PIP3 had obvious effects on KIR whereas both were much less effective on KV. This difference may be explained by the stronger influence that phosphoinositides exert over inwardly rectifying potassium channels versus outward potassium channels (Suh & Hille, 2008). Structural differences of the KIR (with two transmembrane domains) and KV (with six transmembrane domains) are likely to play important roles. Polybasic domains (for KIR just after the second transmembrane segment and for KV just after the sixth transmembrane segment) are the putative interactive residues of the ion channels with PIP2. This same phenomenon could underlie the greater incidence of adaption observed when measuring KIR (58%) when compared to KV (31%) currents in general.
The increased number of adaptive cells observed when levels of PIP2 are elevated suggests that PIP2 acts to stabilize both KIR and KV potassium channels, consistent with the notion that this phospholipid acts to maintain their open state (e.g. Logothetis et al. 2007; Tucker & Baukrowitz, 2008), thus mitigating the inhibition that otherwise would have resulted from caffeine stimulation. In general PIP2 regulation of KV channels is much less studied than regulation of KIR (e.g. Bian & McDonald, 2007). PIP2 regulation of KV channels may differ in that both regulation of inactivation and regulation of activation could be occurring. In general it does not appear that regulation of inactivation of KV is occurring in TRCs. Outward potassium currents in TRCs consist of inactivating and sustained components with time constants on the order of several seconds over several seconds (Chen et al. 1996). When caffeine was applied after having evoked outward KV currents for an extended duration (e.g. 90 s), and hence recording only sustained potassium current, caffeine produced inhibitions with adaptation (personal observations). Since caffeine would be inhibiting potassium current with no inactivation under these conditions, it would suggest that removing inactivation is not the underlying mechanism for PIP2 modulation. It has been demonstrated that with KCNQ channels, a type of delayed rectifier channel with very slow inactivation kinetics, PIP2 acts to stabilize the open state of the channel (Loussouarn et al. 2003).
That PIP2 and PIP3 exerted parallel actions on KIR adds to a growing list of ion channels where these two phosphoinositides act agonistically rather than antagonistically. Both PIP2 and PIP3 may promote PIP2 synthesis by activating PI4P5-kinase (which synthesizes PIP2) via small GTPase ADP ribosylation factor (ARF) proteins (Czech, 2000; Skippen et al. 2002). As well, both connect to pathways that tend to inhibit PI3K (Czech, 2000). Although it is difficult to make generalizations about the actions of PIP2 and PIP3 on different ion channels or transporters, both also have similar actions on many TRP channels, such as the TRPC6 channel where PIP3 may activate TRPC6 current by displacing Ca2+–calmodulin thereby increasing the current (Kwon et al. 2007). These results suggest that PIP2 and PIP3 regulation of potassium channels in TRCs may be more similar to their reported modulatory roles in TRP channels than to their reported modulatory roles for other types of ion channels. For example, in olfactory receptor neurons PIP2 and PIP3 have opposing actions on CNG channels (Zhainazarov et al. 2004; Brady et al. 2006). As well, application of exogenous PIP2 must be affecting numerous membrane signalling pathways in the membrane that could differentially affect these types of potassium channels.
PIP2 regulation may be subject to modulation since activation of the regulatory kinase PKC increased adaptation. The exact mechanism of PKC modulation of adaptation isn't yet understood. Based on other systems, it is likely that the catalytic activity of PLCβ2 is feedback regulated by PKC phosphorylation. PKC, once activated, would act to phosphorylate PLCβ2 in turn reducing its activity, and hence maintaining higher levels of PIP2 with the continuance of adaptation. Additionally, PIP5K, synthetic to PIP2, can be regulated positively by a PKC-mediated pathway (Park et al. 2001). These events will keep the PIP2 levels high after PI4K was activated by calcium entry. Although activation of PKC increased adaptation for both KIR and KV currents, control experiments with the inactive analogue of the PKC activation, 4α-PDD, failed when tested on KV currents. Although this calls into question results with PKC modulation of PIP2 activation with KV currents, other experiments on KV currents conducted with 50 nm bisindolylmaleimide, an antagonist of PKC, acted to eliminate adaptation (data not shown). These results suggest that 4α-PDD could produce unknown actions on KV channels. Further study is required before any conclusion can be made on PKC modulation of the PIP2 influence on adaption for KV currents.
Finally, the molecular identity of the ion channels underlying these currents is not yet known. KIR was among the first of any ion channels documented to be influenced by PIP2 levels and remains the best analysed (e.g. Xie et al. 2007). Receptor-induced depletion of PIP2 can inhibit KIR channels and its resynthesis stabilize the channel in the open configuration by interacting with basic residues on the channel. Rundown is accelerated by manipulations that lower PIP2 (e.g. PIP2 antibodies, PLCβ2 activation, or polycations) and is slowed or reversed by manipulations that increase PIP2 such as phosphatase inhibitors or PIP2 itself. The channel types KIR1 (ROMK), KIR2, KIR3 (GIRK) and KIR6 (KATP) are all activated by PIP2. Although KIR currents in TRCs have been characterized (Sun & Herness, 1996) the molecular identity of these channels remains unknown. The molecular identity of KV type channels in TRCs is less well studied, thought KV1.5 and KV3.1 are well expressed (Liu et al. 2005). Whether these channel types are modulated by PIP2 remains an open question. Outside of a report that interaction of PIP2 with outwardly rectifying potassium channels can produce a functional change from inactivation to non-inactivation (i.e. A-type or delayed rectified type; Oliver et al. 2004) there is little in the literature on KV type channels and PIP2. Thus, our data may represent fertile ground for future study.
PIP2 and adaptation in gustatory receptor cells
The mechanisms of gustatory adaptation at the periphery are incompletely understood. Peripheral adaptation was first documented with the first chorda tympani electrophysiological recording (Pfaffmann, 1941). Early studies on adaptation focused on the decline of the chorda tympani neural response to maintained gustatory stimulation (Smith et al. 1978). Time constants of the decline of the chorda tympani response and those describing the decline of perception were in close agreement suggesting the temporal pattern of action potentials did represent a neural correlate of perception. Although there was initial debate as to whether adaptation observed in the chorda tympani firing pattern was produced by adaptation within the primary receptor cell (or, e.g. at the receptor cell:nerve synapse), recordings of the gustatory action potential in taste receptor cells to sustained stimulation firmly established the phenomenon occurred in the receptor cell (e.g. Béhé et al. 1990). With more recent discoveries of primary transduction pathways, the study of adaptation has received comparatively little attention. Our results suggest that at least one component of the adaptation process resides in the TRC itself. However, since only one stimulus has been tested, it is premature to generalize these effects to other bitter or sweet stimuli.
Collectively the experiments presented here present a unified view for the role of PIP2 resynthesis in the adaptation process to caffeine. Pharmacological manipulations that elevated PIP2 levels enhanced adaptation whereas those manipulations that acted to decrease PIP2 levels had the converse effect. It is probable that one mechanism does not account for the entire adaptation mechanistic scheme. Other events, such as receptor and/or enzyme phosphorylation or the influence of PIP2 resynthesis on other target proteins (such as TRPM5, hemichannels, and/or the IP3 receptor) are likely to participate in this process within the TRC. Additionally, the effects of PIP2 are not restricted to adaptation. Nevertheless, potassium channels in TRCs are clearly modulated by PIP2 resynthesis, a novel observation in itself, and restoring them to their open state appears a likely substrate for adaptation. The participation of extracellular calcium in this process and its route of ingress are the subjects for future investigation.
Acknowledgments
This work was supported by NIH NIDCD DC00401.
References
- Avenet P, Lindemann B. Patch-clamp study of isolated taste receptor cells of the frog. J Membr Biol. 1987;97:223–240. doi: 10.1007/BF01869225. [DOI] [PubMed] [Google Scholar]
- Béhé P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J-S, McDonald TV. Phosphatidylinositol 4,5-bisphosphate interactions with the HERG K+ channel. Pflugers Arch. 2007;455:105–113. doi: 10.1007/s00424-007-0292-5. [DOI] [PubMed] [Google Scholar]
- Brady JD, Rich ED, Martens JR, Karpen JW, Warnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2006;103:15635–15640. doi: 10.1073/pnas.0603344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit α-gustducin in taste cell responses to bitter stimuli. J. Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJB, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chen Y, Herness S. Electrophysiological actions of quinine on dissociated rat taste receptor cells. Pflugers Arch. 1997;434:215–226. doi: 10.1007/s004240050388. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun X-D, Herness MS. Characteristics of the action potentials and their underlying outward currents in rat taste cells. J Neurophysiol. 1996;75:820–831. doi: 10.1152/jn.1996.75.2.820. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Kinnamon SC. Apical K+ channels in Necturus taste cells. J Gen Physiol. 1992;99:591–613. doi: 10.1085/jgp.99.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP. PIP2 and PIP3: Complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. PLCβ2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Sens. 2005;30:593–600. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A. The science and complexity of bitter taste. Nutrition Rev. 2001;59:163–169. doi: 10.1111/j.1753-4887.2001.tb07007.x. [DOI] [PubMed] [Google Scholar]
- Etkovitz N, Rubinstein S, Daniel L, Breitbart H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol Reprod. 2007;77:263–273. doi: 10.1095/biolreprod.106.056705. [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Target-specific PIP2 signalling: how might it work? J Physiol. 2007;582:967–975. doi: 10.1113/jphysiol.2007.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilberston TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10:519–527. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD. Taste transduction: appetizing times in gustation. Neuroreport. 2003;14:905–911. doi: 10.1097/01.wnr.0000074346.81633.76. [DOI] [PubMed] [Google Scholar]
- Herness S. A dissociation procedure for mammalian taste cells. Neurosci Lett. 1989;106:60–64. doi: 10.1016/0304-3940(89)90202-4. [DOI] [PubMed] [Google Scholar]
- Herness S. Researching isolated taste receptor cells: deciphering transduction cascades with patch-clamp and calcium-imaging techniques. In: Simon SA, Nicolelis MA, editors. Methods and Frontiers in Neuroscience: Methods in Chemosensory Research. Boca Raton, FL, USA: CRC Press; 2002. pp. 169–206. [Google Scholar]
- Herness MS, Sun X-D, Chen Y. Cyclic AMP and forskolin inhibit potassium currents in rat taste cells by different mechanisms. Am J Physiol Cell Physiol. 1997;272:C2005–C2018. doi: 10.1152/ajpcell.1997.272.6.C2005. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao F-L, Kaya N, Shen T, Lu S-G, Cao Y. Communication routes within the taste bud by neurotransmitters and neuropeptides. Chem Senses. 2005;30(Suppl. 1):i37–i38. doi: 10.1093/chemse/bjh101. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Science STKE 2001. 2001. p. RE19. [DOI] [PubMed]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Sato M. Neural responses and aversion to bitter stimuli in rats. Chem Senses. 1981;6:119–128. [Google Scholar]
- Keast RSJ, Roper J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem Senses. 2007;32:245–253. doi: 10.1093/chemse/bjl052. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC, Margolskee RF. Mechanisms of taste transduction. Curr Opin Neurobiol. 1996;6:506–513. doi: 10.1016/s0959-4388(96)80057-2. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol. 2005;289:C868–C880. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A. Phosphoinositide-mediated gating of inwardly rectifying K+ channels. Pflugers Arch. 2007;455:83–95. doi: 10.1007/s00424-007-0276-5. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Park K-H, Bellocq C, Baró Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TM, Munger SD, Boughter JD. Haplotypes at the Tas2r locus on distal chromosome 6 vary with quinine taste sensitivity in inbred mice. BMC Genet. 2005;6:32. doi: 10.1186/1471-2156-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Lien C-C, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectified K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- Park SJ, Itoh T, Takenawa T. Phosphatidylinositol 4-phosphate 5-kinase type I is regulated through phosphorylation response by extracellular stimuli. J Biol Chem. 2001;276:4781–4787. doi: 10.1074/jbc.M010177200. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Ashot Kozak J, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, Nir S, Naim M. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Physiol. 2000;278:C17–C25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- Pfaffmann C. Gustatory afferent impulses. J Cell Comp Physiol. 1941;17:243–258. [Google Scholar]
- Roper SD. Cell communication in taste buds. Cellular Mol Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, Yan W, Dasso M, Spielman AI. Possible novel mechanism for bitter taste mediated through cGMP. J Neurophysiol. 1999;81:1661–1665. doi: 10.1152/jn.1999.81.4.1661. [DOI] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase Cβ subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Seto E, Hayahi Y, Mori T. Patch clamp recording of the responses to three bitter stimuli in mouse taste cells. Cell Mol Biol. 1999;45:317–325. [PubMed] [Google Scholar]
- Skippen A, Jones DH, Morgan CP, Li M, Cockcroft S. Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-disphosphate synthesis in HL60 cells. J Biol Chem. 2002;277:5823–5831. doi: 10.1074/jbc.M110274200. [DOI] [PubMed] [Google Scholar]
- Smith DV, Bealer SL, van Buskirk RL. Adaptation and recovery of the rat chorda tympani response to NaCl. Physiol Behav. 1978;20:629–636. doi: 10.1016/0031-9384(78)90256-1. [DOI] [PubMed] [Google Scholar]
- Smith DV, Margolskee RF. Making sense of taste. Sci Am. 2001;284:32–39. doi: 10.1038/scientificamerican0301-32. [DOI] [PubMed] [Google Scholar]
- Spielman A, Huque T, Nagal H, Whitney G, Brand JG. Generation of inositol phosphates in bitter taste transduction. Physiology & Behavior. 1994;56:1149–1155. doi: 10.1016/0031-9384(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Straub SG, Mulvaney-Musa J, Yajima H, Weiland GS, Sharp GW. Stimulation of insulin secretion by denatonium, one of the most bitter-tasting substances known. Diabetes. 2003;52:356–364. doi: 10.2337/diabetes.52.2.356. [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:307–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X-D, Herness S. Characterization of inwardly-rectifying potassium currents from dissociated rat taste receptor cells. Am J Physiol Cell Physiol. 1996;271:C1221–C1232. doi: 10.1152/ajpcell.1996.271.4.C1221. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Baukrowitz T. How highly charged anionic lipids bind and regulate ion channels. J Gen Physiol. 2008;131:431–438. doi: 10.1085/jgp.200709936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L-H, John SA, Ribalet B, Weiss JN. Activation of inwardly rectifying potassium (Kir) channels by phosphatidylinosital-4,5,bisphosphate (PIP2): Interaction with other regulatory ligands. Prog Biophys Mol Biol. 2007;94:320–335. doi: 10.1016/j.pbiomolbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by PtdIns(3,4,5)P3. J Membr Biol. 2004;201:51–57. doi: 10.1007/s00232-004-0707-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F-L, Lu S-G, Herness S. Dual actions of caffeine on voltage-dependent currents and intracellular calcium in taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R115–R129. doi: 10.1152/ajpregu.00410.2001. [DOI] [PubMed] [Google Scholar]
- Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Tirosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:C483–C492. doi: 10.1152/ajpcell.00547.2004. [DOI] [PubMed] [Google Scholar]