Abstract
Physiological changes in the nervous system occur with ageing. Both a decline of function and a decrease in the number of C-fibres in the skin have been reported for healthy aged subjects. With the use of microneurographic recordings from single C-fibres in humans we have compared the sensory and axonal properties of these neurones in young and aged healthy subjects. A total of 146 C-fibres were recorded from the common peroneal nerve in young subjects (mean age 24.7 years) and 230 C-fibres were recorded in aged subjects (mean age 56.2 years). In aged subjects, changes were found in the composition of the C-fibre population and in sensory and axonal properties. The relative incidence of afferent to efferent C-fibres was relatively constant independent of the age of subjects. The ratio of mechano-responsive to mechano-insensitive nociceptors was approximately 8 : 2 in the young controls while in aged subjects it was 7 : 3. In aged subjects 13% of the fibres showed atypical discharge characteristics, while this was not observed in young subjects. Spontaneous activity, sensitization and loss of sensory function were found regularly. Changes in functions of the conductile membrane were also observed in fibres from aged subjects. The degree of activity-dependent conduction velocity slowing in response to high frequency stimulation (2 Hz) was more pronounced, while the normalization of conduction velocity subsequent to high frequency stimulation was protracted. We found that both sensitization and desensitization or degeneration of afferent C-fibres occur with age, but are still rare compared to patients with neuropathy. The changes in the axonal properties of C-fibres in aged subjects are compatible with hypoexcitability of the fibres. These findings are important for the understanding and differential diagnoses regarding pathological processes and normal ageing.
The peripheral and central nervous systems change with age. Functional and morphological studies of the peripheral nervous system have shown decreased function responses of C-fibres and a degeneration of C-fibre endings in aged. Afferent C-fibres have functional deficits as demonstrated by increased temperature thresholds in psychophysical tests in healthy elderly compared with young subjects (Claus et al. 1987; Rolke et al. 2006), and diminished axon-reflex flare size (Helme & McKernan, 1986; Munce & Kenney, 2003; Namer et al. 2004). Efferent sympathetic C-fibres also have a decreased function in aged persons as shown by reduced quantitative sudomotor axon reflexes (QSART-test) (Foster et al. 1976; Low et al. 1990, 1997).
Some studies have also shown morphological changes such as a degeneration of C-fibre endings resulting in a lower intra-epidermal nerve fibre count in aged subjects (McArthur et al. 1998). Even in sural nerve, biopsies reveal a change of morphology of unmyelinated fibres (Kanda et al. 1991) and a decrease in the absolute number of C-fibres in healthy aged subjects (Jacobs & Love, 1985). In some studies, however, no obvious morphological changes were reported (Kanda et al. 1991; Lauria et al. 1999).
All these results have been obtained either by indirect functional methods (QSART) or by morphological analysis. Microneurography is the only method that assesses directly the function of both afferent and efferent C-fibres in awake human subjects. Until now the classification and understanding of function of C-fibres with microneurography have mainly been based on studies in young healthy subjects. However, patients with neuropathy are often over 40 years of age and many of these patients suffer from neuropathic pain. These patients have changes of C-fibre function that can be observed in microneurography recordings (Ochoa et al. 2005; Bostock et al. 2005; Orstavik et al. 2006), and also in non-invasive tests (Low et al. 1983) and skin biopsies (McArthur et al. 1998). To evaluate these pathological changes, it is important to understand the physiological changes of C-fibre function that occur as a result of ageing itself.
Methods
Subjects
Thirty four healthy subjects aged 21–67 years took part in the microneurography experiments. According to their anamnesis the subjects were free of any neurological disease or symptoms. They did not use any medication known to influence the nervous system.
All participants gave their written informed consent. The study was approved by the local ethic committees of the Universities of Erlangen, Oslo and Uppsala and conformed with the declaration of Helsinki.
The method of microneurography has been described in detail elsewhere (Vallbo & Hagbarth, 1968; Torebjork & Hallin, 1974; Schmelz et al. 1995). The recording electrode was inserted into the common peroneal nerve at the level of the fibular head. When the needle was inserted into a fascicle containing C-fibres, the receptive field of a single C-fibre was searched with transcutaneous electrical stimulation. C-fibres were identified by their low conduction velocity (< 2 m s−1), which was assessed from the latency of an electrically evoked action potential after a rest period of at least 2 min. A pair of thin stimulation needles (0.2 mm diameter) was inserted into the receptive field in a spot with low electrical threshold. Through these needles the C-fibres were stimulated repetitively (0.25 Hz; 0.5 ms, 1–30 mA) with a constant current stimulator (Digitimer DS7, Digitimer Ltd, Welwyn Garden City, UK).
Following repetitive electrical stimulation at a fixed frequency from the skin, action potentials of individual C-fibres can be registered with the recording electrode at stable conduction latencies. An increase in conduction latency (‘marking’) is observed when the stimulation frequency at the skin is increased or after the afferent fibre has been additionally activated, e.g. by natural stimuli (Torebjork & Hallin, 1974). Marking is due to activity-dependent slowing of conduction in C-fibres, i.e. conduction of an action potential renders the axonal membrane of afferent C fibres less excitable for tens of seconds and thus slows down conduction velocity of subsequent action potentials (Schmelz et al. 1995).
In this study, the marking technique was used to assess the activity-dependent conduction velocity slowing to repetitive electrical stimulation, the responsiveness to mechanical and heat stimuli and to detect spontaneous activity (Torebjork & Hallin, 1974).
Activity-dependent conduction velocity slowing
After a rest period of at least 2 min, an electrical protocol was performed for each stimulation site at the foot; for this, 20 electrical stimuli were applied intracutaneously at 0.125 Hz, immediately followed by a second train of 20 pulses at 0.25 Hz and a third of 30 pulses at 0.5 Hz. Changes in latency were calculated relative to the initial latency (i.e. that immediately following the 120 s rest period). After this test, the stimulation frequency was set to 0.25 Hz for the rest of the experiments. At the end of some experiments, in which the recording conditions were still good, we performed a second stimulation protocol which consisted of a train of pulses at 2 Hz for 3 min (Serra et al. 1999), which was applied after a new rest period of 2 min.
Afferent and efferent stimulation
To assess the mechanical responsiveness we stimulated the skin with a stiff von Frey filament (750 mN) repetitively all over an area around the stimulation site (10 cm in diameter), to include innervation territories of both mechano-responsive and mechano-insensitive C-fibres (Schmidt et al. 1997, 2002). With a halogen lamp, feed-back controlled by a thermocouple attached to the skin, we assessed the heat responsiveness by increasing skin temperature from 32°C to 50°C at 0.25°C s−1. The subjects were instructed to switch off the heat lamp when the heat became too painful.
Sympathetic fibres were identified by their marking response to arousal stimuli, e.g. an unexpected loud noise, mental stress, or during deep breath intake (Hallin & Torebjork, 1970; Hagbarth et al. 1972). This was performed after the step-wise electrical protocol and the efficacy of these manoeuvers was controlled by recording background activity of sympathetic burst discharges.
Classification of C-fibres
All C-fibres were classified according to their sensory (mechanical) responsiveness and axonal properties (activity-dependent slowing behaviour) in sympathetic, mechano-responsive, mechano-insensitive fibres as previously described (Serra et al. 1999, 2004; Orstavik et al. 2006). Fibres that did show a response to sympathetic stimulation and no response to mechanical or heat stimulation, and those that showed the typical pattern of slowing for sympathetic C-fibres in the 3 min 2 Hz electrical stimulation protocol (Campero et al. 2004), were classified as sympathetic fibres (symp). Fibres that did not respond to mechanical stimulation (750 mN) and showed a characteristic activity-dependent conduction velocity slowing to repetitive electrical stimulation of more than 5% were classified as mechano-insensitive (CMi) (Orstavik et al. 2006). Fibres that did respond to mechanical stimulation (750 mN) and showed an activity-dependent conduction velocity slowing to repetitive electrical stimulation less than 5% were classified as mechano-responsive (CM) (Orstavik et al. 2006).
Fibres that did not match these classification parameters were defined as ‘atypical’.
Data analyses and statistics
Microneurography signals were amplified, processed online and stored to disk using custom-written Spike2 software and a micro1401 DAC (CED, Cambridge UK). Values are given as mean ±s.e.m.P values < 0.05 were considered statistically significant. The time constants were compared by Student's t tests and for comparing the ratio of CM/CMi between old and young subjects the Fisher exact test was used. All other comparisons were computed using an ANOVA design and post hoc tests where necessary (LSD, Scheffé).
Results
Subjects
The group of young healthy subjects consisted of 19 persons (6 female and 13 male) with a mean age of 24.7 years (± 0.8 years) (total range 21–36). Fifteen subjects (6 female and 9 male) with a mean age of 56.2 years (± 2.6 years) (total range 41–67) formed the group of aged healthy subjects. Some subjects took part in more than one experimental session.
Fibre classes
In the group of healthy young subjects, 146 fibres were recorded. Four of these fibres could not be classified, since they were lost before the characterization was finished. Hence, the sample of classified fibres consisted of 142 units: 83 fibres (58.5%) were characterized as mechano-responsive (CM); 22 fibres (15.5%) were characterized as mechano-insensitive (CMi); 37 fibres (26.1%) were classified as sympathetic units due to their responsiveness to sympathetic arousal stimulation and their characteristic slowing pattern in the 3 min 2 Hz stimulation.
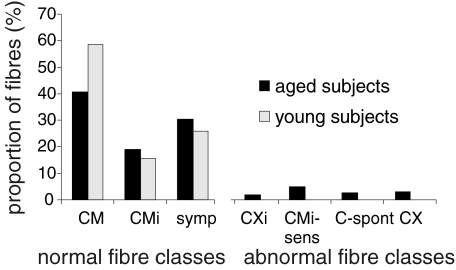
In healthy aged subjects a total of 230 C-fibres could be identified. Twenty eight of these fibres could not be classified for technical reasons. Hence, the total sample of units used for classification consisted of 202 units: 80 (39.6%) were characterized as mechano-responsive (CM) and 37 fibres (18.3%) as mechano-insensitive (CMi) according to the parameters described in the Methods. Sixty fibres were classified as sympathetic (symp) (29.7%). The proportion of efferent to afferent fibres was not changed by ageing (young: 26% symp and 74 afferent; ageing: 30% symp and 70% afferent (P > 0.05 Fisher exact test) (see Fig. 1).
Figure 1. Proportion of fibres in healthy aged (black) and healthy young (light grey) subjects.
In healthy young subjects all tested fibres could be classified as mechano-sensitive (CM), mechano-insensitive (CMi) or efferent sympathetic (symp) fibres, where the majority were CM fibres. In healthy aged subjects, less normal CM fibres were found. Additionally, abnormal fibre classes were observed: (1) mechano- and heat-insensitive fibres with electrical properties of mechano-sensitive fibres (CXi); (2) mechanically sensitized originally mechano-insensitive fibres (CMi-sens); (3) spontaneously active fibres (C-spont); and (4) fibres which showed several abnormalities and could not be classified into classes (1)–(3) (CX).
Atypical fibres
In the group of aged subjects, 25 units (12.4%) could not be classified according to the parameters described above. They were classified as afferent and not efferent C-fibres according to their sensory functions (e.g. mechanical or heat responses) and according to the slowing pattern to the 3 min 2 Hz electrical stimulation. Such atypical afferent C-fibres have been found previously in patients with neuropathy or pain states and were then classified as pathologic (Orstavik et al. 2006). Five of these fibres (2.5%) showed spontaneous activity. Ten fibres (5%) had the classical slowing pattern of mechano-insensitive C-fibres, but did respond to mechanical stimulation. These were regarded as sensitized mechano-insensitive C-fibres (CMi-sens) assuming that the axonal excitability patterns were more pertinent and durable for a nociceptor class than its mechanical sensitivity (Orstavik et al. 2003, 2006). Four fibres (2%) did not respond to mechanical stimulation, but had a slowing in the range of mechano-responsive fibres far below 5% (CM). These fibres were regarded as fibres that presumably have lost their receptive endings and thus lost their ability to transmit sensory stimuli (CXi).
Six fibres showed a mixed pattern of spontaneous activity, sensitization and other distinctive features, so that they could only be categorized as abnormal afferent fibres, but could not be categorized further (X-fibres) (see Fig. 1).
Sensory properties
Fewer polymodal nociceptors with a mechanical response were found in ageing subjects in comparison to young subjects. The ratio of normal mechano-responsive fibres to mechano-insensitive fibres (CM/CMi) was 68.4% CM to 31.6% CMi in aged subjects and 79% CM to 21% CMi in young subjects (P= 0.0495 Fisher exact test) (see Fig. 1).
The number of CM fibres, which did also respond to heating up to 50°C, (CM(H)) was similar in young and aged subjects. In young subjects 34 of the CM units were tested for heat responsiveness (up to 50°C). Twenty five of them were heat responsive (73.5%). In aged subjects 29 CM units were tested for heat responsiveness and 79% were found responsive. Similarly the proportion of heat responsive (CH) and unresponsive mechano-insensitive fibres (CMiHi) was not different in young and aged subjects (heat responsive in young: 4 of 7 tested, in aged 8 of 11 tested.
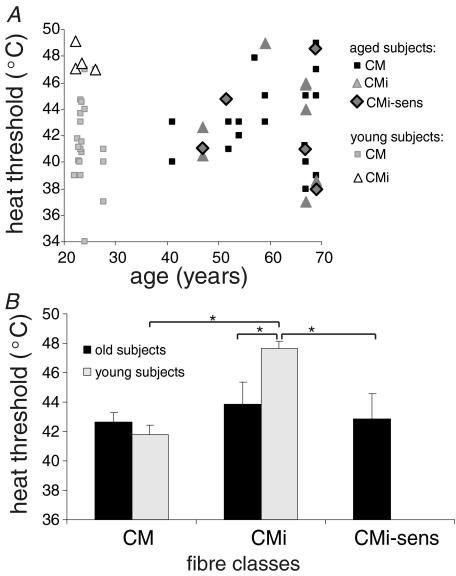
There was no overall significant correlation between heat thresholds and age (Fig. 2A). However, distinct changes in heat responsiveness were found within the different fibre classes in aged subjects. The significant difference in heat thresholds between CM(H) and CMi fibres found in young healthy subjects (41.8 ± 0.7°C versus 47.7 ± 0.5°C, P= 0.001, ANOVA post hoc LSD), which had been encountered also in previous studies (Weidner et al. 1999), was no longer present in aged subjects (Fig. 2B). In aged subjects the heat thresholds of CM(H) (42.7 ± 0.6°C) and CMi (43.9 ± 1.5°C) were not significantly different, since the heat thresholds of CMi in aged (43.9°C) were significantly lower than in young subjects (47.7°C) and therefore more in the range of CM(H) fibres of young subjects (41.8°C) (P= 0.02, ANOVA post hoc LSD). In addition, the mean heat threshold of CM(H) fibres was slightly, but insignificantly, elevated in aged (42.7°C) when compared to young subjects (41.8°C, n.s.). (Fig. 2A and B).
Figure 2. Heat thresholds of mechano-responsive (CM), mechano-insensitive (CMi) and sensitized CMi units (CMi-sens) in young and aged subjects.
A, heat thresholds are shown for the different fibre classes (CM, CMi, CMi-sens; y-axis) in relation to the age of the subjects (x-axis) from whom the respective fibres were recorded. There was no overall correlation between age and heat threshold in fibre class. However, the clear distinction between high heat thresholds of mechano-insensitive (CMi) units and mechano-responsive (CM) units seen in young subjects is blurred in aged mostly due to lowered thresholds of CMi units. B, mean heat thresholds of young (light grey) and aged (black) subjects. Values are given as mean ±s.e.m. Asterisks indicate significant difference (P < 0.05). In aged subjects, heat thresholds of mechano-insensitive (CMi) fibres and sensitized CMi fibres (CMi-sens) were significantly lower when compared to young subjects.
The atypical fibres that were classified according to their conductive properties as CMi fibres, but responding to mechanical stimuli (CMi-sens), had significantly lower heat thresholds than normal CMi fibres of young subjects, (42.9 ± 1.7°C; P= 0.03; ANOVA post hoc LSD) supporting the hypothesis that they were also sensitized to heat (Fig. 2A and B).
The fibres that had no mechanical responsiveness despite conductive properties typical for CM(H) fibres (CXi) did not show any heat response to stimulation of up to 50°C.
When comparing the mean heat threshold of all heat-sensitive fibres, irrespective of their classification, there was a very similar overall mean threshold in young (42.6°C) and aged subjects (42.9°C).
Axonal properties
Conduction velocity
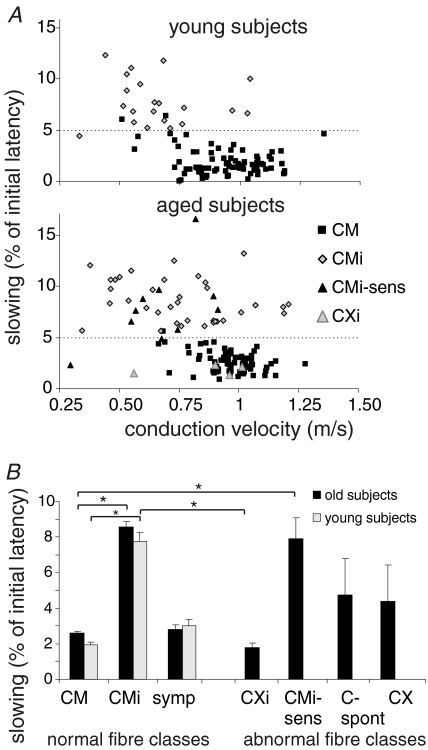
The conduction velocity was slower in CMi units than in CM units in both the aged (CM 0.94 ± 0.02 m s−1; CMi 0.64 ± 0.04 m s−1) and young subject group (CM 0.97 ± 0.01 m s−1; CMi 0.77 ± 0.04 m s−1) (for young subjects P < 0.001; for aged subjects P < 0.001; ANOVA post hoc LSD). There were no significant differences in conduction velocities of normal CM and CMi fibres between aged and young healthy subjects (Fig. 3A).
Figure 3. Axonal properties of the different C-fibre classes in young and aged subjects.
A, slowing to the electrical stimulation protocol from different fibre classes (CM, CMi, CMi-sens, CXi) plotted versus conduction velocities. In the upper panel, in young healthy subjects, the two normal fibre classes of mechano-responsive (CM) and mechano-insensitive (CMi) are divided into two clusters by the 5% slowing line. CM units slow less and CMi units more than 5% of their unconditioned latency (after 2 min without stimulation). Two CM units showed more than 5% slowing and 1 CMi less than 5% slowing. These 3 units were classified according to additional classifying properties as conduction velocity, slowing during 0.125 Hz stimulation and electrical thresholds. CMi units tend to have slower conduction velocities than CM units. In the lower panel, in aged subjects, in addition to the above described fibre classes of CM and CMi units, fibres with the classical electrical properties of CMi were found, which showed mechanical responsiveness (CMi-sens: black triangles). Also fibres with properties of CM without any mechanical or heat responsiveness were observed (CXi: grey triangles). CMi-sens had conduction velocities in the range of normal CMi fibres and CXi units had conduction velocities in the range of normal CM units. B, slowing in the different fibre classes in young (light grey) and aged (black) healthy subjects. Values are given as mean ±s.e.m. Asterisks indicate significant difference (P < 0.05). In both young and old subjects, normal CMi fibres slow significantly more than normal CM fibres. CXi fibres differ significantly from normal CMi fibres and CMi-sens differ significantly from normal CM fibres.
Sensitized CMi units (CMi-sens) had conduction velocities in the normal range of CMi units (0.67 ± 0.06 m s−1). Their conduction velocities were significantly different from the higher conduction velocities of CM units (for young subjects P < 0.001; for aged subjects P < 0.001; ANOVA post hoc LSD) (Fig. 3A and B).
The desensitized CXi units had conduction velocities (0.86 ± 0.1 m s−1) in the range of CM fibres and were significantly faster conducting than CMi fibres (for young subjects P < 0.001; for aged subjects P= 0.004; ANOVA post hoc LSD) (Fig. 3A).
Slowing
According to our classification parameters, the activity-dependent conduction velocity slowing in the electrical stimulation protocol with increasing stimulation pulse frequencies (0.125 Hz, 0.25 Hz and 0.5 Hz) was significantly larger in CMi units than in CM units in both aged and young subjects. Sensitized CMi fibres (CMi-sens) showed slowing in the range of normal CMi units (Fig. 3). The slowing of sympathetic fibres ranged between the slowing of CM and CMi units similarly in young and aged subjects.
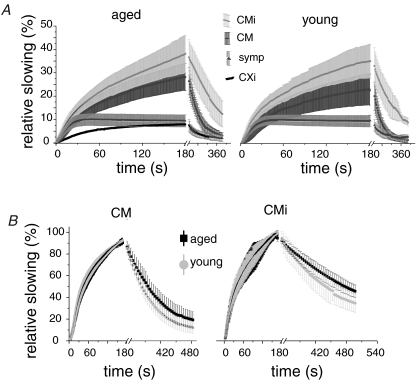
Generally, when stimulating with higher frequencies (2 Hz 3 min) the amount of slowing showed a tendency to be more pronounced in aged subjects. The CM units of aged subjects showed significantly more slowing (28.2 ± 5.9%) than the CM fibres in young subjects (23.1 ± 6.7%) (t test; P= 0.02). In the CMi fibre class the differences were not that pronounced (CMi: aged 38.1 ± 8%, young 35 ± 8.8%) (Fig. 4A). The normalization of conduction velocity during 0.25 Hz stimulation after 2 Hz stimulation was significantly different in the two subject groups. Both CM and CMi in aged had significantly longer time constants of restoration (t test; CM: aged 72.5 ± 4.1 s, young 52 ± 2.1 s, P < 0.001; CMi: aged 185 ± 31.3 s, young 86.2 ± 15.3 s, P= 0.04) (see Fig. 4B).
Figure 4. Activity-dependent conduction velocity slowing in different fibres classes in young and aged subjects to 3 min 2 Hz stimulation.
Values are given as mean ±s.d.A, slowing of the three normal fibre classes and the abnormal fibre class of desensitized fibres (CXi) are plotted against time (s) for aged (left panel) and young (right panel) subjects. The normal CM units of aged subjects showed significantly more slowing than the CM fibres in young subjects (t test: P= 0.02). In the CMi fibre class the differences were not that pronounced. B, the restoration of conduction velocity of CM (left panel) and CMi (right panel) during 0.25 Hz stimulation after 2 Hz stimulation was significantly different in the two subject groups of aged (black) and young subjects (light grey). The time constants of normalization were significantly longer in aged subjects.
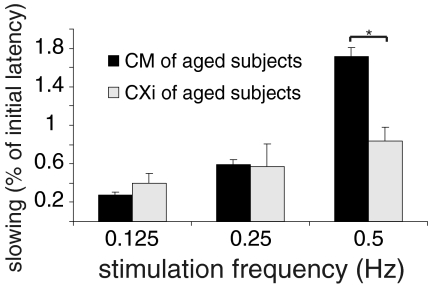
Desensitized CM units (CXi) that were found only in the elderly had a total slowing in the range of normal CM fibres (Fig. 3). However, the slowing to different stimulation frequencies differed from that of normal CM fibres. To low stimulation frequencies (0.125 Hz and 0.25 Hz) the slowing was slightly enhanced but still in the range of normal CM fibres (0.125 Hz: CM: 0.22 ± 0.02%; CXi: 0.4 ± 0.1%) (0.25 Hz: CM: 0.46 ± 0.04%; CXi: 0.57 ± 0.2%) (Fig. 5). For 0.5 Hz the slowing was significantly less in CXi fibres than in CM fibres (0.5 Hz: CM: 1.6 ± 0.04%; CXi: 0.8 ± 0.2%) (ANOVA post hoc LSD P= 0.046) (Fig. 5). The 2 Hz 3 min protocol showed that to higher stimulation frequencies the slowing was decreased even more in the CXi fibres (Fig. 4). Thus, increasing the stimulation frequency led to a bigger difference in the amount of slowing between CXi and CM units.
Figure 5. Activity-dependent conduction velocity slowing in normal (CM) and desensitized mechano-responsive fibres (CXi) of aged subjects to different frequencies of electrical stimulation.
Values are given as mean ±s.e.m. Asterisk indicates significant difference (P < 0.5). The slowing in CXi fibres (light grey) increases with increasing stimulation strength much less than in normal mechano-responsive fibres (black). The difference between normal CM and CXi gets more pronounced with higher stimulation frequencies: CXi slow significantly less than CM at a stimulation rate of 0.5 Hz.
Discussion
Although people at an age of 56 years are not ‘old’ by contemporary standards, almost all previous microneurography studies have been performed on subjects in their mid-twenties. Functional changes that might occur in unmyelinated axons in this long time span cannot be studied in any feasible animal model. A knowledge of the effects associated with ‘normal’ ageing is, however, important for the understanding of the pathology of neuropathies that typically have a higher incidence with age. It is further important for the understanding of normal ageing processes.
Distinct changes in the C-fibre population and also changes of sensory and axonal properties of individual C-fibres were found in healthy aged subjects. The ratio of CM to CMi fibre types was shifted in favour of the CMi units. At the level of individual C-units the heat thresholds of mechano-insensitive CMi fibres were lower. In addition, changes in axonal properties were observed in aged subjects, mainly in C-units with inconspicuous sensory functions, such as a more pronounced activity-dependent conduction velocity slowing.
Further, ‘atypical fibres’ were encountered, which have previously been regarded as ‘pathological’ in patients with neuropathic pain (e.g. spontaneously active and/or sensitized units). This is remarkable since the aged subjects included in this study did not show any symptoms of neuropathy or neuropathic pain.
Fibre classes
Our study confirms the previously reported ratio of CM and CMi fibres of 8 : 2 in young healthy subjects (Schmidt et al. 1995). In a previous microneurographic study on patients suffering from painful diabetic neuropathy, this ratio was fundamentally changed and reversed to 3 : 7 (Orstavik et al. 2006). In the same study, the aged controls had a ratio of 6.9 : 3.1, which is rather similar to the results of the present study. This confirms the trend of a change in the C-fibre population with ageing towards a higher incidence of CMi fibres relative to CM. Microneurographic experiments can only reveal changes in the ratio of fibre classes but do not provide information on the absolute number of C-fibres in a peripheral nerve. Some histological studies suggest that the intra-epidermal nerve fibre count and thin fibre density in the distal peripheral nerve decreases with ageing (Jacobs & Love, 1985; Verdu et al. 2000), while other studies do not support this hypothesis or find only minor changes in subjects older than 75 years (Kanda et al. 1991; Lauria et al. 1999). However, functional measurements of thin nerve fibres such as QST (quantitative sensory testing), axon reflex flare measurements and QSART (quantitative sudomotor axon reflex test) show elevated thermal thresholds (Claus et al. 1987; Rolke et al. 2006), decreased flare size (Helme & McKernan, 1986; Minson et al. 2002; Munce & Kenney, 2003; Namer et al. 2004) and reduced peripheral sweating (Low et al. 1983) in aged populations. Together these findings support the hypothesis that afferent and efferent C-fibres are reduced in number in aged nerves. Mechano-insensitive afferent C-units (CMi) seem to be less affected by this loss.
This hypothesis is further supported by our finding of fibres that did not react to mechanical or heat stimulation, but had activity-dependent slowing of conduction velocity (slowing) in the range of mechano-responsive CM fibres. In a previous study these CXi fibres were abundant in patients with diabetic neuropathy and were interpreted to be fibres with degenerated endings (Orstavik et al. 2006). Thus, a changed CM/CMi ratio and the finding of some CXi fibres in aged subjects would be consistent with a preferential degeneration of mechano-responsive C-fibre endings. Thus, mechano-responsive C-fibres seem to be more prone to degeneration than mechano-insensitive C-fibres. Assuming that CM fibres could be more superficially located in the epidermis than the CMi fibres, the constant pressure to regenerate within the changing epidermal layer may make them more vulnerable to age-related changes. Alternatively, CM and CMi degenerate equally, but increased local availability of Nerve growth factor (NGF) promotes the regeneration mainly of CMi fibres.
Age-related changes in receptive properties
The heat thresholds of mechano-responsive C-fibres have been found to be around 41–42°C in young adults (Weidner et al. 1999). Using comparable heat ramp stimuli, heat pain thresholds assessed psychophysically in humans are around 42–44°C (Tillman et al. 1995; Lautenbacher et al. 2005; Rolke et al. 2006). Assuming that it is not the first action potential that leads to a sensation of heat pain but that a certain frequency of action potentials is required to evoke a sensation of heat pain, it is likely that the class of mechano-responsive C-fibres (i.e. CMH units) is crucial in the determination of the psychophysically assessed heat pain threshold (Van Hees & Gybels, 1981; Tillman et al. 1995). In contrast, individual mechano-insensitive fibres (CMi units) in young subjects usually have high heat thresholds (∼46–48°C) clearly above the human heat pain threshold (Weidner et al. 1999). The current study shows a relative loss of mechano-responsive fibres with age and slightly elevated heat thresholds in the remaining normal CMHs. A decrease in the number of CMH units coupled with an increase in their heat thresholds might result in an increase of heat pain threshold assessed psychophysiologically. However, the psychophysically assessed temperature thresholds do not change dramatically in people between 40 and 60 years (Lautenbacher et al. 2005; Rolke et al. 2006). Interestingly, the mechano-insensitive C-fibres (CMi) in aged subjects had significantly lower heat thresholds than those in younger subjects. As a consequence, the mean heat threshold of the whole population of heat responsive C-fibres was only very slightly elevated in aged subjects (42.9°C) when compared to young subjects (42.6°C). Thus, it could be assumed that heat-sensitized mechano-insensitive fibres with low heat thresholds compensate for lost or desensitized CMH units resulting in unchanged heat pain thresholds in aged subjects.
However, in healthy young subjects a lowering of thresholds of CMi units is related to hyperalgesia and inflammatory pain (Schmelz et al. 1994, 2003) and one may ask why older people do not suffer from heat hyperalgesia. In this context, one possibility is that very slow plastic changes in the course of ageing prevent central sensitization processes.
Possible mechanisms of degeneration and sensitization
The above reported changed ratio of CM to CMi and the occurrence of CXi fibres point towards a degeneration of C-fibres in aged as discussed above. Surprisingly, not only degeneration was seen in healthy aged subjects but also both sensitized and spontaneously active C-fibres were found regularly in healthy aged, but not in young subjects. The percentage of those ‘pathological’ fibres in healthy aged subjects was clearly lower than that of patients with diabetic neuropathy (13%versus 50%) (Orstavik et al. 2006) and the aged subjects in this study did not suffer from pain or hyperalgesia. Indeed, there could be a connection between degeneration and sensitization. Not much is known about the general changes in NGF level in human skin of young and aged people. However, the general level of NGF in the skin may not be the crucial factor in respect to degeneration and sensitization, but the amount of NGF which is available for each fibre. Degenerated endings of nerve fibres do not take up the NGF provided, e.g. by keratinocytes. This may result in a relative surplus of NGF in the epidermis in case of age-dependent reduction of epidermal fibre density (Umapathi et al. 2006).
NGF is known to cause (via changed gene expression) mechanical and heat hyperalgesia (Lewin et al. 1993; Davis et al. 1993, 1997), lowered heat thresholds and spontaneous activity in C-fibres (Stucky et al. 1999). In aged subjects the surplus of NGF caused by the degeneration of mechano-responsive C-fibres could cause lowered heat thresholds in CMi, sensitize CMi to mechanical stimuli and evoke spontaneous activity. In patients with diabetic neuropathy much more desensitized CXi fibres were observed and at the same time more sensitized and spontaneously active fibres (Orstavik et al. 2006). Perhaps there is a balance of degeneration and sensitization preserving normal function in healthy aged, but resulting in neuropathy and pain when the altered and degenerating fibres together with the sensitized surviving units exceed a threshold. Indeed, it has been assumed that a higher incidence of C-fibre degeneration as concluded from elevated heat pain thresholds in diabetic patients correlates with more pain (Kramer et al. 2004).
In healthy aged people the amount of changes in C-fibre function in the direction of hypo- or hyperfunction are obviously below the threshold to cause symptoms of hypo- or hyperalgesia or spontaneous pain. However, the age-related changes may render the C-fibre population more vulnerable to disease processes leading to neuropathies and could worsen their symptoms. Obviously neuropathies, e.g. post herpetic neuralgia or diabetic neuropathy, become much more frequent with age (Johnson et al. 2007). Vincristin therapy causes more severe neuropathy in aged than in young patients (personal communication of E. Jorum, Department of Neurophysiology, Rikshospitalet Oslo).
Axonal properties
Conduction velocity in C-fibres was normal in aged subjects whereas lower conduction velocity of myelinated fibres is found regularly in aged people (Bouche et al. 1993; Verdu et al. 2000). A-fibres are probably more prone to changes in conduction velocity due to their vulnerable myelin sheath, as seen in mice (Robertson et al. 1993).On the other hand, we observed clear changes in activity-dependent slowing of afferent C-fibre conduction in aged subjects. Predominantly, the mechano-responsive C-fibres (CM units) of aged subjects showed more slowing to high frequency stimulation (3 min 2 Hz). This shows again that CM fibres are more prone to changes under stressful conditions. The fibres of aged also showed longer time constants for the normalization of conduction velocity after high frequency stimulation.
The mechanism of activity-dependent conduction velocity slowing is not fully understood. It has been suggested that conduction velocity in unmyelinated axons is predominantly a function of sodium channel availability (De Col et al. 2008). If this were the case, then age-related expression of different sodium channel subtypes (Wang et al. 2006) could contribute to the changes in slowing profiles observed between young and older subjects. Previously sodium–potassium pump activity had been deemed casual for activity-related conduction velocity slowing; however, pump blockade such as that during ouabain treatment or substrate reduction increases the amount of slowing (De Col et al. 2008). In the course of ageing, metabolic changes resulting in reduced ATP supply might lead to decreased sodium–potassium pump activity (Verdu et al. 2000), perhaps resulting from an altered lipid microenvironment (Tanaka & Ando, 1990). Indeed in aged mice, sodium–potassium pump activity is reduced in the sciatic nerve and dorsal root (Robertson et al. 1993). In our study the most likely mechanism responsible for the observed increase in slowing in axons in older subjects is reduced sodium–potassium pump activity. A decreased basal level of pump activity would result in a relatively depolarized membrane potential and a higher proportion of inactivated sodium channels. This would account for hypoexcitability of fibres to sensory stimuli. Since membrane potential affects sodium channel inactivation, the magnitude of hyperpolarizing current attributable to Na+,K+-ATPase activity acts to counter inactivation and thereby limit axonal conduction velocity slowing. Accordingly, a progressive reduction in pump activity with ageing is consistent with the observed exacerbation of slowing and protracted recovery from slowing in aged subjects. However, membrane mechanisms underlying the observed activity-dependent slowing of conduction velocity and those underlying axonal hypoexcitability may not be identical. Thus, the relation of activity-dependent slowing and modulation of axonal excitability awaits further studies.
Conclusions
In this study we found signs of degeneration as well as signs of C-fibre sensitization and spontaneous activity in healthy aged subjects compared to young subjects. In addition activity-dependent conduction velocity slowing was enhanced in the unmyelinated axons in aged subjects. We interpret the lowering of the heat thresholds in mechano-insensitive C-units and the appearance of spontaneously active fibres as discrete signs of compensatory processes. It is of major importance to quantify these age-dependent changes in order to separate them from neuropathy-related processes: the number of pathologically changed C-fibres was much lower than that found in patients with neuropathy and their occurrence therefore does not pose a major problem for our subjects. However, as sensitization and desensitization phenomena already are present, this might make aged people more vulnerable to additional noxious events affecting the peripheral nervous system such as neurotrophic viruses, metabolic changes or chemotherapy (e.g. vincristin).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft grant HA 831 14/1 and 14/2.
References
- Bostock H, Campero M, Serra J, Ochoa JL. Temperature-dependent double spikes in C-nociceptors of neuropathic pain patients. Brain. 2005;128:2154–2163. doi: 10.1093/brain/awh552. [DOI] [PubMed] [Google Scholar]
- Bouche P, Cattelin F, Saint-Jean O, Leger JM, Queslati S, Guez D, Moulonguet A, Brault Y, Aquino JP, Simunek P. Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol. 1993;240:263–268. doi: 10.1007/BF00838158. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol Scand. 2004;182:305–311. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- Claus D, Hilz MJ, Hummer I, Neundorfer B. Methods of measurement of thermal thresholds. Acta Neurol Scand. 1987;76:288–296. doi: 10.1111/j.1600-0404.1987.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387:489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Davis BM, Lewin GR, Mendell LM, Jones ME, Albers KM. Altered expression of nerve growth factor in the skin of transgenic mice leads to changes in response to mechanical stimuli. Neuroscience. 1993;56:789–792. doi: 10.1016/0306-4522(93)90127-2. [DOI] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr RW. Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol. 2008;586:1089–1103. doi: 10.1113/jphysiol.2007.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KG, Ellis FP, Dore C, Exton-Smith AN, Weiner JS. Sweat responses in the aged. Age Ageing. 1976;5:91–101. doi: 10.1093/ageing/5.2.91. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjork HE. Microneurographic analysis of the fibre spectrum of human sensory nerve fascicles. Acta Physiol Scand. 1970;80(4):24–25. doi: 10.1111/j.1748-1716.1970.tb04848.x. [DOI] [PubMed] [Google Scholar]
- Helme RD, McKernan S. Effects of age on the axon reflex response to noxious chemical stimulation. Clin Exp Neurol. 1986;22:57–61. [PubMed] [Google Scholar]
- Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Wasner G, Saddier P, Baron R. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother. 2007;7:1581–1595. doi: 10.1586/14737175.7.11.1581. [DOI] [PubMed] [Google Scholar]
- Kanda T, Tsukagoshi H, Oda M, Miyamoto K, Tanabe H. Morphological changes in unmyelinated nerve fibres in the sural nerve with age. Brain. 1991;114:585–599. doi: 10.1093/brain/114.1.585. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27:2386–2391. doi: 10.2337/diacare.27.10.2386. [DOI] [PubMed] [Google Scholar]
- Lauria G, Holland N, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. J Neurol Sci. 1999;164:172–178. doi: 10.1016/s0022-510x(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Low PA, Opfer-Gehrking TL, Proper CJ, Zimmerman I. The effect of aging on cardiac autonomic and postganglionic sudomotor function. Muscle Nerve. 1990;13:152–157. doi: 10.1002/mus.880130212. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Munce TA, Kenney WL. Age-specific skin blood flow responses to acute capsaicin. J Gerontol A Biol Sci Med. 2003;58:304–310. doi: 10.1093/gerona/58.4.b304. [DOI] [PubMed] [Google Scholar]
- Namer B, Bickel A, Kramer H, Birklein F, Schmelz M. Chemically and electrically induced sweating and flare reaction. Auton Neurosci. 2004;114:72–82. doi: 10.1016/j.autneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve. 2005;32:459–472. doi: 10.1002/mus.20367. [DOI] [PubMed] [Google Scholar]
- Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jorum E, Torebjork HE. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci. 2006;26:11287–11294. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jorum E, Handwerker H, Torebjork E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- Robertson A, Day B, Pollock M, Collier P. The neuropathy of elderly mice. Acta Neuropathol. 1993;86:163–171. doi: 10.1007/BF00334883. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjork HE. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Exp Brain Res. 1995;104:331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Ringkamp M, Handwerker HO, Torebjork HE. Sensitization of insensitive branches of C nociceptors in human skin. J Physiol. 1994;480:389–394. doi: 10.1113/jphysiol.1994.sp020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Ringkamp M, Handwerker HO, Torebjork HE. Innervation territories of mechanically activated C nociceptor units in human skin. J Neurophysiol. 1997;78:2641–2648. doi: 10.1152/jn.1997.78.5.2641. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Weidner C, Handwerker HO, Torebjork HE. Innervation territories of mechano-insensitive C nociceptors in human skin. J Neurophysiol. 2002;88:1859–1866. doi: 10.1152/jn.2002.88.4.1859. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Two types of C nociceptors in human skin andtheir behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–27781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19:8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Ando S. Synaptic aging as revealed by changes in membrane potential and decreased activity of Na+,K+-ATPase. Brain Res. 1990;506:46–52. doi: 10.1016/0006-8993(90)91197-o. [DOI] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: correlation with pain threshold in humans. J Physiol. 1995;485:767–774. doi: 10.1113/jphysiol.1995.sp020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork HE, Hallin RG. Identification of afferent C units in intact human skin nerves. Brain Res. 1974;67:387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Umapathi WL, Tan WL, Tan NC, Chan YH. Determinants of epidermal nerve fiber density in normal individuals. Muscle Nerve. 2006;33(6):742–746. doi: 10.1002/mus.20528. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry. 1981;44:600–607. doi: 10.1136/jnnp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]