Abstract
The aim of this study was to analyse neurotransmitter content, projection areas and target cells of commissural interneurons with input from group I and/or II muscle afferents in lumbar segments in the cat. Axonal projections of 15 intracellularly labelled commissural interneurons were reconstructed. Ten interneurons (nine located in laminae VI–VII, one in lamina VIII) were glutamatergic; only one interneuron (located in lamina VIII) was glycinergic. Contralateral terminal projections were found both in motor nuclei and within laminae VI–VIII. In order to identify target cells of commissural interneurons, effects of stimulation of contralateral group I and II muscle afferents were investigated on interneurons within these laminae. Three tests were used: intracellular records from individual interneurons, modulation of probability of activation of extracellularly recorded interneurons and modulation of their actions on motoneurons using disynaptic PSPs evoked in motoneurons as a measure. All these tests revealed much more frequent and/or stronger excitatory actions of contralateral afferents. The results indicate that commissural interneurons with input from contralateral group I and II afferents target premotor interneurons in disynaptic pathways from ipsilateral group I and II afferents and that excitatory disynaptic actions of contralateral afferents on these interneurons are mediated primarily by intermediate zone commissural interneurons. A second group of commissural interneurons activated by reticulospinal neurons, previously described, frequently had similar, but occasionally opposing, actions to the cells described here, thus indicating that these two subpopulations may act on the same premotor interneurons and either mutually enhance or counteract each other's actions.
Commissural interneurons are a highly non-homogeneous population of spinal neurons. Their common feature is that they project to the contralateral grey matter, as first described by Ramon y Cajal (1909), but they may be activated or inhibited under different circumstances and contribute to the coordination of muscle activity on both sides of the body via a variety of other neurons. Two populations of feline commissural interneurons have been investigated in considerable detail. The first population consists of lamina VIII interneurons with monosynaptic input from reticulospinal neurons (Bannatyne et al. 2003; Jankowska et al. 2003, 2005, 2006; Jankowska, 2008; Matsuyama et al. 2004, 2006; Cabaj et al. 2006). These include excitatory and inhibitory interneurons and have both motoneurons and other interneurons as target cells as do their probable analogues in the neonatal rat (Kiehn & Butt, 2003; Kiehn, 2006). The second population of commissural interneurons consists of neurons located in the dorsal horn (see, e.g. Cajal, 1909; Scheibel & Scheibel, 1966). Neurons belonging to this population are activated by skin and group II muscle spindle afferents (Edgley & Jankowska, 1987b; Bras et al. 1989; Jankowska et al. 1993; Edgley et al. 2003; Edgley & Aggelopoulos, 2006) and have recently been shown to be inhibitory and to terminate in the contralateral intermediate zone and the ventral horn (including motor nuclei; Bannatyne et al. 2006). A third population of commissural interneurons is located in the intermediate zone (see, e.g. Cajal, 1909; Scheibel & Scheibel, 1966). Some of these neurons were previously found to be coactivated by group I and II muscle afferents (Czarkowska et al. 1981; Bras et al. 1989) and the results presented in the companion paper (Bannatyne et al. 2009) revealed that commissural interneurons in this population are exclusively excitatory. They might thus be of particular importance for crossed excitatory reflex actions of group II muscle afferents. However, the question of whether they operate jointly with excitatory lamina VIII commissural interneurons activated by group II afferents remained an open question as the transmitter phenotype of this latter population of interneurons had not been defined. The main aims of the present study were therefore threefold: (i) to compare the properties of intermediate zone and lamina VIII commissural interneurons with input from group II muscle afferents and, in particular, to establish whether the latter include both excitatory and inhibitory interneurons, (ii) to define the contralateral target cells of intermediate zone and lamina VIII commissural interneurons with group II input, and (iii) to investigate which crossed actions of group II afferents on motoneurons are likely to be relayed by intermediate zone interneurons and which are mediated by dorsal horn and lamina VIII commissural interneurons.
In order to answer these questions, we extended the analysis of axonal projections of intermediate zone interneurons, reported in the companion paper, to lamina VIII commissural interneurons with group II input and, where possible, analysed the transmitter phenotypes of these interneurons. The identity of neurons with input from contralateral group II afferents located within terminal projection areas of the labelled neurons was analysed in electrophysiological experiments with particular reference to types of spinal interneurones identified previously. Potential effects of various subpopulations of commissural interneurons on premotor interneurons were also investigated by analysing how PSPs evoked by group I and II afferents in hindlimb motoneurons were affected by conditioning stimulation of contralateral group II afferents.
Methods
Experiments were performed on a total of 13 young adult cats under deep anaesthesia. All procedures were approved by the Göteborg University Ethics Committee and complied with US National Institutes of Health and European Union guidelines. These procedures are described in detail in the companion paper (Bannatyne et al. 2009). Briefly, general anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–2 h, up to 55 mg kg−1, i.v.) following cannulation of the trachea, a carotid artery and both cephalic veins. Additional doses of α-chloralose were given when increases in the blood pressure or heart rate (which were continuously monitored) occurred, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4%. Experiments were terminated by a lethal dose of pentobarbital and/or formalin perfusion resulting in cardiac arrest.
The third to seventh lumbar (L3–L7), and low thoracic (Th11–Th13) segments were exposed by laminectomy. Neurons in the lumbar segments were approached through small holes (about 1–2 mm2) made in the dura mater. At the Th12 level two pairs of stimulating electrodes were put in contact with the lateral funiculus to allow detection of neurons with axons ascending either ipsilaterally or contralaterally beyond this level by antidromic activation. Additionally, in electrophysiological experiments in which the ipsilateral medial longitudinal fascicle (MLF) was stimulated in the medulla, the spinal cord was hemisected on the side opposite to that of the location of the commissural interneurons, in order to prevent actions of contralaterally descending fibres activated inadvertently. Three (quadriceps, Q, sartorius, Sart and gastrocnemius–soleus, GS) contralateral hindlimb nerves and a number of ipsilateral nerves were transected and mounted on stimulating electrodes. The latter included Q, Sart, the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius and soleus (GS), plantaris (Pl), flexor digitorum and hallucis longus (FDL) and deep peroneal (DP) nerves. Nerves were stimulated by constant voltage 0.2 ms long current pulses, with intensity expressed in multiples of threshold (T) for the most excitable fibres in the nerve. In most experiments a tungsten electrode (impedance 40–250 kΩ) was placed in the contralateral MLF after exposure of the caudal part of the cerebellum. The electrode was inserted at an angle of 30 deg (with the tip directed rostrally). The initial target was at Horsley–Clarke co-ordinates P9, L0.6, H–5 but its final position was adjusted on the basis of records of descending volleys from the surface of the lateral funiculus at the Th11 level. The site of the tip of the electrode was marked by an electrolytic lesion (0.4 mA constant current passed for 10 s) and subsequently verified on 100 μm thick frontal sections of the brainstem, cut in the plane of insertion. Either single or trains of 2–5 stimuli (≤ 100 μA, 0.2 ms, 300 Hz) were applied through these electrodes. For details see the results.
Sample of interneurons
The sample of interneurons included: (i) nine labelled laminae VI–VII commissural interneurons in which the transmitter content was defined as described in the companion paper (Bannatyne et al. 2009); they were identified as glutamatergic; (ii) two labelled commissural interneurons located in lamina VIII or at the border between laminae VII and VIII; they were identified as glutamatergic or glycinergic; (iii) four labelled laminae VI–VIII commissural interneurons for which transmitter content could not be defined; (iv) 24 intermediate zone interneurons that were injected with tetramethylrhodamine-dextran but not recovered and which were investigated only electrophysiologically; (v) 39 intracellularly recorded intermediate zone interneurons; and (vi) 16 extracellularly recorded intermediate zone interneurons investigated only electrophysiologically. These interneurons were selected on the basis of monosynaptic input from group II or both group I and II muscle afferents and axonal projections restricted to the lumbosacral enlargement (cells antidromically activated from the last thoracic segment were excluded from the sample).
A search for interneurons to be labelled was conducted primarily in laminae VI–VII and VIII (as judged by extracellular field potentials from group I and group II muscle afferents) in the L3–L7 segments using 5T stimulation of the Q nerve as a search stimulus. In order to avoid the risk of penetrating dorsal horn group II interneurons, the areas explored were ventral and medial to the areas in which dorsal horn field potentials are evoked from group II muscle afferents (Edgley & Jankowska, 1987a). Two of the 15 successfully labelled interneurons and 12 of the 24 unsuccessfully labelled ones were identified as commissural by antidromic activation from the contralateral gastrocnemius–soleus motor nuclei localized as described by (Jankowska et al. 2005), where stimuli (0.2 ms, 5–100 μA) were applied via a thin tungsten electrode (100–300 kΩ). Contralateral projections of the remaining labelled interneurons were only established morphologically. Records from these neurons were obtained with micropipettes with tips broken to about 2 μm and impedances of 12–20 MΩ, filled with the marker solution (see next section). They were obtained just before and after penetration, before, during and following ejection of the marker.
Interneurons that were analysed electrophysiologically only were searched for in the same regions of the grey matter but using other search stimuli (see results). They were recorded from with micropipettes with tips broken to 1.5–2 μm, filled with solutions of 2 m potassium citrate (for intracellular recording) or 2 m NaCl (for extracellular recording).
Labelling and morphological and immunocytochemical analysis
Selected interneurons were labelled intracellularly with a mixture of equal parts of 5% tetramethyl rhodamine-dextran (Molecular Probes, Eugene, Oregon, USA) and 5% Neurobiotin (Vector Laboratories, Peterborough, UK) in saline (pH 6.5). The marker was ejected by passing 5 nA positive constant current for 5–8 min (up to 30–40 nA × min). Sections containing neurons were reacted as described in the companion paper (Bannatyne et al. 2009), where details of perfusion, preparation of the tissue and confocal microscopy are also given. Axonal projections were mapped for 15 commissural interneurons including nine analysed by Bannatyne et al. (2009), and six either located more ventrally or where only more proximal axonal branches could be visualized. The list of these neurons is in Table 1.
Table 1.
List of bilaterally and contralaterally projecting labelled commissural interneurons
| Soma | Axon | Gr I | Gr II | MLF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neuron no. 1 | Transmitter 2 | Segment 3 | Laminae 4 | Ipsi laminae 5 | Contra laminae 6 | EPSP (ms) 7 | IPSP (ms) 8 | EPSP (ms) 9 | IPSP (ms) 10 | EPSP (ms) 11 |
| A. Bilaterally projecting | ||||||||||
| 1 (N) | Glut | L7 | VI | VII, IX | VII | 0.9 | 1.5 | 3.5 | 4.3 | — |
| 2 (M) | Glut | L5 | VII | VI–VII | wm* | — | — | 2.5 | 3.2 | nt |
| 3 (I) | Glut | L5 | VII | VI–VII | VI–VII* | 1.1 | 1.8 | 2.4 | 3.7 | nt |
| 4 (F) | Glut | L4 | VII | VII | VII–VIII | 0.9 | 1.5 | 2.6 | 3.0 | — |
| 5 (G) | Glut | L4 | VII | VII–VIII | VII–VIII | 1.2 | 1.4 | 3.8 | 3.3 | |
| 6 (H) | Glut | L5 | VII | VIII–IX | VI–VIII | 0.9 | 1.6 | 2.6 | 3.3 | 3.9 |
| 7 | Unid | L6 | VII–VIII | VIII | wm | 0.7 | — | 2.4 | 3.7 | 3.8 |
| 8 | Glut | L3 | VII–VIII | VII–IX | VI–IX | — | — | 1.9 | 3.3 | 3.1 |
| B. Contralaterally projecting | ||||||||||
| 9 (L) | Glut | L7 | VI–VII | — | VIII | 0.5 | 1.4 | 3.4 | 4.6 | — |
| 10 (K) | Glut | L7 | VII | — | VII–VIII | 0.8 | 1.5 | 2.8 | 4.1 | 3.2 |
| 11 | Unid | L6 | VII | — | VII–IX | 0.8 | 1.2 | 2.8 | 3.5 | 3.1 |
| 12 (J) | Glut | L4 | VII | — | VIII–IX | 1.9 | 1.3 | 3.3 | — | — |
| 13 | Unid | L4 | VII–VIII | — | VIII–IX | — | — | 2.3 | — | 3.1 |
| 14 | Gly | L5 | VIII | — | VI–IX | — | 1.4 | 2.1 | 2.6 | 3.9 |
| 15 | Unid | L5 | VIII | — | VIII–IX | — | — | 2.3 | 3.4 | — |
In columns from the left to the right are as follows. 1, successive numbers of interneurons (with letters in parentheses corresponding to letters designating neurons in Fig. 6 in the companion paper). 2, transmitter phenotype; glut, glutamatergic; gly, glycinergic; unid, transmitter not identified. 3, segmental location of somata. 4, location of somata according to Rexed's laminae. 5 and 6, Rexed's laminae in which terminal projections were found ipsilaterally and/or contralaterally; wm, white matter. Asterisks in column 6 indicate projections to contralateral GS motor nuclei confirmed by antidromic activation of the interneurons by electrical stimuli applied within these nuclei. 7–11, latencies of EPSPs and/or IPSPs evoked from ipsilateral group I, group II and the descending volleys evoked by MLF stimulation. In column 11 ‘nt’ indicates that input from the MLF was not tested. EPSPs evoked at latencies ≥ 3.8 ms from the descending had features of disynaptically evoked PSPs.
Electrophysiological analysis of target neurons of commissural interneurons with input from muscle afferents
Target cells of commissural interneurons were analysed in one series of experiments by recording from interneurons in reflex pathways from group Ia, Ib and II afferents and investigating the effects of contralateral muscle afferents on them; some of these interneurons could be identified as last order premotor interneurons by criteria established previously (see Results). In other experiments recordings from hindlimb α-motoneurons were used to monitor changes in the disynaptic EPSPs and IPSPs evoked in them from group I and II afferents by conditioning stimulation of contralateral group II afferents. Effects evoked by contralateral group II afferents were compared with those evoked from the contralateral MLF in order to differentiate between effects mediated by commissural interneurons with monosynaptic input from muscle afferents and those relaying reticulospinal actions. Both original records and averages of 20–40 records made on-line were stored using a sampling and analysis system developed by E. Eide, T. Holmström and N. Pihlgren, Göteborg University. Data are expressed as means ±s.e.m. Differences between data sets were assessed for statistical significance by using Student's t test for paired or unpaired samples.
Results
General characteristics of commissural interneurons with input from group I and/or group II muscle afferents
Location and input. The majority of labelled commissural interneurons with monosynaptic input from group I and/or group II muscle afferents were located in lamina VII and within the border zone between laminae VII and VIII (cells 2–8 and 10–13 in Table 1). Two cells were located more dorsally (1 and 9 in Table 1) and two more ventrally (14 and 15 in Table 1). As they were labelled in experiments where we searched for commissural, other intermediate zone and ventral horn interneurons (see the companion paper: Bannatyne et al. 2009), this suggests that commissural interneurons with monosynaptic input from group I and II afferents are preferentially located within lamina VII. In contrast, commissural interneurons with monosynaptic input from the MLF are preferentially located within lamina VIII (Bannatyne et al. 2003). Locations of interneurons that were analysed electrophysiologically could only be defined approximately. However, those that were antidromically activated from the ipsilateral GS motor nuclei (n= 34) were found most frequently within the region in which focal field potentials were evoked by both group I and II afferents and those that were antidromically activated from the contralateral GS motor nuclei (n= 14) were found within more ventral regions where the field potentials were evoked only by group II afferents, these two regions overlapping approximately with laminae VI–VII and lamina VIII, respectively (see Edgley & Jankowska, 1987a).
In interneurons located in laminae VI, VII and at the border between laminae VII and VIII, monosynaptic EPSPs were evoked from both group I and II afferents (at segmental latencies of 0.5–1.2 ms and 2.1–2.6 ms, respectively) as well as from the MLF (at latencies 3.1–3.3 ms from the stimuli, see Table 1, which corresponded to < 1 ms from the earliest components of the descending volleys). In the two interneurons penetrated within lamina VIII, monosynaptic EPSPs were evoked from group II but not group I afferents or the MLF (Table 1) as previously described by Jankowska et al. (2005). It will be noted that monosynaptic input from both group II afferents and the MLF in interneurons located in lamina VII contrasts with more selective monosynaptic input from either group II afferents or the MLF to lamina VIII commissural interneurons (Jankowska et al. 2005).
Transmitter content
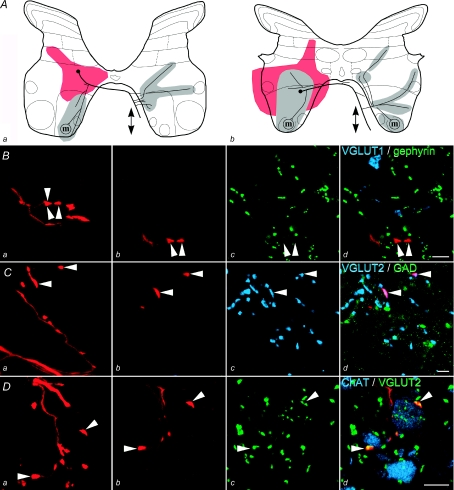
It was possible to identify the neurotransmitter type for 11 of the 15 labelled interneurons but poor tissue immunoreactivity or insufficient labelling of terminals precluded identification of the neurotransmitter in the four remaining interneurons. The majority of the interneurons (10/11) formed axon terminals which were immunoreactive for VGLUT2. Examples of glutamatergic commissural interneurons are shown in Fig. 1. Glutamatergic commissural interneurons were identified by the presence of VGLUT2 immunoreactivity in their terminals (Fig. 1D) and the absence of GAD immunoreactivity (Fig. 1C) or any association with gephyrin puncta (Fig. 1B). Only one interneuron (no. 14 in Table 1) formed axon terminals that were apposed to gephyrin immunoreactive puncta. These terminals were not immunoreactive for GAD or either of the vesicular glutamate transporters and hence the neuron was likely to be glycinergic (Fig. 2B–D). This neuron was located at the border between laminae VII and VIII and was penetrated within the region (depth 3.2 mm from the surface) where field potentials were evoked by group II but not group I afferents. Furthermore, it was monosynaptically excited by group II but not by group I afferents or by stimulation of the MLF, i.e. it had functional properties that were typical of lamina VIII interneurons (Jankowska et al. 2005) rather than those characteristic of intermediate zone interneurons (Edgley & Jankowska, 1987b; Davies & Edgley, 1994). For these reasons this cell was not included in the sample of intermediate zone interneurons described in the companion paper (see second section of Methods in Bannatyne et al. 2009).
Figure 1. Identification of glutamatergic interneurons.
A, summary diagram of the soma position, area of dendritic spread (red) and axonal projections (grey: locations of terminals for all rostrocaudal levels where terminals were observed) of two glutamatergic interneurons, no. 6 (a) and no. 8 (b) in Table 1. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma and ‘m’ indicates the presence of synaptic contacts with motoneurons demonstrated as in D. B–D, series of confocal microscope images of interneuron no. 6: panel a in each case shows a projected image of a length of labelled axon (red); panels b–d show single optical sections illustrating axon terminals (red, arrowheads) and immunolabelling for neurotransmitter markers; the sequence in Bb–d shows that they do not contain VGLUT1 (shown in blue) or are associated with gephyrin (shown in green) while Cb–d demonstrates that the terminals are immunoreactive for VGLUT2 (blue) but not GAD (green). D, contacts between labelled interneuronal terminals positively labelled for VGLUT2 (green) and ipsilateral motoneuron dendrites visualized with anti-ChAT antibodies (shown in blue). Scale bars: B–D 5 μm.
Figure 2. Morphology of a glycinergic interneuron.
A, summary diagram of the soma position, area of dendritic spread (red) and axonal projections (grey: locations of terminals for all rostrocaudal levels where terminals were observed) for interneuron no. 14 in Table 1. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma. B–D, series of confocal microscope images; panel 1 in each case shows a projected image of a section of labelled axon (red); panels b–d show single optical sections illustrating axon terminals (red, arrowheads) and immunolabelling for neurotransmitter markers. B shows that gephyrin (green) is associated with labelled terminals but VGLUT1 (blue) immunoreactivity is not present within them; C, gephyrin positive terminals associated with a proximal dendrite and cell body of a gephyrin-rich cell in contralateral lamina VIII; D shows that the terminals are not immunoreactive for VGLUT2 (blue) or GAD (green). Scale bars: Ba 5 μm; Bb–d 2 μm; Ca–d 10 μm; Da–d 5 μm.
Terminal projection areas
Contralateral terminal projection areas of bilaterally and contralaterally projecting intermediate zone commissural interneurons were generally similar, as they extended over several laminae and over a length of the spinal cord (Fig. 6 in the companion paper, Fig. 3). As axons of both bilaterally and contralaterally projecting interneurons descended and/or ascended within the contralateral ventral funiculus, interneurons of both these kinds would operate as propriospinal neurons. Antidromic activation from the GS motor nuclei (see below and Jankowska et al. 2005) of two of the labelled interneurons (no. 2 and 3 in Table 1) and of several of the interneurons investigated only electrophysiologically indicate in addition that some of these neurons project along the whole length of the lumbosacral enlargement. In contrast, ipsilateral axon collaterals of bilaterally projecting neurons branched exclusively within the grey matter and none entered the white matter; therefore they would have local actions only.
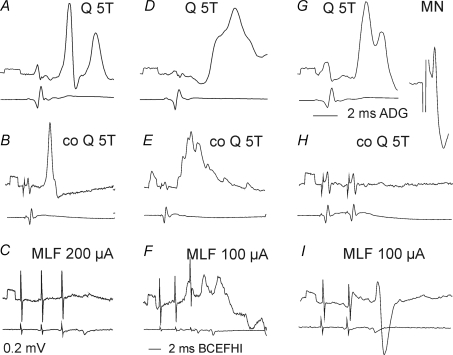
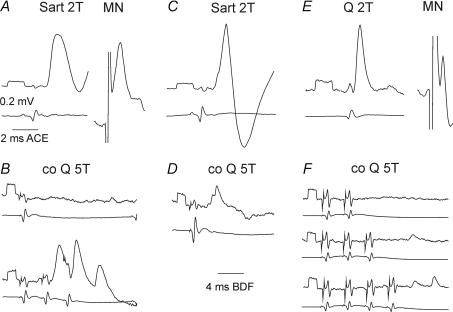
Figure 6. Examples of synaptic actions from contralateral group II afferents and the MLF on 3 group II excited interneurons.
Left (A–C), middle (D–F) and right (G–H) panels show intracellular records from three interneurons (upper traces) and the corresponding records of afferent and descending volleys from the cord dorsum (lower traces). A, D and G illustrate EPSPs evoked from ipsilateral group II afferents and antidromic activation of one of these interneurons from the GS motor nucleus (MN). B, E and H show effects of stimuli applied to the contralateral Q nerve at intensity near-maximal for group II afferents (5T) and C, F and I effects evoked from the MLF. Note in C that hardly any PSPs were evoked by even 200 μA stimuli. Voltage calibrations (0.2 mV) are at the beginning of intracellular records. Time calibration in G is for the records in A, D, G and that in I for the remaining records.
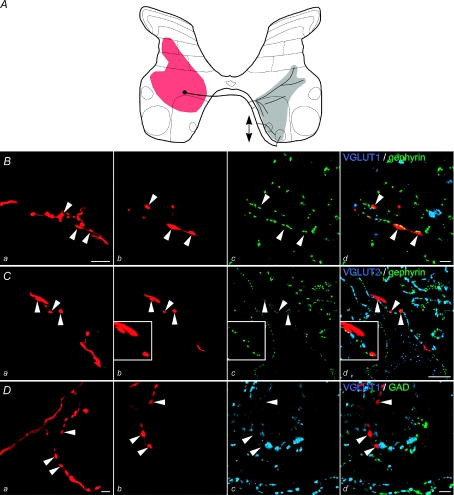
Figure 3. Projection areas of commissural interneurons for which the neurotransmitter could not be identified.
Reconstruction of stem axon, collateral branching patterns and terminal projection areas of contralaterally projecting commissural interneurons nos 7, 11, 13 and 15 in Table 1. Circles represent somata and thick lines stem axons, and shading summarizes locations of terminals for all rostrocaudal levels where terminals were observed. Arrows indicate rostral and/or caudal projections where the axon could be followed more than 250 μm in either direction from the soma.
Although bilaterally and contralaterally projecting commissural interneurons project to similar areas of the grey matter, some differences in the patterns of their terminal projections were observed. For instance, as shown in Table 1 (columns 5 and 6) a higher proportion of contralaterally as opposed to bilaterally projecting neurons was found to project to contralateral motor nuclei. In fact none of the bilaterally projecting interneurons located in lamina VII was found to project to contralateral lamina IX and the only interneuron with a terminal projection area in contralateral motor nuclei (no. 8 in Table 1; illustrated in in Fig. 1Dd) was located within the border zone between laminae VII and VIII, and therefore might be considered to be a lamina VIII rather than an intermediate zone commissural interneuron. However, it should be noted that terminal projection areas of this and three other bilaterally projecting interneurons (no. 4–6 in Table 1, F, G, H in Fig. 6 in the companion paper) and of all but one of the contralaterally projecting interneurons (10–15 in Table 1, illustrated in Fig. 3 and in Fig. 6 in the companion paper) extended to areas just outside contralateral motor nuclei in the L3–L6 segments. Both bilaterally and contralaterally projecting commissural interneurons therefore could have terminated on dendrites of contralaterally located motoneurons.
In the L4 segment, terminations were found in the proximity of pelvic limb motor nuclei, including the iliopsoas, pectineus and adductor longus motor nuclei. In the L5 segment, they were present medially close to sartorius and gracilis motor nuclei and laterally to quadriceps motor nucleus (Vanderhorst & Holstege, 1997). Projections to more caudally located motor nuclei could not be revealed morphologically but were found electrophysiologically for two interneurons (indicated by asterixes in Table 1) which were antidromically activated by stimuli applied in contralateral GS nuclei in the L7 segment.
Terminal projection areas outside motor nuclei showed three main features. Firstly, they often covered considerable parts of the contralateral grey matter. The majority of interneurons were found to project to more than one contralateral lamina in addition to lamina IX and therefore may contact a great number of neurons in these laminae. Terminal projection areas were most often found in laminae VII and VIII but some interneurons also projected to lamina VI (see Table 1, Figs 3 and 6 in the companion paper). In all of these laminae, terminations were found predominantly in central regions but a few interneurons projected medially and some projections extended into lateral parts of laminae VI–VII. Secondly, terminal projection areas of individual commissural interneurons varied, showing that these neurons do not replicate each other's actions but may target various sets of contralateral interneurons. Thirdly, axon collaterals of bilaterally projecting commissural interneurons tended to terminate, at least partly, within the same laminae on either side of the spinal cord, e.g. within the dorsal part of lamina VIII in Fig. 1A, in addition to their terminations within different laminae.
Identification of target cells of commissural interneurons with group II input in electrophysiological experiments
Bruggencate & Lundberg (1974) have shown that some subpopulations of commissural interneurons may target distinct types of premotor interneurons because contralateral high threshold muscle afferents (often referred to as flexor reflex afferents, FRA, including group III in addition to group II afferents) act on premotor interneurons mediating inhibition of flexor but not of extensor motoneurons from the ipsilateral vestibular nucleus and from group Ia afferents.
One of the aims of the present study was therefore to find out whether the two subpopulations of commissural interneurons, with input from either group I and/or II afferents or from the MLF, target the same premotor interneurons in reflex pathways from ipsilateral muscle afferents (as shown schematically in Fig. 4).
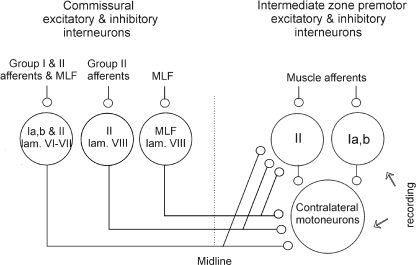
Figure 4. Relationships between commissural interneurons and interneurons in disynaptic pathways between ipsilateral group Ia, group Ib and group II afferents and motoneurons investigated in this study.
Circles to the left of the dotted line represent intermediate zone commissural interneurons with input from group I and/or group II afferents and lamina VIII commissural interneurons with input from either group II or the MLF. Circles to the right represent potential contralateral target cells of these interneurons. Ia, Ib and II denote input from these groups of afferent fibres. Arrows to the right indicate recording sites (from interneurons and motoneurons).
Postsynaptic potentials from contralateral group II afferents and from the MLF in interneurons with excitatory input from ipsilateral group I and/or group II afferents
Interneurons tested for input from contralateral afferents were located within lamina VI or the dorsal part of lamina VII. Most of these interneurons were likely to be premotor interneurons, as 33 of 39 of them were antidromically activated from ipsilateral GS motor nuclei in the L7 or S1 segments. Whenever possible, antidromic activation was tested both before and after penetration of the interneurons. In either situation, this was confirmed by a short and stable latency (0.6–1.3 ms) of action potentials evoked from the GS motor nucleus, illustrated in Fig. 5A and E and Fig. 6G and/or by collision with action potentials evoked by peripheral stimuli.
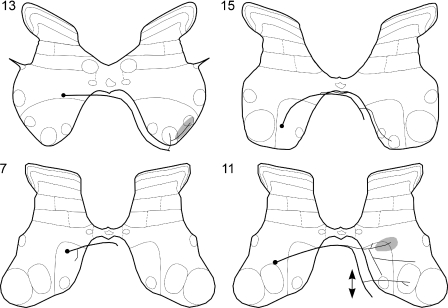
Figure 5. Examples of synaptic actions from contralateral group II afferents on three Ib interneurons.
Left (A and B), middle (C and D) and right (E and F) panels show intracellular records from three interneurons (upper traces) and the corresponding records of afferent volleys from the cord dorsum (lower traces). A, C and E illustrate PSPs evoked from ipsilateral group I afferents, with blocked antidromic spikes evoked from the contralateral motor nuclei (MN) shown in A and E. B, D and F show effects of single, two, three and four stimuli applied to the contralateral Q nerve at intensity near-maximal for group II afferents. EPSPs in B and D were classified as evoked disynaptically and those in F as evoked either di- or trisynaptically. Note the slower time base of records in B, D and F. Rectangular pulses at the beginning of the traces are calibration pulses (0.2 mV). In this and the following records negativity is downwards in intracellular records and upwards in records from the cord dorsum.
In 24 interneurons the dominating peripheral monosynaptic input was drawn from group Ib afferents. Most interneurons with this type of input that project to ipsilateral motor nuclei are reported to be inhibitory (Brink et al. 1983; Hongo et al. 1983; Jankowska, 1992). The majority of these neurons would therefore be likely to mediate non-reciprocal inhibition of hindlimb motoneurons from tendon organ afferents. For the remaining 15 interneurons, the dominating peripheral ipsilateral monosynaptic input was from group II afferents. In previous studies such neurons were found to include both excitatory and inhibitory interneurons (Cavallari et al. 1987; Bannatyne et al. 2009) and it is likely that this was also the case for the present sample.
Interneurons with input from ipsilateral group I afferents were infrequently affected by stimulation of contralateral group I and II afferents. EPSPs evoked at segmental latencies < 3.5 ms from the effective stimulus were found in only 3/24 interneurons. They are illustrated in the left and middle panels of Fig. 4. EPSPs evoked at such latencies were considered to be compatible with disynaptic actions relayed by commissural interneurons with monosynaptic input from these afferents (circles to the left in Fig. 4) because they were within the range of latencies for disynaptic actions of group II afferents on ipsilaterally located neurons (see Fig. 2 in Jankowska et al. 2005 and Fig. 6 in Lundberg et al. 1987; Bajwa et al. 1992). In a further three interneurons, EPSPs were evoked at longer latencies (3.5–5 ms; see right panels in Fig. 4), most likely di- or trisynaptically. IPSPs compatible with disynaptic actions relayed by commissural interneurons were found in only one interneuron.
In interneurons with input from ipsilateral group II afferents EPSPs from contralateral group II afferents were found more frequently (in 5/15 interneurons) but IPSPs were found in only one of these cells. As these PSPs were evoked at latencies < 3.5 ms they were compatible with disynaptic actions mediated by commissural interneurons monosynaptically activated by group II afferents.
Excitatory disynaptic actions from contralateral group II afferents occurring more frequently than inhibitory ones would be indicative of predominant actions of excitatory intermediate zone rather than of other commissural interneurons with group II input on premotor interneurons.
Disynaptic EPSPs from the MLF were evoked in a larger proportion of group Ib interneurons than from contralateral group II afferents (15/24 versus 3/24), while they were found in a similar proportion of group II interneurons (6/15 versus 5/15). Disynaptic IPSPs from the contralateral MLF were evoked in larger proportions of both group Ib and group II interneurons (14/24 and 5/15) than from contralateral group II afferents (in single neurons). The different proportions suggest effects mediated by distinct populations of commissural interneurons, which is further supported by a comparison of input from group II afferents and the MLF in individual interneurons. Disynaptic EPSPs from group II afferents were associated with disynaptic EPSPs from the MLF in only 3/39 interneurons (Fig. 6E and F showing an example of this). In all the remaining interneurons PSPs were evoked from either contralateral group II afferents (Fig. 6B and C) or the MLF (Fig. 6H and I). Excitatory actions of contralateral group II afferents could be mediated by some intermediate zone commissural interneurons coexcited by these afferents and by reticulospinal neurons descending in the MLF (see Table 2 in the companion paper) but more frequently by separate interneurons. In contrast lamina VIII commissural interneurons with input from the MLF would mediate inhibition from reticulospinal neurons.
Modulation of activation of interneurons with input from ipsilateral group I and group II afferents following conditioning stimulation of contralateral group II afferents
Intracellular records described in the preceding section were supplemented with analysis of effects of conditioning stimulation of contralateral group II afferents and the MLF on 27 extracellularly recorded interneurons (14 activated by group I and 13 by group II afferents). These interneurons were activated by stimulation of muscle nerves at submaximal stimulus intensities and responded in only 10–75% of trials to allow the detection of both increases or decreases in the number of responses evoked by a series of stimuli. Examples of the tested responses and of peri-stimulus time histograms and cumulative sums of responses evoked by 20 stimuli are shown in Fig. 7A and B. The neurons responded with single spikes at segmental latencies < 1.8 ms for group I excited interneurons and 2–5 ms for group II excited interneurons.
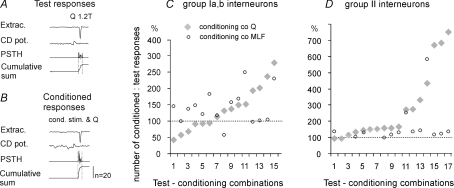
Figure 7. Modulation of activation of interneurons with input from ipsilateral group I and group II afferents by conditioning stimulation of contralateral group II afferents, compared with effects of stimulation of the MLF.
A and B, examples of extracellular records from an interneuron and cord dorsum (CD) potentials aligned with peristimulus time histograms (PSTHs) and cumulative sums of responses to 20 stimuli in a series, when these stimuli were applied alone (test responses) or were preceded by conditioning stimuli (conditioned responses). The counts were made within time windows indicated by vertical dotted lines. For technical details see Jankowska et al. (1997). C and D, comparison of mean numbers of responses to 20 successive stimuli (20 responses equivalent to 100%). Data for 13 group I excited interneurons (15 test–conditioning combinations, as in 2 interneurons test and conditioning stimuli were applied to two pairs of peripheral nerves rather than one) and 13 group II excited interneurons (17 test–conditioning combinations). Ordinate: percentages of responses following conditioning stimulation compared with test responses alone. Conditioning stimuli were delivered to the MLF or to group II afferents in the contralateral quadriceps (Q) nerve at 5T intensity. The data are ranked from the weakest to the strongest conditioning effects of group II afferents.
The sample of Ib interneurons included five neurons located in the L6 segment, likely to be inhibitory as they were antidromically activated from the ipsilateral lateral funiculus at the border between the L3 and L4 segments (Hongo et al. 1983) and were activated from nerves (Q, GS, Pl or FDL) that are the main source of inhibitory actions of Ib afferents on motoneurons (Eccles et al. 1957). The remaining interneurons were activated by stimulation of the same nerves and were recorded at similar locations. All of the group II interneurons were antidromically activated from the ipsilateral GS motor nucleus; group II input to these interneurons was from the Q, Sart, FDL and/or DP nerves.
Figure 7 shows that, in keeping with the results of intracellular recording described in the preceding section, facilitation was the dominant effect of conditioning stimulation of contralateral group II afferents; activation of only three interneurons (1–3 in Fig. 7C) was depressed. The mean facilitation of interneurons with group I input was from 7.9 ± 1.1 (mean ±s.e.m.) to 10.5 ± 1.1 responses per 20 stimuli, i.e. to 132%. The mean depression was from 12.2 ± 0.9 to 8.3 ± 0.8 responses, i.e. to 68%. These differences were not statistically significant for the whole sample of data in Fig. 7C (paired t test P < 0.05). The mean facilitation of interneurons with group II input was from 9.3 ± 1.2 to 15.5 ± 0.9 responses per 20 stimuli, i.e. to 166% and highly statistically significant (P < 0.001).
Facilitation evoked by conditioning stimulation of group II afferents was much more potent than facilitation from the MLF in the majority of interneurons activated by group II afferents (the overall differences between data points in Fig. 7D being highly statistically significant). In contrast it was either stronger, weaker, or similar on interneurons activated by group I afferents (Fig. 7C) and no statistically significant differences were found between overall effects of stimulation of contralateral group II afferents and of the MLF on these interenurons.
Frequent similar facilitatory effects of conditioning stimulation of group II afferents and of the MLF on individual interneurons are compatible with actions of contralaterally or bilaterally projecting excitatory intermediate zone interneurons coexcited by group II afferents and reticulospinal neurons (see companion paper: Bannatyne et al. 2009). The only indications for selective actions of either group II or MLF excited commissural interneurons are opposite actions of conditioning stimulation of group II afferents and of the MLF (on neurons 1, 3 and 8 in Fig. 7C) and much stronger facilitatory effects of these afferents (on neurons 12–14 in Fig. 7C and 12, 13 and 15, 16, 17 in Fig. 7D).
Modulation of synaptic actions of group I and group II afferents on α-motoneurons
In order to compare effects of various subpopulations of commissural interneurons on premotor interneurons, effects of conditioning stimulation of contralateral group II afferents and the MLF were also investigated by using changes in disynaptic PSPs evoked in motoneurons by ipsilateral group I and II afferents. The analysis included EPSPs from group II afferents and IPSPs from group Ib and group II afferents; effects of conditioning stimuli could not be tested on EPSPs of group I origin because these were not sufficiently distinct.
Increases (up to about 200%) in areas of both IPSPs and EPSPs following conditioning stimulation of contralateral group II muscle afferents were found more frequently than decreases (down to 40%). They are illustrated in Fig. 8A–D and summarized for the total sample of tested motoneurons in Fig. 8E–G. The plots show also that the effects of conditioning stimulation of muscle afferents and of the MLF were often similar and no statistically significant differences were found between the two whole sets of data points in Fig. 8E–G (P > 0.05 t test for paired data). Only differences between effects of group II and the MLF stimulation in a subset of the motoneurons 8–17 in the plot in Fig. 8F were statistically significant (P < 0.02).
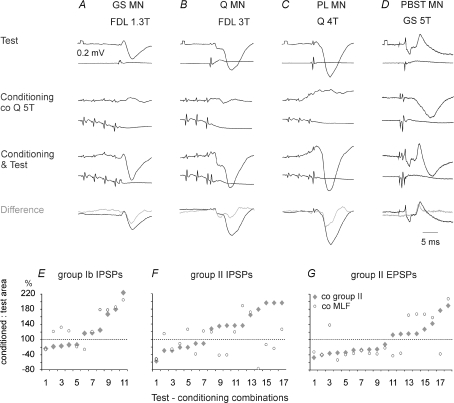
Figure 8. Facilitation of IPSPs from group Ib afferents and of IPSPs and EPSPs from group II afferents evoked by conditioning stimulation of contralateral group II afferents.
A–D, records from four motoneurons (GS, Q, Pl and PBST) where stimulation of contralateral group II afferents preceded stimulation of ipsilateral group I and/or II afferents. Upper row, test PSPs. Second row, effects of conditioning stimuli alone; IPSPs in B and C were evoked at latencies of 3.3 and 3.4 ms from group I volleys. Third row, PSPs evoked by joint application of the conditioning and test stimuli. Fourth row, differences between the latter and sums of PSPs evoked by separate test and conditioned stimuli [conditioning & test – (test + conditioning)] (grey traces) superimposed on test IPSPs (black traces). Voltage calibration pulses (0.2 mV) are at the beginning of the top records in each column. Time calibration at the bottom right is for all records. E–G, comparison of effects of conditioning stimulation of contralateral group II afferents and the MLF on IPSPs evoked by group Ib and II afferents and EPSPs evoked by group II afferents in the same motoneurons. Ordinates, areas of the conditioned PSPs in percentages of areas of the test PSPs. The data are ranked from the weakest to the strongest conditioning effects of group II afferents.
The most potent facilitation or depression was seen at conditioning–testing intervals corresponding to intervals of 0.5–1.5 ms between test and conditioned afferent volleys, as expected of direct actions of both excitatory and inhibitory commissural interneurons on premotor interneurons. Nevertheless both facilitation and depression occurred at longer (up to 2–4 ms) intervals and might have also depended on polysynaptic actions.
Discussion
Transmitter content of interneurons
The results of this study show that commissural interneurons with input from group I and/or II afferents, and with somata located in the intermediate zone of the adult cat lumbar spinal cord are predominantly glutamatergic and therefore have excitatory actions. Only one commissural interneuron located at the dorsal border of lamina VIII was glycinergic and no evidence of GABAergic commissural projections was found. Our results therefore are in agreement with previous findings for electrophysiologically defined excitatory commissural neurons in the adult lamprey (Buchanan & Grillner, 1987) and neonatal mammalian spinal cord (Kjaerulff & Kiehn, 1997; Butt & Kiehn, 2003; Quinlan & Kiehn, 2007). However, our findings are at variance with a recent anatomical study of commissural cells, where anterograde labelling techniques were used in neonatal rats, which concluded that a subpopulation of commissural cells is GABAergic (Weber et al. 2007). It is not possible to determine if this discrepancy is related to methodological differences resulting from labelling of undefined populations of neurons, to changes occurring during development or to other factors.
Bilateral or contralateral axonal projections
Our finding, reported in the companion paper (Bannatyne et al. 2009), that a considerable proportion of excitatory laminae VII and VIII interneurons with monosynaptic input from group I and II muscle afferents form bilateral projections contrasts with our previous findings for dorsal horn interneurons with group II input where inhibitory, but not excitatory, cells form bilateral projections (Bannatyne et al. 2006). Projections of the current sample of interneurons are also significantly different from the almost exclusive contralateral projections of lamina VIII commissural interneurons found both in the present study and by Bannatyne et al. (2003, 2009). Taken together the differential projections of these three populations of commissural interneurons provide a strong indication that these neurons play different roles in coordinating neuronal activity on both sides of the spinal cord (see the last section of the Discussion). Whether all, or only some of them are of particular importance for postural adjustments and for rhythmic locomotor, or other motor activity, remains to be established.
The observations on different patterns of axonal projections of commissural interneurons, described above, were made in adult cats and it is of interest that these findings are at variance with bilateral projections of lamina VIII commissural neurons in the neonatal mouse or kitten reported by Ramon y Cajal (1909) and Scheibel & Scheibel (1966) but consistent with bilateral projections of dorsal horn interneurons found by them. However, a recent study of commissural interneurons, located in lamina VII, VIII and X in the neonatal mouse, likewise revealed predominant contralateral projections (Quinlan & Kiehn, 2007) and therefore the question of whether the bilaterally projecting lamina VIII commissural neurons previously described represent particular functional subpopulations of commissural interneurons, immature, or atypical neurons is still open.
Projections to motor nuclei
We were not able to show that axons of commissural interneurons with input from group I and II muscle afferents which project to contralateral motor nuclei formed contacts with motoneurons. However, as discussed in the companion paper, contacts with motoneurons might be formed at distances longer than labelled axons could be traced. Indeed this is very likely because several interneurons with input from group I and II muscle afferents were antidromically activated by stimuli applied in contralateral motor nuclei located in lower lumbar segments.
Our observations of projections of commissural interneurons with group I input to motor nuclei appear to be in disagreement with results of electrophysiological experiments where EPSPs from group I afferents were rarely found to be evoked in contralateral motoneurons and, if present, were evoked at long latencies (Harrison & Zytnicki, 1984; Arya et al. 1991). However, EPSPs evoked by group I afferents in commissural interneurons were often very small, both in the present sample and in lamina VIII commissural interneurons with monosynaptic input from the MLF (Jankowska et al. 2005). Hence, group I actions on commissural interneurons may be sufficient to activate these interneurons only under some particular conditions, e.g. temporal facilitation of effects of long trains of nerve impulses in group I afferents might be needed to induce action potentials in these interneurons, as indicated by crossed facilitatory actions of weak muscle stretches likely to activate group Ia muscle spindle afferents (Perl, 1959). The fact that weak muscle stretches and weak electrical stimulation of muscle nerves not only excited but also inhibited contralateral motoneurons (Perl, 1958, 1959) does not contradict the conclusion that intermediate zone interneurons include only excitatory commissural interneurons with group I input because crossed inhibition might occur as a consequence of activation of local inhibitory interneurons by excitatory commissural interneurons (Fig. 9C). Crossed inhibition of group I origin could also be mediated by direct actions of lamina VIII commissural interneurons coexcited by group I afferents and by reticulospinal and vestibulospinal tract neurons (Jankowska et al. 2005), although not by inhibitory commissural interneurons located in the dorsal horn as the latter are not coexcited by group I afferents (Edgley & Jankowska, 1987b; Bannatyne et al. 2006).
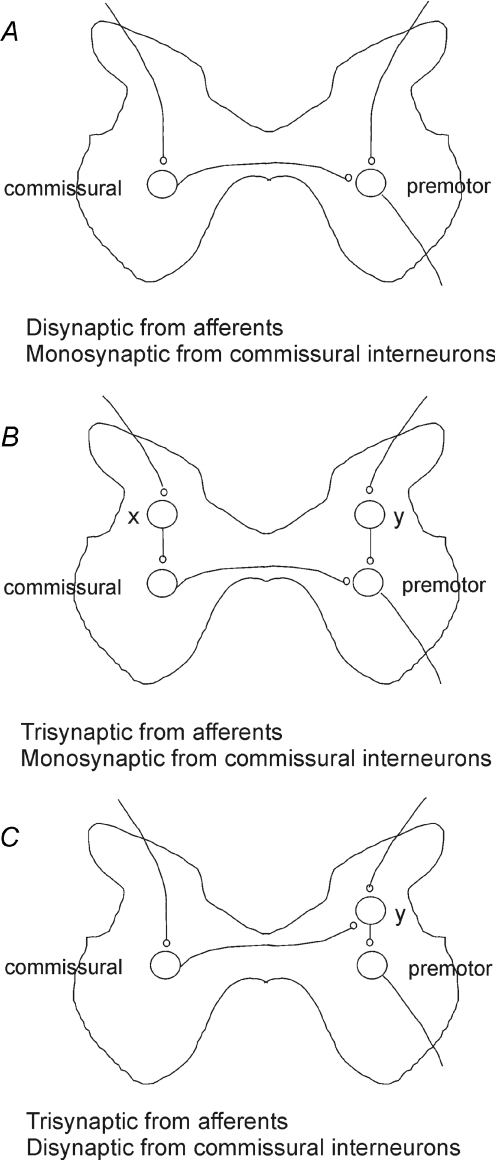
Figure 9. Hypothetical connections between ipsilateral peripheral afferents and contralaterally located premotor interneurons.
Modified diagrams of pathways proposed by Bajwa et al. (1992); see their Fig. 6 to explain either disynaptic (A) or trisynaptic (B and C) coupling between group I or II afferents providing input to commissural interneurons (left) and premotor interneurons (right). The annotation was added by us with permission of the authors. X, neurons relaying excitation to commissural interneurons. Y, neurons relaying excitation or inhibition to premotor interneurons from both ipsilateral afferents and from commissural interneurons.
However, projections of commissural interneurons with group II input to motor nuclei are consistent with electrophysiological records showing PSPs of group II origin in contralateral motoneurons. The shortest segmental latencies of both IPSPs and EPSPs (≤ 3 ms; Arya et al. 1991) were only marginally longer than latencies of disynaptic PSPs from group II afferents evoked in ipsilateral motoneurons (Edgley & Jankowska, 1987b; Lundberg et al. 1987). Such short latencies leave room for only one interposed interneuron between group II afferents and contralateral motoneurons, i.e. the commissural interneurons that form synaptic contacts with these motoneurons. Nevertheless, the relative contribution of intermediate zone and lamina VIII excitatory commissural interneurons to such disynaptic EPSPs and the contribution of dorsal horn and lamina VIII inhibitory commissural interneurons to disynaptic IPSPs remains an open question. Both intermediate zone and excitatory lamina VIII commissural interneurons activated by group II afferents during stronger muscle stretches (Perl, 1959) might also contribute to polysynaptic actions of these afferents mediated by other locally operating excitatory interneurons.
Projections and target cells outside motor nuclei
Bajwa et al. (1992) reported that a large majority of midlumbar neurons with input from group II, or both group I and II afferents located in laminae VI–VII were influenced by stimulation of contralateral group II afferents. Findings showing that interneurons in reflex pathways from group Ia and Ib afferents are affected by contralateral muscle afferents are also consistent with their results (Harrison & Zytnicki, 1984). The conclusions drawn from these studies are summarized in the diagrams of Fig. 9 adapted from Fig. 6 of Bajwa et al., which illustrate disynaptic (A) or trisynaptic (B and C) coupling between afferents and premotor interneurons. Disynaptic coupling could occur through direct contacts between commissural interneurons monosynaptically activated by group I and/or II afferents and premotor interneurons. Trisynaptic coupling was proposed to be via interneurons (X) which mediate disynaptic excitation of commissural interneurons or via interneurons (Y) which mediate indirect actions of commissural interneurons.
The electrophysiological observations made in the present study extend these findings. Firstly, they show that premotor interneurons contacted by commissural interneurons include both excitatory and inhibitory interneurons in disynaptic reflex pathways from group Ib or both Ia and Ib afferents and from group II afferents to hindlimb motoneurons. This was shown by direct records from interneurons that were monosynaptically excited by these afferents (Figs 5–7) as well as by modulation of their actions on motoneurons (Fig. 8).
Secondly, they show that commissural interneurons with input from group II afferents may mediate not only disynaptic excitation but also disynaptic inhibition of contralaterally located premotor interneurons, as in Fig. 8B and D. Disynaptic excitation could be mediated by both intermediate zone interneurons and lamina VIII interneurons and disynaptic inhibition by both dorsal horn interneurons and lamina VIII interneurons. This does not exclude the trisynaptic inhibition that was proposed by Bajwa et al.(1992) to be evoked via additional excitatory interneurons (X in Fig. 9B) activating inhibitory lamina VIII commissural interneurons, or inhibitory interneurons (Y in Fig. 9C) activated by excitatory laminae VI–VII or VIII commissural interneurons. Trisynaptic excitation would be evoked by excitatory commissural interneurons and additional excitatory interneurons (X in pathways B or Y in pathways C).
Thirdly, our results show that crossed synaptic actions of group I and II afferents may be mediated by the same intermediate zone commissural interneurons rather than by separate subpopulations of these cells because a high proportion of interneurons in our sample had input from both group I and group II afferents.
Fourthly, facilitation of reflex actions of ipsilateral muscle afferents on motoneurons by conditioning stimulation of contralateral group II afferents along with the MLF indicates that input to premotor interneurons is provided by commissural interneurons activated by group II afferents in addition to commissural interneurons activated by reticulospinal neurons with axons descending in the MLF. Group II afferents and reticulospinal neurons acting through commissural interneurons thus can jointly adjust the degree of activation of contralateral muscles. One notable example of such adjustments is the resetting of muscle activity from flexion to extension when contralateral FRA (including group II muscle afferents) are stimulated in conjunction with stimulation of the MLF during locomotion (Leblond et al. 2000). Joint actions of various subpopulations of commissural interneurons are secured by projections of these neurons to similar areas of the contralateral grey matter (Bannatyne et al. 2003, 2006, 2009).
Bilateral projections of individual commissural interneurons would assist in coordinating activation of premotor interneurons on both sides. However, other neurons also might be used to this end; they are represented by cells labelled X in Fig. 9B. For instance dorsal horn interneurons might relay input from group II afferents to both ipsilaterally projecting intermediate zone interneurons and contralaterally projecting commissural interneurons (Jankowska et al. 2002, 2003; Bannatyne et al. 2006). The coordinating neurons might also include neurons of intrinsic spinal networks that are involved in centrally initiated phasic or rhythmic movements, as well as a number of descending tract neurons, in particular reticulospinal and vestibulospinal tract neurons (Davies & Edgley, 1994; Jankowska et al. 2003; Krutki et al. 2003). Neurons of this type would be of particular importance for coordinating activity of commissural interneurons which discharge rhythmically during fictive locomotion in cats and rodents (Kiehn & Kullander, 2004; Matsuyama et al. 2004, 2006; Kiehn, 2006) as well as in the lamprey and tadpole (for references see Buchanan & Einum, 2008; Roberts et al. 2008). However, they could also coordinate activity of commissural interneurons with more selective input from group II afferents (see Jankowska et al. 2005) and integrate their actions with actions of other neurons including commissural interneurons activated by reticulospinal neurons.
Comparisons with commissural interneurons in other species
In other species relatively little attention has been paid to commissural interneurons with peripheral input and to the range of functions of commissural interneurons. In several preparations, inhibitory commissural interneurons have been proposed to be particularly important for ensuring rhythmic alternating activity of muscles on both sides of the body (Buchanan, 1996, 1999; Grillner, 2003) and for adjusting its timing. However, an analysis of the activity of individual inhibitory commissural interneurons and paired records from these interneurons and their target motoneurons revealed that a considerable proportion of them are active throughout the swimming cycle or in phase, rather than out of phase with contralateral ventral root activity, thereby possibly contributing to shaping the pattern of swimming and postural adjustments needed to control body orientation (Biro et al. 2008). Excitatory commissural interneurons have been found to be involved to an even greater extent in patterning of activation of the myotomal and fin motoneurons (Mentel et al. 2008). In the tadpole and zebrafish it has long been known that commissural interneurons are activated by peripheral stimuli. However, it has only been demonstrated recently that different kinds of skin stimulation activate different classes of tadpole excitatory commissural interneurons, in association with swimming or with struggling (Li et al. 2007). In zebrafish larvae, activity of only one class of excitatory commissural interneurons has been related so far to a particular type of reaction (Ritter et al. 2001). It was concluded that these interneurons were of particular importance for maintaining intersegmental phase coordination.
Newly reported observations on excitatory commissural interneurons in rodents similarly draw attention to their coordinative functions, ‘coordinating rostral–caudal left–right synergies’ and not only to alternating but also to synchronous activity at the segmental levels (Butt & Kiehn, 2003; Kiehn et al. 2008).
In the turtle, no differentiation has yet been made between excitatory and inhibitory commissural interneurons but those analysed were found to be active during different variants of scratch and withdrawal reactions, and during either only one or both of these reactions (Berkowitz, 2005). The turtle commissural interneurons thus appear to be as highly specialized as in the cat with respect to the stimuli to which they respond and the movements with which they are associated. As axon terminals of these neurons were found both in the ventral and the dorsal horn and in the intermediate zone, the properties of turtle commissural interneurons, at least those active during scratching, show several features in common with those in the cat.
Taken together, observations on commissural interneurons made in different species agree in that, even in relatively simple preparations, functions of commissural interneurons go far beyond a mere rhythm generation and that more complex movement synergies are mediated by highly specialized subpopulations of these neurons. We have previously analysed differentiation of feline commissural interneurons with respect to their location, input, output, and various control systems. The reported results extend this analysis to target cells of both excitatory and inhibitory commissural interneurons with input from muscle afferents and to their relationships to other neurons in feline spinal interneuronal networks. It is our hope that the knowledge of these relationships should help in further studies of neuronal networks of these neurons.
Acknowledgments
The study was supported by grants from NINDS/NIH (R01 NS040863) and from the Swedish Research Council (for I.H.). We wish to thank Mrs Rauni Larsson for her invaluable assistance and T. T. Liu for her participation in one of the experiments
References
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S, Edgley SA, Harrison PJ. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol. 1992;455:205–217. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurones in pathways from group I and II afferents: differences in axonal projections and inputs. J Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J Neurophysiol. 2005;94:4455–4470. doi: 10.1152/jn.00229.2005. [DOI] [PubMed] [Google Scholar]
- Biro Z, Hill RH, Grillner S. The activity of spinal commissural interneurons during fictive locomotion in the lamprey. J Neurophysiol. 2008;100:716–722. doi: 10.1152/jn.90206.2008. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989;290:1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Brink E, Harrison PJ, Jankowska E, McCrea DA, Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggencate G, Lundberg A. Facilitatory interaction in transmission to motoneurones from vestibulospinal fibres and contralateral primary afferents. Exp Brain Res. 1974;19:248–270. doi: 10.1007/BF00233233. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Lamprey spinal interneurons and their roles in swimming activity. Brain Behav Evol. 1996;48:287–296. doi: 10.1159/000113207. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Commissural interneurons in rhythm generation and intersegmental coupling in the lamprey spinal cord. J Neurophysiol. 1999;81:2037–2045. doi: 10.1152/jn.1999.81.5.2037. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Einum JF. The spinobulbar system in lamprey. Brain Res Rev. 2008;57:37–45. doi: 10.1016/j.brainresrev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib and II afferents and crossed reticulospinal actions. J Neurophysiol. 2006;95:3911–3922. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Histologie Du Systeme Nerveux de L'homme and Des Vertebres. Madrid: Instituto Ramon y Cajal; 1909. [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska J, Jankowska E, Sybirska E. Common interneurones in reflex pathways from group 1a and 1b afferents of knee flexors and extensors in the cat. J Physiol. 1981;310:367–380. doi: 10.1113/jphysiol.1981.sp013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synpatic actions in motoneurons caused by impulses in Gligi tendon afferents. J Physiol. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Aggelopoulos NC. Short latency crossed inhibitory reflex actions evoked from cutaneous afferents. Exp Brain Res. 2006;171:541–550. doi: 10.1007/s00221-005-0302-9. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from group II muscle afferents. J Physiol. 2003;552:961–974. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Zytnicki D. Crossed actions of group I muscle afferents in the cat. J Physiol. 1984;356:263–273. doi: 10.1113/jphysiol.1984.sp015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo LZ, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Szabo Lackberg Z, Hammar I. Morphology of interneurones in pathways from group II muscle afferents in sacral segments of the cat spinal cord. J Comp Neurol. 1993;337:518–528. doi: 10.1002/cne.903370312. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol. 2002;542:301–314. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson L-G, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt S. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–321. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Quinlan KA, Restrepo CE, Lundfald L, Borgius L, Talpalar AE, Endo T. Excitatory components of the mammalian locomotor CPG. Brain Res Rev. 2008;57:56–63. doi: 10.1016/j.brainresrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci. 1997;17:9433–9447. doi: 10.1523/JNEUROSCI.17-24-09433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond H, Menard A, Gossard JP. Bulbospinal control of spinal cord pathways generating locomotor extensor activities in the cat. J Physiol. 2000;525:225–240. doi: 10.1111/j.1469-7793.2000.t01-1-00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi S, Aoki M. Projection patterns of lamina VIII commissural neurons in the lumbar spinal cord of the adult cat: an anterograde neural tracing study. Neuroscience. 2006;140:203–218. doi: 10.1016/j.neuroscience.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: Morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Mentel T, Cangiano L, Grillner S, Buschges A. Neuronal substrates for state-dependent changes in coordination between motoneuron pools during fictive locomotion in the lamprey spinal cord. J Neurosci. 2008;28:868–879. doi: 10.1523/JNEUROSCI.4250-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl ER. Crossed reflex effects evoked by activity in myelinated afferent fibers of muscle. J Neurophysiol. 1958;21:101–112. doi: 10.1152/jn.1958.21.2.101. [DOI] [PubMed] [Google Scholar]
- Perl ER. Effects of muscle stretch on excitability of contralateral motoneurones. J Physiol. 1959;145:193–203. doi: 10.1113/jphysiol.1959.sp006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci. 2007;27:6521–6530. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DA, Bhatt DH, Fetcho JR. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev. 2008;57:22–28. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Spinal motorneurons, interneurons and Renshaw cells. A Golgi study. Arch Ital Biol. 1966;104:328–353. [Google Scholar]
- Vanderhorst VGJM, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindllimb, pelvic floor and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Weber I, Veress G, Szucs P, Antal M, Birinyi A. Neurotransmitter systems of commissural interneurons in the lumbar spinal cord of neonatal rats. Brain Res. 2007;1178:65–72. doi: 10.1016/j.brainres.2007.06.109. [DOI] [PubMed] [Google Scholar]