Abstract
Whilst calsequestrin (CSQ) is widely recognized as the primary Ca2+ buffer in the sarcoplasmic reticulum (SR) in skeletal muscle fibres, its total buffering capacity and importance have come into question. This study quantified the absolute amount of CSQ isoform 1 (CSQ1, the primary isoform) present in rat extensor digitorum longus (EDL) and soleus fibres, and related this to their endogenous and maximal SR Ca2+ content. Using Western blotting, the entire constituents of minute samples of muscle homogenates or segments of individual muscle fibres were compared with known amounts of purified CSQ1. The fidelity of the analysis was proven by examining the relative signal intensity when mixing muscle samples and purified CSQ1. The CSQ1 contents of EDL fibres, almost exclusively type II fibres, and soleus type I fibres [SOL (I)] were, respectively, 36 ± 2 and 10 ± 1 μmol (l fibre volume)−1, quantitatively accounting for the maximal SR Ca2+ content of each. Soleus type II [SOL (II)] fibres (∼20% of soleus fibres) had an intermediate amount of CSQ1. Every SOL (I) fibre examined also contained some CSQ isoform 2 (CSQ2), which was absent in every EDL and other type II fibre except for trace amounts in one case. Every EDL and other type II fibre had a high density of SERCA1, the fast-twitch muscle sarco(endo)plasmic reticulum Ca2+-ATPase isoform, whereas there was virtually no SERCA1 in any SOL (I) fibre. Maximal SR Ca2+ content measured in skinned fibres increased with CSQ1 content, and the ratio of endogenous to maximal Ca2+ content was inversely correlated with CSQ1 content. The relative SR Ca2+ content that could be maintained in resting cytoplasmic conditions was found to be much lower in EDL fibres than in SOL (I) fibres (∼20 versus, >60%). Leakage of Ca2+ from the SR in EDL fibres could be substantially reduced with a SR Ca2+ pump blocker and increased by adding creatine to buffer cytoplasmic [ADP] at a higher level, both results indicating that at least part of the Ca2+ leakage occurred through SERCA. It is concluded that CSQ1 plays an important role in EDL muscle fibres by providing a large total pool of releasable Ca2+ in the SR whilst maintaining free [Ca2+] in the SR at sufficiently low levels that Ca2+ leakage through the high density of SERCA1 pumps does not metabolically compromise muscle function.
Calsequestrin (CSQ) is the most abundant Ca2+-binding protein in the sarcoplasmic reticulum (SR) in skeletal muscle (MacLennan & Wong, 1971; Ikemoto et al. 1972; Beard et al. 2004). Calsequestrin in mammalian fast-twitch skeletal muscle is predominantly or exclusively the CSQ1 isoform. Slow-twitch mammalian skeletal muscle contains both CSQ1 and the cardiac isoform, CSQ2. Calsequestrin 1 is nevertheless still the predominant isoform, with the ratio of CSQ1:CSQ2 in rabbit soleus muscle reported as being ∼3:1 (Damiani et al. 1990) or greater (Fliegel et al. 1989), although the precise value is likely to depend on the proportion of fast- and slow-twitch fibres present in the given muscle. Original estimates of maximal Ca2+ binding to CSQ were ∼40–50 mol mol−1 (MacLennan & Wong, 1971; Ikemoto et al. 1972), with a more recent estimate being ∼80 mol mol−1 for CSQ1 and ∼60 mol mol−1 for CSQ2 (Park et al. 2004).
In vertebrate adult skeletal muscle fibres, contraction depends almost exclusively on Ca2+ released from the SR and not on the influx of extracellular Ca2+ (Melzer et al. 1995), and traditionally CSQ has been regarded as having the paramount role in the storage and release of SR Ca2+. However, recent findings have raised questions about whether CSQ, in particular CSQ1, does indeed have such a paramount role. For example, it has been found in C2C12 myotubes that knock-down of CSQ1 did not lead to reduction in SR Ca2+ storage or release whereas knock-down of CSQ2 did (Wang et al. 2006). Furthermore, CSQ1-null mice are viable, and their fast-twitch muscles display little if any change in twitch and tetanic peak force and only moderate slowing of the rise time and decay of the twitch, although the peak size of the intracellular Ca2+ transient to single electrical stimuli was reduced by ∼40% (Paolini et al. 2007). There was no upregulation of CSQ2 in the fast-twitch fibres, nor apparent change in the amount of SERCA1, the fast-twitch muscle sarco(endo)plasmic reticulum Ca2+-ATPase isoform, or in the total volume of the SR. There was, however, a twofold increase in the number of ryanodine receptor Ca2+-release channels (RyRs) and in the number of triad junctions, where the transverse tubules appose the SR, as well as a doubling of the number of mitochondria (Paolini et al. 2007). Thus, fast-twitch muscle can function without any CSQ, though various compensatory changes may be necessary to achieve near-normal contractile performance.
A further difficulty with the proposal that CSQ is the major Ca2+ storage protein is that the published values for the amount of CSQ in fast- and slow-twitch mammalian muscle are approximately three to sixfold too low to account for the measured maximal Ca2+ storage capacities. The CSQ contents have been reported to be ∼250 and 130 μg (g muscle wet weight)−1 in fast- and slow-twitch rabbit muscle, respectively (Leberer & Pette, 1986), which correspond to only ∼7 and ∼3.5 μm CSQ (‘μm’ denotes μmol per litre fibre volume, not water volume; see Discussion for other details of calculation). At maximal binding (80 Ca2+ ions per CSQ molecule), these amounts would account for only ∼0.56 and 0.3 mm of Ca2+ storage, respectively, which are much lower than the maximal SR Ca2+ content values of ∼3.7 and 1.1 mm measured in fast-twitch (EDL) and slow-twitch [soleus type I; SOL (I)] fibres of the rat (in the same units; Fryer & Stephenson, 1996). A similar mismatch is also apparent between measured CSQ amounts (Volpe & Simon, 1991) and SR Ca2+ capacity (Pape et al. 2007) in frog muscle fibres.
Fryer & Stephenson (1996) assayed the maximal SR Ca2+ content by lysing skinned fibres, which permitted only a single measurement on each fibre. They also assayed the endogenous SR Ca2+ content in similar fibres, yielding values of ∼1.0 and 1.1 mm in EDL and soleus type I fibres, respectively, with a similar value being found in toad iliofibularis fibres (∼1.1 mm; Owen et al. 1997; all values corrected for small Ca2+ content of transverse tubular system). These values for the endogenous Ca2+ content are very similar to those found by other methods in human muscle (∼1 mm, in same units, allowing for extracellular volume; Overgaard et al. 2004) and frog fibres (∼1.2 mm; Somlyo et al. 1981). It was also found that virtually all of the SR Ca2+ in the skinned fibres could be released either by fibre depolarization or by applying a caffeine-containing low-[Mg2+] solution to potently activate the RyRs (Fryer & Stephenson, 1996; Owen et al. 1997; Posterino & Lamb, 2003), offering an alternative, non-destructive way in which to assay relative endogenous and maximal SR Ca2+ content in the same fibre.
The present study used a novel quantitative Western blot method to assay the entire constituents of muscle fibre samples for CSQ, in order to examine whether Ca2+ binding to CSQ is indeed the major determinant of maximal SR Ca2+ content in fast- and slow-twitch fibres. The study also verified the important but frequently neglected finding that fast-twitch mammalian fibres are loaded endogenously at only a small fraction of their maximal SR Ca2+ capacity (Fryer & Stephenson, 1996) and investigated whether the divergence in Ca2+-loading properties of fast- and slow-twitch fibres was attributable to differences in the isoforms and amounts of CSQ and SERCA pumps present in the two fibre types.
Methods
Preparations
All animal experiments were approved by the La Trobe University Animal Ethics Committee. Male Long–Evans hooded rats (∼5–8 months old) were killed by overdose with fluothane (2% v/v) in a restricted air space. The extensor digitorum longus (EDL), soleus and red gastrocnemius muscles were rapidly excised and either homogenized for biochemical analysis or pinned at resting length under paraffin oil and kept cool (∼10°C) on an ice pack for fibre skinning. Cane toads (Bufo marinus) were killed by pithing, and the iliofibrilaris muscles removed. Single muscle fibres were mechanically skinned with fine jeweller's forceps as previously described (Lamb & Stephenson, 1994). When used to assay SR Ca2+ content, a skinned fibre segment was attached to a force transducer (AME801, SensoNor, Horten, Norway), set at 120% of resting length and pre-equilibrated for 2 min in ‘intracellular’ solution (see ‘Solutions’ below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, NSW, Australia). All force recordings were made at ∼23 ± 2°C.
Solutions
All chemicals were obtained from Sigma (St Louis, MO, USA), unless specified otherwise. The standard ‘intracellular’ solution used to bathe rat skinned fibres contained (mm): K+, 126; Na+, 36; 1,6-diaminohexane-N,N,N′,N′,-tetraacetic acid (HDTA2−; Fluka, Buchs, Switzerland) 50; total ATP, 8; total magnesium, 8.5; creatine phosphate, 10; total EGTA, 0.10; and Hepes, 90; pH 7.10 ± 0.01 and pCa (=−log10[Ca2+]) 7.1. This solution had 1 mm free Mg2+ and an osmolality of 295 ± 10 mosmol (kg H2O)−1. The solution for toad fibres was similar but contained only 60 mm Hepes and 117 mm K+ and had an osmolality of 255 ± 10 mosmol (kg H2O)−1. Solutions used to load the SR were made from the standard K-HDTA solution by adding small amounts of the 50 mm EGTA and CaEGTA solutions (see below) to give a total EGTA–CaEGTA of 1.0 mm with the pCa set at 6.4, 6.7 or 7.3. The solution used for equilibration for 2 min under resting cytoplasmic conditions was made similarly with 1 mm total EGTA and pCa 7.3 and with 3 mm added creatine so as to set the free [ADP] at ∼10 μm via the creatine kinase reaction (based on the presence of 8 mm ATP and 10 mm creatine phosphate and equilibrium constant of 270; see Macdonald & Stephenson, 2001).
Maximal Ca2+-activated force production by the contractile apparatus was assessed with solutions with very heavy Ca2+ buffering, which were similar to the standard HDTA solution but with all HDTA replaced with 50 mm EGTA; the relaxing solution had no added Ca2+ (pCa > 10) and the maximal activation solution (‘max’) had 49.5 mm added Ca2+ (pCa 4.7), with total magnesium of 10.3 and 8.1 mm, respectively, to maintain the free [Mg2+] at 1 mm (Lamb & Stephenson, 1994). Free [Ca2+] of solutions in the range pCa 3.0–7.3 was measured with a Ca2+-sensitive electrode (Orion Research Inc., Boston, MA, USA). The Sr2+ sensitivity of fibres was tested with a similar EGTA-based solution, with Sr2+ added to give pSr (=−log10[Sr2+]) of 5.3 (see Trinh & Lamb, 2006).
Assessment of endogenous and maximal SR Ca2+ content
Since fibres were skinned under paraffin oil, the SR initially retained its endogenous level of Ca2+. Each fibre segment was first fully depleted of the releasable SR Ca2+ present endogenously by exposing the fibre to the full release solution, which was similar to the standard K-HDTA solution but with 30 mm caffeine and only 0.05 mm free Mg2+ and with 0.5 mm EGTA present to chelate the released Ca2+ (Lamb et al. 2001). The skinned fibre was then subjected to repeated cycles in which the SR was loaded with Ca2+ to various levels and then depleted again with the full release solution. The sequence of solution changes used in this cycle was as follows: step (1) pre-equilibrate for 10 s (HDTA solution with 0.5 mm EGTA, pCa 8); step (2) empty SR of Ca2+ in full release solution (60 s), with the force response as an assay of Ca2+ content; step (3) wash for 60 s (solution similar to step (1)); and step (4) reload for set time (load solution with 1 mm total CaEGTA–EGTA at pCa 6.7). In experiments where fibres were equilibrated in resting cytoplasmic conditions (as in Figs 6 and 7A; solution details above) the following additional step was included: step (5) equilibration at pCa 7.3 for 2 min (solution with 1 mm total EGTA with/without 3 mm creatine).
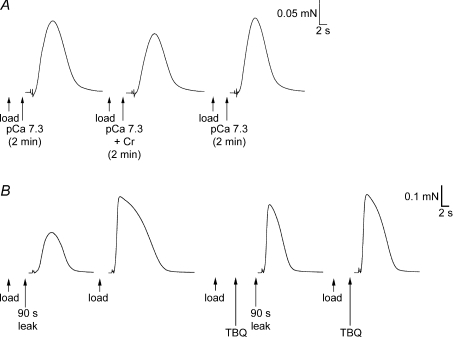
Figure 6. Changes in SR Ca2+ content in skinned fibres maintained at pCa 7.3.
A, force responses in a skinned EDL fibre upon emptying SR of Ca2+ in repeated load–release cycles as in Fig. 4. The SR was loaded to the same level (approximately endogenous level) on each repetition shown; the reduced area of the force response in the middle trace indicates that the SR lost net Ca2+ during the 2 min period in solution at pCa 7.3 (1 mm total EGTA; Cr added to buffer ADP at ∼10 μm; see main text). B, similar responses in a SOL (I) fibre, which displayed a small increase in SR Ca2+ content after 2 min in the pCa 7.3 solution. The EDL and SOL (I) fibres shown were typical of type: CSQ1 75 and 23% of EDL average, CSQ2 absent and present, and SERCA1 amounts 135 and ∼1% of EDL average, respectively.
Figure 7. Effects on SR Ca2+ leakage of raising [ADP] with Cr or blocking SERCA with TBQ.
A, force responses in a skinned EDL fibre upon emptying the SR of Ca2+. On each load–release cycle the SR was loaded to the same set level (approximately the endogenous level), and then equilibrated in solution at pCa 7.3 (1 mm total EGTA) with or without 3 mm Cr present. Following exposure to the solution with Cr present (which raised [ADP] to ∼10 μm), the SR Ca2+ content was relatively reduced. B, force responses in another EDL skinned fibre in which the SR was loaded with Ca2+ (by a 30 s period followed by a further 10 s period), with or without a subsequent 90 s leakage period (in a solution at ∼pCa 9 with 2 mm free EGTA; see two left-most traces). The procedure was then repeated with 25 μm TBQ present during the leakage period (TBQ added during the second, 10 s, part of the load procedure). Loss of SR Ca2+ during the 90 s leakage period was greatly reduced in the presence of TBQ. The TBQ was removed between each cycle (see Methods). Bracketing control and other load–leak cycles in the sequence are not shown.
In the experiments with 2,5-di-tert-butyl-1,4-hydroquinone (TBQ; e.g. Fig. 7B), a stock of 25 mm TBQ was made in DMSO, and diluted 1000-fold (to give 25 μm) in appropriate solutions as needed, with an equal amount of DMSO added to all other solutions used in that experiment. The skinned fibre was loaded in pCa 6.7 solution for 30 s without TBQ and then for a further 10 s in a similar load solution with TBQ, immediately left to leak in a solution with 2 mm free EGTA (∼pCa 9) for a set period, and then Ca2+ released as in steps (1) and (2). The subsequent wash period [step (3)] was modified to include two 1 min periods in which the skinned fibre was returned to paraffin oil to aid in removal of TBQ from the fibre, as previously described (Bakker et al. 1996).
Before assaying endogenous SR Ca2+ content, each skinned fibre was initially equilibrated for 2 min in a solution with very little contaminating Ca2+ and very weak Ca2+ buffering (0.1 mm total EGTA, ∼pCa 7.3), so that the SR could not take up any substantial net amount of Ca2+ from the bathing solution but nevertheless could still readily recover most or all of any Ca2+ that leaked from the SR into the cytoplasmic space within the fibre. The initial 2 min equilibration period was necessary to ensure that all diffusible Ca2+ buffers present endogenously in the cytoplasm were washed out of the fibre, ensuring that cytoplasmic conditions were similar on all repetitions. In preliminary experiments, it was found that the SR Ca2+ content remained approximately unchanged in this solution, whereas if the total [EGTA] were instead increased to 0.2 mm (no added Ca2+, pCa ∼7.6) there was a net loss of Ca2+ from the SR over the 2 min period. These findings were made by loading the SR to a set level followed by a 2 min equilibration in the solution of interest, and then comparing the force response upon emptying the SR of Ca2+ to that found with the same loading but no equilibration period; this procedure is similar to that shown in Fig. 6 for an equilibration solution with the free Ca2+ more strongly buffered with EGTA (1 mm total EGTA).
The estimate of SR Ca2+ content in EDL fibres took into account that a threshold level of loading was needed for any force response to be elicited upon emptying the SR (see Fig. 4 and Results). The relative amount of this non-detected SR Ca2+ was determined by back-extrapolation of the area–load time curve to zero load time; this amount, typically ∼10% of the maximal SR Ca2+ content, was added to the measured values.
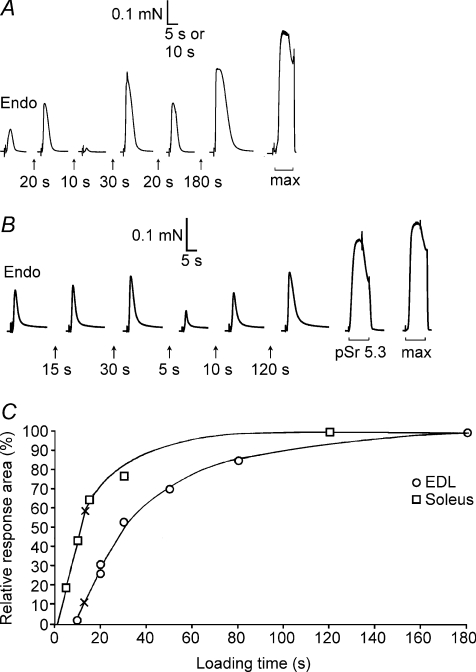
Figure 4. Assay of SR Ca2+ content and loading properties in fibres with known CSQ.
A, force responses of skinned EDL fibre segment upon releasing all SR Ca2+ by exposure to 30 mm caffeine–low [Mg2+] solution. First response (‘Endo’) reflects release of endogenous Ca2+ content. The SR was reloaded by bathing the segment for the indicated time in solution at pCa 6.7 with 1.0 mm total EGTA. Maximal Ca2+-activated force (in response to ‘max’ solution) is shown on a slower time scale. The fibre segment had ∼120% of average EDL CSQ1 content, no CSQ2 and typical EDL SERCA1 content. B, force responses in SOL (I) fibre; high relative force response in solution with pSr 5.3 indicates type I fibre. Calsequestrin 1 content was ∼20% of EDL average, typical SOL level of CSQ2, and SERCA1 was not determined. C, time integral of force responses versus load time for fibres in A and B, expressed as a percentage of the maximal value. Crosses indicate the time required to reload SR Ca2+ to the level present endogenously. In A and B, the force artefacts upon changing solution (start of each record) are shown on a compressed time scale.
Sarcoplasmic reticulum preparations
Skeletal SR vesicles were prepared from EDL muscle from Long–Evans hooded rats using the methods of Saito et al. (1984) and Ahern et al. (1994), which were further purified to obtain junctional face membrane, as previously described (Costello et al. 1986), with minor changes (Beard et al. 2002).
Preparation of pure CSQ
Rat skeletal CSQ1 was purified from junctional face membrane (Beard et al. 2002; Saito et al. 1984). Recombinant rabbit skeletal muscle CSQ1 was expressed as Glutathione-S-transferase (GST) fusion proteins and purified as previously described (Beard et al. 2005). Human recombinant CSQ2 was expressed as fusion proteins containing a HIS tag (consisting of six consecutive histidine residues) and expressed as for rabbit skeletal CSQ1. For CSQ2 purification, following cell sedimentation, cells were resuspended in a solution containing (mm): NaH2PO4, 50; imidazol, 10; and β-mercaptothenol, 10 (pH 8.0) and disrupted using a French press. Following centrifugation at ∼5000g, disrupted cells were incubated with a Nickelnitroacetic acid agarose matrix and washed with 4 × 15 volumes of (mm): NaH2PO4, 50; imidazol, 20; and β-mercaptothenol, 10 (pH 8.0). Calsequestrin 2 was eluted in a buffer containing (mm) NaH2PO4, 50; imidazol, 500; and β-mercaptothenol, 10 (pH 8.0). Calsequestrin 2 was dialysed against a buffer containing (mm): Mops, 20; NaCl, 150; and CaCl2, 1 (pH 7.4) and stored in small aliquots at −70°C. For both CSQ1 and CSQ2, protein purity was assessed by applying the pure protein samples to 10% SDS-PAGE followed by detection of proteins by GelCode SilverSNAP Stain Kit II (Pierce, Rockford, IL, USA) as previously described (Verburg et al. 2005). Protein content was determined using Quant-iT protein assay (Invitrogen, Sydney, NSW, Australia).
Muscle homogenates
For Western blotting, EDL and soleus rat skeletal muscle was homogenized (10:1 w/v) in a physiological solution containing (mm): K+, 129; Na+, 36; free Mg2+ 1 (10.3 total Mg2+); Hepes, 90; and EGTA, 50 (pH 7.10) with or without the addition of protease inhibitor cocktail (PIC, COMplete, Roche Diagnostics, Sydney, NSW, Australia). There was no apparent difference in the amount of either CSQ1 or CSQ2 between muscle homogenates prepared with or without PIC. In some instances, homogenates were prepared in a solution similar to that described above, but where the [Na+] was 165 mm and there was no K+ present. Homogenates were subsequently diluted in the physiological solution and added (2:1 v/v) to SDS loading buffer (0.125 m Tris-HCl, 10% glycerol, 4% SDS, 4 m urea, 10% mercaptoethanol and 0.001% Bromophenol Blue, pH 6.8) to give a final concentration of ∼1.7 μg muscle μl−1. Preparations were heated to 95°C for 4 min and stored at −20°C until analysed by Western blotting. For Stains-All, rat EDL and soleus muscles were homogenized (4:1 w/v) in 2.5 mm NaOH and spun (10 000g, 3 min). Supernatants were adjusted to pH 6.8, filtered (Whatman no. 2) and spun (14 100g, 30 min). The pellet was resuspended in a solution containing (mm): KCl 150; and Mes, 20 and re-spun (14 100g, 30 min). The final pellet was resuspended in this same solution and diluted to 4 μg protein μl−1 following a protein assay (Quant-iT).
Western blotting
Whole muscle homogenates (4–32 μg total muscle mass, equivalent to ∼1–8 μg total protein) and individual fibre segments (∼8–16 μg muscle mass, equivalent to ∼2–4 μg total protein) from rat EDL and soleus skeletal muscle were analysed for CSQ1 and CSQ2 protein content by Western blotting using a similar protocol to that previously described (Murphy et al. 2006). Protein from muscle preparations as well as pure CSQ protein samples were separated on 10% SDS-PAGE gels and probed with antibodies against CSQ1 (1 in 2000, mouse monoclonal VIIID12 clone, ab2824, Abcam, Cambridge, UK, which does not detect CSQ2), CSQ1 and 2 (1 in 2000, rabbit affinity isolated polyclonal, ab3516, Abcam, which detects CSQ2 with greater efficacy than CSQ1), SERCA1 (1 in 200, mouse monoclonal CaF2-5D2 clone, Developmental Studies Hybridoma Bank, University of Iowa, IA, USA), SERCA2a (1 in 1000, mouse monoclonal 2A7-A1 clone, MA3-919, Affinity Bioreagents, Golden, CO, USA) or actin (1 in 200, rabbit polyclonal, affinity isolated, A2066, Sigma) all diluted in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) with 0.025% Tween (PBST). Following transfer, the SDS-PAGE gel was stained with BioSafe Coomassie Stain (Bio-Rad, Hercules, CA, USA) for detection of myosin heavy chain (MHC), used as an indicator for the relative amount of protein in fibre segments (Murphy et al. 2006). Images were collected following exposure to chemiluminescent substrate (Pierce) using a CCD camera attached to a ChemiDoc XRS (Bio-Rad, USA) and using Quantity One software (Bio-Rad). Densitometry was performed with the Quantity One software.
For absolute quantification of CSQ1 and CSQ2, standard curves were plotted [density versus known amounts of pure protein (CSQ1 or CSQ2)] and the amount of CSQ1 or CSQ2 in a given sample determined from standard curve(s) on the same Western blot. The absolute amount of CSQ1 in rat skeletal muscle samples was determined using pure CSQ1 prepared from skeletal muscle from the same rat strain. Samples were sometimes run with recombinant rabbit CSQ1. The anti-CSQ1 antibody detected rat CSQ1 with approximately twofold greater sensitivity, and this difference was taken into account when quantifying the absolute amounts of CSQ1 in rat skeletal muscle samples when using the pure rabbit CSQ1. Owing to difficulties in preparing rat cardiac CSQ2, rat samples were quantified using recombinant human CSQ2. To verify the quantitative nature of the Western blotting technique, pure protein (CSQ1 or CSQ2) and whole muscle preparations were loaded either in separate lanes or together in the same lane, separated by SDS-PAGE and examined for CSQ1 and CSQ2 protein by Western blotting. Using this approach, the total density of the immunoreactive bands could be used as a measure of the transfer efficiency of the CSQ present in the samples.
For relative quantification of the amounts of CSQ1 present in EDL and soleus muscle fibre segments, the density of the given band was converted to an amount of CSQ1 based on a calibration curve derived from purified CSQ1 amounts run on the same Western blot. This CSQ1 amount was then normalized by the relative amount of MHC in that fibre segment, and then expressed as a percentage of the average value found across all the EDL fibres on the same gel. In a similar manner, the normalized amount of CSQ2 present in each fibre was derived and expressed relative to the average amount of CSQ2 present in soleus fibres on the same gel. For SERCA1, the density of each band was normalized by the relative amount of MHC in that fibre and expressed relative to the average value obtained across all EDL fibres on that gel.
Stains-All
Stains-All (4,5,4′,5′-Dibenzo-3,3′-diethyl-9-methylthiocarbocyanine bromide; Sigma) is a cationic carbocyanine dye, which stains sialoglycoproteins and phosphoproteins blue and almost all other proteins red. Thus, Stains-All dye binds to anionic sites on the protein and stains negatively charged Ca2+-binding proteins blue or purple. The colour of protein–dye complex reflects the degree of Ca2+-binding capacity of the protein. Fifty micrograms of protein from final resuspended pellets (see ‘Muscle homogenates’) and 5 μg each of purified rat CSQ1 and human recombinant CSQ2 (same lane) were separated on a 8% SDS-PAGE and, following washes in 25% isopropanol to remove SDS (4 × 30 min), exposed to Stains-All solution (0.025% Stains-All, 7.5% formamide, 25% isoproponal, 30 mm Tris-base, pH 8.8) overnight in a container protected from light. Following staining, the gel was washed extensively in 25% isopropanol to destain. Stains-All-stained gels were photographed with a digital camera (Panasonic Lumix) to obtain the colour image and with the ChemiDoc XRS (Bio-Rad) to allow analyses of the bands corresponding to CSQ1 and CSQ2 using Quantity One software.
Results
Quantification of amount of CSQ1 present in homogenates of different muscles
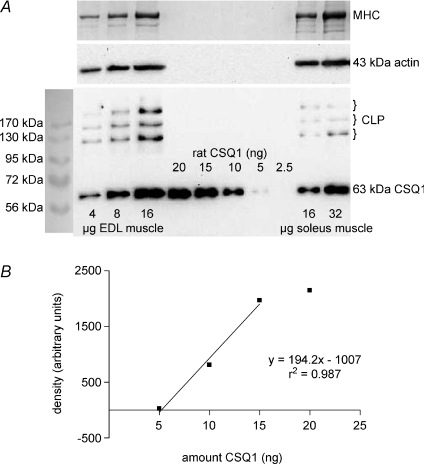
The amount of CSQ1 present in rat EDL and soleus muscles was assessed as in Fig. 1, by running very small amounts of total muscle homogenate samples and purified rat CSQ1 on the same SDS-PAGE gel and comparing the quantified density of the relevant bands after transfer to a nitrocellulose membrane and Western blotting. Importantly, the entire constituents of the muscle homogenates were run, without any spinning and fractionation, in order to ensure that no CSQ1 was omitted. The CSQ1 was detected at an apparent molecular weight of approximately 63 kDa. A range of homogenate and purified CSQ1 amounts were run on the same gel so as to ensure that they could be compared over a range where band intensity varied approximately linearly with the amount of purified CSQ1 (see Fig. 1B; note that the parameters do not vary precisely in proportion and that a threshold amount of CSQ1 is required for detection). Two different primary antibodies were examined, one detecting only CSQ1 and the other detecting both CSQ1 and CSQ2 (see Methods), and similar values for the amount of CSQ1 present in a homogenate were obtained using either antibody. The mean amount of CSQ1 found in EDL muscle (9 measurements, 3 rats) was 1.4 ± 0.1 mg (g muscle wet weight)−1, and in soleus muscle (9 measurements, 4 rats) it was 0.43 ± 0.03 mg (g muscle wet weight)−1.
Figure 1. Quantification of CSQ1 in rat EDL and soleus muscle homogenates.
A, protein from rat EDL and soleus whole muscle homogenates (4–32 μg muscle wet weight, as indicated) and 2.5–20 ng purified CSQ1 (prepared from rat skeletal muscle), were separated on a 10% SDS-PAGE gel and CSQ1 detected by Western blotting (anti-CSQ1). To verify relative amounts of muscle loaded, membranes were reprobed for actin (middle panel), and also gels were stained with Coomassie Blue following transfer to identify myosin heavy chain (MHC, top panel). Calsequestrin-like proteins (CLP) were also observed in all homogenate samples. B, density of band for purified CSQ1 plotted against amount of protein. Amount of CSQ1 in EDL and soleus homogenates (1.55 and 0.56 ng (μg muscle wet weight)−1, respectively) determined from intensity of bands that fell within linear range for samples of purified CSQ1 on the same gel (shown in A).
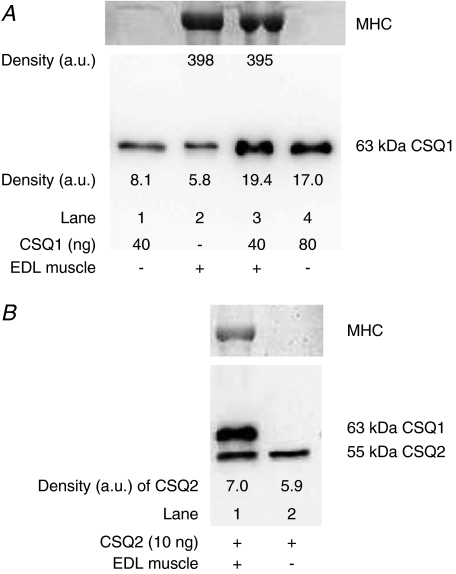
The above method of quantification is only valid if the purified CSQ1 and the CSQ1 present in the muscle sample both: (i) run on the gel; (ii) transfer to the nitrocellulose; and (iii) are detected by the Western blotting, with equal overall efficacy. This was directly tested by comparing the band intensities of Western blots for lanes containing purified CSQ1 alone (Fig. 2A, lane 1), muscle homogenate alone (Fig. 2A, lane 2), and muscle homogenate with added purified CSQ1 (Fig. 2A, lane 3). Conversion of band intensity to a final amount of CSQ1 had to take into account the fact that the density was not directly proportional to the amount of CSQ1 (e.g. see Fig. 1B). In five independent repetitions, the estimate of total CSQ1 present when purified CSQ1 was run with muscle homogenate was not significantly different from the sum of two samples run separately (mean ±s.e.m. value, 118 ± 15%), demonstrating the reliability of the quantification method. Calsequestrin 2 could also be reliably quantified by the same method, as indicated in Fig. 2B. It is important to note that if substantially larger amounts of muscle homogenates were run in a given lane (e.g. >100 μg wet muscle weight, that is >23 μg total protein), the CSQ was no longer transferred and immunodetected with the same efficacy as purified CSQ run alone, leading to substantial underestimation of the amount of CSQ present in the muscle sample.
Figure 2. Verification of CSQ quantification by adding pure CSQ to muscle homogenates.
A, lower panel shows Western blot (probed with anti-CSQ1) of lanes containing purified CSQ1 alone (40 ng, lane 1; 80 ng, lane 4), EDL muscle homogenate alone (lane 2), or the same amount of muscle homogenate with 40 ng added CSQ1 (lane 3). Post-transfer Coomassie Blue staining of the gel for MHC (upper panel) verified that lanes with muscle homogenate had a similar amount loaded. Density values for CSQ1 and MHC are indicated. Band densities indicate that the mixture of purified CSQ1 and muscle homogenate CSQ1 was transferred, detected and quantified with similar efficacy as purified CSQ1 run by itself (also see Results). B, similar to A, but with 10 ng pure CSQ2 added to lanes 1 and 2 and EDL muscle homogenate present in lane 1 only (probed with anti-CSQ1 and 2). The EDL muscle contains virtually no CSQ2, and it is apparent that the detection of the exogenous CSQ2 was not hindered by the proteins present in the whole muscle sample; CSQ1 was also detected in the muscle sample.
In the Western blots for CSQ1, bands of higher molecular weight were also detected, there typically being three distinct bands at ∼130, 170 and 230 kDa (e.g. Fig. 1A). These bands, which have been observed previously in both skeletal and cardiac muscle and called ‘CSQ-like proteins’ (Cala et al. 1990; Culligan et al. 2002), were present in EDL and soleus muscle in approximately fixed proportion with CSQ1. In EDL muscle the three CLP bands together had a summed intensity equivalent to 57 ± 4% of the CSQ1 band in the same sample (n= 10).
Measurement of CSQ1 and CSQ2 in individual fibres
In order to establish the range and variability of CSQ content in different individual fibres, the same technique was used with segments of single fibres from EDL, soleus and red gastrocnemius muscles of the rat. The muscle mass of each fibre segment (∼3 mm long and ∼8–15 μg) was in the range found suitable for CSQ quantification (see previous subsection and Fig. 1). Every EDL fibre examined contained a substantial amount of CSQ1, which on average was about three-to-four times more than that present in a typical soleus fibre (values normalized by amount of fibre present, as gauged either from MHC or actin content; Fig. 3A). Soleus muscle of the rat contains both type I [SOL (I)] and type II fibres ([SOL (II)]; ∼80 and 20%, respectively). Fibres from soleus muscle were typed by mounting the fibre segment on a force transducer and determining the relative force response to a Sr2+-based solution at pSr 5.3 (see Methods); type I fibres give >70% of maximal force at such pSr (e.g. Fig. 4B) whereas type II fibres give <10% of maximal force (Bortolotto et al. 2000; Trinh & Lamb, 2006). The sensivity to Sr2+ is determined by the presence of fast or slow isoforms of troponin C (Bortolotto et al. 2000), which in rat muscle fibres is very tightly associated with the presence of fast and slow MHC isoforms, respectively (O'Connell et al. 2004).
Figure 3. Calsequestrin 1, CSQ2 and SERCA1 in segments of individual muscle fibres.
A, entire constituents of a segment of an individual EDL fibre (extreme left) and a soleus fibre (SOL, extreme right) were separated on a 10% SDS-PAGE gel, and CSQ1 and CSQ2 detected by Western blotting (anti-CSQ1 and 2). The blot was reprobed for SERCA1, and also for actin as an indicator of the relative amount of fibre added. Post-transfer Coomassie Blue staining for MHC (top panel) also confirmed that approximately equal amounts of the EDL and SOL fibre were run. Calsequestrin 1 content was much greater in the EDL fibre than in the SOL fibre, whereas CSQ2 was present only in the SOL fibre. B, Western blot comparing CSQ1 and CSQ2 contents in segments of 7 SOL (I) fibres and 2 SOL (II) fibres, calibrated by indicated amounts of purified CSQ1 (5–15 ng, rabbit) and CSQ2 (2–10 ng, human); (anti-CSQ1 and 2 and anti-CSQ1). The relative amount of fibre present is indicated by MHC stain (top panel). Fibre type was determined beforehand from the contractile response of the fibre segment to pSr 5.3 solution (see Methods and Fig. 4). The SOL (II) fibres contained more CSQ1 than SOL (I) fibres, and had a high density of SERCA1 but no CSQ2. C, relative amounts of CSQ1 in individual fibres from different muscles. Amounts of CSQ1 were derived from the standard curve for purified CSQ1 on the same gel, normalized by MHC content and expressed as a percentage of the mean value for EDL fibres on the same gel. In two SOL (II) cases (crossed circles) there were no EDL fibres on the same gel (shown in B), and values were first normalized to the mean of SOL (I) fibres on that gel and then rescaled by the ratio of mean values of EDL to SOL (I) fibres. The SOL (I) fibres were significantly different from EDL, RG (II) and SOL (II), and the SOL (II) and RG (II) fibres were significantly different from EDL fibres; one-way ANOVA with Newman–Keuls post hoc analyses. D, 8% gel Stains-All of 30 μg of protein loaded for EDL (2nd lane), soleus (3rd lane) and heart muscle preparations (4th lane; see Methods). Purified CSQ1 and CSQ2 (5 μg each) were loaded in the same lane. The sizes of two different molecular weight markers, applied to either side of the gel, are indicated (MWt).
Soleus type I fibres invariably contained both CSQ1 and CSQ2 (in all 37 fibres examined), with the proportion of CSQ2 to CSQ1 being broadly similar in each (e.g. Fig. 3B). In contrast, SOL (II) fibres contained very little or no CSQ2 (4 fibres with none, one fibre with ∼5% of that in SOL (I) fibres), and on average had roughly twofold more CSQ1 than did SOL (I) fibres (Fig. 3C). Type II fibres from red gastrocnemius muscle were very similar to SOL (II) fibres, both in the amount of CSQ1 present (Fig. 3C) and the fact that none had any CSQ2 (6 fibres). Of the 39 EDL fibres examined, only one had any detectable CSQ2 and in that case the amount present was only ∼10% of the average present in SOL (I) fibres. The EDL fibres were not type tested with Sr2+ but almost certainly all or virtually all were type II fibres, since previous such Sr2+ testing of rat EDL muscle did not find any type I fibres in more than 80 fibres examined (Bortolotto et al. 2000; Trinh & Lamb, 2006).
Density of SERCA pumps
Western blotting of individual fibres also demonstrated that the SR calcium pump protein SERCA1 was present in comparable amounts in every EDL fibre examined. In contrast, SERCA1 was absent or at very low relative density (<2% of that in EDL fibres) in every SOL (I) fibre examined (e.g. Fig. 3A). Soleus type II fibres were similar to EDL fibres in that SERCA1 was present at relatively high density in all four fibres examined (e.g. Fig. 3B). Red gastrocenemius type II [RG (II)] fibres were not tested for SERCA1. Western blotting of soleus muscle homogenates indicated ∼20% as much SERCA1 as in EDL homogenates, consistent with the fibre type distribution in soleus muscle. Similar blots for SERCA2a showed a strong signal in soleus homogenates and no detectable signal in EDL homogenates, indicating that there was little or no SERCA2a in the latter. The SERCA2a antibody was not sufficiently sensitive for use on single fibres.
Stains-All
Evaluation of the absolute amount of CSQ2 in fibres was not possible because efforts to obtain purified CSQ2 from rat cardiac muscle were unsuccessful and Western blots of rat muscle samples could be compared only to purified recombinant human CSQ2, which may well be detected with different sensitivity. Instead, the relative amount of CSQ2 to CSQ1 present in rat soleus muscle was determined from staining gels of SR preparations with Stains-All (Fig. 3D; see Methods) and comparing the intensities of the bands to those for purified CSQ2 and CSQ1, respectively. This relies on the reasonable assumption that Stains-All stains rat and human CSQ2 with similar efficacy. Such analysis indicated that the CSQ2:CSQ1 ratio in rat soleus muscle on average was 0.47:1. The Stains-All staining also indicated that the total amount of all SERCA isoforms present (seen at ∼100 kDa) was many-fold higher in EDL muscle than in soleus muscle of rats. Additionally, there was very little Stains-All staining of the CSQ-like proteins in the EDL and soleus samples, despite the fact that Western blotting (not shown) demonstrated that they were present in all fractions at approximately the same ratio to CSQ1 as seen in total homogenates (Fig. 1).
Endogenous and maximal SR Ca2+ content of skinned fibre segments: relationship to CSQ
The relative amount of releasable Ca2+ in the SR of each skinned fibre segment was ascertained from the time integral (‘area’) of the force response upon fully depleting the SR of Ca2+ (see Methods), as previously described (Lamb et al. 2001; Trinh & Lamb, 2006). Each fibre segment was subjected to repeated cycles in which the SR was emptied of Ca2+ and then partly or maximally reloaded by bathing the segment in a load solution for a set period (e.g. Fig. 4). The amount of free EGTA in the release solution was substantial (0.5 mm EGTA), in order to ensure that the resulting force response never reached the saturating force level, which for the caffeine-containing, low [Mg2+] release solution was ∼80% of maximal Ca2+-activated force (shown as ‘max’ response in Fig. 4A and B). In SOL (I) fibres in these conditions, the area of the force response upon depleting the SR increased approximately in a linear manner with loading time for shorter loading times (e.g. Fig. 4B and C), indicating that the area of the response could be used as a relative measure of the amount of Ca2+ loaded into the SR. The relationship was similar in EDL fibres except that the fibres had to be loaded to a threshold level before any force response was observed upon emptying the SR in the standard release solution (e.g. Fig. 4A and C). This threshold behaviour simply reflects that the Ca2+ sensitivity of the contractile apparatus in EDL fibres is lower than in SOL (I) fibres (Trinh & Lamb, 2006) and that the EDL fibres have a higher density of additional Ca2+-binding sites, in particular more SR Ca2+ pumps (Fryer & Stephenson, 1996; also see Fig. 3D); the threshold level could be reduced by lowering the amount of free EGTA present (not shown). The threshold load level was ascertained in each EDL fibre and taken into account when quantifying the endogenous and maximal SR load levels (see Methods).
Importantly, since the experiments used fresh muscle fibres that were mechanically skinned under paraffin oil, the SR of each fibre segment initially contained the level of Ca2+ present in vivo, referred to here as the ‘endogenous’ Ca2+ content. In SOL (I) fibres (e.g. Fig. 4B and C), this endogenous Ca2+ content was a relatively large proportion of the maximal Ca2+ level that could be loaded into the SR (the level reached with long loading times), which was found to be approximately the same regardless of whether the fibre was loaded in a solution at pCa 6.7 or at pCa 6.4 (not shown; see also Fryer & Stephenson, 1996). In contrast, the endogenous Ca2+ content of EDL fibres was found to be only a relatively small proportion of the maximal content (e.g. Fig. 4A and C). On average, the endogenous Ca2+ content as a percentage of maximal content (‘Endo/Max’) was 22 ± 2% in EDL fibres (n= 8) and 68 ± 8% in SOL (I) fibres (n= 9; significantly different, Student's unpaired t test, P < 0.05).
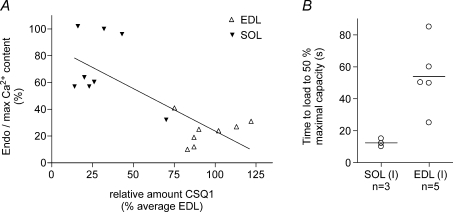
The fibre type of every SOL (I) fibre was established by Sr2+ testing. Each of these EDL and SOL (I) fibre segments was then individually examined for CSQ1, CSQ2 and SERCA1 by Western blotting, with the values found being typical of those throughout the whole study (Fig. 3). Linear regression analysis indicated an inverse relationship between the relative level of endogenous Ca2+ loading (Endo/Max) and the amount of CSQ1 in a fibre segment (Fig. 5A); the apparent relationship was not solely the result of comparing the two disparate types of fibres [SOL (I) versus EDL], since it was also seen in the sample of SOL (I) fibres examined.
Figure 5. Relationship between CSQ1 amount and Ca2+ content in single fibres.
A, endogenous Ca2+ content present in SR in a fibre segment expressed as a percentage of maximal Ca2+ content (see Fig. 4) plotted against the amount of CSQ1 in the fibre segment. Calsequestrin 1 in the fibre segment is expressed relative to the mean for EDL fibres on the same gel (see Fig. 3). Linear regression analysis indicates a significant inverse relationship (r2= 0.55, P < 0.005). B, time taken to reload SR to 50% of maximal capacity (see Fig. 4; load solution at pCa 6.7 with moderate Ca2+ buffering; 0.5 mm CaEGTA, 0.5 mm free EGTA). Lines indicate means for the two fibre types. Calsequestrin 1 was present in EDL fibres at 83–102% of EDL average, and in soleus fibres at 14–32%. In both A and B, all soleus fibres were shown to be type I by Sr2+ sensitivity. Calsequestrin 2 was present in all SOL (I) fibres and absent in all EDL fibres. SERCA1 was present at high density in all EDL fibres and negligible in all SOL (I) fibres; not measured in one fibre of each type in B.
EDL and SOL (I) fibres have similar absolute amounts of Ca2+ present in the SR endogenously (∼1.0–1.1 mm; Fryer & Stephenson, 1996), and the low Endo/Max value in EDL fibres observed here is primarily indicative of the high maximal SR Ca2+ capacity of such fibres. This was also evident from the fact that when SR in each fibre was loaded maximally, the time integral of the force response upon releasing the Ca2+ was over two times greater in the EDL fibres than in the SOL (I) fibres (e.g. Fig. 4A and B; 549 ± 120, n= 8; 262 ± 35, n= 9, respectively; in units of percentage maximal force × seconds; P < 0.05, Student's two-tailed unpaired t test). In each fibre, the force response was expressed relative to the maximal Ca2+-activated force measured in the same segment, and Ca2+ release was elicited in the presence of identical Ca2+ buffering in all fibres. Consequently, given that: (i) the Ca2+ sensitivity of the contractile apparatus is lower in EDL fibres than in SOL (I) fibres (Trinh & Lamb, 2006); and (ii) EDL fibres have more Ca2+-binding sites (on both troponin C and SERCA Ca2+ pumps) than SOL (I) fibres, the amount of Ca2+ contained in the SR and released must have been more than twofold higher in the EDL fibres than in the SOL (I) fibres. Furthermore, the relative rates of Ca2+ loading also indicated that the EDL fibres had a larger maximal SR Ca2+ capacity than the SOL (I) fibres. With the particular level of Ca2+ buffering used in the loading solution here (0.5 mm CaEGTA, 0.5 mm free EGTA, pCa 6.7), the absolute rate of Ca2+ uptake was determined primarily by the rate of diffusion of CaEGTA into the skinned fibre and not by the density and properties of the Ca2+ pumps nor by the small differences in fibre diameter (Trinh & Lamb, 2006). Consequently, the finding that the time to load to half-maximal capacity was on average approximately three times longer in EDL fibres than in SOL (I) fibres (Fig. 5B) indicates that the maximal SR Ca2+ content in EDL fibres was larger by a broadly similar factor.
Net Ca2+ uptake/leak in resting cytoplasmic conditions
The EDL fibres evidently have considerably more SERCA pumps (see Fig. 3D) (mostly SERCA1) and CSQ1 in the SR than do SOL (I) fibres, which one might presume would result in EDL fibres having a large endogenous SR Ca2+ content in resting conditions. However, this is not what was found in the present study (e.g. Fig. 5A), nor in the study of Fryer & Stephenson (1996) which assessed the endogenous Ca2+ content by a different method. In order to investigate this further, an experiment was designed to assess the overall effect on net SR Ca2+ uptake of the different densities and isoforms of SERCA and CSQ present in the two fibre types. Each skinned segment of an EDL or SOL (I) fibre was first emptied of its endogenous Ca2+ and then subjected to repeated load–release cycles in which it was reloaded to a fixed level similar to that present endogenously [loaded in standard pCa 6.7 solution for 20 s in EDL fibres and for a set time in the range 12–15 s in SOL (I) fibres]. As shown in Fig. 6, on one load–release cycle the skinned fibre segment was additionally left to equilibrate for 2 min in a solution with the free [Ca2+] buffered at 50 nm (pCa 7.3), close to the level thought to be present in resting fibres, and with the free [ADP] set at ∼10 μm, close to level in a rested fibre. The [ADP] in the cytoplasm has been shown to affect the level of Ca2+ leakage through the SERCA pumps (Macdonald & Stephenson, 2001, 2006), and the [ADP] was set here, as in those previous studies, by fixing the relative levels of ATP, creatine (Cr) and creatine phosphate in the bathing solution (see Methods). Of the five EDL fibres examined, four showed a decrease in SR Ca2+ content after the 2 min in the pCa 7.3 conditions relative to the bracketing control measurements (e.g. Fig. 6A) and one fibre showed an increase in content. As assessed by Student's paired t test, the SR content of the EDL fibres on average was not significantly changed from the initial level (88 ± 15%; see Table 1). In contrast, all six SOL (I) fibres examined showed a modest, but significant, net increase in SR Ca2+ content after 2 min in the same pCa 7.3 conditions (e.g. Fig. 6B and Table 1), even though each had relatively little CSQ1 and was already loaded at a substantial proportion of maximal capacity (∼60%, in contrast to ∼18% in EDL fibres). [Note that the EDL and SOL (I) fibres were examined alternately, using the same solutions.] These results show that the relative level of Ca2+ loading of the SR that can be maintained in the presence of near-resting cytoplasmic conditions is much lower in EDL fibres than in SOL (I) fibres.
Table 1.
Change in SR Ca2+ content in skinned fibres equilibrated in pCa 7.3 solution
| Initial Ca2+ content | Ca2+ content after equilibration | ||||||
|---|---|---|---|---|---|---|---|
| (% of maximal) | (% of endogenous) | (% of endogenous) | (% of Initial) | CSQ1 (% av EDL) | CSQ2 (% av SOL) | SERCA1 (% av EDL) | |
| EDL fibres (n= 5) | 18 ± 4 | 124 ± 11 | 110 ± 18 | 88 ± 15 | 75–102 | 0 | 80–140 |
| SOL (I) fibres (n= 6) | 60 ± 2† | 99 ± 7 | 121 ± 8 | 123 ± 3* | 16–43 | 70–130 | 0–1 |
As in Fig. 6, each EDL or SOL (I) fibre was loaded to a set level (initial Ca2+ content), similar to that present endogenously, and the change in SR Ca2+ content after a 2 min equilibration period at pCa 7.3 was assessed from the area of the force response upon emptying the SR (relative to bracketing control measurements). † Maximal content was not measured in 2 SOL fibres, and indicated mean value is for 4 fibres only. Ranges of CSQ1, CSQ2 and SERCA1 amounts found in each segment are shown relative to average level in EDL or SOL (I) fibres. Calsequestrin and SERCA1 densities were not measured in 1 EDL fibre. Soleus type I fibres significantly increased SR Ca2+ content in the pCa 7.3 solution (*P < 0.05, Student's paired t test), whereas EDL fibres showed no significant change. Note that ‘% av’ is the percentage of the average value found across all the EDL or SOL fibres on the same gel.
In agreement with the findings of Macdonald & Stephenson (2001), the leakage of Ca2+ out of the SR in the EDL fibres was found to be increased by the presence of 3 mm creatine in the pCa 7.3 equilibration solution, as shown in Fig. 7A. Similar results were found in all four EDL fibres examined in this way (time integral of force response after equilibration period with Cr present was 81 ± 4% of the bracketing responses without Cr present, P < 0.05). This indicates that even resting levels of cytoplasmic ADP (∼10 μm) cause an increase in the leakage of Ca2+ from the SR, presumably through the SERCA pumps (Macdonald & Stephenson, 2001). This was verified by examining the effect of the SERCA blocker, TBQ, on SR Ca2+ leakage; TBQ potently blocks SERCA but has no evident effects on either the contractile apparatus (Bakker et al. 1996) or the calcium release channels (Posterino & Lamb, 2003). In the fibre shown in Fig. 7B, Ca2+ loss from the SR during a 90 s leakage period (in a solution with 2 mm free EGTA, pCa ∼9) caused a large reduction in SR Ca2+ content (time integral of force response reduced to 38% of that of bracketing control responses with no leak period), but when TBQ was present during the leakage period to substantially block SERCA, the loss of SR Ca2+ was greatly reduced (time integral 64% of bracketing control responses with no leak period). Similar results were found in the other EDL fibre examined in this same way. [Note that TBQ was added during the final stage of loading the SR in order to facilitate its blocking action and to ensure its presence through the subsequent leakage period. As to be expected, less total Ca2+ was loaded into the SR during the overall loading procedure than when TBQ was absent (compare fourth with second force response in Fig. 7B), which emphasizes how relatively little Ca2+ was lost from the SR in the presence of TBQ (compare third with fourth force response in Fig. 7B).] These results indicate that much of the Ca2+ leakage in the EDL fibres occurred though SERCA, even in the virtual absence of any ADP.
Calsequestrin and SR Ca2+ content in toad fibres
Finally, for comparison, measurements of SR Ca2+ content were also made in skinned fibres from the iliofibularis muscle of the toad. In general, the relative endogenous SR content and the Ca2+ reloading behaviour more closely resembled those of rat EDL fibres than of SOL (I) fibres, though there appeared to be some disparity between fibres. In five toad iliofibularis fibres, the endogenous SR content was 31–47% of the maximal content, whereas in the other fibre examined it was 70%; the overall mean value was 43 ± 6% (n= 6). The fibres all took between 50 and 70 s in a load solution at pCa 6.7 to reach half-maximal SR content; maximal loading was achieved after 3 min in pCa 6.4 solution, and the level reached was ∼22% more than that after 3 min at pCa 6.7. The antibody to mammalian CSQ1 was found by Western blotting also to detect CSQ in toad iliofibrilaris muscle (not shown), but the absolute amount of CSQ present in toad muscle was not quantified because purified toad CSQ was unavailable.
Discussion
Calsequestrin amounts and maximal SR Ca2+ content in fast- and slow-twitch fibres
This study demonstrates that the amounts of CSQ1 present in both fast- and slow-twitch mammalian muscle fibres are much higher than previously reported (Leberer & Pette, 1986) and that the amount of CSQ1 is likely to be the major determinant of the maximal SR Ca2+ capacity in such fibres. Calsequestrin 1 content measured in homogenates of rat EDL and soleus muscle corresponded to 1.4 ± 0.1 and 0.43 ± 0.3 mg (g muscle wet weight)−1, respectively. Taking into account the CSQ1 molecular weight (47.8 kDa), the mass to volume ratio for muscle (1.06 g ml−1) and the volume of extracellular fluid in whole muscles (∼20% in both EDL and soleus muscle of rat; Clausen et al. 2004), these values correspond to average fibre contents of ∼36 ± 2 and 11 ± 1 μm CSQ1, respectively (‘μm’ denoting μmoles per litre total fibre volume). Soleus muscle in rats consists of ∼80% SOL (I) and 20% SOL (II) fibres (results here and see Trinh & Lamb, 2006) and so, given that SOL (II) fibres on average had twice as much CSQ1 per unit volume as did SOL (I) fibres (Fig. 3C), it can be inferred from the homogenate data that SOL (I) fibres on average contain ∼9.6 μm CSQ1. This would imply that the ratio of CSQ1 content in EDL fibres compared with SOL (I) fibres is 1:0.27, which is in good agreement with the average ratio found when directly examining individual segments of single EDL and SOL (I) fibres (1:0.30; Fig. 3C).
Given that CSQ1 can bind up to 80 mol Ca2+ per mole (Park et al. 2004), the above values indicate that the CSQ1 present in EDL and SOL (I) fibres could account for storage of up to 2.9 and 0.77 mm Ca2+, respectively. In the study of Park et al. (2004), maximal binding occurred only at a free [Ca2+] of 10 mm, though this was in the presence of unphysiologically high [KCl] (300 mm), and CSQ polymerization has been observed to occur at lower free [Ca2+] in the other conditions (150 mm NaCl; Beard et al. 2008). At maximal active loading, the free [Ca2+] in the SR can reach as high as ∼10 mm (Inesi & de Meis, 1989; Kurebayashi & Ogawa, 1998) and so, given that the SR volume is ∼9.3 and 5.5% of total fibre volume in fast- and slow-twitch mammalian muscle, respectively (Eisenberg, 1983), this could increase the total Ca2+ content by ∼0.93 and 0.55 mm, respectively. When the SR is near maximal capacity, Ca2+ binding to the high density of SERCA1 proteins present in EDL fibres could account for a further ∼0.15 mm Ca2+ (Baylor et al. 1983), and maximal binding to CSQ2 could account for an additional ∼0.27 mm in SOL (I) fibres. Thus, these figures imply maximal Ca2+ content values of ∼4.1 and 1.5 mm in EDL and SOL (I) fibres, respectively, without taking into account possible additional Ca2+ binding to other less abundant proteins, such as sarcalumenin (Leberer et al. 1990), histidine-rich Ca2+-binding protein (Sacchetto et al. 2001), junctate (Divet et al. 2007) and the CSQ-like proteins (Cala et al. 1990; Culligan et al. 2002). Thus, it appears that Ca2+ binding to CSQ is likely to account for a large proportion of the maximal SR Ca2+ content values of ∼3.7 and 1.1 mm measured in the rat EDL and SOL (I) fibres, respectively, in this laboratory (Fryer & Stephenson, 1996).
The present study also demonstrates that in normal adult rat muscle, fast- and slow-twitch fibres fall into separate homogeneous populations with stereotyped properties. Specifically, every EDL fibre and every type II fibre from soleus and red gastrocenemius (RG) muscle had: (i) substantial amounts of CSQ1; (ii) no or very little CSQ2; (iii) a high density of SERCA1; and (iv) no detectable SERCA2a, the last condition being the case at least in EDL fibres where it could be ascertained from whole muscle homogenates. One area of variability, however, was that the CSQ1 content in the EDL fibres sampled was significantly higher than in the other two groups of type II fibres (Fig. 3C), which might reflect differences between subgroups of type II fibres, since EDL muscle contains primarily IIB and IIX fibres, whereas SOL (II) fibres are largely or exclusively IIA fibres and RG (II) fibres are mostly type IIA and IIX (Delp & Duan, 1996; Goodman et al. 2003). In contrast to the type II fibres, SOL (I) fibres had: (i) less CSQ1, on average only ∼30% as much as EDL fibres; (ii) substantial amounts of CSQ2; (iii) no or very little SERCA1; and (iv) SERCA2a present, albeit at a much lower density than for SERCA1 in EDL fibres (Fig. 3D; also see Wu & Lytton, 1993).
Endogenous Ca2+ content and CSQ
The present study also confirmed the findings of Fryer & Stephenson (1996) that the endogenous SR Ca2+ content in rat EDL fibres is only a relatively small proportion of the maximal content, whereas in SOL (I) fibres it is a much higher proportion. Furthermore, the parallel measurements of CSQ1 in the same fibres indicated that this difference was primarily the result of the presence of relatively large amounts of CSQ1 in EDL fibres (Fig. 5A). Fryer & Stephenson (1996) found that the absolute values of endogenous Ca2+ content were very similar in EDL and SOL (I) fibres (∼1.0 and 1.1 mm, respectively), with the latter being close to the maximal content of the SOL (I) fibres. However, the findings of the present study suggest that the endogenous Ca2+ content of the SOL (I) fibres may have been overestimated in the study of Fryer & Stephenson (1996), which assayed the content after initially equilibrating each skinned fibre segment for 1 min in a Ca2+-buffered solution at pCa 7.1 (with 2.3 mm total BAPTA). It was found here that skinned SOL (I) fibres readily accumulate net Ca2+ into the SR in Ca2+-buffered solutions even at pCa 7.3 (Fig. 6B and Table 1), making it likely that the true endogenous SR Ca2+ content of the SOL (I) fibres is somewhat lower than that reported by Fryer & Stephenson (1996). In the present study, the endogenous Ca2+ content of SOL (I) was estimated to be ∼68% of maximal content, and this was assayed after equilibrating the fibres in a solution containing virtually no Ca2+ (and little buffering; see Methods), so that the SR would neither take up nor lose much net Ca2+. Another study (Nielsen et al. 2007) has estimated endogenous SR content in skinned SOL (I) fibres of the rat as being ∼45% of maximal content, but this is likely to be an underestimate because the fibres were initially equilibrated at pCa 8 (1 mm EGTA), which was found here to cause net loss of Ca2+ from the SR in SOL (I) fibres (see Methods). In any case, all studies agree that the ratio of endogenous-to-maximal content is low in EDL fibres and much higher in SOL (I) fibres (see Results and Fryer & Stephenson, 1996; Trinh & Lamb, 2006; Nielsen et al. 2007). In comparison, the endogenous SR Ca2+ content in toad fibres (∼1.1 mm in absolute terms; Owen et al. 1997) is ∼40% of the maximal level, similar to that recently found in frog fibres using a different technique (Pape et al. 2007).
Sarcoplasmic reticulum free Ca2+, SERCA and Ca2+ leakage
Importantly, it can be concluded that in normal muscle at rest, the free [Ca2+] in the SR must be considerably lower in EDL fibres than in SOL (I) fibres, because the ratio of total SR Ca2+ to total Ca2+-binding sites on CSQ is only ∼0.34 in EDL fibres (i.e. 1 mm Ca2+ and 2.9 mm sites on CSQ1) whereas it is ∼0.7 or greater in SOL (I) fibres (= 0.75 mm Ca2+ and 0.77 mm+ 0.3 mm sites on CSQ1 and CSQ2) and, furthermore, the SR volume is nearly twice as large in EDL fibres.
It may thus seem surprising then that when the cytoplasmic conditions were set close to normal resting levels (pCa 7.3, 10 μm ADP) and the SR loaded at close to normal endogenous Ca2+ content levels, EDL fibres were less able than SOL (I) fibres to maintain their SR Ca2+ content (Fig. 6 and Table 1), despite the EDL fibres evidently having a lower free [Ca2+] in the SR and also having a far higher SERCA pump density. In fact, it appears that the high SERCA density is a prime underlying cause of the relative SR leakiness of the EDL fibres. The cytoplasmic Ca2+ affinity of SERCA1 (here referring to the adult isoform, SERCA1a) is reported to be very similar to or even higher than that of SERCA2a (Lytton et al. 1992; Wolosker et al. 1997), the primary isoform present in SOL (I) fibres (Periasamy & Kalyanasundaram, 2007) and, if anything, this should aid the Ca2+ uptake in EDL fibres. [SERCA2b pumps are also reported to be present at low or very low density in both fast- and slow-twitch fibres and to have a higher Ca2+ affinity than either SERCA1a or SERCA2a (Periasamy & Kalyanasundaram, 2007), but their possible contribution to Ca2+ uptake at resting cytoplasmic Ca2+ is presently unknown.] However, as described by Inesi & de Meis (1989) and Macdonald & Stephenson (2001), the SERCA pumps are themselves a very significant pathway for leakage of Ca2+ out of the SR, with this leakage being increased in the presence of cytoplasmic ADP (e.g. Fig. 7A) and blocked at least partly by application of the SERCA blocker TBQ (e.g. Fig. 7B; see also Launikonis et al., 2005). The precise proportion of the total leakage occurring through SERCA in the present experiments is unclear, because there is no certainty that all SERCA pumps were completely blocked by TBQ throughout the entire leakage period. It is nevertheless clear that much of the leakage was through SERCA (Fig. 7B) and that this proportion is increased by elevated [ADP] (Fig. 7A; and Macdonald & Stephenson, 2001). Certainly, little if any of the Ca2+ leakage out of the SR occurs through the RyRs if the cytoplasmic [Ca2+] is near resting levels (pCa 7.3), as evidenced by the fact that it is unaffected by the RyR blocker, Ruthenium Red (Launikonis & Stephenson, 1997; Lamb & Cellini, 1999; Macdonald & Stephenson, 2006). Some additional Ca2+ leakage may occur through a ‘ryanodine-insensitive’ pathway related to the RyRs that has been reported in SR vesicles (Pessah et al. 1997), but that pathway has not been reported as yet in other preparations. In summary: (i) the relatively low SR Ca2+ content in EDL fibres in resting cytoplasmic conditions must be reflecting the point where the total Ca2+ leakage out of the SR matches the (low) level of Ca2+ uptake by the pumps at pCa 7.3; and (ii) much of that leakage occurs through the SERCA pumps, at least in EDL fibres.
New perspective of the role of CSQ1
The present study offers a new perspective on one of the major roles of CSQ1 in fast-twitch muscle. By necessity, fast-twitch fibres need to have a substantial amount of Ca2+ stored in the SR that can be released rapidly upon stimulation (Beltrán et al. 2006) in order to saturate the Ca2+-binding sites on troponin C. They must also have a high density of SERCA pumps in order to be able to rapidly resequester the Ca2+ for fast relaxation. The high density of SERCA pumps not only increases the number of cytoplasmic Ca2+-binding sites and consequent Ca2+ release requirement, but also greatly increases the number of leak pathways out of the SR. Although it might be argued that the Ca2+ uptake ability of the SR is increased in tandem with the increase in leakage pathways, there would a very high energetic cost if the resting equilibrium situation involved a continuous large leak of SR Ca2+ that was only kept in balance by an equally large amount of ATP-consuming Ca2+ uptake. This would be particularly problematic in fast-twitch fibres, which have fewer mitochondria, hence more limited aerobic capacity. At normal endogenous SR load levels, the rate constant of Ca2+ leakage from the SR in EDL fibres is low and virtually the same as that in SOL (I) fibres (∼0.008 s−1; Macdonald & Stephenson, 2001, 2006). Importantly, this low level of Ca2+ leakage in the EDL fibres is evidently achieved in large part by having a high concentration of CSQ1 present in the SR so as to keep the free [Ca2+] comparatively low and yet still have a large total amount of Ca2+ available for rapid release. Based on the relationship found between free and total SR Ca2+ in rabbit SR vesicles (Donoso et al. 1995), an endogenous content of ∼20% of maximal would be indicative of a free SR [Ca2+] of ∼0.3–0.4 mm (expressed in relation to SR volume), consistent with the in vivo level estimated in fast-twitch fibres of mouse (Rudolf et al. 2006). Having this relatively low free [Ca2+] in the SR in fast-twitch fibres would also help reduce or delay the occurrence of calcium phosphate precipitation in the SR, a phenomenon that may lead to muscle fatigue by reducing the Ca2+ available for rapid release, particularly in fast-twitch fibres where inorganic phosphate reaches high levels in the cytoplasm during vigorous activity (Allen et al. 2008).
The role and importance of CSQ1 described above also help in understanding the findings in CSQ1-null mice (Paolini et al. 2007). Given the considerable volume of the SR in fast-twitch fibres and the presence of other possible Ca2+-buffering proteins besides CSQ (see first subsection of Discussion), the SR can still contain sufficient Ca2+ to activate troponin C even without CSQ1, since this requires <0.3 mm total Ca2+ (Fryer & Stephenson, 1996), less than one-third of the normal SR Ca2+ content and less than one-tenth of normal maximal SR capacity. Although it was not described as such, Paolini et al. (2007) found that the maximal Ca2+ content of the SR in fast-twitch fibres in CSQ1-null mice was ∼20% of that in wild-type mice, this being apparent from responses observed when emptying the SR of Ca2+ after prolonged loading of skinned fibres at pCa 6.45 (Fig. 8E in Paolini et al. 2007). Based on the present results, this suggests that the maximal SR Ca2+ content in CSQ-null fibres was ∼0.8 mm (assuming that mouse and rat fibres are comparable), well in excess of that needed for activation of troponin C. It would nevertheless mean that the free [Ca2+] in the SR must be much higher in CSQ1-null mice than in normal mice; how this could occur needs further investigation, with it perhaps involving an increased role of SERCA2b pumps or modulation of SERCA1 properties.
The above perspective also readily explains the potentially catastrophic metabolic consequences of CSQ1 ablation. If sufficient Ca2+ is stored in the SR for near-normal contractile performance in fast-twitch fibres, the free SR [Ca2+] must be substantially raised and, consequently, there will be increased Ca2+ leak from SR through SERCA and any other leak pathways, with a concomitant increase in Ca2+ uptake and ATP metabolism. The latter would be likely also to increase cytoplasmic [ADP] and further increase SR Ca2+ leakage through the pump. In mice with a RyR mutation, such increased SR leak has been found to readily trigger a feedforward cycle of ATP usage, heat production and increased Ca2+ leak, with the result that heat stress readily leads to hyperthermia and death (Durham et al. 2008). In complete accord, CSQ1-null mice have recently been reported to be highly susceptible to a lethal crisis induced by heat stress or anaesthesia (Dainese et al. 2008), highlighting the great importance of having a large amount of CSQ1 in the SR so that the free [Ca2+] can be kept relatively low.
Finally, we note that the SOL (I) fibres were the only fibres which were endogenously loaded at a high proportion of maximal SR capacity and that every one of these fibres was found to have CSQ2 present, whereas CSQ2 was effectively absent in every type II fibre examined. This may be indicative that CSQ2 acts to reduce Ca2+ leakage from the SR, as has been suggested for CSQ2 in cardiac muscle cells (Chopra et al. 2007). Additionally, CSQ2 might be needed in SOL (I) muscle fibres because its polymerization and Ca2+-buffering properties differ somewhat from those of CSQ1 (Park et al. 2004), and this may help smooth out changes in SR free [Ca2+] during rapid release. A further difference of SOL (I) fibres could be that Ca2+ sequestration into the SR is aided by an interaction between sarcalumenin and SERCA2a, as has recently been reported to occur in cardiac cells (Shimura et al. 2008).
In summary, this study has established that CSQ1 indeed plays a vital role in fast-twitch mammalian muscle, acting as the primary Ca2+ buffer in the SR, which enables the free [Ca2+] there to be kept sufficiently low that Ca2+ leakage through the SERCA1 pumps does not metabolically compromise muscle function.
Acknowledgments
The SERCA1 monoclonal antibody developed by D. Fambrough was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. We thank Maria Cellini, Heidy Latchman and Dr Travis Dutka for assistance. This work was supported by National Health and Medical Research Council of Australia grants (280623 and 433034) and Fellowship to R.M.M. (380842), Clive and Vera Ramaciotti Establishment Grant (RA042/06) and Australian Research Council grant (DP0773683).
References
- Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J Muscle Res Cell Motil. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Chandler WK, Marshall MW. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Casarotto MG, Wei L, Varsángi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophysical J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Beard NA, Sakowska MM, Dulhunty AF, Laver DR. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Wei L, Cheung SN, Kimura T, Varsanyi M, Dulhunty AF. Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium. 2008;44:363–373. doi: 10.1016/j.ceca.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Beltrán M, Barrientos G, Hidalgo C. Fast kinetics of calcium dissociation from calsequestrin. Biol Res. 2006;39:493–503. doi: 10.4067/s0716-97602006000300011. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca2+- or Sr2+-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–C1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Cala SE, Scott BT, Jones LR. Intralumenal sarcoplasmic reticulum Ca2+-binding proteins. Seminars Cell Biol. 1990;1:265–275. [PubMed] [Google Scholar]
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- Clausen T, Overgaard K, Nielsen OB. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol Scand. 2004;180:209–216. doi: 10.1111/j.0001-6772.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- Costello B, Chadwick C, Saito A, Chu A, Maurer A, Fleischer S. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J Cell Biol. 1986;103:741–753. doi: 10.1083/jcb.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K, Banville N, Dowling P, Ohlendieck K. Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J Appl Physiol. 2002;92:435–445. doi: 10.1152/japplphysiol.00903.2001. [DOI] [PubMed] [Google Scholar]
- Dainese M, Quarta M, Paolini C, Canato M, Allen PD, Reggiani C, Protasi F. Heat and anesthesia cause a lethal crisis in calsequestrin-1 null mice. Biophys J. 2008;94:2687. [Google Scholar]
- Damiani E, Volpe P, Margreth A. Coexpression of two isoforms of calsequestrin in rabbit slow-twitch muscle. J Muscle Res Cell Motil. 1990;11:522–530. doi: 10.1007/BF01745219. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Divet A, Paesante S, Grasso C, Cavagna D, Tiveron C, Paolini C, Protasi F, Huchet-Cadiou C, Treves S, Zorzato F. Increased Ca2+ storage capacity of the skeletal muscle sarcoplasmic reticulum of transgenic mice over-expressing membrane bound calcium binding protein junctate. J Cell Physiol. 2007;213:464–474. doi: 10.1002/jcp.21121. [DOI] [PubMed] [Google Scholar]
- Donoso P, Prieto H, Hidalgo C. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys J. 1995;68:507–515. doi: 10.1016/S0006-3495(95)80212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachy LD, Adrian RH, Geiger SR, editors. Handbook of Physiology. Bethesda, MD, USA: American Physiological Society; 1983. pp. 73–112. section 10, Skeletal Muscle. [Google Scholar]
- Fliegel L, Leberer E, Green NM, MacLennan DH. The fast-twitch muscle calsequestrin isoform predominates in rabbit slow-twitch soleus muscle. FEBS Lett. 1989;242:297–300. doi: 10.1016/0014-5793(89)80488-0. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C, Patterson M, Stephenson G. MHC-based fiber type and E-C coupling characteristics in mechanically skinned muscle fibers of the rat. Am J Physiol Cell Physiol. 2003;284:C1448–C1459. doi: 10.1152/ajpcell.00569.2002. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Bhatnagar GM, Nagy B, Gergely J. Interaction of divalent cations with the 55,000-dalton protein component of the sarcoplasmic reticulum. Studies of fluorescence and circular dichroism. J Biol Chem. 1972;247:7835–7837. [PubMed] [Google Scholar]
- Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Effect of luminal calcium on Ca2+ release channel activity of sarcoplasmic reticulum in situ. Biophys J. 1998;74:1795–1807. doi: 10.1016/S0006-3495(98)77890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA. High intracellular [Ca2+] alters sarcoplasmic reticulum function in skinned skeletal muscle fibres of the rat. J Physiol. 1999;519:815–827. doi: 10.1111/j.1469-7793.1999.0815n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. J Physiol. 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Rios E. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence. J Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles of defined fiber composition. Eur J Biochem. 1986;156:489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Leberer E, Timms BG, Campbell KP, MacLennan DH. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J Biol Chem. 1990;265:10118–10124. [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effect of ADP on slow-twitch muscle fibres of the rat: implications for muscle fatigue. J Physiol. 2006;573:187–198. doi: 10.1113/jphysiol.2006.105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of mu-calpain in rat skeletal muscle. J Physiol. 2006;576:595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JS, Sahlin K, Ortenblad N. Reduced sarcoplasmic reticulum content of releasable Ca2+ in rat soleus muscle fibres after eccentric contractions. Acta Physiol (Oxf) 2007;191:217–228. doi: 10.1111/j.1748-1716.2007.01732.x. [DOI] [PubMed] [Google Scholar]
- O’Connell B, Nguyen LT, Stephenson GM. A single-fibre study of the relationship between MHC and TnC isoform composition in rat skeletal muscle. Biochem J. 2004;378:269–274. doi: 10.1042/BJ20031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K, Fredsted A, Hyldal A, Ingemann-Hansen T, Gissel H, Clausen T. Effects of running distance and training on Ca2+ content and damage in human muscle. Med Sci Sports Exerc. 2004;36:821–829. doi: 10.1249/01.mss.0000126468.65714.60. [DOI] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. J Physiol. 1997;498:571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape PC, Fenelon K, Lamboley CR, Stachura D. Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J Physiol. 2007;581:319–367. doi: 10.1113/jphysiol.2006.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and the ir calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Molinski TF, Meloy TD, Wong P, Buck ED, Allen PD, Mohr FC, Mack MM. Bastadins relate ryanodine-sensitive and -insensitive Ca2+ efflux pathways in skeletal SR and BC3H1 cells. Am J Physiol Cell Physiol. 1997;272:C601–C614. doi: 10.1152/ajpcell.1997.272.2.C601. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Magalhaes PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetto R, Damiani E, Turcato F, Nori A, Margreth A. Ca2+-dependent interaction of triadin with histidine-rich Ca2+-binding protein carboxyl-terminal region. Biochem Biophys Res Commun. 2001;289:1125–1134. doi: 10.1006/bbrc.2001.6126. [DOI] [PubMed] [Google Scholar]
- Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura M, Minamisawa S, Takeshima H, Jiao Q, Bai Y, Umemura S, Ishikawa Y. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc Res. 2008;77:362–370. doi: 10.1093/cvr/cvm019. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Gonzalez-Serratos HG, Shuman H, McClellan G, Somlyo AP. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981;90:577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh HH, Lamb GD. Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol. 2006;33:591–600. doi: 10.1111/j.1440-1681.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation–contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol. 2005;564:775–790. doi: 10.1113/jphysiol.2004.082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P, Simon BJ. The bulk of Ca2+ released to the myoplasm is free in the sarcoplasmic reticulum and does not unbind from calsequestrin. FEBS Lett. 1991;278:274–278. doi: 10.1016/0014-5793(91)80134-o. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L, Duan H, Pasek DA, Eu JP, Meissner G. Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J Biol Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Rocha JB, Engelender S, Panizzutti R, De Miranda J, de Meis L. Sarco/endoplasmic reticulum Ca2+-ATPase isoforms: diverse responses to acidosis. Biochem J. 1997;321:545–550. doi: 10.1042/bj3210545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KD, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca2+-ATPase isoforms in rat muscles. Am J Physiol Cell Physiol. 1993;264:C333–C341. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]