Abstract
The aim of the present study was to compare properties of excitatory and inhibitory spinal intermediate zone interneurons in pathways from group I and II muscle afferents in the cat. Interneurons were labelled intracellularly and their transmitter phenotypes were defined by using immunocytochemistry. In total 14 glutamatergic, 22 glycinergic and 2 GABAergic/glycinergic interneurons were retrieved. All interneurons were located in laminae V–VII of the L3–L7 segments. No consistent differences were found in the location, the soma sizes or the extent of the dendritic trees of excitatory and inhibitory interneurons. However, major differences were found in their axonal projections; excitatory interneurons projected either ipsilaterally, bilaterally or contralaterally, while inhibitory interneurons projected exclusively ipsilaterally. Terminal projections of glycinergic and glutamatergic cells were found within motor nuclei as well as other regions of the grey matter which include the intermediate region, laminae VII and VIII. Cells containing GABA/glycine had more restricted projections, principally within the intermediate zone where they formed appositions with glutamatergic axon terminals and unidentified cells and therefore are likely to be involved in presynaptic as well as postsynaptic inhibition. The majority of excitatory and inhibitory interneurons were found to be coexcited by group I and II afferents (monosynaptically) and by reticulospinal neurons (mono- or disynaptically) and to integrate information from several muscles. Taken together the morphological and electrophysiological data show that individual excitatory and inhibitory intermediate zone interneurons may operate in a highly differentiated way and thereby contribute to a variety of motor synergies.
It has long been established that interneurons in excitatory and inhibitory reflex pathways from muscle afferents are under the control of different supraspinal systems (Holmqvist & Lundberg, 1959; Holmqvist & Lundberg, 1961; Lundberg, 1982; Aggelopoulos et al. 1996) and recent studies show that activation of excitatory and inhibitory interneurons is regulated differentially in some behavioural contexts. For instance, inhibition of motoneurons dominates under resting conditions but is depressed during locomotion (Gossard et al. 1994; McCrea et al. 1995; Perreault et al. 1995, 1999; Quevedo et al. 2000) or following spinal cord injury, when excitation is released (Arya et al. 1991; Aggelopoulos & Edgley, 1995; Aggelopoulos et al. 1996). However, the extent to which this differential regulation might depend on properties of premotor interneurons mediating excitation or inhibition of motoneurons, or on neurons that provide input to premotor interneurons, has not been established.
Excitatory and inhibitory interneurons that are activated by primary afferents clearly play different roles in motor behaviour and it is of fundamental importance that we know what differences exist between them in terms of input properties, morphology and, especially, axonal projections and target cells. However, until recently, possibilities to compare properties of excitatory and inhibitory premotor interneurons have been limited. In the cat, a number of extracellularly recorded interneurons inducing EPSPs or IPSPs in motoneurons were identified by using spike-triggered averaging (Brink et al. 1981, 1983; Cavallari et al. 1987), but as these interneurons could only be penetrated in exceptional circumstances (see Fig. 1 in Cavallari et al. 1987), this precluded a systematic comparison of excitatory and inhibitory interneurons according to input, morphology and immunohistochemistry. Recently, spike-triggered averaging has been used to analyse actions of premotor interneurons on motoneurons in lamprey (see, e.g. Biro et al. 2008), frog tadpole (see, e.g. Li et al. 2007), zebra fish (see, e.g. Ritter et al. 2001) and neonatal rats and mice in vitro (Butt et al. 2002; Butt & Kiehn, 2003; Kiehn & Butt, 2003; Kiehn, 2006; Quinlan & Kiehn, 2007). Records from these interneurons were obtained by using whole-cell-tight-seal recording that also permitted the use of intracellular markers and subsequent visualization of recorded cells. Comparison of morphology of excitatory and inhibitory subpopulations of these neurons involved mapping cell locations and projections of stem axons. The pharmacology of their actions on motoneurons was analysed by using antagonists to putative neurotransmitters and examining firing patterns during fictive locomotion or other forms of rhythmic activity. However in vitro preparations provide limited opportunities to analyse input to neurons and to differentiate between input–output related functional subpopulations. In the present study we used immunohistochemistry to identify excitatory and inhibitory intermediate zone interneurons in reflex pathways from group I and II muscle afferents which were labelled intracellularly (Bannatyne et al. 2003, 2006; Stecina et al. 2008).
Figure 1. An ipsilaterally projecting glutamatergic interneuron.
A, a reconstruction of the soma, dendrites and initial course of the axon of interneuron C in Fig. 6. B and C, series of confocal microscope images showing terminals of this interneuron and their relationships with immunoreactivity for neurotransmitter markers. Ba and Ca show projected images of the axon (red) through a number of optical sections; panels b–d show single optical sections illustrating axon terminals (red, arrowheads) and neurotransmitter markers: the sequence in Bb–d shows that the terminals are immunoreactive for VGLUT2 (blue) but not GAD (green). Cb–d, no association was found with either VGLUT1 (shown in blue) or gephyrin (shown in green). Da–d, a series of single optical sections showing contacts between the terminals of this axon (red, arrows) and the soma (a and b) and dendrites (c and d) of motoneurons labelled with antibodies raised against ChAT (green) in the lateral motor nucleus of the L6 segment. VGLUT2 immunoreactivity is shown in blue. Scale bars: A 100 μm; Ba–d 5 μm Ca 5 μm; Cb–d 2 μm; Da, Dc 5 μm; Db, Dd 2 μm.
Methods
Preparation
The experiments were performed on a total of 13 young adult cats under deep anaesthesia. All procedures were approved by the Göteborg University Ethics Committee and complied with US National Institutes of Health and European Union guidelines. General anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose as required to maintain full anaesthesia (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–2 h, up to 55 mg kg−1, i.v.) after cannulation of the trachea, one carotid artery and both cephalic veins. Blood pressure and heart rate were monitored continuously. Additional doses of α-chloralose were given when increases in blood pressure or heart rate were evoked by noxious stimulation, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 37.5°C by servo-controlled infrared lamps. Experiments were terminated by a lethal dose of pentobarbital followed by perfusion with a solution of paraformaldehyde.
The third to seventh lumbar (L3–L7), and low thoracic (Th11–Th13) segments were exposed by laminectomy. Neurons in the lumbar segments were approached through small holes (about 1–2 mm2) made in the dura mater. Two pairs of stimulating electrodes were placed in contact with the left and right lateral funiculi at low thoracic levels to allow detection of neurons with axons ascending beyond this level by antidromic activation using stimulus intensities of 1mA. Two contralateral hindlimb nerves (the quadriceps, Q and sartorius, Sart) and a number of ipsilateral nerves were transected and mounted on stimulating electrodes. The latter included Q, Sart, the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius and soleus (GS), plantaris (Pl) and deep peroneal (DP) nerves. The nerves were stimulated by constant voltage 0.2 ms long current pulses, with the intensity expressed in multiples of threshold (T) for the most excitable fibres in a given nerve. In most experiments a tungsten electrode (impedance 40–250 kΩ) was placed in the ipsilateral medial longitudinal fascicle (MLF) after having exposed the caudal part of the cerebellum. The electrode was inserted at an angle of 30 deg (with the tip directed rostrally). The initial target was at Horsley–Clarke co-ordinates P9, L0.6, H–5 but its final position was adjusted on the basis of records of descending volleys from the surface of the lateral funiculus at the Th11 level. The site of the tip of the electrode was marked by an electrolytic lesion (0.4 mA constant current passed for 10 s) and subsequently verified on 100 μm thick frontal sections of the brainstem, cut in the plane of insertion of the electrodes using a freezing microtome.
Cells to be labelled were selected on the basis of input from group II or both group I and II afferents and axonal projections restricted to the lumbosacral enlargement (cells antidromically activated form the last thoracic segment were not included in the sample). A search was made in the L3–L7 segments, within regions 1–2 mm apart, where distinct field potentials were evoked from both group I and II afferents (Edgley & Jankowska, 1987a,b). The sample was intentionally non-homogeneous and included interneurons projecting ipsilaterally as well as contralaterally (i.e. intermediate zone commissural interneurons). Short distance projections (ipsilateral and/or contralateral) were defined by reconstructing the trajectories of the axons. Longer distance projections were established by antidromic activation of some of the interneurons from the area between the gastrocnemius–soleus and posterior semitendinosus motor nuclei where stimuli (0.2 ms, 5–100 μA) were applied via a thin tungsten electrode (100–300 kΩ).
Electrophysiological analysis of the labelled interneurons and their classification
Micropipettes with tips broken to about 2 μm, filled with a mixture of tetramethylrhodamine-dextran and Neurobiotin in saline (see next section) with impedances of 12–20 MΩ were used. Recording from neurons commenced prior to penetration, provided that extracellular spike potentials were sufficiently distinct. At this stage it was verified that the neurons were (1) activated by stimulation of the Q nerve and/or other muscle nerves at 5T and at latencies compatible with oligosynaptic input, (2) that they were activated antidromically from the contralateral G-S motor nuclei (when tested), and (3) that they were not activated antidromically by stimuli applied to the lateral funiculi at the low thoracic level. A collision test with synaptically evoked spikes was used in order to evaluate whether the neurons were activated antidromically. After penetration, effects of the same stimuli were rapidly scanned to ensure that we had penetrated the same neuron; thereafter the ejection of the marker was started by passing a depolarizing current. More detailed analysis of the peripheral and descending input to the labelled neurons was made while the depolarizing current was passed. To this end, PSPs evoked by stimulation of all of the dissected nerves were first recorded while using 5T stimuli and graded stimuli were subsequently applied only to the effective nerves. If the condition of a neuron allowed, recording was repeated after labelling had been completed but the best quality records were usually obtained just after the penetration (when the injury discharges stopped) and at the beginning of the injection. Unless stated otherwise, all of the records illustrated were obtained at the beginning of depolarization. In cases where the electrode penetrated an interneuron which had not been analysed extracellularly, characterization of the neuron was made intracellularly and the beginning of the ejection of the marker was delayed. Both original records and averages of 10–20 records made on-line were stored using a sampling and analysis system of E. Eide, T. Holmström and N. Pihlgren, Göteborg University.
The aim of the study was to compare excitatory and inhibitory intermediate zone interneurons. However, in order to achieve this, it was necessary to differentiate these cells from two other populations of interneurons that have been previously described: (1) dorsal horn interneurons located more dorsally and (2) lamina VIII interneurons located more ventrally (Edgley & Jankowska, 1987b; Jankowska et al. 2005) both of which have input from group II but not from group I afferent fibres. When somata of labelled interneurons were located within laminae VI–VII, IV–V or VIII, classification was straightforward. However, additional criteria were required to establish the identity of neurons located within the border zones between laminae V and VI and between laminae VII and VIII where different cell categories may be intermixed. In such cases additional criteria based on established properties of larger samples of interneurons at various locations were used. ‘Typical’ dorsal horn or lamina VIII interneurons were not found to be coexcited by group I afferents and those which were coexcited were classified as intermediate zone interneurons even if their somata were located in the most ventral part of lamina V or the most dorsal part of lamina VIII. Conversely, interneurons located within the same border zones but not coexcited by group I afferents or showing typical properties of lamina VIII interneurons were classified as belonging to that class. Such properties included selective input from either MLF or group II afferents to lamina VIII interneurons (Jankowska et al. 2003, 2005). After applying these principles, only one inhibitory interneuron located within the lamina VII–VIII border zone was classified as not belonging to the group of intermediate zone interneurons (see interneuron no. 14 in the companion paper: Jankowska et al. 2009) and three excitatory interneurons were classified as belonging to the group of intermediate zone interneurons because they were coexcited by group I and II afferents (interneurons B and D in Fig. 6 and G in Fig. 7) or by group II afferents and from the MLF (interneuron P in Fig. 7).
Figure 6. Projection areas of excitatory interneurons.
A–N Circles represent somata and thick lines stem axons, and shading summarizes locations of terminals for all rostrocaudal levels where terminals were observed. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma. ‘m’ indicates presence of synaptic contacts with interneurons. Cells are grouped depending on whether the axonal projections were ipsilateral (A–E), bilateral (F–I, M, N) or contralateral (J–L) and also on the basis of their dominant excitatory input from group I or from group II afferents. gr I < gr II and gr I > gr II at the bottom of each frame indicate that in neurons depicted in this frame monosynaptic EPSPs evoked from group I afferents were smaller or larger than EPSPs from group II afferents.
Figure 7. Projection areas of inhibitory interneurons.
A–V, projection areas of 22 interneurons grouped depending on their dominant excitatory input from group I (A–K) or II afferents (L–V). Circles represent somata and thick lines stem axons, and shading summarizes locations of terminals for all rostrocaudal levels where terminals were observed as in Fig. 6. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma.
Labelling and morphological and immunocytochemical analysis of the interneurons
Commissural interneurons were labelled intracellularly with a mixture of equal parts of 5% tetramethyl-rhodamine-dextran (Molecular Probes, Eugene, OR, USA) and 5% Neurobiotin (Vector Laboratories, Peterborough, UK) in saline (pH 6.5). The marker was applied by passing 5 nA positive constant current for 5–8 min. At the end of the experiment the animals were perfused through the descending aorta with a solution containing 4% paraformaldehyde in phosphate buffer. Sections containing labelled interneurons were reacted firstly with avidin–rhodamine (1 : 1000, Jackson Immunoresearch, Luton, UK) and the transmitter content of terminals was identified by incubating sections containing axonal processes in a combination of antibodies for either vesicular glutamate transporter 1 (VGLUT1, 1 : 5000; Chemicon, Harlow, UK) and gephyrin (1 : 100, Synaptic Systems, Gottingen, Germany), or a second combination containing antibodies for glutamic acid decarboxylase (GAD; recognizing both 65 and 67 isoforms, 1 : 2000; Sigma, Poole, UK) and vesicular glutamate transporter 2 (VGLUT2, 1 : 5000; Chemicon) as previously described (Bannatyne et al. 2006). Axonal contacts formed with motoneurons were revealed by using antibodies for choline acetyltransferase (ChAT 1 : 100, Chemicon). The sections were scanned with a confocal laser scanning microscope (Biorad 1024, Zeiss, Hemel Hempstead, UK) and reconstructions of somata and dendritic trees were drawn using Neurolucida for Confocal (MBF Bioscience, Williston, Vermont, USA). Axonal projections, including terminal branches and/or terminals, were mapped for the total sample of 38 labelled neurons (see Table 1).
Table 1.
Association of terminals of GABAergic/glycinergic interneurons with VGLUT1-immunoreactive axons
| Cell Ref. in Fig. 8 | No. of terminals analysed | No. associated with VGLUT1 terminals |
|---|---|---|
| A | 110 | 60 |
| B | 105 | 10 |
Results
Identification of excitatory and inhibitory interneurons
Transmitter content could be defined for terminals of 38 labelled interneurons analysed in this study. VGLUT2 was found in terminals of 14 interneurons and therefore these cells were glutamatergic and excitatory. Twenty-two interneurons had terminals that apposed gephyrin puncta but were not immunoreactive for GAD and hence were glycinergic. Terminals from the remaining two interneurons were immunolabelled for GAD and apposed gephyrin puncta and thus were likely to contain a mixture of GABA and glycine.
Glutamatergic interneurons
The sample of glutamatergic interneurons included five ipsilaterally projecting, six bilaterally projecting and three contralaterally projecting interneurons. Interneurons with contra- or bilateral projections are described in more detail in the accompanying publication (Jankowska et al. 2009). Figure 1 shows details of a typical ipsilaterally projecting glutamatergic interneuron.
Glycinergic interneurons
All 22 glycinergic cells had ipsilateral projections (see below). Axon terminals of these cells apposed gephyrin puncta but were not immunoreactive for VGLUT1, VGLUT2 or GAD. Properties of a typical glycinergic interneuron are shown in Fig. 2.
Figure 2. An ipsilaterally projecting glycinergic interneuron.
A, reconstruction of the soma, dendrites and initial course of the axon of interneuron M in Fig. 7. B–C, series of confocal microscope images showing terminals of this interneuron and their relationship with immunoreactivity for neurotransmitter markers. Panel a in each case shows a projected image of the axon (red) through a number of optical sections; panels b–d show single optical sections illustrating axon terminals (red, arrowheads) and neurotransmitter markers. The sequence in Bb–d shows that the terminals are apposed to gephyrin puncta (green) but do not contain VGLUT1 (blue) and the images in Cb–d confirm that there is no association with either VGLUT2 (shown in blue) or GAD (shown in green). Scale bars: A 100 μm; Ba, Ca 10 μm; Bb–d 5 μm; Cb–d 2 μm.
Target cells of glutamatergic and glycinergic interneurons
The majority (4/5) of glutamatergic cells with ipsilateral terminations within motor nuclei formed contacts with cell bodies and dendrites of motoneurons which were identified by using ChAT immunoreactivity (Fig. 1D). In addition, many terminals were located in regions outside motor nuclei where they formed contacts with unidentified neurons. However, none of the contralaterally projecting interneurons could be shown to form contacts with motoneurons, although one out of the three cells labelled had terminations within motor nuclei. Finally, only one of the six bilaterally projecting interneurons made contacts with ipsilateral motoneurons. Findings from electrophysiological studies to investigate target cells of group I and II activated commissural interneurons are discussed in the accompanying paper (Jankowska et al. 2009).
Glycinergic interneurons (9/22) also made contacts with cell bodies and dendrites of motoneurons within ipsilateral motor nuclei and with cells in areas surrounding them (Fig. 3A). Motoneurons could readily be identified by the presence of ChAT immunoreactivity and, as gephyrin is found at postsynaptic densities (e.g. see Todd et al. 1995), this confirms that such contacts are synaptic. On a limited number of occasions we were able to discern characteristics of other target cells of the labelled interneurons in addition to motoneurons. Firstly, they formed contacts with gephyrin-rich cells located at the border of laminae VII and IX (Fig. 3B). They also contacted cells with concentrations of VGLUT1 terminals on proximal dendrites (Fig. 3C).
Figure 3. Postsynaptic targets of inhibitory interneurons.
Series of images showing contacts formed by terminals of inhibitory interneurons J, D and M in Fig. 7 with ventral horn neurons. Note that all of the putative contacts are associated with gephyrin immunoreactivity, thus confirming that they are synaptic in nature. A, a motoneuron which is labelled positively for ChAT (blue). B, a gephyrin rich cell at the border between lamina VII and IX. C, a lamina VII cell with a high density of VGLUT1 terminals on proximal areas of the dendritic tree. Left hand panels (a) show images projected from several sections; small panels (b–d, e–g) show details of the axon (red), apposed gephyrin puncta (green) and either ChAT (A, blue) or VGLUT1 (C, blue). Insets Ba and Ca show projected images of the axon collaterals present in each section. Scale bars: Aa, Ba, Ca 5 μm; panels b–d and e–g 2 μm.
GABAergic/glycinergic interneurons
Two of the cells in the sample had axon terminals that apposed gephyrin puncta but were also immunoreactive for GAD. Properties of one of these cells are shown in Fig. 4. Neither cell had terminals that were immunoreactive for VGLUT1 or VGLUT2. However, their terminals formed associations with other axon terminals that were immunoreactive for VGLUT1, which is localized principally within large myelinated primary afferent fibres (Varoqui et al. 2002; Todd et al. 2003). Approximately 50% of the terminals of the first cell and 10% of the second formed close appositions with VGLUT1 immunoreactive terminals (Table 1) but no appositions were formed with VGLUT2-containing axons. Gephyrin immunoreactivity was not present at the site of contact between interneuron and VGLUT1 terminals but was present at other locations (Fig. 4D), on the same interneuron terminals which were probably sites of contact with dendrites or cell bodies of other cells.
Figure 4.
Immunocytochemistry of a GABAergic/glycinergic interneuron A, reconstruction of the soma, dendrites and initial course of the stem axon (arrow) of one of the interneurons (A in Fig. 8). The axon of this cell entered the dorsal column (border indicated by grey line and arrowheads on right). B–D, series of confocal microscope images showing terminals of the interneuron and their relationships with immunoreactivity for neurotransmitter markers. Panel a in each case shows a projected image of the axon (red) through a number of optical sections; panels b–d show single optical sections illustrating axon terminals (red, arrowheads). Terminals of this interneuron were immunoreactive for GAD (B and C; green) and associated with gephyrin puncta (D; green). Terminals were frequently observed apposed to profiles that were immunoreactive for VGLUT1 (Bd, Cd; blue). Scale bars: A 100 μm; Ba, Ca 10 μm; Bb–d 5 μm; Cb–d 2 μm.
Comparison of location, somata and dendritic trees of excitatory and inhibitory interneurons
A search was made for interneurons to be labelled primarily in the L4–L6 segments but some were penetrated in the L3 and L7 segments. In all of these segments, cell bodies of the current sample of interneurons were located within laminae VI–VII. No obvious differences were observed between the locations of inhibitory versus excitatory interneurons, which were intermixed in all laminae (Fig. 5), or in the organization of their dendritic trees, which ramified within laminae IV to IX but rarely extended into the white matter; cf. e.g. dendritic trees of interneurons illustrated in Fig. 1A and Fig. 2A and of interneurons illustrated in Figs 1A and 2A in the companion paper. Cell bodies of glycinergic interneurons tended to be smaller than those of glutamatergic interneurons, with diameters 39.4 ± 11.9 μm and 48.1 ± 14.3 μm, respectively (mean ± standard deviation; glycinergic n= 13, glutamatergic n= 8) but this difference was not statistically significant (Student's t test; P < 0.05). The diameters were measured as diameters of equivalent circles from projected images using ImageJ software (available from NIH, USA).
Figure 5. Location of excitatory and inhibitory interneurons.
Location of cell bodies of labelled interneurons. Circles, squares and stars represent cells with ipsilateral, contralateral and bilateral projections, respectively; open symbols denote glutamatergic, filled black symbols glycinergic and filled grey symbols GABAergic cells.
Comparison of axonal projections and terminal projection areas of glutamatergic and glycinergic interneurons
In contrast to the similarities in location of cell bodies and morphology of dendritic trees, major differences were found in axonal projections of glutamatergic and glycinergic intermediate zone interneurons. As stated above, excitatory interneurons were found to project ipsilaterally, bilaterally or contralaterally while axonal projections of inhibitory interneurons were exclusively ipsilateral. Projections of individual excitatory and inhibitory interneurons are shown in Figs 6 and 7. For further details of projections of commissural interneurons of our sample as well as of more ventrally located interneurons, see the accompanying paper (Jankowska et al. 2009).
Figure 6 shows that stem axons of ipsilaterally projecting interneurons run towards the white matter within a short distance from the soma and enter either the middle or the ventral part of the lateral funiculus, or the ventral funiculus, after giving off a number of initial axon collaterals. A similar pattern of projection was found for ipsilaterally projecting glycinergic interneurons (Fig. 7). Both figures show also that on entering the white matter the stem axons commonly bifurcated and projected both rostrally and caudally. Axonal projections of all contralaterally projecting glutamatergic interneurons were similar; their stem axons were directed towards the ventral commissure where they crossed the midline within about 0.5 mm distance from the soma and entered the contralateral ventral funiculus. The most strongly labelled axons could be followed both caudally and rostrally (see arrows in Fig. 6) for up to 1–2 mm total distances and gave rise to axon collaterals that ramified within the contralateral ventral horn. Stem axons of bilaterally projecting glutamatergic interneurons behaved in the same way as the contralaterally projecting ones. Their ipsilateral projections were found exclusively within the grey matter and none entered the ipsilateral lateral or ventral funiculi, which indicates that they might have affected contralaterally located neurons over a length of spinal cord but act fairly locally ipsilaterally.
Although stem axons of ipsilateral glycinergic and glutamatergic cells had different trajectories, their terminal projection areas were similar. These included motor nuclei as well as other regions of the grey matter, principally the intermediate region and lamina VII and VIII. The distribution of terminal projection areas appeared in part to be related to the location of interneurons as there was a tendency for interneurons located in the L3 and 4 segments to terminate primarily in laminae VI–VIII and for interneurons located in the L5 and L6 segments to extend their projection areas to lamina IX. However, only the terminal branches of the earliest axon collaterals could be reconstructed and therefore we cannot exclude the possibility that more caudally located motoneurons are the main targets of these interneurons.
Axonal projections of GABAergic/glycinergic interneurons
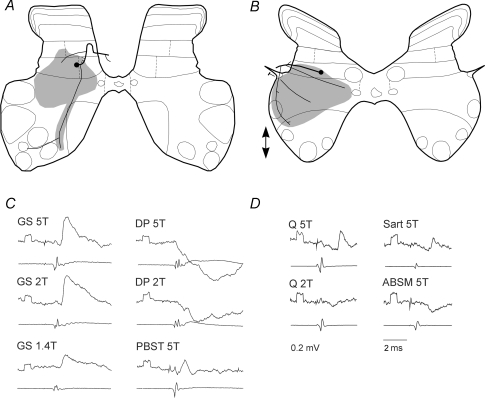
Projection areas of the two cells that had GAD-immunoreactive axons and apposed gephyrin puncta are shown in Fig. 8. The cell body of the first interneuron was located in the central part of lamina VI with the major dendritic fields confined laterally and ventrally in the grey matter of laminae V–VII (Fig. 4A). The axon gave rise to several collaterals before entering the ipsilateral dorsal column. The axon collaterals terminated within the envelope of the dendritic tree and deeper within lamina VII and IX. The soma and dendrites of the second cell were also located within lamina VI and its axon could be followed to the dorsal part of the lateral funiculus where it bifurcated to run both rostrally and caudally. Collateral axons were observed in the intermediate grey matter and also more ventrally. Records show that neuron A was excited by group I afferents of gastrocnemius soleus but not by group II afferents because the amplitude of the EPSPs evoked by near maximal stimulation of group I afferents did not increase when the intensity of the stimuli was increased from 2T to 5T to include group II afferents. In contrast EPSPs evoked in neuron B appeared only when the intensity of the stimuli was increased from 2T to 5T. However, in both neurons IPSPs were apparently evoked from both group I and II afferents as indicated both by threshold and latency. Axonal projections of these neurons were located in regions that would be predicted for interneurons mediating presynaptic inhibition of group Ia, Ib and II afferents (for references see Jankowska, 1992 and Rudomin & Schmidt, 1999).
Figure 8. Axonal projection areas of GABAergic/glycinergic interneurons.
A and B, location and reconstruction of axonal projections of two interneurons. Circles represent somata and thick lines stem axons, and shading summarizes locations of terminals for all rostrocaudal levels where terminals were observed. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma of interneuron B. C and D, intracellular records from these interneurons obtained at the beginning of the injection of the marker by passing 5 nA depolarizing current (upper traces) and records from the cord dorsum of the afferent volleys (lower traces). They show that the first interneuron was excited by group I afferents of gastrocnemius–soleus (GS) and posterior biceps–semitendinosus (PBST) and the second by group II afferents of quadriceps (Q) and sartorius (Sart) and that they were inhibited by group I and II afferents of DP and by group II afferents of anterior biceps–semimembranosus (ABSM), respectively. The latencies of the EPSPs were 0.9 ms in C and 2.7 and 2.9 ms in D, in both cases from group I afferent volleys, and compatible with monosynaptically evoked actions of group I and group II afferents, respectively. 5T, 2T and 1.4T, stimulus intensity expressed in multiples of threshold intensities. In this and in the following figures all the records are averages of 10 or 20 consecutive single sweeps. Negativity is downwards in intracellular records and upwards in records of afferent volleys. Rectangular pulses at the beginning of the records are calibration pulses (0.2 mV). Time calibration (2 ms) in D is for all of the records.
Comparison of input to excitatory and inhibitory interneurons
Figure 9 and Table 2 show that group I and group II afferents provided input to all three subgroups of excitatory interneurons as well as to inhibitory interneurons. Peripheral nerves were stimulated with different stimulus intensities to allow differentiation of synaptic actions from group Ia muscle afferents and group Ib tendon organ afferents (pooled as group I afferents) and from group II muscle spindle afferents. PSPs evoked by stimuli not exceeding 2T (at latencies not exceeding 1.1 ms for monosynaptic EPSPs and 1.8 ms for disynaptic EPSPs or IPSPs) were classified as evoked by group I afferents whereas PSPs evoked at thresholds between 2T and 4T (at latencies ranging between 2 and 4.5 ms) were classified as evoked from group II afferents.
Figure 9. Examples of PSPs evoked in four excitatory interneurons and four inhibitory interneurons.
Upper and lower traces in each panel are averaged intracellular records (negativity downwards; obtained during passage of 5 nA of depolarizing current) and records from the cord dorsum (negativity upwards), respectively. Intracellular records from the top to bottom are from excitatory interneurons labelled K, B, H and E in Fig. 6 and from inhibitory interneurons labelled G, E, K and T in Fig. 7, as indicated in each panel. Amplitudes of these records have been normalized to make it easier to compare the declining phases of EPSPs in the left and right columns. In the three top rows, amplitudes of EPSPs evoked from group I afferents exceeded those from group II afferents; they were evoked in excitatory interneurons projecting contralaterally, ipsilaterally and bilaterally, respectively, and in three ipsilaterally projecting inhibitory interneurons. In contrast, in the bottom row EPSPs evoked from group I afferents were smaller than those from group II afferents; they were evoked in ipsilaterally projecting excitatory and inhibitory interneurons. First dotted lines indicate the arrival of afferent volleys from group I afferents. Second and third dotted lines indicate the approximate onset of EPSPs from group I and from group II afferents in the left and right columns and of IPSPs of group I origin and EPSPs of group II origin in the middle row. Rectangular pulses at the beginning of records are voltage calibration pulses (0.2 mV). Time calibration (4 ms) is for all records.
Table 2.
Distribution of monosynaptic excitatory and inhibitory input from group I and II afferents and from the ipsilateral MLF to excitatory and inhibitory interneurons
| Glutamatergic | |||||
|---|---|---|---|---|---|
| Ipsilateral | Bilateral | Contralateral | Glycinergic Ipsilateral | GABA/glycine Ipsilateral | |
| n | 5 | 6 | 3 | 22 | 2 |
| Excitatory input | |||||
| Group III | 3 | 4 | 3 | 11 | 1 |
| Group I<II | 2 | 2 | 0 | 11 | 1 |
| MLF mono | 4 of 4 | 1 of 4 | 1 of 3 | 8 of 18 | |
| MLF disyn | 3 of 4 | 5 of 18 | |||
| No MLF | 0 | 0 | 2 of 3 | 5 of 18 | 2 |
| Inhibitory input | |||||
| Group I and II | 5 | 6 | 2 | 18 | 2 |
| No IPSPs | 0 | 0 | 1 | 4 | |
| MLF disyn | 2 of 4 | 3 of 4 | 2 of 3 | 7 of 18 | |
| No MLF | 0 | 0 | 1 of 3 | 11 of 18 | 2 |
The subgroups of interneurons are those illustrated in Figs 6–8. Excitatory input is classified as stronger either from group I (I > II) or from group II (I < II) afferents on the basis of comparison of amplitudes of monosynaptic EPSPs evoked by these afferents. Interneurons with input apparently from only group I afferents were included in the first group and interneurons with input apparently from only group II afferents were included in the second group. Input from the MLF was classified as monosynaptic when EPSPs were evoked at latencies not exceeding 0.9 ms from the first component of descending volleys evoked by stimulation of the MLF and did not show temporal facilitation when 2 or 3 as opposed to single stimuli were used (see Jankowska et al. 2003). EPSPs and IPSPs were classified as evoked disynaptically if they appeared at latencies 1–2 ms from descending volleys.
For the majority of neurons, quantitative estimates of the relationships between excitatory input from group I and II afferents to individual neurons were approximate because comparisons of amplitudes of EPSPs evoked from these afferents were hampered by several factors. There was overlap between EPSPs and IPSPs that either followed monosynaptic EPSPs from group I afferents or both preceded and followed EPSPs from group II afferents. Another factor was rapidly progressive deterioration of the majority of the neurons caused by penetration and the resulting depolarization (with frequent injury discharges) which was exacerbated by the passage of current needed to inject the marker and an increase in the volume of the neurones. However, the passage of 5 nA depolarizing current would not have interfered with detection of EPSPs by itself. This was indicated by records from neurones that were only moderately affected by the penetration (as judged by their membrane potential and amplitudes of action potentials) where amplitudes of monosynaptic EPSPs decreased by only some 20–30% during 5–10 min of passage of 5 nA. This interpretation is supported by previous studies where considerably stronger depolarizing current was needed to bring the neurones to the reversal potential of EPSPs (see, e.g. Flatman et al. 1982) or where amplitudes of EPSPs remained substantially unchanged during passage of 20–40 nA under similar experimental conditions (see, e.g. Fetz et al. 1979). For these reasons we were only able to estimate whether input from group I afferents appeared stronger or weaker than input from group II afferents. In addition, we could not exclude the possibility that small EPSPs evoked from group I and II afferents were undetectable with the recording methods described or that there was input from group I and II afferents in untested peripheral nerves. Therefore, the numbers of neurons with inputs from these groups of afferents may be underestimated in Table 2.
Notwithstanding these limitations, excitatory input from group I afferents was generally stronger to excitatory interneurons, especially those projecting contralaterally, since monosynaptic EPSPs evoked from group I afferents were larger in amplitude than those from group II afferents in a greater proportion of excitatory interneurons (Table 2).
The presence of IPSPs following EPSPs was estimated from the extent of the declining slope of the EPSPs. As illustrated in the first and third columns in Fig. 9, IPSPs might have been minimal in the top records but the larger IPSPs could not only increase the declining slope of the EPSPs but also start during the rising slope of these EPSPs and prevent the full development of the EPSP (Fig. 10C). EPSPs evoked from group II afferents not only could be cut short by IPSPs that followed them, but were also often preceded by IPSPs; examples of this are shown in the middle column in Fig. 9, where the approximate onset of the IPSPs is indicated by the second dotted line and of the EPSPs by the third dotted line.
Figure 10. Examples of reversal of IPSPs at the end of labelling.
A and B–D, upper traces: averaged intracellular records from two interneurons (A in Fig. 7 and H in Fig. 6); lowest traces in each panel are from the cord dorsum. Black records were taken during ejection of the marker by passage of 5 nA depolarizing current and grey records after the current was reduced to 0.5 or 0 nA and the cells repolarized. Dotted lines in A, B and C indicate afferent volleys and the onset of monosynaptic EPSPs and disynaptic IPSPs from group I afferents. Note large IPSPs following EPSPs during the depolarization and their subsequent reversal. Note also that IPSPs were evoked not only from group I afferents (B and C) and group II afferents (A and B) but also from the MLF (D).
In previous studies, evidence for IPSPs associated with faster decays was provided by reversal following Cl− injection or hyperpolarization of the neurons. Such tests could not be made during labelling associated with passage of the depolarizing current, but reversal was occasionally seen when the cells remained in a relatively good state after the injection of Neurobiotin had been terminated.
Table 3 shows that no obvious differences occurred between sources of peripheral input to inhibitory and to ipsilaterally and contralaterally projecting excitatory neurons, as in all these subpopulations EPSPs and IPSPs were evoked from the same muscle nerves. In individual interneurons both EPSPs and IPSPs were most often evoked from several nerves (1–3 nerves and 1–5 nerves, respectively). In some cases both excitation and inhibition could be evoked from the same nerve while only IPSPs were evoked from other nerves.
Table 3.
Proportions of glutamatergic and glycinergic interneurons in which monosynaptic EPSPs and oligosynaptic IPSPs were evoked by stimulation of group I and II afferents of various nerves
| Group I | Group II | |||||||
|---|---|---|---|---|---|---|---|---|
| Excitatory | Inhibitory | Excitatory | Inhibitory | |||||
| Ipsilateral | Bilateral | Contralateral | Ipsilateral | Ipsilateral | Bilateral | Contralateral | Ipsilateral | |
| n | 5 | 6 | 3 | 22 | 5 | 6 | 3 | 22 |
| EPSPs from nerves: | ||||||||
| Q | 2 | 5 | 1 | 7 | 4 | 2 | 0 | 13 |
| Sart | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 7 |
| PBST | 2 | 1 | 1 | 3 | 0 | 0 | 0 | 0 |
| ABSM | 2 | 1 | 0 | 3 | 0 | 0 | 0 | 0 |
| GS | 2 | 4 | 1 | 1 | 0 | 0 | 0 | 0 |
| DP | 0 | 1 | 1 | 2 | 3 | 1 | 2 | 10 |
| IPSPs from nerves: | ||||||||
| Q | 4 | 4 | 2 | 14 | 1 | 5 | 0 | 9 |
| Sart | 5 | 2 | 0 | 2 | 1 | 4 | 0 | 4 |
| PBST | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 1 |
| ABSM | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| GS | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| DP | 1 | 3 | 2 | 2 | 4 | 5 | 2 | 6 |
| From same nerve as EPSP | 4 | 4 | 2 | 10 | 3 | 5 | 1 | 10 |
| From other nerves | 11 | 6 | 2 | 14 | 4 | 11 | 1 | 10 |
PSPs evoked by stimuli not exceeding 2T were classified as being evoked from group I afferents, and as monosynaptic or disynaptic if the latencies were less than 1.1 ms (EPSPs) or 1.8 ms (EPSPs and IPSPs), respectively. PSPs evoked at thresholds 2–4T and latencies ranging from 2 to 4.5 ms were classified as being evoked from group II afferents.
Reticulospinal fibres likewise were found to provide input to all three subgroups of excitatory interneurons as well as to inhibitory interneurons. The proportions of interneurons in which monosynaptic and disynaptic EPSPs and disynaptic IPSPs were evoked are given in Table 2 and an example of this is shown in Fig. 9D. These observations suggest that monosynaptic input from the MLF was strongest to ipsilaterally projecting excitatory interneurons but MLF fibres did not appear either to favour or to counteract any particular subpopulations of intermediate zone interneurons which might assist the selection of excitatory interneurons during locomotion or after spinal cord lesions.
Discussion
Properties of intermediate zone interneurons
The most striking observations made in the present study were the major differences in axonal projections of the three classes of interneuron (defined according to their transmitter content), with direct input from group I and II muscle afferents. They show that excitatory intermediate zone interneurons not only target cells ipsilaterally or contralaterally but also bilaterally in the spinal grey matter. In contrast, inhibitory intermediate zone interneurons were found to project exclusively to ipsilateral target cells. This statement is valid for all cells classified as intermediate zone interneurons according to the criteria outlined in Methods. It should nevertheless be noted that we encountered one inhibitory interneuron with crossed projections that was located within the border zone between laminae VII and VIII (see interneuron no. 14 in the companion paper) which therefore could be considered as either an intermediate zone or a borderline lamina VIII interneuron. However, as its properties conformed with those of lamina VIII interneurons (Jankowska et al. 2003, 2005; see Methods), it was classified as lamina VIII rather than an aberrant intermediate zone interneuron.
The majority of glutamatergic interneurons that had projections which were exclusively ipsilateral formed direct contacts with motoneurons. It is therefore likely that most ipsilaterally projecting excitatory interneurons activated monosynaptically by group I and II primary afferent axons have direct actions on motoneurons, thus corroborating conclusions from previous electrophysiological experiments (Edgley & Jankowska, 1987b; Cavallari et al. 1987). However, it is likely that the numbers of cells making such contacts and the numbers of contacts themselves were underestimated because labelling of the finest and most distal axon branches and terminals was incomplete even though stem axons were well labelled and could often be traced over several hundred micrometres.
The extent of terminal projection areas of glutamatergic interneurons of our sample shows that these interneurons formed terminations not only within motor nuclei but also within surrounding regions of the ventral horn and in the intermediate zone. Therefore, in addition to their actions on motoneurons, individual interneurons should provide excitatory input to other interneuronal or projection systems.
Axonal projections and target cells of bilaterally and contralaterally projecting glutamatergic interneurons are discussed in detail in the accompanying paper (Jankowska et al. 2009). Briefly, one out of the sample of six bilaterally projecting interneurons was found to form contacts with ipsilateral but not contralateral motoneurons and none of the three glutamatergic interneurons that had projections that were exclusively contralateral formed direct contacts with motoneurons. However, all these cells had terminations within the ipsilateral intermediate zone, and contralaterally in a region that ‘mirrored’ ipsilateral terminations suggesting that these interneurons target similar types of neuron on both sides of the spinal cord and hence are well suited to be involved in left–right co-ordination of activity at a premotor level.
If contralaterally and bilaterally projecting intermediate zone interneurons form only sparse synaptic contacts with contralateral motoneurons, this would explain why interneurons located in laminae VI–VII were not labelled, or were labelled only occasionally, in experiments where retrograde transport (of either WGA-HRP or latex microspheres) from within contralateral motor nuclei led to labelling of interneurons located in lamina VIII (Alstermark & Kummel, 1986; Harrison et al. 1986; Jankowska & Skoog, 1986; Hoover & Durkovic, 1992).
All of the glycinergic interneurons in our sample gave rise to axonal projections that were exclusively ipsilateral but the termination regions of individual neurons varied. Some projected to motor nuclei and regions of lamina VII surrounding them whereas others were found to project only to regions outside motor nuclei, in particular within the intermediate zone itself. This observation is in good agreement with previous descriptions of terminal projection areas of intermediate zone interneurons of unknown transmitter phenotype that were labelled with HRP (Czarkowska et al. 1976; Bras et al. 1989) and of IPSPs recorded from the ventral roots by using spike-triggered averaging of population PSPs evoked by single interneurons (Brink et al. 1981, 1983; Cavallari et al. 1987).
Although we did not specifically search for other target cells of glycinergic interneurons, we noted that some of them made contact with cells that were rich in gephyrin and were located at the border of laminae VII and IX. These cells are in a similar location to Renshaw cells which are also rich in gephyrin (Alvarez et al. 1997). However, the pattern of gephyrin labelling associated with cells described here appears to differ from that described by Alvarez et al. who showed that Renshaw cells are associated with intense rings of immunoreactivity (see their Fig. 1B). Furthermore, according to Gonzalez-Forero et al. (2005) most of the inhibitory cells that form synaptic contacts with Renshaw cells release a mixture of GABA and glycine. None of the terminals that formed contacts with gephyrin-rich cells contained GAD and therefore these cells are unlikely to be Renshaw cells. We also saw inhibitory contacts on a type of cell that has large numbers of VGLUT1-immunoreactive axons on proximal dendrites, and this will form the basis of a future report. We know that this type of cell receives powerful monosynaptic input from group I and II muscle afferents but that some of them are inhibitory whereas others are excitatory. The fact that they also have input from glycinergic cells that are activated by the same classes of afferent, provides an anatomical basis for the primary afferent-evoked disynaptic IPSPs that are frequently observed to follow monosynaptic EPSPs recorded from intermediate zone interneurons in the present and other studies (see, e.g. Brink et al. 1983; Edgley & Jankowska, 1987b).
Consistent differences were not found between termination patterns of excitatory and inhibitory interneurons, or between termination patterns of interneurons with dominating input from group I or from group II afferents, but considerable variability was found in each of these interneuronal subpopulations. The variability of both input and output in these subpopulations appears to be an intrinsic property of intermediate zone interneurons and is essential for them to fulfil their integrative roles in sensorimotor networks. This would be in keeping with previous electrophysiological evidence that subsets of excitatory and inhibitory interneurons in reflex pathways from group I and/or II afferents contribute to different motor synergies and may be either coexcited or counteract each other's actions depending on the behavioural context (see, e.g. Lundberg et al. 1987; Jankowska, 1992).
The final group of interneurons provided perhaps the most novel information. These two cells had axons which were immunoreactive for GAD and apposed gephyrin puncta. They are therefore likely to contain a mixture of GABA and glycine (e.g. see Todd & Spike, 1993). These are the first two examples of GABAergic interneurons that we have seen in the studies we have performed to date (Bannatyne et al. 2003, 2006; Stecina et al. 2008; Jankowska et al. 2009). The cells had discrete axonal projections that ramified ipsilaterally in the deep laminae of the dorsal horn and intermediate zone, although one cell had an axon collateral that projected towards the ipsilateral motor nucleus. Terminals of these cells apposed other axons containing VGLUT1 that are likely to originate from large myelinated primary afferent fibres (Varoqui et al. 2002; Todd et al. 2003). It was also observed that gephyrin was not present at the point of contact between interneuron axon terminals and those labelled for VGLUT1; this is consistent with observations made by Todd et al. (1995) who showed that gephyrin was not present at axo-axonic synapses formed by axons that contained a mixture of GABA and glycine. Gephyrin was present, however, at other sites of contact, which were presumably dendrites of other neurons. It is very likely that the contacts between interneuron axon terminals and VGLUT1-labelled axons are axo-axonic synapses (see Hughes et al. 2005) and that these cells are involved in presynaptic inhibition associated with primary afferent depolarization (PAD), i.e. they are PAD interneurons. As far as we are aware, these are the first two examples of PAD interneurons in higher vertebrates to be characterized electrophysiologically and labelled intracellularly. However as they also contact dendrites it seems that they not only mediate presynaptic inhibition but also exert postsynaptic inhibitory control over other neurons in the deep parts of the dorsal horn and intermediate zone as proposed by Rudomin (see, e.g. Rudomin & Schmidt, 1999). Furthermore ultrastructural studies of identified group I (Watson & Bazzaz, 2001) and group II afferents (Maxwell & Riddell, 1999) show that presynaptic boutons which contain a mixture of GABA and glycine not only form axo-axonic synapses with primary afferent terminals but frequently also form synapses with the same dendrite that is postsynaptic to the primary afferent, i.e. they form synaptic triads.
Limited information is available about input to interneurons mediating primary afferent depolarization. The two cells identified in the present study were monosynaptically activated by group I and group II primary afferents, respectively. However according to the classical studies of Eccles et al. (1962), measurements of the latency of PAD in large muscle afferents suggest that they are not driven monosynaptically by primary afferents but are last order neurons in trisynaptic or short polysynaptic pathways. Disynaptic coupling was proposed for PAD interneurons with input from group I afferents (see, e.g. Rudomin & Schmidt, 1999), but this might not apply to those excited by slower conducting group II afferents (cf. latencies of EPSPs in Fig. 8C and D), which may be excited monosynaptically although at longer latencies. In addition, in the dorsal horn, candidate PAD neurons which contain a mixture of GABA and acetylcholine are known to receive direct input from primary afferent axons (Olave et al. 2002). It should be borne in mind that the classical studies of PAD were focused on the ventral horn, and the circuitry proposed by Eccles and colleagues may only be valid for PAD interneurons which form axo-axonic synapses with group Ia terminals in lamina IX, which probably constitute a particular subset of PAD interneuron as they do not contain glycine (see Hughes et al. 2005). The PAD interneurons we have identified belong to a group of cells that contain a mixture of GABA and glycine. Presynaptic axo-axonic terminals on Ia and group II afferents outside motor nuclei contain a mixture of GABA and glycine (Maxwell & Riddell, 1999; Watson & Bazzaz, 2001) and therefore the interneurons described here may be the origin of such terminals.
Comparison of axonal projections of intermediate zone interneurons with input from group I and/or group II afferents and of interneurons with similar inputs at other locations
Diagrams in Fig. 11 summarize differences in axonal projections between subpopulations of intermediate zone interneurons and dorsal horn interneurons and lamina VIII interneurons analysed previously (Bannatyne et al. 2003, 2006). The three groups of interneurons are represented in left, middle and right hand diagrams, respectively, and their ipsilateral and/or contralateral axonal projections to the left and right.
Figure 11. Main projection areas of excitatory and inhibitory intermediate zone interneurons compared with projection areas of dorsal horn and lamina VIII interneurons.
Circles represent excitatory and inhibitory interneurons located in the dorsal horn (left), the intermediate zone (middle) and lamina VIII (right). Different shades are used for the three subpopulations of intermediate zone interneurons (projecting bilaterally, ipsilaterally or contralaterally). Rectangular spaces to the left and right of these circles represent ipsilateral (i) and contralateral (co) projection areas within the dorsal horn, intermediate zone and the ventral horn, including motor nuclei. Data for dorsal horn interneurons are from Bannatyne et al. (2006) and those for ventral horn lamina VIII interneurons were reported previously (Bannatyne et al. 2003) and in the companion paper (Jankowska et al. 2009).
The results of the present study show that projections and target cells of intermediate zone excitatory and inhibitory interneurons differ from projections of other interneurons in several respects. Firstly, bilateral projections are characteristic of a subtype of excitatory intermediate zone interneuron but are a distinguishing feature of inhibitory dorsal horn interneurons. Secondly, bilaterally projecting excitatory intermediate interneurons and contralaterally projecting lamina VIII interneurons terminate within laminae VII–IX, whereas bilateral projections of dorsal horn interneurons also extend to laminae IV–VI. Thirdly, excitatory and inhibitory intermediate zone interneurons but only inhibitory dorsal interneurons were found to project to ipsilateral motor nuclei. These differences are likely to reflect the various roles played by intermediate zone, dorsal horn and lamina VIII interneurons in coordination of movement.
On factors favouring activation of excitatory or inhibitory intermediate zone interneurons
As pointed out above, one of the main findings of this study has been that a considerable proportion of excitatory but not inhibitory intermediate zone interneurons with group I and/or II input project bilaterally or contralaterally, which makes these interneurons particularly well suited to co-ordinate activity on both sides of the spinal cord. Excitatory intermediate zone interneurons might thus play a key role in movements for which such coordination is of particular importance, e.g. locomotion. The higher probability of activation of inhibitory interneurons with group I input under resting conditions and of excitatory interneurons during locomotion induced by supraspinal stimulation (see Introduction and, e.g. Angel et al. 2005 for a review) and differential recruitment during the stance and swing phases of the locomotor cycle (Gossard et al. 1994; McCrea et al. 1995; Angel et al. 1996, 2005) might depend on the balance between peripheral and descending inhibitory and excitatory input to intermediate zone interneurons under different circumstances. As centrally initiated locomotion depends to a great extent on reticulospinal neurons (Jordan et al. 2008), it was of particular interest to compare input from reticulospinal neurons with that from peripheral nerves to the excitatory and inhibitory interneurons. However, no consistent differences were found in effects of reticulospinal neurons with axons descending in the medial longitudinal fascicle (MLF) on excitatory and inhibitory interneurons of our sample, indicating that other descending neurones might be more essential in this respect. Monoamines were found to facilitate responses of some intermediate zone interneurons with input from group I and II muscle afferents but depress responses of other interneurons (Jankowska et al. 2000). Although it was not possible to link these effects to either excitatory or inhibitory interneurons, monoaminergic neurons thus could play a decisive role in their selection. Models of neuronal circuitry draw particular attention to connections between neurons initiating locomotion and excitatory interneurons (see Rybak et al. 2006a,b). When more is known about connections between neurons initiating locomotion and excitatory and inhibitory intermediate zone interneurons in pathways from group I and II afferents, models of neuronal circuitry of locomotion (see McCrea & Rybak, 2007, 2008) might use the knowledge of these connections to explain the release of actions of excitatory interneurons on motoneurons during locomotion and thereby to deepen our understanding of its mechanisms.
Acknowledgments
The study was supported by a grant from NINDS/NIH (R01 NS040863) and from the Swedish Research Council (to I.H.). We wish to thank Mrs Rauni Larsson for her invaluable assistance.
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurons from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60–64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kummel H. Transneuronal labelling of neurones projecting to forelimb motoneurones in cats performing different movements. Brain Res. 1986;376:387–391. doi: 10.1016/0006-8993(86)90205-2. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe REW. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jimenez T, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol. 1996;494:851–861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Jankowska E, McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol. 2005;563:597–610. doi: 10.1113/jphysiol.2004.076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro Z, Hill RH, Grillner S. The activity of spinal commissural interneurons during fictive locomotion in the lamprey. J Neurophysiol. 2008;100:716–722. doi: 10.1152/jn.90206.2008. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Janowska E, Kubin L. Morphology of midlumbar interneurons relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Brink E, Harrison PJ, Jankowska E, McCrea DA, Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E, Jankowska E, McCrea D, Skoog B. Use of sucrose gap for recording postsynaptic population potentials evoked by single interneurones in spinal motoneurones. Brain Res. 1981;223:165–169. doi: 10.1016/0006-8993(81)90817-9. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci. 2002;22:9961–9971. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left–right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska J, Jankowska E, Sybirska E. Axonal projections of spinal interneurones excited by group I afferents in the cat, revealed by intracellular staining with horseradish peroxidase. Brain Res. 1976;118:115–118. doi: 10.1016/0006-8993(76)90844-1. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962;160:62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Jankowska E, Johannisson T, Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman JA, Engberg I, Lambert JD. Reversibility of Ia EPSP investigated with intracellularly iontophoresed QX-222. J Neurophysiol. 1982;48:419–430. doi: 10.1152/jn.1982.48.2.419. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Pastor AM, Geiman EJ, Benítez-Temiño B, Alvarez FJ. Regulation of gephyrin cluster size and inhibitory synaptic currents on Renshaw cells by motor axon excitatory inputs. J Neurosci. 2005;25:417–429. doi: 10.1523/JNEUROSCI.3725-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol. 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. On the organization of the supraspinal inhibitory control of interneurones of various spinal reflex arcs. Arch Ital Biol. 1959;97:340–356. [Google Scholar]
- Holmqvist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiol Scand Suppl. 1961;186:1–15. [PubMed] [Google Scholar]
- Hoover JE, Durkovic RG. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens Mot Res. 1992;9:211–226. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci U S A. 2005;102:9038–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Liu TT, Cabaj A, Stecina K, Hammar I, Maxwell DJ. Commissural interneurones with input from muscle afferents in midlumbar segments in the cat; axonal projections, transmitter content and target cells. J Physiol. 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Skoog B. Labelling of midlumbar neurones projecting to cat hindlimb motoneurones by transneuronal transport of a horseradish peroxidase conjugate. Neurosci Lett. 1986;71:163–168. doi: 10.1016/0304-3940(86)90552-5. [DOI] [PubMed] [Google Scholar]
- Jordan L, Liu J, Hedlund P, Akay T, Pearson K. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Ann Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Li WC, Cooke T, Sautois B, Soffe SR, Borisyuk R, Roberts A. Axon and dendrite geography predict the specificity of synaptic connections in a functioning spinal cord network. Neural Develop. 2007;2:17. doi: 10.1186/1749-8104-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways. In: Sjölund B, Björklund A, editors. Brain Stem Control of Spinal Mechnisms. Amsterdam: Elsevier Biomedical Press; 1982. pp. 179–225. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways form group II muscle afferent. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS. Axoaxonic synapses on terminals of group II muscle spindle afferent axons in the spinal cord of the cat. Eur J Neurosci. 1999;11:2151–2159. doi: 10.1046/j.1460-9568.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog Brain Res. 2007;165:235–253. doi: 10.1016/S0079-6123(06)65015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave MJ, Puri N, Kerr R, Maxwell DJ. Myelinated and unmyelinated primary afferent axons form contacts with cholinergic interneurons in the spinal dorsal horn. Exp Brain Res. 2002;145:448–456. doi: 10.1007/s00221-002-1142-5. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault MC, Shefchyk SJ, Jimenez I, McCrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. J Physiol. 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Fedirchuk B, Gosgnach S, McCrea DA. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol. 2000;525:549–564. doi: 10.1111/j.1469-7793.2000.t01-1-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci. 2007;27:6521–6530. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DA, Bhatt DH, Fetcho JR. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol. 2006a;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol. 2006b;577:641–658. doi: 10.1113/jphysiol.2006.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson L-G, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol. 2008;586:557–574. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–638. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Chong D, Neilson M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur J Neurosci. 1995;7:1–11. doi: 10.1111/j.1460-9568.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/Pi transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AHD, Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol. 2001;433:335–348. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]