Abstract
In order to overcome the relatively long delay in processing visual feedback information when pursuing a moving visual target, it is necessary to predict the future trajectory of the target if it is to be tracked with accuracy. Predictive behaviour can be achieved through internal models, and the cerebellum has been implicated as a site for their operation. Purkinje cells in the lateral cerebellum (D zones) respond to visual inputs during visually guided tracking and it has been proposed that their neural activity reflects the operation of an internal model of target motion. Here we provide direct evidence for the existence of such a model in the cerebellum by demonstrating an internal model of a moving external target. Single unit recordings of Purkinje cells in lateral cerebellum (D2 zone) were made in cats trained to perform a predictable visually guided reaching task. For all Purkinje cells that showed tonic simple spike activity during target movement, this tonic activity was maintained during the transient disappearance of the target. Since simple spike activity could not be correlated to eye or limb movements, and the target was familiar and moved in a predictable fashion, we conclude that the Purkinje cell activity reflects the operation of an internal model based on memory of its previous motion. Such a model of the target's motion, reflected in the maintained modulation during the target's absence, could be used in a predictive capacity in the interception of a moving object.
The cerebellum is essential for accurate visually guided movement (Holmes, 1917 see also; Miall et al. 1986; Stein & Glickstein, 1992; Ebner & Fu, 1997), but exactly how it controls limb movements to external objects is still vigorously debated. However, much routine behaviour is thought to depend on the cerebellum operating in a way that involves prediction.
Predictive behaviour can be achieved through internal models (Miall et al. 1987; Wolpert et al. 1995; Desmurget & Grafton, 2000; Wolpert & Flanagan, 2001; Glasauer, 2003). They can be of two types: forward and inverse. For limb movements, forward models could predict the position or velocity of the limb, whereas inverse models could transform the desired trajectory into appropriate joint forces/torques. There is disagreement over whether cerebellar models are forward or inverse. Purkinje cell simple spike activity patterns in cerebellar regions concerned with eye movements have been interpreted as representing inverse dynamic models (Shidara et al. 1993; Gomi et al. 1998), but this is not universally accepted (Ostry & Feldman, 2003; Pasalar et al. 2006; Green et al. 2007).
Whereas previous studies have concentrated on internal modelling of own body movements (eye, head or limb) here we address a separate but related question: whether the cerebellum contains internal models of the movement of external objects in the outside world which can predict a familiar object's future position and velocity. Indirect evidence for internal models of external objects and tools comes from cerebellar imaging studies (Imamizu et al. 2000, 2003, 2004; Higuchi et al. 2007) but to obtain direct evidence, recordings from cerebellar neurones are required. Previous studies confirm that the lateral cerebellum is intimately involved in visually guided movement, with Purkinje cell simple spike activity found to precisely signal visual events, and encode target motion during visually guided reaching (Miles et al. 2006). If an external target moves in a predictable fashion, consistent features might be built into an internal model of its motion. However, neuronal activity previously found to be related to target movement has two possible interpretations: either it represents coding of actual target motion driven by the visual stimulus or, since the target was familiar, a model of its motion could have been constructed, so that it represents the active operation of an internal model closely simulating target motion.
Temporary visual denial of the target can distinguish between these two possibilities. Whilst invisible, the tonic increase in discharge rate seen whilst the visible target is moving should disappear if the cells are being passively driven by the visual target, whereas such activity would be expected to survive temporary absence of the target if centrally generated. The present study recorded Purkinje cell simple spike activity in the D2 zone in the lateral cerebellum that matched the second possibility. These experiments therefore provide strong evidence for a cerebellar internal model of an external object's motion.
Methods
All experimental and surgical procedures were approved and performed in accordance with local animal welfare guidelines and the UK Animals (Scientific Procedures) Act 1986.
Surgery and implants
Three purpose-bred adult male cats (4–6 kg) selected for their cooperative nature and propensity to use their left forepaw were trained to perform a visually guided reach towards a moving target containing a food reward. No aversive training techniques were used. Following a 6–8 week training period, a recording chamber was implanted over the left cerebellum under full surgical anaesthesia. Surgical procedures have been described in full elsewhere (Marple-Horvat & Criado, 1999; Miles et al. 2006). In brief, anaesthesia was induced and maintained by a continuous infusion of propofol (0.05 ml min−1i.v.; Schering-Plough, Welwyn Garden City, UK) following premedication with medetomidine hydrochloride (Domitor, 150 μg kg−1s.c.; Pfizer, UK). A single dose of Atropine (0.5 ml s.c.; Animalcare, Dunnington, UK) was given to prevent excessive secretion in the respiratory passages and a broad-spectrum antibiotic (0.2 mg kg−1s.c.; Amfipen LA, Intervet, Milton Keynes, UK) was administered as a precautionary measure, pre- and postoperatively. The temperature of the animal was kept within physiological limits with the aid of a thermostatically controlled heating blanket. Post-operative analgesic (buprenorphine 0.01 mg kg−1i.m.; Temgesic; Reckitt Benckiser, Slough, UK) was administered and maintained for 24 h. All three animals recovered uneventfully.
Surgery was carried out with aseptic precautions and a craniotomy was made which exposed part of the left cerebellar hemisphere, including crus I of the ansiform lobule. A lightweight titanium chamber was placed over the four folia of crus I. Keyhole slots were drilled to gain access to the frontal sinuses and electro-oculography (EOG) electrodes positioned on the bony orbital surface. To ensure the chamber was firmly fixed, a T-bolt was anchored to the skull which also served as an indifferent connection for differential recording (see below). A lead was routed subcutaneously down the left forelimb to permit detection of paw lift during reaching. The frontal sinuses were filled with dental acrylic, and a headpiece fashioned so as to incorporate the titanium chamber, and terminal connectors for EOG, contact signals, earth and indifferent.
Visually guided reaching task
The task was similar to that previously described (Miles et al. 2006). Briefly, the cat was connected via a harness to a Perspex enclosure consisting of an adjustable back and side walls with an open front and an open top. The cat was loosely restrained with a natural seated posture. The head was unrestrained, which meant that the animal was as natural and free to move as possible. The target for reach consisted of a Perspex tube 30 mm in diameter and dimly lit by a ring of LEDs. A vertical rod barred access to the tube. The tube was initially stationary 7 cm to the left of centre (as viewed by the cat) at a comfortable height for reaching. The tube moved at a constant velocity of 6.2 cm s−1 (25 deg s−1) rightwards across the cat's visual field. This velocity was chosen for two reasons: (1) in a previous study from our laboratory, Purkinje cell recordings obtained from an identical region displayed tonically altered discharge at a ‘preferred’ target velocity of 6.2 cm s−1 when tested against three target velocities (Miles et al. 2006), and (2) it represents the optimum velocity for accumulation of sufficient trials for statistical analysis of spike trains, whilst maintaining the cat's interest in the task. For the amplitude and speed of our target, the cats did not use the head to track. Rather, the head was clearly and voluntarily ‘locked’ in a stationary position. When occasionally between trials cats did turn their head, we invariably saw a gross change in the recorded extracellular potentials, or complete loss, or impalement and cell death. By marked contrast the cats sat so still during task performance we typically recorded stable ‘artefact free’ single unit activity for 30–45 min.
The cue to reach with the left forepaw and retrieve the food reward (the ‘go’ signal) was a brightening of the LEDs and the concurrent withdrawal of the rod by a solenoid, and was given 600 ms after the start of target movement, calculated so that addition of typical reaction and reach times meant the target would be intercepted at an approximately central location. On entry of the cat's paw into the tube, an infrared beam spanning the mouth of the tube was interrupted and halted its movement to permit comfortable retrieval of the food reward. Within a block of 20 trials, the ‘go’ signal was randomly delivered in 14 trials. The tube subsequently returned leftwards to the start position at a constant velocity of 12.4 cm s−1. In trials where the ‘go’ signal was not given (‘no-go’ trials) the LEDs did not brighten; instead the tube continued rightwards until the end of the track (14 cm from the start) and the cat made no reach. After a delay of approximately 1 s the tube returned leftwards to the start position at 12.4 cm s−1.
Experiments were conducted without ambient illumination in a light-proof room. Thus, the only source of visual information available to the cat was from the target LEDs. At defined stages of the task, illumination of the tube LEDs was temporarily extinguished (‘denial’) for 200 or 300 ms, during which time the animal was in total darkness. Denial trials were only introduced to the animal once recording sessions had commenced (i.e. once the animal was fully trained). The 300 ms denial period was introduced in later experiments to determine whether a longer period of LED extinction could be more effective at altering neuronal activity. Importantly, denial was always presented in ‘go’ trials well before any limb movement or related postural adjustments occurred (see Discussion).
In trials where illumination of the LEDs disappeared for 200 ms, denial was randomly delivered at 200 or 400 ms after the start of target movement. In trials where the LEDs were extinguished for 300 ms, the denial period began 200 ms after the start of target movement in 50% of the trials, i.e. 10 within a block of 20 trials. For the remaining 10 trials, the LEDs were not extinguished in order to compare denial versus non-denial trials. In ‘no-go’ trials, a further two possible denial points were used; as well as one of those described above, denial was randomly delivered either as the target continued to move rightwards past the centre, or during the return of the target to its initial start position. Only one denial period was ever delivered in each ‘go’ trial; two were delivered in ‘no-go’ trials. Cat B and Cat F were presented with 200 and 300 ms denial trials, respectively. Cat P was presented with both 200 and 300 ms denial trials but these were never mixed within an experimental session. To determine whether the temporary extinction and re-illumination of the target had any effect on neuronal activity, a denial point was also delivered in between every trial when the target was stationary.
Recording arrangements
Extracellular single unit recordings confined to the superficial cerebellar cortical layers were obtained in the awake animal during the visually guided reaching task using custom-made glass-insulated tungsten microelectrodes (impedance 2–6 MΩ). At the beginning of each recording session, the microelectrode was advanced into the cerebellar cortex with a microprocessor controlled stepping microdrive mounted onto an X-Y stage that was attached to the recording chamber. Purkinje cells were identified by the presence of complex spikes and on the basis of their interspike interval distribution and spike duration (Eccles et al. 1964; Thach, 1967; Armstrong & Rawson, 1979). Signals from the microelectrode were relayed through a FET preamplifier stage to a unit amplifier (gain × 1000, bandpass filtered 0.5–10 kHz) and monitored on an oscilloscope and an audio amplifier. EOG signals were relayed through an isolating preamplifier (×100) and a high cut filter (500 Hz). Paw lift-off from the ground was continuously monitored using a high-frequency carrier signal applied to a copper contact plate. A potentiometer monitored the position of the tube and transition from a high to low voltage level signalled the extinguishing of the dimly illuminated LED ring. All data were stored on digital audio tape (DAT) for off-line analysis. Attempts were made to define any peripheral receptive field for somatic afferent inputs generated by manually delivered stimuli such as brushing of hairs, tapping of skin, palpation of muscle, and the passive movement of joints. For subsequent computer analysis of the data, all signals were digitized off-line with customized Spike2 software running on a CED 1401Plus computer interface unit (Cambridge Electronic Design, Cambridge, UK). Discrimination of unitary potentials was performed offline using the Multiple Spike Detector System (Alpha Omega Engineering, Nazareth, Israel). The spike trains were converted to standard transistor to transistor logic (TTL) pulses and the resulting event data digitized at 1 kHz. All other signals were sampled at 200 Hz. EOG recordings were digitally bandpass filtered between 1 and 10 Hz. Spike2 software was used to compile peri-event time histograms (PETHs).
Analysis
PETHs were time aligned to the denial of the target during and in between trials, onset of target movement, termination of target movement, footlift, and eye movements. Only PETHs generated from recording sessions involving > 20 trials were analysed. As in previous studies (Marple-Horvat & Criado, 1999; Miles et al. 2006), a cell was judged to be significantly modulated if the discharge frequency in at least two successive 10 ms bins was ± 2 s.d. from the mean level of discharge in the preceding 200 ms ‘control’ period immediately prior to the event. Onset latency was calculated to the leading edge of the first significant bin. Latency to onset and peak, duration of modulation, and frequency change from mean level in the control period were calculated. All mean values given in Results are expressed ±s.e.m.
In order to determine whether PETHs aligned to target movement onset displayed tonic changes in neuronal firing rate when the target was moving compared to when it was stationary, the relationship between tonic discharge rate and ongoing target movement was analysed. The mean discharge rate during a 200 ms period immediately preceding the start of target movement was compared with the mean discharge rate for the period 100–300 ms into its movement (a time window which both excluded any initial phasic response, and preceded delivery of the ‘go’ signal). The mean discharge rate during the 200 ms period immediately preceding start of target movement was also compared with the mean discharge rate during the 100–300 ms period after the target stopped moving at the end of each trial.
Cells whose neuronal activity was modulated significantly (Student's paired t test, P < 0.05) during ongoing target movement were analysed further to examine their activity in both the presence and the absence of a visible target. In the case of the 200 ms target denial, PETHs aligned to target movement were calculated separately for the 200 and 400 ms denial point deliveries, with each PETH divided into three 200 ms time segments. For the 200 ms denial point delivery the three segments were: (1) 0–200 ms, after the onset of target motion but before the occurrence of the denial period; (2) 200–400 ms, the 200 ms epoch between LED disappearance and reappearance which occurred 200 ms after target movement; and (3) 400–600 ms, when the target was again visible 400 ms after target onset but preceding the delivery of the ‘go’ signal. For the 400 ms time denial point delivery, the three segments were: (1) 0–200 ms, first 200 ms after the onset of target motion; (2) 200–400 ms, the 200 ms epoch before the denial period; and (3) 400–600 ms denial, the 200 ms epoch between LED disappearance and reappearance. For cells tested with the denial period of 300 ms occurring 200 ms after the onset of target motion, the three time segments were altered to reflect the longer duration of LED extinction; the three time segments consisted of (1) 0–200 ms, a 200 ms period after the onset of target motion but before the occurrence of the denial period; (2) 200–500 ms, the 300 ms epoch between LED disappearance and reappearance which occurred 200 ms after target movement; and (3) 500–600 ms, the 100 ms period when the target was again visible 500 ms after target onset but preceding the delivery of the ‘go’ signal. One-way ANOVA (P < 0.05) was used to compare the mean discharge rates across the three periods during target motion to determine if neuronal firing during visual denial was altered when compared to periods when the target was visible. Post hoc comparisons were made using Tukey's multiple comparison test.
Histological verification of recording sites and retrograde labelling studies
Following a period of productive single unit recording (between 8 and 12 weeks) all three cats were re-anaesthetized as described above, and red fluorescently tagged latex microspheres (Lumafluor, Naples, Florida, USA) were microinjected into the tips of the folia in the region of cerebellar cortex in which most of the microelectrode tracks had been made during the recording sessions. The beads were delivered hydraulically via a glass micropipette attached to a 1 μl Hamilton syringe. In each case 150–200 nl of beads were delivered 0.5–1 mm below the cortical surface. Following a survival period of 7–11 days to allow axonal transport of tracer to occur, the animals were re-anaesthetized with sodium pentobarbitone (Euthatal, 100 mg kg−1, i.p.; Rhone Merieux, UK), and perfused transcardially with 0.9% heparinized saline rinse followed by 4% paraformaldehyde fixative and 0.2 m phosphate buffer (PB; pH 7.4) containing 10% sucrose. The cerebellum and brainstem were removed and the dorsal surface photographed before being stored in 30% sucrose PB at 4°C. A freezing microtome was used to cut the cerebellum and brainstem into 50 μm sagittal and transverse sections, respectively. Two series of sections were collected: one was for analysis of fluorescent material, and the other was counterstained with cresyl violet and used to permit reconstruction of microelectrode tracks.
For mapping of fluorescent retrograde cell labelling and injection sites, sections were viewed with a Zeiss Axioskop II Mot microscope (Zeiss, Germany) fitted with a 100 W mercury UV light source and viewed with a no. 15 filter set (Dichroic mirror 580 nm, BP 546/12 nm, LP 590 nm). The numbers of retrogradely labelled cells in the inferior olive were counted and their location charted onto standard transverse diagrams (Trott & Armstrong, 1987; Edge et al. 2003). In one animal (Cat B) retrograde cell labelling did not occur. In this case, the cerebellum was cut at 50 μm intervals in the sagittal plane and the tissue counterstained for reconstruction of microelectrode tracks.
Results
Stable electrophysiological recordings were obtained from a total of 92 lateral cerebellar cortical neurones during the performance of a visually guided reaching task (38 cells in Cat B, 34 cells in Cat P, 20 cells in Cat F). Sixty-nine of these cells were identified as Purkinje cells by the presence of complex spikes. The remaining 23 cells were unidentified cortical neurones. As the recordings were made in behaving animals, to avoid the possibility of mechanical stimulation of neurones by the microelectrode tip we did not attempt to maximize spike heights once a satisfactory signal to noise ratio for Purkinje cell simple spike activity was obtained. Thus, discrimination of complex spikes was often not possible during task performance, and complex spikes are therefore not considered further. Despite careful inspection, none of the 69 Purkinje cells displayed any detectable changes in simple spike activity in response to peripheral stimuli such as brushing of hairs, skin taps, palpation of muscles and passive movement of joints of the limbs and trunk.
Localization of neurones
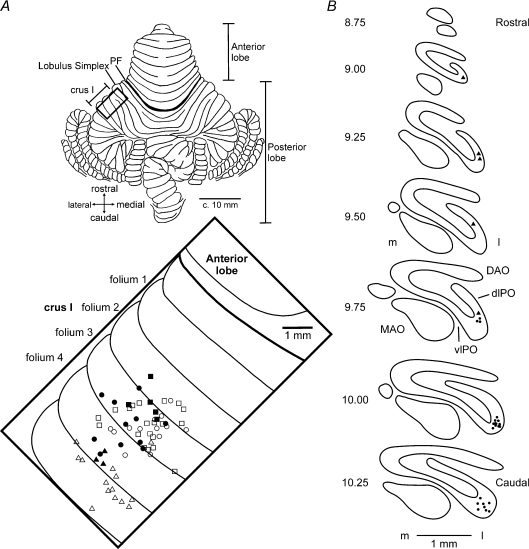
Recordings were made mainly from the most superficial Purkinje cell layer in the rostral part of folia 2–4 of crus I. The approximate locations of individual recordings obtained from cells tonically responsive to target motion are shown in Fig. 1A projected onto the surface of the lateral cerebellum. Responsive cells (filled symbols) were intermingled with unresponsive cells (open symbols) and located mainly within folia 3 and 4 of crus I. At the end of the recording period, all three cats received microinjections of red fluorescently tagged latex microspheres into the cerebellar cortex, approximately centred where the majority of recordings were obtained in each animal. Successful retrograde transport to the inferior olive occurred in two cats (P and F). Retrograde cell labelling was confined to the contralateral inferior olive, entirely within the principal olive. In Cat P, cell labelling (indicated by circles) was mainly in the lateral bend of the principal olive, while in Cat F labelling (triangles) was in the dorsal lamella of the principal olive. This pattern of retrograde cell labelling indicates that in both animals recordings were made mainly (perhaps entirely) from the medial aspect of the D2 zone (Herrero et al. 2006). Since recording tracks for the third animal (Fig. 1A, squares) were in a very similar region of crus I it seems reasonable to conclude that recordings in this experiment were also obtained from the same zone.
Figure 1. Location of recording tracks in the lateral cerebellum.
A, dorsal view of the unfolded cat cerebellum (modified from Larsell, 1953) indicating the approximate positions of the recording chamber (boxed region), with expanded view of the ansiform lobule (crus I) showing the locations of the electrode penetrations in Cat B (squares), Cat P (circles) and Cat F (triangles). Filled symbols indicate tracks in which Purkinje cells were found with tonic changes in simple spike activity during target motion. Open symbols indicate location of remaining (unresponsive) Purkinje cells. Note, for 3 electrode tracks, more than one cell was recorded (2 filled symbols, 1 unfilled symbol). B, distribution of retrogradely labelled olive cells after a tracer injection of red latex microspheres was made into crus I coinciding with the parts of the cerebellar cortex where most of the microelectrode tracks were made. Equally spaced standard transverse outlines of the inferior olive between AP levels 10.25 and 8.75. Each circle and triangle corresponds to one retrogradely labelled cell in Cat P and Cat F, respectively. DAO, dorsal accessory olive; dLPO, dorsal lamella of the principal olive; l, lateral; m, medial; MAO, medial accessory olive; PF, primary fissure; vlPO, ventral lamella of the principal olive.
Modulation to target movement and effects of visual denial
Nearly half of the 69 Purkinje cells (32, 46.4%) displayed statistically significant (P < 0.05) increases or decreases in simple spike firing rate relative to background discharge during target movement in the rightwards direction. The remaining cells were unrelated in any way to the task, apart from three cells which responded to the ‘go’ signal.
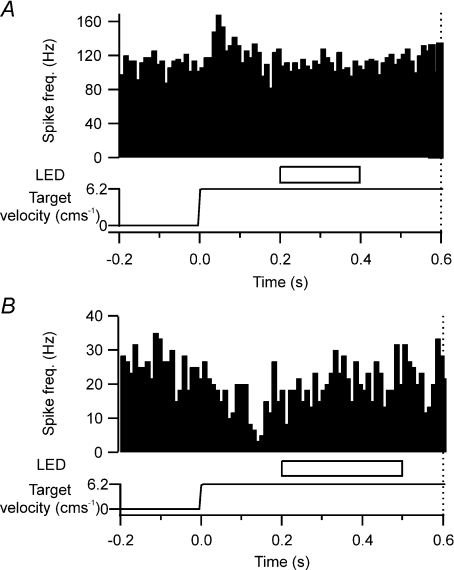
Eleven of the target-related cells (11/32, 34.4%) responded vigorously to the onset of target movement with a phasic response in simple spike activity. The majority of phasic cells (8/11) significantly increased their discharge rate (P < 0.05; Fig. 2A) whilst the remaining three cells significantly reduced their discharge rate (P < 0.05; Fig. 2B). Onset latencies and duration for phasic responses were 45 ± 10 ms and 63 ± 12 ms, respectively. The average pre-target movement firing rate for phasic increases and decreases in simple spike activity were 34.9 ± 10.9 impulses s−1 and 28.4 ± 4.3 impulses s−1, respectively. During target movement, peak modulation was often a doubling or halving of discharge rate, with an increase of 31.2 ± 6.1 impulses s−1 and a decrease of 20.4 ± 0.9 impulses s−1, respectively.
Figure 2. Purkinje cell simple spike phasic activity in relation to target motion.
A, peri-event time histogram (PETH) showing an example of a phasic increase in simple spike activity in response to the onset of target motion. Target motion started at time zero. Target was occluded at 200 ms after the start of target onset for a duration of 200 ms (horizontal open bar, labelled LED). Dotted vertical line at 0.6 s after onset of target movement represents the time point of the ‘go’ signal. Time course of target velocity is represented in the bottom trace. B, same as A, but representative example of a phasic decrease in simple spike activity in response to the onset of target motion and the target occluded for 300 ms.
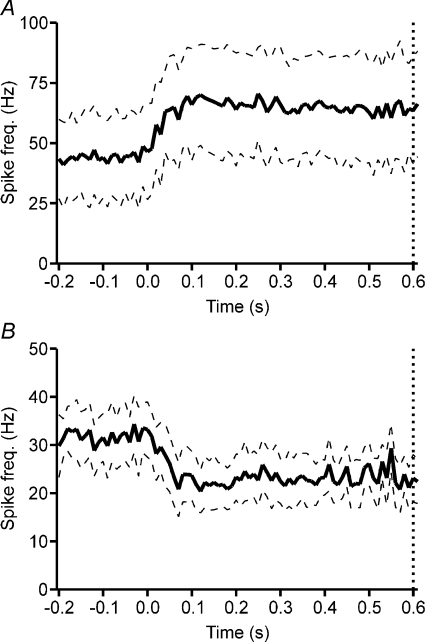
However, the most common finding for target-related cells (21/32, 65.6%) was a tonic change in simple spike activity, with a statistically significant difference in mean firing rate while the target was moving compared to when it was stationary (Student's paired t test, P < 0.05). Over half (12/21, 57.1%) of such responses were a sustained increase. Figure 3A illustrates the population average PETH for this group of cells (n= 12). Among these cells, onset latency was 60 ± 15 ms. The average simple spike firing rate before the onset of target movement was 43.9 ± 17.5 impulses s−1, while the average firing rate during target movement increased to 68.6 ± 21.7 impulses s−1. The average firing rate after the target stopped moving was 48.4 ± 17.5 impulses s−1. The remaining tonically modulated cells (9/21, 42.9%) displayed a significant reduction in activity (Fig. 3B, n= 9). Onset latencies for cells displaying a tonic reduction in activity were 78 ± 14 ms. The average simple spike rate before the onset of target movement was 32.0 ± 5.5 impulses s−1 while the firing rate during target movement decreased to 22.6 ± 4.9 impulses s−1. The average firing rate after the target stopped moving was 30.6 ± 5.4 impulses s−1.
Figure 3. Tonic simple spike activity in relation to target motion.
A, population average PETH for all Purkinje cells which displayed a tonic increase in simple spike activity in relation to target motion (n= 12). B, population average PETH for all Purkinje cells which displayed a tonic decrease in simple spike activity in relation to target motion (n= 9). Target motion aligned at time zero. Dotted vertical line at 0.6 s after onset of target movement represents the time point of the ‘go’ signal. Dashed lines represent ±s.e.m.
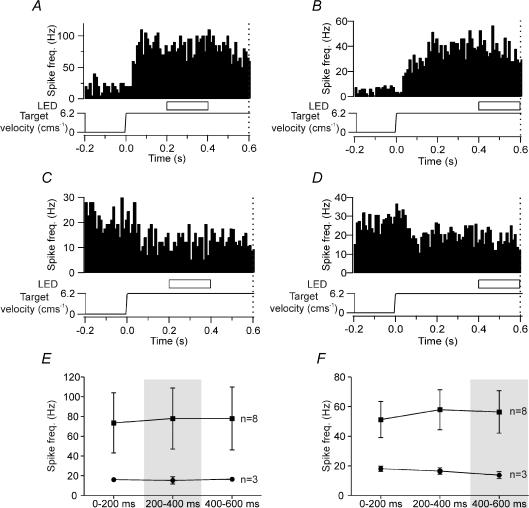
The effect of temporarily extinguishing the visual target for 200 ms (200 or 400 ms after the start of target movement) was tested for 11 of the 21 tonically active Purkinje cells. Eight of these 11 cells showed a sustained increase in neuronal activity while the remaining three cells displayed a sustained decrease in firing rates. Figure 4A–D illustrates PETHs of four such examples in which Purkinje cells exhibited a sustained increase (Fig. 4A and B) or sustained decrease (Fig. 4C and D) in neuronal activity during target movement in the rightwards direction. Under conditions of 200 ms temporary visual denial, no statistically significant change in neuronal activity compared to the level during visible target motion was found in any of the 11 Purkinje cells tested, regardless of whether the denial point was delivered 200 ms (Fig. 4A and C) or 400 ms (Fig. 4B and D) after the onset of target motion during ‘go’ trials, or whether the cells exhibited a sustained increase or decrease in their firing rate (one-way ANOVA, P > 0.05). Figure 4E and F summarizes the mean firing rates for all 11 cells during periods of target denial compared to when the target was visible whilst the target was moving. Figure 4E shows data when the 200 ms denial period starts 200 ms after onset of target motion, and Fig. 4F shows data when the 200 ms denial period starts 400 ms after onset of target motion. It is evident that all the Purkinje cells maintained their neuronal activity compared to levels during visible target motion, irrespective of the denial time point, and whether they displayed a tonic increase or decrease in activity during target motion (one-way ANOVA, P > 0.05).
Figure 4. Effect of 200 ms duration target denial on Purkinje cell simple spike activity during target motion.
A and B, examples of two Purkinje cells which displayed a tonic increase in simple spike activity in relation to target motion. Target denial occurred 200 ms (A) and 400 ms (B) after onset of target motion. C and D, examples of two Purkinje cells which displayed a tonic reduction in simple spike activity during target motion. Target denial occurred 200 ms (C) and 400 ms (D) after onset of target motion. Dotted vertical line at 0.6 s after onset of target movement represents the time point of the ‘go’ signal. E, comparison of average firing rate comparing epochs when the target was visible (0–200 ms, 400–600 ms) with target denial delivered 200–400 ms after the onset of target motion (one-way ANOVA, P > 0.05). F, comparison of average firing rate comparing epochs when the target was visible (0–200 ms, 200–400 ms) with target denial delivered 400–600 ms after the onset of target motion (one-way ANOVA, P > 0.05). ▪, Purkinje cells with an increase in simple spike activity, n= 8; •, Purkinje cells with a decrease in simple spike activity, n= 3.
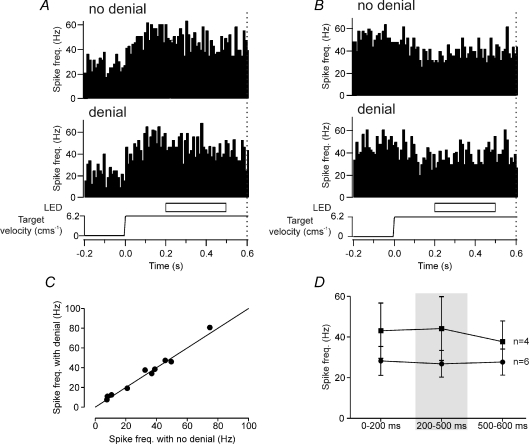
We also examined the effect of a longer period of LED occlusion for the 10 additional Purkinje cells that were also tonically active during target motion. Four of these cells displayed a tonic increase to target movement whilst the remaining six cells displayed a tonic decrease. In half of the trials, the target was occluded 200 ms after the onset of target movement for a duration of 300 ms. Figure 5A and B illustrates two representative examples. Trials with target denial showed no significant change in neuronal activity compared to trials with no target denial. Figure 5C quantifies this by comparing the neuronal activity for the 200–500 ms epoch in trials with the denial period and those without it. The scatterplot shows that all the data points lie close to the line of equality, and no statistically significant difference in neuronal activity was found between denial and non-denial trials (Student's paired t test, P > 0.05). Figure 5D summarizes the mean firing rates for the 10 cells tested with the longer period of target denial compared to when the target was visible whilst it was moving. As was evident for the 200 ms denial period, no statistically significant difference in neuronal activity was found for the 300 ms denial period in any of the 10 Purkinje cells when compared to the levels during visible target motion, irrespective of whether they displayed a tonic increase or decrease in activity (one-way ANOVA, P > 0.05). Thus, all 21 Purkinje cells that displayed a tonic change in simple spike activity during target motion did not significantly alter their activity during temporary visual denial of the target prior to the ‘go’ signal.
Figure 5. Purkinje cell simple spike response to 300 ms duration target denial during target motion.
A and B, two example Purkinje cells. In one half of the trials (denial), the moving target disappeared for 300 ms during target motion. Target denial occurred 200 ms after the onset of target motion. In the other half of the trials (no denial), the moving target was visible throughout. Dotted vertical line at 0.6 s represents ‘go’ signal. C, comparison of responses during target denial with no denial control. No significant change was found (Student's paired t test, P > 0.05, n= 10). Line represents unity. D, comparison of average firing rate comparing epochs when the target was visible (0–200 ms, 500–600 ms) with target denial delivered 200–500 ms after the onset of target motion (one-way ANOVA, P > 0.05). ▪, Purkinje cells with an increase in simple spike activity, n= 4. •, Purkinje cells with a decrease in simple spike activity, n= 6.
Concerning target movement in the reverse direction (i.e. back towards its start location) it was clear that the animals often were not attending to or looking at the target during return travel. Nevertheless, we did on one occasion see a clear example of sustained modulation throughout target motion (a period of 1 s). As with other tonically active cells, temporary disappearance of the target did not alter simple spike activity (one-way ANOVA, P > 0.05). Moreover, the tonic increase in relation to target movement could not have been related to any limb movement or related postural adjustments as the animal did not receive, nor was it expecting, a reward and the neuronal activity returned to baseline levels once the target stopped moving.
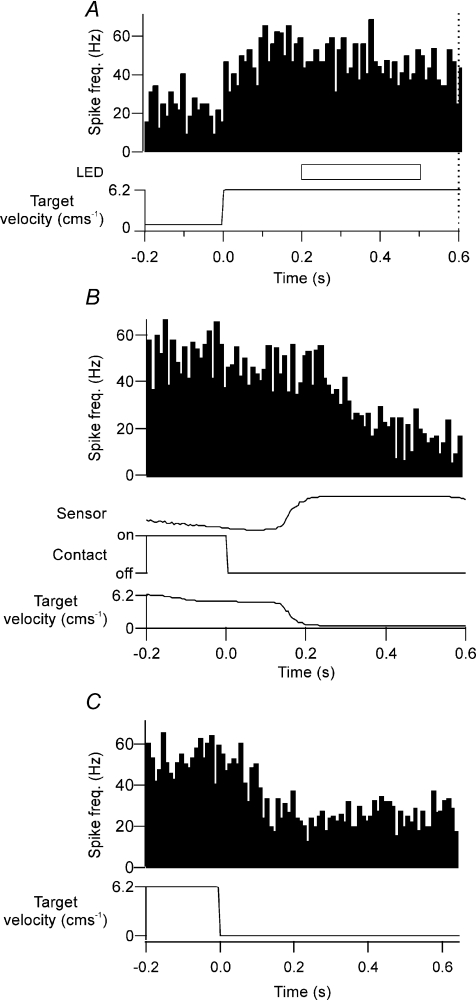
Similarly that the denial points during ‘go’ trials always occurred before delivery of the ‘go’ signal, i.e. well before the cat's subsequent reaching movement (see Discussion). None of the 21 tonically active Purkinje cells displayed a significant change in activity when PETHs were aligned to movement of paw lift (Student's paired t test, P > 0.05). Moreover, the change in neuronal activity was coupled with motion of the target and returned to baseline levels once the target stopped moving (Fig. 6). Mean discharge rate after the target stopped moving was not significantly different from the mean discharge rate before the target started moving (Student's paired t test, P > 0.05). We therefore conclude that the tonic change in neuronal activity is related to ongoing target motion and not due to any limb movement during task performance.
Figure 6. Purkinje cell simple spike activity and cessation of target motion.
A, an example Purkinje cell showing a tonic increase in simple spike activity in response to the onset of target motion. As with other tonically active cells, temporary disappearance of the target (horizontal open bar, labelled LED) did not alter simple spike activity. Dotted vertical line at 0.6 s after onset of target movement represents time point of ‘go’ signal. Time course of target velocity is represented in the bottom trace. B, PETH for same cell as A, averaged in relation to time of paw lifting off the contact plate (time zero). In this and all other cells that displayed tonic activity in relation to target motion, no statistically significant change in activity in relation to paw lift was found (Student's paired t test, P > 0.05). The target stopped moving upon entry of the cat's paw into the tube, which was detected by an infrared beam (sensor trace) spanning the mouth of the tube entrance and allowed the cat to retrieve its food reward. C, PETH for same cell as A and B averaged in relation to termination of target movement. Increased neuronal activity was coupled with motion of the target and returned to baseline levels once the target stopped moving.
Could Purkinje cell activity be related to concurrent eye movements?
In addition to the well established eye movement related areas of the cerebellum in the paraflocculus and flocculus (Noda & Suzuki, 1979; Noda & Mikami, 1986; Fujikado & Noda, 1987; Noda & Fujikado, 1987; Stone & Lisberger, 1990; Shidara & Kawano, 1993; Krauzlis & Miles, 1998), others have suggested that Purkinje cells in crus I can also show eye movement-related activity (Cohen et al. 1965; Ron & Robinson, 1973). It was therefore important to determine whether any of the Purkinje cells in our study were also responsive to eye movements.
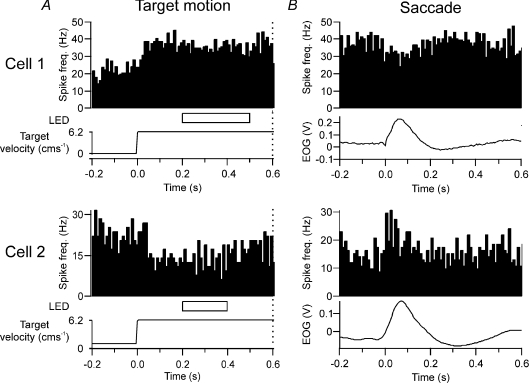
Of our sample of 69 Purkinje cells, only 14 (20%) were found to be modulated in relation to both spontaneous and visually triggered saccades with a change in simple spike activity 80 ± 17 ms prior to saccade onset and a duration of 116 ± 13 ms. Six of these saccade-related Purkinje cells also displayed tonic activity in response to target movement. Of these six Purkinje cells, two were responsive only to leftward eye movements. Since this is opposite to the direction (rightwards) of the target motion to which they were also responsive, any eye movement-related activity in these cells is unlikely to have influenced our findings. However, for the remaining four cells, saccade-related activity was in the same direction as target movement, raising the possibility that the sustained simple spike activity during denial was related to concurrent eye movements. Figure 7 illustrates two such examples in which the Purkinje cell exhibited tonic activity in response to rightwards target movement (Fig. 7A). Because these cells modulated their activity in relation to eye movements in the same direction, PETHs were also aligned on saccades that occurred during target motion onset (Fig. 7B). However, both cells displayed changes in activity opposite to that during target movement, e.g. cell 2 tonically reduced its discharge rate during target motion, but transiently increased its firing rate in relation to eye movement. Similar findings were obtained for the other two Purkinje cells. We therefore conclude that for the four Purkinje cells in which saccade-related modulation also occurred in the same direction as target motion, such activity could not have driven the tonic activity, but rather it would have detracted from it.
Figure 7. Tonic simple spike activity during target motion in relation to concurrent eye movements.
A, two examples of Purkinje cells that exhibited tonic activity in response to rightwards target movement. Dotted vertical line at 0.6 s represents ‘go’ signal. B, same Purkinje cells as A, but PETHs aligned to rightward saccades during target movement.
Using the methods for monitoring eye movement in the present study, we were not able to monitor smooth pursuit. However, smooth pursuit in cats is considered to be less developed than in the primate (Evinger & Fuchs, 1978; Missal et al. 1995; de Brouwer et al. 2001). Pursuit gain is variable and much lower in cats (∼0.5). As a result, smooth pursuit of a target is not sustained and is frequently interrupted with ‘catch-up’ saccades (Missal et al. 1995; de Brouwer et al. 2001; Klam et al. 2001). Consistent with this, the probability of a saccade occurring increased during target motion for the four Purkinje cells in which both tonic activity and saccade-related phasic activity were both found. An average of one saccade per trial was found, with up to four saccades per trial in some instances (not illustrated). It is now recognized that the smooth pursuit system collaborates with the saccadic system in order to optimize visual tracking of a moving target (e.g. Krauzlis, 2004; Orban de Xivry et al. 2006; Orban de Xivry & Lefevre, 2007). It should therefore be sufficient in our experiments to monitor saccades to identify any significant eye movement-related activity. Since in the large majority of our sample of Purkinje cells no such activity could be found (and in the few exceptions the modulation was opposite to the target motion-related activity), we therefore conclude that eye movements did not play a major part in generating the sustained neural activity we observed during visual denial of the target's motion.
Discussion
The present study tests whether Purkinje cell simple spike activity in the lateral cerebellum during a visually guided reach retrieval task is consistent with an internal model representation of a moving target. For 66% of our sample of target-related Purkinje cells, simple spikes displayed tonically altered activity during ongoing target movement. The key finding was that the tonic activity was not altered by visual extinction of the target during any stage of the task prior to the ‘go’ signal to make a reach. This supports the hypothesis that a model of target movement has been constructed. Tracing of the olivo-cerebellar connections of the cortical area where the recordings were made demonstrated that the cells lay mainly within the lateral cerebellar D2 zone. This cerebellar cortical zone projects to rostral dentate (Voogd & Bigaré, 1980; Herrero et al. 2006) which in turn has major connections, via the thalamus, with motor and premotor areas of cerebral cortex (Hoover & Strick, 1999; Middleton & Strick, 2001).
Target motion versus eye or limb movements
Target-related simple spike activity began with the onset of its motion, persisted for as long as the target was moving, and ceased when the target stopped moving. It might be argued that the maintained activity was related to limb movements, but we consider this unlikely. Firstly, none of these cells had forelimb (or any other) peripheral receptive fields, whereas these have been readily identified in other cerebellar zones. For example, the C3 and C2 zones which lie adjacent to the D zones have well-defined forelimb peripheral receptive fields (Edgley & Lidierth, 1988; Garwicz et al. 1998). Secondly, the tonic discharge was observed in the 600 ms interval between onset of target movement and the ‘go’ signal to reach, when the animal was watching the target but not reaching or about to reach; postural changes in cats occur 85–150 ms after a ‘go’ signal (Alstermark & Wessberg, 1985; Schepens & Drew, 2003; see also Greger et al. 2004). Finally, none of the cells modulated in relation to paw lift, consistent with a previous study in this cerebellar region in which Purkinje cells displayed little or no limb movement-related activity (Miles et al. 2006).
We also consider it unlikely that the tonic activity of Purkinje cells responding to horizontal target movement can be fully explained by eye movements: (i) stimulation of crus I in cat can evoke eye movements but only in an upward rotary direction (Cohen et al. 1965); (ii) in terms of smooth pursuit eye movements, recordings in the primate from superficial layers of crus I have failed to find any Purkinje cells with such activity (Mano et al. 1991); (iii) smooth pursuit in cats is limited compared to primates (Missal et al. 1995; de Brouwer et al. 2001). As a result, cats generate several catch-up saccades (de Brouwer et al. 2001; Klam et al. 2001), as seen in the current study; and (iv), results from the present study have found that only 4/21 Purkinje cells displayed modulation to both rightwards saccades and target motion. In each of these four cells the saccade-related activity was phasic and opposite to the direction of change in tonic discharge. We therefore conclude that the tonic simple spike activity most likely encoded the visual target's motion rather than eye or limb movements.
The few cells that displayed phasic activity during target motion might encode target acceleration at movement onset, since this had a similar duration of approximately 65 ms. And, concerning unresponsive cells, individual Purkinje cell simple spike discharge appears tuned to a particular combination of speed and direction, or preferred velocity (Marple-Horvat & Stein, 1990; Fu et al. 1997; Coltz et al. 1999; Johnson & Ebner, 2000; cf. Gomi et al. 1998 in relation to eye movements). Our unresponsive cells might therefore encode other horizontal velocities (cf. Miles et al. 2006) or indeed any vertical component of target velocity, such that the whole population of Purkinje cells in this part of the D2 zone encodes a range of velocities in the X-Y plane of target motion.
Internal models
The simple spike tonic discharge of our sample of target-related Purkinje cells was unaffected by temporary visual denial of the target, which is a necessary feature if internally generated control mechanisms are operating, in which a predictive model of the target's motion with at least a 200–300 ms operating range has been constructed. Humans and monkeys accurately infer the motion of an occluded visual target providing indirect evidence in favour of an actively generated internal model of a moving target that survives its temporary absence (Assad & Maunsell, 1995; Filion et al. 1996; Barborica & Ferrera, 2003, 2004; Zago et al. 2004). Since the target in our study was encountered many times, a model of its kinematics could have been constructed such that lateral cerebellar Purkinje cells in the D2 zone output modelled target movement rather than on-line feedback of its movement.
An alternative explanation is that the visual occlusion was too brief, so that neuronal activity represents a persistent passive description of recent target motion. This seems unlikely, however, because visual feedback delays of > 100 ms during manual tracking tasks are known to disrupt performance (Miall et al. 1985; Foulkes & Miall, 2000; Miall & Jackson, 2006): in the present experiments visual feedback was disrupted for 2–3 times longer.
Neuronal evidence of an internal model in the lateral cerebellum has been reported previously during a visually guided cursor-tracking task in monkeys, in an area of the cortex that lacked limb receptive fields or activity related to eye movement (Miall, 1998; Liu et al. 2003). In this case the neuronal signal was thought to represent the accurately predicted visual sensory outcome of limb movement. Additional evidence for neuronal encoding of internal models within the cerebellum has also been obtained in relation to eye movements in the flocculus and paraflocculus (Suh et al. 2000).
However, both these studies differ from the present findings in fundamental ways: (1) neither provides evidence for an internal cerebellar model of an external moving visual target; and (2) our sample of Purkinje cells was recorded from a very different region of cerebellar cortex, with different input–output connections and so inevitably a different functional role (see below). Our finding is therefore novel in that the neuronal activity we report does not depend at all on movement by the animal (of eyes or limbs). On the contrary, the activity of the neurones reported here reveals an internal model of the motion of an external visual target.
Cerebellar microcomplexes
The differences in what is being modelled in the studies of Suh et al. (2000), Liu et al. (2003) and the present findings probably arise because of differences in localization. Although the zonal identity of the recordings of Liu et al. (2003) was not reported, their electrode tracks were made mainly in lateral parts of lobule IV to VI (and included Purkinje cells with arm movement-related activity) whereas our recordings were made mainly from the anatomically defined D2 zone in crus I. The recordings of Suh et al. (2000) were in an altogether different cerebellar region, i.e. the flocculus and ventral paraflocculus.
A salient feature of cerebellar organization is its division into discrete microcomplexes, defined by structure–function relationships, and thought to represent fundamental operational units (Ito, 1990; Apps & Garwicz, 2005). Imaging studies have found that internal models for two novel tools may reside in spatially segregated areas within the cerebellum (Imamizu et al. 2003, 2004). It remains to be seen whether other classes of predictable target motion can be modelled and represented in lateral cerebellar neurones. But at least one other frequently encountered trajectory, the parabola, described by an object falling under gravity, is a likely candidate (Zago & Lacquaniti, 2005).
Concluding comments
Although the present data are consistent with Purkinje cell simple spike activity representing an internal model of a moving target, the results do not address acquisition of the model (which must have occurred before the recording sessions, during training), nor do they exclude the possibility that Purkinje cells in other regions provide inverse and/or forward models of body movements. The present study also does not address whether the Purkinje cells we studied are downstream from brain site(s) where the internal model has been constructed. For example, the lateral cerebellum receives inputs via the pontine nuclei from visual association areas, including the posterior parietal cortex (Mower et al. 1980). Neurones in this region are active during visual guidance tasks and may be concerned with localization of visual targets in visual/egocentric reference frames (Andersen & Gnadt, 1989; Stein & Glickstein, 1992). These neurones also remain active during occlusion of visual targets (Sakata et al. 1983; Assad & Maunsell, 1995), indicating an internal representation of the target.
Irrespective of these considerations, the present study does, however, provide direct electrophysiological evidence for the operation of an internal model that simulates an external object's motion, expressed in Purkinje cell simple spike activity within the lateral cerebellar D2 zone. Such a predictive capacity is likely to be a key feature of executing interceptive actions – a cardinal feature of normal cerebellar function.
Acknowledgments
This work was supported by a BBSRC grant held jointly by D.M.-H. and R.A. We thank Professor David M. Armstrong for his help with surgery and Rachel Bissett, Clare Everard and Steve Gilbey for their technical assistance.
References
- Alstermark B, Wessberg J. Timing of postural adjustment in relation to forelimb target-reaching in cats. Acta Physiol Scand. 1985;125:337–340. doi: 10.1111/j.1748-1716.1985.tb07724.x. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Gnadt JW. Posterior parietal cortex. Rev Oculomot Res. 1989;3:315–335. [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Rawson JA. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Maunsell JH. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature. 1995;373:518–521. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci. 2003;6:66–74. doi: 10.1038/nn990. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Modification of saccades evoked by stimulation of frontal eye field during invisible target tracking. J Neurosci. 2004;24:3260–3267. doi: 10.1523/JNEUROSCI.4702-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Goto K, Shanzer S, Weiss AH. Eye movements induced by electric stimulation of the cerebellum in the alert cat. Exp Neurol. 1965;13:145–162. doi: 10.1016/0014-4886(65)90105-6. [DOI] [PubMed] [Google Scholar]
- Coltz JD, Johnson MT, Ebner TJ. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci. 1999;19:1782–1803. doi: 10.1523/JNEUROSCI.19-05-01782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Lefevre P. Role of retinal slip in the prediction of target motion during smooth and saccadic pursuit. J Neurophysiol. 2001;86:550–558. doi: 10.1152/jn.2001.86.2.550. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Fu Q. What features of visually guided arm movements are encoded in the simple spike discharge of cerebellar Purkinje cells? Prog Brain Res. 1997;114:431–447. doi: 10.1016/s0079-6123(08)63379-8. [DOI] [PubMed] [Google Scholar]
- Eccles J, Llinas R, Sasaki K. Excitation of cerebellar Purkinje cells by the climbing fibres. Nature. 1964;203:245–246. doi: 10.1038/203245a0. [DOI] [PubMed] [Google Scholar]
- Edge AL, Marple-Horvat DE, Apps R. Lateral cerebellum: functional localization within crus I and correspondence to cortical zones. Eur J Neurosci. 2003;18:1468–1485. doi: 10.1046/j.1460-9568.2003.02873.x. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Lidierth M. Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J Physiol. 1988;401:399–415. doi: 10.1113/jphysiol.1988.sp017169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger C, Fuchs AF. Saccadic, smooth pursuit, and optokinetic eye movements of the trained cat. J Physiol. 1978;285:209–229. doi: 10.1113/jphysiol.1978.sp012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion CM, Washburn DA, Gulledge JP. Can monkeys (Macaca mulatta) represent invisible displacement? J Comp Psychol. 1996;110:386–395. doi: 10.1037/0735-7036.110.4.386. [DOI] [PubMed] [Google Scholar]
- Foulkes AJ, Miall RC. Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res. 2000;131:101–110. doi: 10.1007/s002219900286. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol. 1997;78:478–491. doi: 10.1152/jn.1997.78.1.478. [DOI] [PubMed] [Google Scholar]
- Fujikado T, Noda H. Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol. 1987;394:573–594. doi: 10.1113/jphysiol.1987.sp016885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwicz M, Jorntell H, Ekerot CF. Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol. 1998;512:277–293. doi: 10.1111/j.1469-7793.1998.277bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer S. Cerebellar contribution to saccades and gaze holding: a modeling approach. Ann N Y Acad Sci. 2003;1004:206–219. doi: 10.1196/annals.1303.018. [DOI] [PubMed] [Google Scholar]
- Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys. J Neurophysiol. 1998;80:818–831. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- Green AM, Meng H, Angelaki DE. A reevaluation of the inverse dynamic model for eye movements. J Neurosci. 2007;27:1346–1355. doi: 10.1523/JNEUROSCI.3822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger B, Norris SA, Thach WT. Spike firing in the lateral cerebellar cortex correlated with movement and motor parameters irrespective of the effector limb. J Neurophysiol. 2004;91:576–582. doi: 10.1152/jn.00535.2003. [DOI] [PubMed] [Google Scholar]
- Herrero L, Yu M, Walker F, Armstrong DM, Apps R. Olivo-cortico-nuclear localizations within crus I of the cerebellum. J Comp Neurol. 2006;497:287–308. doi: 10.1002/cne.20976. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Imamizu H, Kawato M. Cerebellar activity evoked by common tool-use execution and imagery tasks: an fMRI study. Cortex. 2007;43:350–358. doi: 10.1016/s0010-9452(08)70460-x. [DOI] [PubMed] [Google Scholar]
- Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535. [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci U S A. 2003;100:5461–5466. doi: 10.1073/pnas.0835746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Yoshioka T, Kawato M. Functional magnetic resonance imaging examination of two modular architectures for switching multiple internal models. J Neurosci. 2004;24:1173–1181. doi: 10.1523/JNEUROSCI.4011-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Ito M. A new physiological concept on cerebellum. Rev Neurol (Paris) 1990;146:564–569. [PubMed] [Google Scholar]
- Johnson MT, Ebner TJ. Processing of multiple kinematic signals in the cerebellum and motor cortices. Brain Res Brain Res Rev. 2000;33:155–168. doi: 10.1016/s0165-0173(00)00027-8. [DOI] [PubMed] [Google Scholar]
- Klam F, Petit J, Grantyn A, Berthoz A. Predictive elements in ocular interception and tracking of a moving target by untrained cats. Exp Brain Res. 2001;139:233–247. doi: 10.1007/s002210100759. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91:591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Role of the oculomotor vermis in generating pursuit and saccades: effects of microstimulation. J Neurophysiol. 1998;80:2046–2062. doi: 10.1152/jn.1998.80.4.2046. [DOI] [PubMed] [Google Scholar]
- Larsell O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953;99:135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Liu X, Robertson E, Miall RC. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol. 2003;89:1223–1237. doi: 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- Mano N, Ito Y, Shibutani H. Saccade-related Purkinje cells in the cerebellar hemispheres of the monkey. Exp Brain Res. 1991;84:465–470. doi: 10.1007/BF00230957. [DOI] [PubMed] [Google Scholar]
- Marple-Horvat DE, Criado JM. Rhythmic neuronal activity in the lateral cerebellum of the cat during visually guided stepping. J Physiol. 1999;518:595–603. doi: 10.1111/j.1469-7793.1999.0595p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple-Horvat DE, Stein JF. Neuronal activity in the lateral cerebellum of trained monkeys, related to visual stimuli or to eye movements. J Physiol. 1990;428:595–614. doi: 10.1113/jphysiol.1990.sp018230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC. The cerebellum, predictive control and motor coordination. Novartis Found Symp. 1998;218:272–284. doi: 10.1002/9780470515563.ch15. [DOI] [PubMed] [Google Scholar]
- Miall RC, Jackson JK. Adaptation to visual feedback delays in manual tracking: evidence against the Smith Predictor model of human visually guided action. Exp Brain Res. 2006;172:77–84. doi: 10.1007/s00221-005-0306-5. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Visuomotor tracking with delayed visual feedback. Neuroscience. 1985;16:511–520. doi: 10.1016/0306-4522(85)90189-7. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Manual tracking of visual targets by trained monkeys. Behav Brain Res. 1986;20:185–201. doi: 10.1016/0166-4328(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Visuo-motor tracking during reversible inactivation of the cerebellum. Exp Brain Res. 1987;65:455–464. doi: 10.1007/BF00236319. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OB, Cerminara NL, Marple-Horvat DE. Purkinje cells in the lateral cerebellum of the cat encode visual events and target motion during visually guided reaching. J Physiol. 2006;571:619–637. doi: 10.1113/jphysiol.2005.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missal M, Lefevre P, Crommelinck M, Roucoux A. Evidence for high-velocity smooth pursuit in the trained cat. Exp Brain Res. 1995;106:509–512. doi: 10.1007/BF00231076. [DOI] [PubMed] [Google Scholar]
- Mower G, Gibson A, Robinson F, Stein J, Glickstein M. Visual pontocerebellar projections in the cat. J Neurophysiol. 1980;43:355–366. doi: 10.1152/jn.1980.43.2.355. [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987;58:359–378. doi: 10.1152/jn.1987.58.2.359. [DOI] [PubMed] [Google Scholar]
- Noda H, Mikami A. Discharges of neurons in the dorsal paraflocculus of monkeys during eye movements and visual stimulation. J Neurophysiol. 1986;56:1129–1146. doi: 10.1152/jn.1986.56.4.1129. [DOI] [PubMed] [Google Scholar]
- Noda H, Suzuki DA. The role of the flocculus of the monkey in fixation and smooth pursuit eye movements. J Physiol. 1979;294:335–348. doi: 10.1113/jphysiol.1979.sp012933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Bennett SJ, Lefevre P, Barnes GR. Evidence for synergy between saccades and smooth pursuit during transient target disappearance. J Neurophysiol. 2006;95:418–427. doi: 10.1152/jn.00596.2005. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Lefevre P. Saccades and pursuit: two outcomes of a single sensorimotor process. J Physiol. 2007;584:11–23. doi: 10.1113/jphysiol.2007.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Feldman AG. A critical evaluation of the force control hypothesis in motor control. Exp Brain Res. 2003;153:275–288. doi: 10.1007/s00221-003-1624-0. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- Ron S, Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973;36:1004–1022. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- Sakata H, Shibutani H, Kawano K. Functional properties of visual tracking neurons in posterior parietal association cortex of the monkey. J Neurophysiol. 1983;49:1364–1380. doi: 10.1152/jn.1983.49.6.1364. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90:3066–3086. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- Shidara M, Kawano K. Role of Purkinje cells in the ventral paraflocculus in short-latency ocular following responses. Exp Brain Res. 1993;93:185–195. doi: 10.1007/BF00228385. [DOI] [PubMed] [Google Scholar]
- Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365:50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72:967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. I. Simple spikes. [DOI] [PubMed] [Google Scholar]
- Suh M, Leung HC, Kettner RE. Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. J Neurophysiol. 2000;84:1835–1850. doi: 10.1152/jn.2000.84.4.1835. [DOI] [PubMed] [Google Scholar]
- Thach WT., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967;30:675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Trott JR, Armstrong DM. The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. Exp Brain Res. 1987;66:318–338. doi: 10.1007/BF00243308. I. Projections from the intermediate region. [DOI] [PubMed] [Google Scholar]
- Voogd J, Bigaré F. Topographical distribution of olivary and cortico nuclear fibers in the cerebellum: a review. In: Courville J, editor. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven; 1980. pp. 207–235. [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Zago M, Bosco G, Maffei V, Iosa M, Ivanenko YP, Lacquaniti F. Internal models of target motion: expected dynamics overrides measured kinematics in timing manual interceptions. J Neurophysiol. 2004;91:1620–1634. doi: 10.1152/jn.00862.2003. [DOI] [PubMed] [Google Scholar]
- Zago M, Lacquaniti F. Visual perception and interception of falling objects: a review of evidence for an internal model of gravity. J Neural Eng. 2005;2:S198–S208. doi: 10.1088/1741-2560/2/3/S04. [DOI] [PubMed] [Google Scholar]