Abstract
Peak aerobic power in humans ( ) is markedly affected by inspired O2 tension (
) is markedly affected by inspired O2 tension ( ). The question to be answered in this study is what factor plays a major role in the limitation of muscle peak
). The question to be answered in this study is what factor plays a major role in the limitation of muscle peak  in hypoxia: arterial O2 partial pressure (
in hypoxia: arterial O2 partial pressure ( ) or O2 content (
) or O2 content ( )? Thus, cardiac output (dye dilution with Cardio-green), leg blood flow (thermodilution), intra-arterial blood pressure and femoral arterial-to-venous differences in blood gases were determined in nine lowlanders studied during incremental exercise using a large (two-legged cycle ergometer exercise: Bike) and a small (one-legged knee extension exercise: Knee) muscle mass in normoxia, acute hypoxia (AH) (
)? Thus, cardiac output (dye dilution with Cardio-green), leg blood flow (thermodilution), intra-arterial blood pressure and femoral arterial-to-venous differences in blood gases were determined in nine lowlanders studied during incremental exercise using a large (two-legged cycle ergometer exercise: Bike) and a small (one-legged knee extension exercise: Knee) muscle mass in normoxia, acute hypoxia (AH) ( ) and after 9 weeks of residence at 5260 m (CH). Reducing the size of the active muscle mass blunted by 62% the effect of hypoxia on
) and after 9 weeks of residence at 5260 m (CH). Reducing the size of the active muscle mass blunted by 62% the effect of hypoxia on  in AH and abolished completely the effect of hypoxia on
in AH and abolished completely the effect of hypoxia on  after altitude acclimatization. Acclimatization improved Bike peak exercise
after altitude acclimatization. Acclimatization improved Bike peak exercise  from 34 ± 1 in AH to 45 ± 1 mmHg in CH (P < 0.05) and Knee
from 34 ± 1 in AH to 45 ± 1 mmHg in CH (P < 0.05) and Knee  from 38 ± 1 to 55 ± 2 mmHg (P < 0.05). Peak cardiac output and leg blood flow were reduced in hypoxia only during Bike. Acute hypoxia resulted in reduction of systemic O2 delivery (46 and 21%) and leg O2 delivery (47 and 26%) during Bike and Knee, respectively, almost matching the corresponding reduction in
from 38 ± 1 to 55 ± 2 mmHg (P < 0.05). Peak cardiac output and leg blood flow were reduced in hypoxia only during Bike. Acute hypoxia resulted in reduction of systemic O2 delivery (46 and 21%) and leg O2 delivery (47 and 26%) during Bike and Knee, respectively, almost matching the corresponding reduction in  . Altitude acclimatization restored fully peak systemic and leg O2 delivery in CH (2.69 ± 0.27 and 1.28 ± 0.11 l min−1, respectively) to sea level values (2.65 ± 0.15 and 1.16 ± 0.11 l min−1, respectively) during Knee, but not during Bike. During Knee in CH, leg oxygen delivery was similar to normoxia and, therefore, also
. Altitude acclimatization restored fully peak systemic and leg O2 delivery in CH (2.69 ± 0.27 and 1.28 ± 0.11 l min−1, respectively) to sea level values (2.65 ± 0.15 and 1.16 ± 0.11 l min−1, respectively) during Knee, but not during Bike. During Knee in CH, leg oxygen delivery was similar to normoxia and, therefore, also  in spite of a
in spite of a  of 55 mmHg. Reducing the size of the active muscle mass improves pulmonary gas exchange during hypoxic exercise, attenuates the Bohr effect on oxygen uploading at the lungs and preserves sea level convective O2 transport to the active muscles. Thus, the altitude-acclimatized human has potentially a similar exercising capacity as at sea level when the exercise model allows for an adequate oxygen delivery (blood flow ×
of 55 mmHg. Reducing the size of the active muscle mass improves pulmonary gas exchange during hypoxic exercise, attenuates the Bohr effect on oxygen uploading at the lungs and preserves sea level convective O2 transport to the active muscles. Thus, the altitude-acclimatized human has potentially a similar exercising capacity as at sea level when the exercise model allows for an adequate oxygen delivery (blood flow × ), with only a minor role of
), with only a minor role of  per se, when
per se, when  is more than 55 mmHg.
is more than 55 mmHg.
It has been reported that during single leg knee extension exercise in normoxia, peak muscle blood flow and  may reach values between 2.5–4 l kg−1 min−1 and 0.35–0.60 l kg−1 min−1, respectively (Andersen & Saltin, 1985; Richardson et al. 1995; Rådegran et al. 1999), implying that the human has the potential to reach
may reach values between 2.5–4 l kg−1 min−1 and 0.35–0.60 l kg−1 min−1, respectively (Andersen & Saltin, 1985; Richardson et al. 1995; Rådegran et al. 1999), implying that the human has the potential to reach  values just by activating maximally ∼10 kg of muscle mass. However, the amount of muscle mass that can be perfused maximally during exercise depends on the maximal cardiac output, which has a finite magnitude (Calbet et al. 2004a). Thus, during exercise with a large muscle mass,
values just by activating maximally ∼10 kg of muscle mass. However, the amount of muscle mass that can be perfused maximally during exercise depends on the maximal cardiac output, which has a finite magnitude (Calbet et al. 2004a). Thus, during exercise with a large muscle mass,  is mainly limited by O2 delivery, which in turn depends on blood flow and the oxygen content of the arterial blood (
is mainly limited by O2 delivery, which in turn depends on blood flow and the oxygen content of the arterial blood ( ). However, during exercise with a small muscle mass,
). However, during exercise with a small muscle mass,  is not limited by cardiac output since, at fatigue, cardiac output has not reached maximal values (Roach et al. 1999).
is not limited by cardiac output since, at fatigue, cardiac output has not reached maximal values (Roach et al. 1999).
During exercise with a large muscle mass in severe acute hypoxia and chronic hypoxia, cardiac output and leg blood flow are reduced (Calbet et al. 2003a,b; Lundby et al. 2006). The combination of a lower peak leg blood flow with a lower  explains the marked reduction in
explains the marked reduction in  observed during exercise in severe acute hypoxia (Calbet et al. 2003a). With altitude acclimatization,
observed during exercise in severe acute hypoxia (Calbet et al. 2003a). With altitude acclimatization,  improves marginally and remains reduced despite a marked recovery of maximal systemic oxygen delivery (Calbet et al. 2003b). This dissociation between maximal systemic O2 delivery and
improves marginally and remains reduced despite a marked recovery of maximal systemic oxygen delivery (Calbet et al. 2003b). This dissociation between maximal systemic O2 delivery and  has been attributed to a ‘peripheral factor’ and it has been suggested that it could be an O2 diffusion limitation or reduced mitochondrial oxidative capacity induced by altitude acclimatization (Cerretelli, 1976; Wagner, 2000). However, an insufficient mitochondrial oxidative capacity as the mechanism behind the persistent reduction of
has been attributed to a ‘peripheral factor’ and it has been suggested that it could be an O2 diffusion limitation or reduced mitochondrial oxidative capacity induced by altitude acclimatization (Cerretelli, 1976; Wagner, 2000). However, an insufficient mitochondrial oxidative capacity as the mechanism behind the persistent reduction of  in chronic hypoxia has been ruled out since a moderate level of hyperoxia is able to re-establish sea level
in chronic hypoxia has been ruled out since a moderate level of hyperoxia is able to re-establish sea level  values (Calbet et al. 2003b; Lundby et al. 2006). Moreover, we have recently observed that maximal mitochondrial respiration remains unaltered after 8–10 days of residence at 4550 m (R. Boushel, E. Gnaiger, C. Wright-Paradis, P. Robach, J. A. Calbet & C. Lundby, unpublished observations) and neither citrate synthase nor 3-hydroxyacil-CoA-dehydrogenase activities are affected by prolonged exposure to high altitude (Mizuno et al. 2008).
values (Calbet et al. 2003b; Lundby et al. 2006). Moreover, we have recently observed that maximal mitochondrial respiration remains unaltered after 8–10 days of residence at 4550 m (R. Boushel, E. Gnaiger, C. Wright-Paradis, P. Robach, J. A. Calbet & C. Lundby, unpublished observations) and neither citrate synthase nor 3-hydroxyacil-CoA-dehydrogenase activities are affected by prolonged exposure to high altitude (Mizuno et al. 2008).
The fact that exercise is interrupted before the maximal cardiac output and muscle blood flow has been reached also in chronic hypoxia (Pugh, 1964; Reeves et al. 1987; Calbet et al. 2002, 2003a,b; Lundby et al. 2008a), a condition in which  is similar to sea level, may suggest that
is similar to sea level, may suggest that  plays a more important role than
plays a more important role than  . This may be tested by studying the exercise response to incremental exercise to exhaustion with a small and a large muscle mass in acute and chronic hypoxia. If the main mechanism causing fatigue is a low
. This may be tested by studying the exercise response to incremental exercise to exhaustion with a small and a large muscle mass in acute and chronic hypoxia. If the main mechanism causing fatigue is a low  , then reducing the size of the muscle mass activated during exercise will have no, or only a minor, effect in terms of attenuating the effect of hypoxia on
, then reducing the size of the muscle mass activated during exercise will have no, or only a minor, effect in terms of attenuating the effect of hypoxia on  and, hence, exercise capacity. By reducing the active muscle mass to the quadriceps muscle of one leg we expected the peak leg blood flow to be maintained or elevated, since the sympathetic activation is low and the maximal (pumping) functional reserve of the heart not fully exploited (Savard et al. 1989; Strange et al. 1993). Moreover, if the main limitation to peak
and, hence, exercise capacity. By reducing the active muscle mass to the quadriceps muscle of one leg we expected the peak leg blood flow to be maintained or elevated, since the sympathetic activation is low and the maximal (pumping) functional reserve of the heart not fully exploited (Savard et al. 1989; Strange et al. 1993). Moreover, if the main limitation to peak  during exercise in hypoxia resides in a limitation to O2 diffusion in the lung and contracting muscles, then there will be no benefit from an elevation of blood flow (or the benefit will be less than expected from the increase in O2 delivery) during exercise with a small muscle mass. In addition, the main mechanism limiting pulmonary gas exchange during exercise in hypoxia is a diffusion limitation (Torre-Bueno et al. 1985; Hammond et al. 1986). Due to lower peak cardiac output and higher pulmonary transit times during exercise with a small compared to a large muscle mass we also expect a better pulmonary gas exchange with the reduction of the active muscle mass (Calbet et al. 2008).
during exercise in hypoxia resides in a limitation to O2 diffusion in the lung and contracting muscles, then there will be no benefit from an elevation of blood flow (or the benefit will be less than expected from the increase in O2 delivery) during exercise with a small muscle mass. In addition, the main mechanism limiting pulmonary gas exchange during exercise in hypoxia is a diffusion limitation (Torre-Bueno et al. 1985; Hammond et al. 1986). Due to lower peak cardiac output and higher pulmonary transit times during exercise with a small compared to a large muscle mass we also expect a better pulmonary gas exchange with the reduction of the active muscle mass (Calbet et al. 2008).
Thus, to test the hypothesis that a low  is not the primary cause of the reduced peak
is not the primary cause of the reduced peak  during exercise at altitude, we studied the influence of the size of the active muscle mass on the cardiovascular and respiratory response to exercise in healthy humans exposed to acute and chronic hypoxia. Altitude-acclimatized humans can reach sea level maximal exercise
during exercise at altitude, we studied the influence of the size of the active muscle mass on the cardiovascular and respiratory response to exercise in healthy humans exposed to acute and chronic hypoxia. Altitude-acclimatized humans can reach sea level maximal exercise 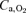 values. This makes it possible to clearly evaluate which factor plays the major role in limiting muscle peak
values. This makes it possible to clearly evaluate which factor plays the major role in limiting muscle peak  : arterial oxygen tension or its content.
: arterial oxygen tension or its content.
Methods
These experiments were carried out during and after the Copenhagen Muscle Research Centre 1998 Expedition to Mount Chacaltaya (Bolivia). They included submaximal and maximal exercise on the cycle ergometer (Bike), as well as on the one-leg knee extension ergometer (Knee), which we report here for the first time. The respiratory, haemodynamic and blood gas responses during submaximal and maximal exercise on the cycle ergometer were previously reported (Boushel et al. 2001; Calbet et al. 2002, 2003a,b, 2004b; Wagner et al. 2002). In these articles a detailed description of the subjects and methods applied can be found.
In the present article we integrate new analyses from the previously published responses on Bike with the current Knee data into a unified article to compare the respiratory, haemodynamic and blood gases responses to large and small muscle mass exercise. Since the focus of the present study is  , we limit our comparisons to the maximal exercise points, except for O2 extraction. The latter allowed for determining if O2 extraction is reduced during exercise with a small muscle mass when O2 delivery is not restricted by blood flow. In addition, new calculations on oxygen conductances and mean capillary
, we limit our comparisons to the maximal exercise points, except for O2 extraction. The latter allowed for determining if O2 extraction is reduced during exercise with a small muscle mass when O2 delivery is not restricted by blood flow. In addition, new calculations on oxygen conductances and mean capillary  for all these conditions are included in the present article. During sea level experiments, valid data were obtained in all subjects; however, due to uncompleted data collection in two subjects, chronic hypoxia results are based on the data obtained from the remaining seven subjects (3 females and 4 males).
for all these conditions are included in the present article. During sea level experiments, valid data were obtained in all subjects; however, due to uncompleted data collection in two subjects, chronic hypoxia results are based on the data obtained from the remaining seven subjects (3 females and 4 males).
Subjects
Nine healthy, physically active, Danish lowlanders (5 males and 4 females) volunteered to participate in these studies. Their mean (±s.e.m.) age, height and weight were 24.3 ± 0.5 years, 176 ± 3 cm and 74 ± 4 kg, and their  averaged 59 ± 2 ml kg−1 min−1 (range: 49–62 ml kg−1 min−1) and 53 ± 4 ml kg−1 min−1 (range: 47–63 ml kg−1 min−1) in the males and females, respectively. All subjects had normal resting ECG, liver, kidney and thyroid functions, as well as fasting plasma glucose and electrolyte concentrations. All subjects were informed about the procedures and risks of the study before giving written informed consent to participate as approved by the Copenhagen and Fredriksberg Ethical Committee. All experiments conformed with the Declaration of Helsinki.
averaged 59 ± 2 ml kg−1 min−1 (range: 49–62 ml kg−1 min−1) and 53 ± 4 ml kg−1 min−1 (range: 47–63 ml kg−1 min−1) in the males and females, respectively. All subjects had normal resting ECG, liver, kidney and thyroid functions, as well as fasting plasma glucose and electrolyte concentrations. All subjects were informed about the procedures and risks of the study before giving written informed consent to participate as approved by the Copenhagen and Fredriksberg Ethical Committee. All experiments conformed with the Declaration of Helsinki.
Experimental preparation
Catheterization
Catheters were inserted percutaneously under local anaesthesia (2% lidocaine) in the femoral vein, an antecubital vein and the femoral artery, as described elsewhere (Calbet et al. 2003a). Briefly, an 18-gauge, 20-cm-long catheter (Hydrocath, Ohmeda, Swindon, UK) was inserted in the femoral vein of either the right or left leg. This catheter was introduced 2 cm below the inguinal ligament and advanced 7 cm distally. The femoral vein catheter was used for venous sampling and downstream cold saline injection. Subsequently, a thin polyethylene-coated thermistor (model 94-030-2.5F T.D. Probe, Edwards Edslab, Baxter, Irvine, CA, USA) was inserted 3 cm below the inguinal ligament and advanced proximally 10 cm into the same femoral vein. A second 18-gauge catheter was placed into the femoral artery 2 cm below the inguinal ligament and advanced 14 cm proximally. An additional catheter was placed in a vein in the left upper arm for the injection of the Cardio-green dye for measurement of cardiac output. After the catheters were placed, the subjects rested in a supine position for 30 min.
Experimental design
Chronic hypoxia tests (CH) were conducted at altitude after 9–10 weeks residence at 5260 m on Mount Chacaltaya, Bolivia. Acute hypoxia tests (AH) at sea level were carried out at least 6 months after the subjects returned to Denmark, after just a few minutes exposure to hypoxic gas.
Subjects first performed leg knee extension exercise, which was followed after a 60 min rest by exercise on the cycle ergometer in the upright position. The exercise tests were carried out at altitude and at sea level with two different fractions of inspired O2 ( ). During the experiments at altitude, the subjects inspired room air (408 mmHg,
). During the experiments at altitude, the subjects inspired room air (408 mmHg,  mmHg) and air from a tank containing 55% O2 in nitrogen (
mmHg) and air from a tank containing 55% O2 in nitrogen ( mmHg). Experiments at sea level were carried out at a barometric pressure of 750–760 mmHg with two different levels of
mmHg). Experiments at sea level were carried out at a barometric pressure of 750–760 mmHg with two different levels of  : 0.210 (room air) and 0.105 (from a pre-mixed tank of O2 in nitrogen). The latter resulted in a
: 0.210 (room air) and 0.105 (from a pre-mixed tank of O2 in nitrogen). The latter resulted in a  similar to that observed at altitude (i.e. 75 mmHg).
similar to that observed at altitude (i.e. 75 mmHg).
Exercise protocol
The exercise protocol is depicted in Fig. 1. One-legged knee extension exercise was performed on a specifically designed leg extension ergometer that confines muscle contractile activity to the quadriceps muscle in each leg extension while the return to the starting position (about 90 deg knee angle) was brought about passively by the crank and fixed gear of the ergometer, as described elsewhere (Andersen & Saltin, 1985). Leg extension exercise consisted of 10 min of continuous exercise at an intensity of 30 W (warm up) followed by a rest period of 3 min. Then an incremental leg knee extension test to exhaustion was performed starting from 30 W. The 30 W intensity was maintained for 2 min, then the load was increased by 10–15 W increments every 2 min until exhaustion. At the end of the leg extension exercise subjects were allowed to rest for 60 min. Thereafter, they performed submaximal exercise on the cycle ergometer (Monark 824E, Varberg, Sweden), which consisted of 10 min constant-intensity exercise at ∼120 W (102–141 W, at 80 r.p.m.). This was followed by a resting period of 10 min in CH and 20–30 min in AH and a maximal exercise test on the cycle ergometer. The latter was started at an initial intensity identical to that used in the submaximal test, which was maintained for 2 min. Exercise intensity was then increased by 20–40 W every minute until reaching the maximal exercise intensity ( ). Load increments were adjusted such that the exercise duration of the incremental exercise tests was approximately 6–7 min in all conditions.
). Load increments were adjusted such that the exercise duration of the incremental exercise tests was approximately 6–7 min in all conditions.
Figure 1. Experimental protocol.
Vertical arrows indicate the time points at which measurements were performed. The same sequence was carried out in normoxia, acute hypoxia and chronic hypoxia. Leg extension exercise was performed first and followed after a 60 min rest by exercise on the cycle ergometer in upright position. The exercise tests were carried out at altitude and at sea level with two different fractions of inspired O2 ( ). During the experiments at altitude, the subjects inspired room air (408 mmHg,
). During the experiments at altitude, the subjects inspired room air (408 mmHg,  mmHg) and air from a tank containing 55% O2 in nitrogen (
mmHg) and air from a tank containing 55% O2 in nitrogen ( mmHg). Experiments at sea level were carried out at a barometric pressure of 750–760 mmHg with two different levels of
mmHg). Experiments at sea level were carried out at a barometric pressure of 750–760 mmHg with two different levels of  : 0.210 (room air) and 0.105 (from a pre-mixed tank of O2 in nitrogen). The latter resulted in a
: 0.210 (room air) and 0.105 (from a pre-mixed tank of O2 in nitrogen). The latter resulted in a  similar to that observed at altitude (i.e. 75 mmHg). In the acute and chronic hypoxic conditions and when the subjects were almost exhausted, the
similar to that observed at altitude (i.e. 75 mmHg). In the acute and chronic hypoxic conditions and when the subjects were almost exhausted, the 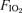 was increased to 0.21 and to 0.55 during acute hypoxia and chronic hypoxia, respectively, as indicated by ‘oxygenation’.
was increased to 0.21 and to 0.55 during acute hypoxia and chronic hypoxia, respectively, as indicated by ‘oxygenation’.
During the exercise tests, subjects breathed through a 2-way valve inspiring room air (CH) or 10.5% O2 (AH) for 5 min before the resting measurements were made (Fig. 1). At the end of exercise with hypoxia, subjects were vigorously encouraged to keep kicking or pedalling while they were switched to breathe hyperoxic air ( ), giving a
), giving a  of about 200 mmHg in CH, or room air at sea level in the AH study. After 2 min at this
of about 200 mmHg in CH, or room air at sea level in the AH study. After 2 min at this  , a further set of measurements was made, and finally the workload was increased again if possible by steps of 10–15 W (knee extension exercise) or 20–40 W (cycle ergometer exercise) every minute until exhaustion. Measurements were performed at ∼90% of
, a further set of measurements was made, and finally the workload was increased again if possible by steps of 10–15 W (knee extension exercise) or 20–40 W (cycle ergometer exercise) every minute until exhaustion. Measurements were performed at ∼90% of  and repeated with each load increment until exhaustion. In this way, the same exercise protocols and O2 exposure profiles were used in both acute and chronic hypoxia.
and repeated with each load increment until exhaustion. In this way, the same exercise protocols and O2 exposure profiles were used in both acute and chronic hypoxia.
During the AH experiments, normoxic and hypoxic submaximal exercise bouts were administered in random order. When submaximal hypoxic exercise was performed first, the recovery periods were extended when needed, to allow venous blood lactate to reach similar values to those observed at rest. After the submaximal exercise, the normoxic maximal tests were carried out and then, after at least 1 h of rest in the semi-recumbent position breathing room air, the acute hypoxia tests were performed. Just at the end of the exercise with hypoxia, subjects were vigorously encouraged to keep kicking or pedalling while they were switched to breathing room air. After 2 min at the  attained in the hypoxic trial, the workload was increased again in steps of 20–40 W until exhaustion.
attained in the hypoxic trial, the workload was increased again in steps of 20–40 W until exhaustion.
Measurements
Respiratory variables
Pulmonary  , CO2 production (
, CO2 production ( ) and expired minute ventilation (
) and expired minute ventilation ( ) were measured with an on-line system (Medical Graphics CPX, Saint Paul, Minneapolis, MN, USA) while the subjects breathed through a low-resistance breathing valve and averaged every 15 s. Gases with known
) were measured with an on-line system (Medical Graphics CPX, Saint Paul, Minneapolis, MN, USA) while the subjects breathed through a low-resistance breathing valve and averaged every 15 s. Gases with known  and
and  concentration (micro-Scholander) were used for calibration of the gas analyser.
concentration (micro-Scholander) were used for calibration of the gas analyser.
Blood flow
Femoral venous blood flow (i.e. leg blood flow) was measured in the femoral vein by constant-infusion thermodilution as described in detail elsewhere (Andersen & Saltin, 1985; Calbet, 2003; Calbet et al. 2003a). At peak effort, the measurements were made within 1 min of exhaustion. When possible, duplicate measurements of leg blood flow and femoral arteriovenous O2 differences were made during the brief period of peak exercise. Heart rate (HR), arterial blood pressure, pulmonary  ,
, 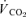 and
and 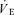 were measured at the same time as leg blood flow and cardiac output.
were measured at the same time as leg blood flow and cardiac output.
Blood pressure and heart rate
Arterial blood pressure was monitored continuously by a disposable transducer (T100209A, Baxter, Unterschleissheim, Germany) placed at the level of the inguinal ligament as reported elsewhere (Calbet, 2003; Calbet et al. 2003a).
Cardiac output
Cardiac output was measured by indocyanine green (ICG, Akorn Inc., IL, USA) dye dilution (Dow, 1956; Boushel et al. 2001).
Blood analysis
Blood haemoglobin concentration ([Hb]) and O2 saturation ( ) were measured with a co-oximeter (OSM 3 Haemoximeter, Radiometer, Copenhagen, Denmark).
) were measured with a co-oximeter (OSM 3 Haemoximeter, Radiometer, Copenhagen, Denmark). 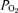 ,
, 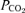 and pH were determined with a blood gas analyser (ABL 5, Radiometer, Copenhagen, Denmark) and corrected for measured femoral vein blood temperature. Haematocrit was determined by microcentrifugation on triplicate samples. Arterial and venous O2 content (
and pH were determined with a blood gas analyser (ABL 5, Radiometer, Copenhagen, Denmark) and corrected for measured femoral vein blood temperature. Haematocrit was determined by microcentrifugation on triplicate samples. Arterial and venous O2 content (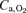 and
and 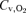 ) were computed from the saturation and [Hb] (i.e.
) were computed from the saturation and [Hb] (i.e.  ). Plasma noradrenaline and adrenaline concentrations were measured by HPLC with electrochemical detection (Hallman et al. 1978).
). Plasma noradrenaline and adrenaline concentrations were measured by HPLC with electrochemical detection (Hallman et al. 1978).
Calculations
Arteriovenous [O2] difference ( ) was calculated from the difference in femoral arterial and femoral venous [O2]. This difference was then divided by arterial concentration to give O2 extraction. Oxygen delivery was computed as the product of blood flow and
) was calculated from the difference in femoral arterial and femoral venous [O2]. This difference was then divided by arterial concentration to give O2 extraction. Oxygen delivery was computed as the product of blood flow and  . Leg
. Leg  was calculated as the product of leg blood flow and
was calculated as the product of leg blood flow and  . Non-leg
. Non-leg 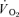 was computed as the difference between pulmonary
was computed as the difference between pulmonary  and either one time leg
and either one time leg  (knee extension exercise) or two times leg
(knee extension exercise) or two times leg 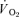 (exercise on the cycle ergometer).
(exercise on the cycle ergometer).
Statistical analysis
Differences in the measured variables among conditions and exercise levels were analysed with two-way ANOVA for repeated measures, with condition and exercise intensity as within-subjects factors, followed by a LSD post hoc test for pair-wise comparisons. Significance was accepted at P < 0.05. Data are reported as means ±s.e.m.
Results
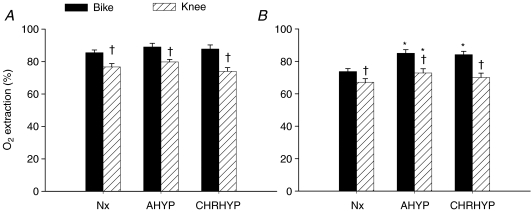
The size of the active muscle mass had a major impact on the tolerance to exercise in hypoxia. In acute hypoxia, reducing the size of the active muscle mass blunted the effect of hypoxia on  by 62% and in chronic hypoxia the effect was completely abolished (Fig. 2). The main reasons accounting for these effects were a better pulmonary gas exchange and less of a shift to the right of the oxygen haemoglobin dissociation curve, which facilitated oxygen uploading in the lungs. The lack of impact of chronic hypoxia on whole-body and leg
by 62% and in chronic hypoxia the effect was completely abolished (Fig. 2). The main reasons accounting for these effects were a better pulmonary gas exchange and less of a shift to the right of the oxygen haemoglobin dissociation curve, which facilitated oxygen uploading in the lungs. The lack of impact of chronic hypoxia on whole-body and leg  during knee extension is explained by the improvement of pulmonary gas exchange combined with the increase of blood haemoglobin concentration elicited by altitude acclimatization. These mechanisms allowed for a higher arterial oxygen content and oxygen delivery to the active muscles, and the other vascular beds. A detailed analysis of the impact of the size of the active muscle mass on the links of the oxygen transport cascade is reported below.
during knee extension is explained by the improvement of pulmonary gas exchange combined with the increase of blood haemoglobin concentration elicited by altitude acclimatization. These mechanisms allowed for a higher arterial oxygen content and oxygen delivery to the active muscles, and the other vascular beds. A detailed analysis of the impact of the size of the active muscle mass on the links of the oxygen transport cascade is reported below.
Figure 2. Effect of hypoxia on peak pulmonary and leg .
.
Drop in pulmonary  during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in acute hypoxia (A) and after 9–10 weeks of residence at 5260 m (C). *Significantly different from normoxia (P < 0.05).
during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in acute hypoxia (A) and after 9–10 weeks of residence at 5260 m (C). *Significantly different from normoxia (P < 0.05).
 and pulmonary gas exchange
and pulmonary gas exchange
Compared to normoxia, acute hypoxia reduced whole-body  by 47 and 18% (Fig. 2) and the maximal load achieved at the end of the incremental exercise to exhaustion by 31 and 8% during Bike and Knee, respectively. After altitude acclimatization, Bike whole-body and leg peak
by 47 and 18% (Fig. 2) and the maximal load achieved at the end of the incremental exercise to exhaustion by 31 and 8% during Bike and Knee, respectively. After altitude acclimatization, Bike whole-body and leg peak  were still reduced by 26 and 21%, respectively (Fig. 2). In contrast, altitude acclimatization allowed for a complete restoration of whole-body and leg peak
were still reduced by 26 and 21%, respectively (Fig. 2). In contrast, altitude acclimatization allowed for a complete restoration of whole-body and leg peak  values during Knee (Fig. 2).
values during Knee (Fig. 2).
Pulmonary gas exchange was impaired during exercise in acute hypoxia but to a greater extent during Bike (Fig. 3). Acclimatization resulted in an improvement in pulmonary gas exchange which was more marked during Knee. The ventilatory response to exercise was exacerbated in hypoxia as reflected by the fact that the 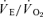 was higher during exercise in hypoxia than in normoxia. However, absolute ventilation values at exhaustion were lower in acute hypoxia than in normoxia when the exercise was performed on the cycle ergometer (Fig. 3). In contrast, exercise hyperventilation reached higher values during Knee in acute hypoxia compared to the normoxic condition (Fig. 3).
was higher during exercise in hypoxia than in normoxia. However, absolute ventilation values at exhaustion were lower in acute hypoxia than in normoxia when the exercise was performed on the cycle ergometer (Fig. 3). In contrast, exercise hyperventilation reached higher values during Knee in acute hypoxia compared to the normoxic condition (Fig. 3).
Figure 3. Pulmonary ventilation and blood gases.
Pulmonary ventilation ( ), alveolar
), alveolar  (
( ), and arterial
), and arterial  (
( ) and
) and  (
( ) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same
) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Compared to normoxia,  was reduced with acute hypoxia to the same extent in both models of exercise (53 and 54%, P= n.s.) (Fig. 3). Compared to acute hypoxia, peak exercise alveolar
was reduced with acute hypoxia to the same extent in both models of exercise (53 and 54%, P= n.s.) (Fig. 3). Compared to acute hypoxia, peak exercise alveolar  was increased with acclimatization by 9 and 7% during Bike and Knee, respectively (Fig. 3). A similar response was observed for
was increased with acclimatization by 9 and 7% during Bike and Knee, respectively (Fig. 3). A similar response was observed for 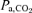 which was reduced by 23 and 20%, during Bike and Knee, respectively (Fig. 3). In the acclimatized subjects,
which was reduced by 23 and 20%, during Bike and Knee, respectively (Fig. 3). In the acclimatized subjects,  was reduced to the same level, i.e. ∼20 mmHg during peak exercise regardless of muscle mass (Fig. 3).
was reduced to the same level, i.e. ∼20 mmHg during peak exercise regardless of muscle mass (Fig. 3).
At peak exercise in normoxia,  was lower during Bike than Knee (102 ± 3 and 110 ± 2 mmHg, respectively) (Fig. 3). Acute hypoxia resulted in a 66% lower
was lower during Bike than Knee (102 ± 3 and 110 ± 2 mmHg, respectively) (Fig. 3). Acute hypoxia resulted in a 66% lower  regardless of the exercise mode. However,
regardless of the exercise mode. However, 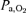 was 3.7 mmHg lower during Bike compared to Knee. Compared to acute hypoxia, acclimatization improved Bike peak exercise
was 3.7 mmHg lower during Bike compared to Knee. Compared to acute hypoxia, acclimatization improved Bike peak exercise  by 34% (from 34 ± 1 to 45 ± 1 mmHg, in acute and chronic hypoxia, respectively) and Knee
by 34% (from 34 ± 1 to 45 ± 1 mmHg, in acute and chronic hypoxia, respectively) and Knee  by 44% (from 38 ± 1 to 55 ± 2 mmHg, in acute and chronic hypoxia, respectively), the improvement being significantly higher for Knee compared to Bike.
by 44% (from 38 ± 1 to 55 ± 2 mmHg, in acute and chronic hypoxia, respectively), the improvement being significantly higher for Knee compared to Bike.
Consequently the  was 8 mmHg higher during Bike exercise in AH compared to normoxia (23 ± 1 and 15 ± 1 mmHg, respectively) (Fig. 4). With acclimatization the maximal Bike exercise
was 8 mmHg higher during Bike exercise in AH compared to normoxia (23 ± 1 and 15 ± 1 mmHg, respectively) (Fig. 4). With acclimatization the maximal Bike exercise  was reduced to 13 ± 1 mmHg. This effect of acclimatization on the
was reduced to 13 ± 1 mmHg. This effect of acclimatization on the 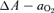 was even more marked during leg extension exercise with a reduction of 13 mmHg (from 15 ± 1 mmHg in AH to just 2 ± 1 mmHg in CH).
was even more marked during leg extension exercise with a reduction of 13 mmHg (from 15 ± 1 mmHg in AH to just 2 ± 1 mmHg in CH).
Figure 4. Pulmonary gas exchange.
Alveolar–arterial  difference (
difference ( ) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same
) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Despite the relatively small influence of exercising muscle mass on  during exercise in acute hypoxia,
during exercise in acute hypoxia,  was about 10 units higher during Knee (66.2 ± 2.7 and 76.5 ± 1.7%, respectively) (Fig. 5). About 40% of the difference in
was about 10 units higher during Knee (66.2 ± 2.7 and 76.5 ± 1.7%, respectively) (Fig. 5). About 40% of the difference in  could be accounted for by the higher arterial pH (+0.23°C) during Knee, and 10% by the higher blood temperature (+0.35°C) during Bike. The rest of the difference in
could be accounted for by the higher arterial pH (+0.23°C) during Knee, and 10% by the higher blood temperature (+0.35°C) during Bike. The rest of the difference in  (45% of it) could be accounted for by the impact of the 3.7 mmHg lower
(45% of it) could be accounted for by the impact of the 3.7 mmHg lower  on
on  during Bike. Consequently at peak exercise in acute hypoxia,
during Bike. Consequently at peak exercise in acute hypoxia,  was 17% higher during Knee than during Bike (Fig. 5). Compared to acute hypoxia, peak exercise
was 17% higher during Knee than during Bike (Fig. 5). Compared to acute hypoxia, peak exercise  was improved dramatically with altitude acclimatization. This effect was more marked during Knee than during Bike (11 and 5 units more, respectively). In CH,
was improved dramatically with altitude acclimatization. This effect was more marked during Knee than during Bike (11 and 5 units more, respectively). In CH,  was also higher during Knee than during Bike (86.1 ± 1.2 and 72.2 ± 2.8%, respectively). Almost 16% of the difference in peak exercise
was also higher during Knee than during Bike (86.1 ± 1.2 and 72.2 ± 2.8%, respectively). Almost 16% of the difference in peak exercise  observed in CH between Bike and Knee could be accounted for by the higher arterial pH (+0.09) during Knee, and 8% by the higher blood temperature (+0.5°C) during Bike. The higher
observed in CH between Bike and Knee could be accounted for by the higher arterial pH (+0.09) during Knee, and 8% by the higher blood temperature (+0.5°C) during Bike. The higher  during Knee explained 55% of the difference in
during Knee explained 55% of the difference in  . At peak Knee in CH, the
. At peak Knee in CH, the  was 53 and 18% higher than during exercise with the same model in AH and normoxia, respectively. Compared to Bike,
was 53 and 18% higher than during exercise with the same model in AH and normoxia, respectively. Compared to Bike, 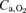 during Knee in CH was 20% higher.
during Knee in CH was 20% higher.
Figure 5. Oxygen content.
Arterial O2 saturation (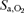 ) and oxygen content (
) and oxygen content ( ) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same
) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Cardiac output and leg blood flow
During Bike, peak cardiac output and peak leg blood flow were reduced by 17 and 22% with acute hypoxia; however, acute hypoxia had no significant effect on peak cardiac output and leg blood flow during Knee (Fig. 6). During Knee and Bike in CH, peak cardiac output was similarly reduced by ∼14 and 15%, respectively. After altitude acclimatization, peak leg blood flow was reduced by 25% during Bike, but during peak Knee no significant differences were observed among the three conditions. At peak Bike effort in AH, heart rate, stroke volume and mean arterial pressure were reduced by 8, 9 and 5%, respectively. In contrast, AH had no significant effect on the heart rate, the stroke volume and the mean arterial blood pressure attained at the end of the Knee. Stroke volume at exhaustion in acute hypoxia was significantly higher during Bike than during Knee (118 ± 4 and 105 ± 7 ml, respectively).
Figure 6. Cardiovascular variables.
Cardiac output, leg blood flow and mean arterial blood pressure (MAP) during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
In CH the heart rate response to Knee was 11 and 9% lower than in normoxia and AH, respectively. Neither  nor altitude acclimatization influenced significantly the stroke volume at peak exercise during Knee. At exhaustion in CH, stroke volume was 46% higher during Bike than during Knee (130 ± 9 and 89 ± 6 ml, respectively).
nor altitude acclimatization influenced significantly the stroke volume at peak exercise during Knee. At exhaustion in CH, stroke volume was 46% higher during Bike than during Knee (130 ± 9 and 89 ± 6 ml, respectively).
Oxygen delivery and oxygen extraction
Acute hypoxia resulted in a reduction of systemic O2 delivery (46 and 21%) and leg O2 delivery (47 and 26%) during Bike and Knee, respectively, almost matching the corresponding reduction in  (Fig. 7). Altitude acclimatization almost restored peak systemic O2 delivery during Bike, which remained 11% lower than during normoxic exercise under control conditions. In contrast, during Knee in CH, systemic O2 delivery (2.69 ± 0.27 and 2.65 ± 0.15 l min−1) and leg O2 delivery (1.28 ± 0.11 and 1.16 ± 0.11 l min−1) was similar to that observed in normoxia. Compared to AH, altitude acclimatization raised peak Knee systemic oxygen delivery by 28% and, hence, peak leg
(Fig. 7). Altitude acclimatization almost restored peak systemic O2 delivery during Bike, which remained 11% lower than during normoxic exercise under control conditions. In contrast, during Knee in CH, systemic O2 delivery (2.69 ± 0.27 and 2.65 ± 0.15 l min−1) and leg O2 delivery (1.28 ± 0.11 and 1.16 ± 0.11 l min−1) was similar to that observed in normoxia. Compared to AH, altitude acclimatization raised peak Knee systemic oxygen delivery by 28% and, hence, peak leg  attained a value similar to normoxia.
attained a value similar to normoxia.
Figure 7. Oxygen delivery.
Systemic and leg O2 delivery during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Regardless of  , O2 extraction was always higher during Bike (Fig. 8A). This was also true during submaximal exercise (Fig. 8B). Peak leg O2 fractional extraction was increased from 85.5 ± 1.7% in normoxia to 89.0 ± 2.3% during Bike in AH. A similar trend was observed during Knee (75.5 ± 2.2 to 78.4 ± 1.9%, in normoxia and AH, respectively, P < 0.10). After altitude acclimatization peak leg O2 fractional extraction was higher during Bike than during Knee (87.7 ± 2.5 and 74.0 ± 2.3%, respectively). Compared to AH, peak leg O2 extraction during Knee tended to be marginally lower after altitude acclimatization (79.7 ± 1.6 and 74.0 ± 2.3%, respectively).
, O2 extraction was always higher during Bike (Fig. 8A). This was also true during submaximal exercise (Fig. 8B). Peak leg O2 fractional extraction was increased from 85.5 ± 1.7% in normoxia to 89.0 ± 2.3% during Bike in AH. A similar trend was observed during Knee (75.5 ± 2.2 to 78.4 ± 1.9%, in normoxia and AH, respectively, P < 0.10). After altitude acclimatization peak leg O2 fractional extraction was higher during Bike than during Knee (87.7 ± 2.5 and 74.0 ± 2.3%, respectively). Compared to AH, peak leg O2 extraction during Knee tended to be marginally lower after altitude acclimatization (79.7 ± 1.6 and 74.0 ± 2.3%, respectively).
Figure 8. Oxygen extraction.
Fractional O2 extraction during maximal (A) and submaximal (B) exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Distribution of blood flow
In AH and in normoxia, the amount of blood flow directed to tissues apart from the active leg was quite similar (5.3–5.7 l min−1 (s.e.m.: 0.8 and 0.6) and 8.0–8.5 l min−1 (s.e.m.: 0.5) l min−1) during Bike and Knee, being significantly higher during Knee than Bike. However, after altitude acclimatization, the amount of blood flow directed to tissues apart from the active leg was similar in Bike and Knee (6.6 ± 0.8 and 6.0 ± 0.7 l min−1). The amount of flow directed to tissues apart from the active leg was significantly lower during Knee after altitude acclimatization compared to AH. This distribution of blood flow combined with the greater  during peak Knee made available almost twice as much O2 delivery for the tissues apart from the active muscles during Knee than during Bike, when exercising in AH (Fig. 9). Moreover, during Knee in hypoxia, oxygen delivery to the tissues apart from the active muscles was similar, if not greater, than that observed at peak Bike in normoxia.
during peak Knee made available almost twice as much O2 delivery for the tissues apart from the active muscles during Knee than during Bike, when exercising in AH (Fig. 9). Moreover, during Knee in hypoxia, oxygen delivery to the tissues apart from the active muscles was similar, if not greater, than that observed at peak Bike in normoxia.
Figure 9. Distribution of blood flow and O2 delivery.
Blood flow directed to tissues apart from the exercising leg/s and oxygen delivery to the tissues apart from the active leg/s during peak exercise on cycle ergometer (Bike) and knee extension ergometer (Knee) in normoxia at sea level (Nx), acute hypoxia at sea level (AHYP) and after 9–10 weeks of residence at 5260 m above sea level (CHRHYP). *Significantly different from normoxia (P < 0.05). †Significantly different from exercise on the cycle ergometer (same  ) (P < 0.05).
) (P < 0.05).
Plasma catecholamines
At exhaustion in normoxia, arterial noradrenaline and adrenaline concentrations were higher during Bike than during Knee (27 ± 4 and 5 ± 1 nmol l−1, respectively). Acute hypoxia had no significant effect on the catecholamine responses to incremental exercise with the two models studied. After altitude acclimatization, the catecholaminergic response to Knee was not different from that observed in AH. The corresponding values for noradrenaline were 7 ± 2 and 5 ± 1 nmol l−1, respectively, and for adrenaline 2 ± 1 and 2 ± 1 nmol l−1, respectively. At exhaustion during Bike after altitude acclimatization, arterial noradrenaline was increased up to 52 ± 7 nmol l−1 and arterial adrenaline concentrations up to 11 ± 3 nmol l−1, values that were greater than those observed during Knee.
Discussion
Summary of key findings
This study demonstrates the marked effect of the size of muscle mass activated during exercise on several links of the O2 transport cascade in acute and chronic hypoxia (Fig. 10). During exhaustive exercise in acute and chronic hypoxia with the knee extensor muscles of one leg compared to ordinary bicycling:
Figure 10. Oxygen cascade from the atmosphere to the femoral vein.
The values of inspiratory O2 pressure ( ), alveolar
), alveolar  (
( ), arterial
), arterial  (
( ), estimated mean capillary
), estimated mean capillary  (
(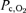 ) and femoral vein
) and femoral vein  (
( ) are represented during exercise on the cycle ergometer (thick lines) and knee extension exercise (thin lines), during normoxia (black lines), acute hypoxia (blue lines) and chronic hypoxia (red lines). Mean capillary
) are represented during exercise on the cycle ergometer (thick lines) and knee extension exercise (thin lines), during normoxia (black lines), acute hypoxia (blue lines) and chronic hypoxia (red lines). Mean capillary 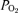 was calculated as previously described (Lundby et al. 2008b).
was calculated as previously described (Lundby et al. 2008b).
the pulmonary gas exchange is less perturbed,
the O2 dissociation curve of the haemoglobin is less shifted to the right,
peak leg blood flow and peak cardiac output are the same as in normoxia.
With altitude acclimatization, pulmonary gas exchange is improved, but more during Knee than Bike, such that  remains above 55 mmHg at exhaustion during hypoxic Knee while it drops below this figure during Bike. The latter combined with the increase in blood haemoglobin concentration results in an unaltered peak
remains above 55 mmHg at exhaustion during hypoxic Knee while it drops below this figure during Bike. The latter combined with the increase in blood haemoglobin concentration results in an unaltered peak  during hypoxic Knee while it remains still reduced by 26% during Bike.
during hypoxic Knee while it remains still reduced by 26% during Bike.
In addition, during Knee in hypoxia the amount of flow available to perfuse other vascular beds apart from the active muscles is about 3 l min−1 higher than during Bike, reducing the likelihood of an insufficient O2 delivery to the central nervous system, myocardium or respiratory muscles.
These experimental data lead to the main conclusion that the main mechanism accounting for the reduction of 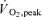 in both acute and chronic hypoxia is the reduction of O2 delivery to the active muscles. In addition, our data demonstrate that even with a
in both acute and chronic hypoxia is the reduction of O2 delivery to the active muscles. In addition, our data demonstrate that even with a  value as low as 55 mmHg, the human skeletal muscle is able to achieve normoxic peak
value as low as 55 mmHg, the human skeletal muscle is able to achieve normoxic peak 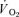 values, if O2 delivery is sufficient (Fig. 1). The latter argues against a major diffusional O2 limitation during maximal exercise in human skeletal muscles, either in hypoxia or normoxia (Wagner, 1993; Calbet et al. 2002; Lundby et al. 2008b).
values, if O2 delivery is sufficient (Fig. 1). The latter argues against a major diffusional O2 limitation during maximal exercise in human skeletal muscles, either in hypoxia or normoxia (Wagner, 1993; Calbet et al. 2002; Lundby et al. 2008b).
Cardiovascular responses
The combination of other studies (Stenberg et al. 1966; Koskolou et al. 1997a,b; Roach et al. 1999; Boushel et al. 2001; Lundby et al. 2008a) and the present investigation show that the reduction in maximal cardiac output during exercise in hypoxia is only observed when the exercise is performed with a large muscle mass and the level of hypoxia is higher than that observed at around 4000 m above sea level. Calbet et al. (2003b) hypothesized that the reduction of maximal cardiac output during exercise in severe hypoxia could be mediated by  , and probably by
, and probably by  and a
and a  sensing mechanisms that regulate the output drive from cardiovascular nuclei in the central nervous system (Sun & Reis, 1994). An alternative explanation is that the cardioinhibitory effect of hypoxia could also have been mediated by activation of the peripheral chemoreceptors, which through the release of NO, may attenuate the activation of presympathetic vasomotor neurones at the rostral ventrolateral medulla during hypoxia (Zanzinger et al. 1998). The current study supports this theory since, although exercise with a small and a large muscle mass was performed with the same level of
sensing mechanisms that regulate the output drive from cardiovascular nuclei in the central nervous system (Sun & Reis, 1994). An alternative explanation is that the cardioinhibitory effect of hypoxia could also have been mediated by activation of the peripheral chemoreceptors, which through the release of NO, may attenuate the activation of presympathetic vasomotor neurones at the rostral ventrolateral medulla during hypoxia (Zanzinger et al. 1998). The current study supports this theory since, although exercise with a small and a large muscle mass was performed with the same level of  (0.105), the
(0.105), the  was 17% and
was 17% and  10 units higher during leg extension exercise, giving values of
10 units higher during leg extension exercise, giving values of  and
and  similar to that observed during peak whole body exercise at ∼4000 m (Lundby et al. 2004) and, hence, peak cardiac output and leg blood flow were not reduced. Despite this marked difference in
similar to that observed during peak whole body exercise at ∼4000 m (Lundby et al. 2004) and, hence, peak cardiac output and leg blood flow were not reduced. Despite this marked difference in  ,
,  differed only by 3.7 mmHg. This could indicate that
differed only by 3.7 mmHg. This could indicate that 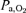 is not as strong a regulator as
is not as strong a regulator as  (or brain O2 delivery) to mediate these putative cardioinhibitory effects on the CNS in severe acute hypoxia. Accordingly, maximal cardiac output is not reduced in acute anaemia during two-legged knee extension exercise (Koskolou et al. 1997b) or bicycling (Ekblom et al. 1976). Neither the combination of acute isovolaemic anaemia (reduction of blood [Hb] by 20%) with acute hypoxia (
(or brain O2 delivery) to mediate these putative cardioinhibitory effects on the CNS in severe acute hypoxia. Accordingly, maximal cardiac output is not reduced in acute anaemia during two-legged knee extension exercise (Koskolou et al. 1997b) or bicycling (Ekblom et al. 1976). Neither the combination of acute isovolaemic anaemia (reduction of blood [Hb] by 20%) with acute hypoxia ( ) (Roach et al. 1999), which decreased acutely systemic O2 delivery at peak two-legged knee extension exercise by 38%, resulted in a lower peak cardiac output compared to that observed in normoxia (Roach et al. 1999). A particular feature of the two-legged knee extension exercise model is that at maximal exercise cardiac output values remain 4–5 l min−1 below those measured during maximal exercise on the cycle ergometer (Roach et al. 1999). In agreement, the difference between the actual maximal cardiac output, i.e. that attained during exercise on the cycle ergometer, and that measured during knee extension exercise in severe acute hypoxia was 9 l min−1 in the present investigation. Thus, during knee extension exercise in acute hypoxia with either one or two legs, O2 delivery and
) (Roach et al. 1999), which decreased acutely systemic O2 delivery at peak two-legged knee extension exercise by 38%, resulted in a lower peak cardiac output compared to that observed in normoxia (Roach et al. 1999). A particular feature of the two-legged knee extension exercise model is that at maximal exercise cardiac output values remain 4–5 l min−1 below those measured during maximal exercise on the cycle ergometer (Roach et al. 1999). In agreement, the difference between the actual maximal cardiac output, i.e. that attained during exercise on the cycle ergometer, and that measured during knee extension exercise in severe acute hypoxia was 9 l min−1 in the present investigation. Thus, during knee extension exercise in acute hypoxia with either one or two legs, O2 delivery and  are reduced in a similar proportion, while peak cardiac output is not increased to compensate for the reduction in
are reduced in a similar proportion, while peak cardiac output is not increased to compensate for the reduction in  , despite ample functional reserve (4–9 l min−1). Taken together, during 1- and 2-leg knee extension, cardiac output is maintained in acute hypoxia while during cycling exercise it is clearly reduced.
, despite ample functional reserve (4–9 l min−1). Taken together, during 1- and 2-leg knee extension, cardiac output is maintained in acute hypoxia while during cycling exercise it is clearly reduced.
The present investigation shows that a major difference between small and large muscle mass exercise in acute hypoxia is that the amount of blood flow available to perfuse vascular beds apart form the active muscles is about 3 l min−1 greater during exercise with a small than a large muscle mass. This value is similar to that observed during combined acute anaemia and hypoxia in the two-legged extension ergometer (Roach et al. 1999). This implies that the amount of oxygen delivery to be shared by the tissues apart from the active muscle, which includes the myocardium, the respiratory muscles and the brain, is much lower during exercise with a large muscle mass. The present study also shows that the perfusion pressure is higher during exercise with a small compared to exercise with a large muscle mass. Therefore, a higher oxygen delivery combined with a higher perfusion pressure minimizes the risk of mismatch between O2 delivery and demand in the myocardium, respiratory muscles and CNS during exercise with a small compared to a large muscle mass.
Our experimental data are compatible with a lower brain O2 delivery during exercise in severe acute hypoxia with a large muscle mass, than during leg extension exercise (Amann & Calbet, 2008). A lower O2 brain delivery could cause a cardioinhibitory reflex, establishing a lower ceiling for the elevation of maximal cardiac output and, hence, leg blood flow, leading ultimately to a reduction of O2 delivery to the active muscle and consequently peak  and exercise performance.
and exercise performance.
Alternatively, low brain oxygenation in severe acute hypoxia could blunt central command (motor output to the active skeletal muscles) but such a possibility is unlikely since: (1) afferent feedback from the respiratory muscles is not able to reduce the central command (Savard et al. 1996); (2) maximal voluntary contraction declines to the same extent during incremental exercise in normoxia and acute hypoxia (Garner et al. 1990; Fulco et al. 1996); (3) the deficit of neural activation observed 2 min after the end of exhausting dynamic exercise with different 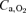 and
and  values is similar (Amann et al. 2006); and (4) neither peak power output nor fatigue index during a Wingate test are affected by severe acute hypoxia (Calbet et al. 2003c).
values is similar (Amann et al. 2006); and (4) neither peak power output nor fatigue index during a Wingate test are affected by severe acute hypoxia (Calbet et al. 2003c).
Sensory input from the fatiguing muscles could also blunt motor output to the active skeletal muscles (Amann & Dempsey, 2008; Amann et al. 2008a,b). Due to better muscle oxygenation during exercise with a small muscle mass, particularly after altitude acclimatization, this potential negative influence of sensory input on motor output is probably weaker when the size of the active muscle mass is small.
Respiratory responses
Pulmonary ventilation
In agreement with previous studies, exercise with a small muscle mass elicited a higher degree of relative hyperventilation than exercise with a large muscle mass (Asmussen & Nielsen, 1946). This effect may be explained by an enhancement of motor drive to the respiratory centres of the brain. If a given metabolic rate should be sustained by a lower muscle mass, then the effort is perceived as being harder and fatigue precipitates (Galbo et al. 1987). Both factors, fatigue and the perception of the exercise as harder, may increase the output drive from the motor cortex to the respiratory centres (Galbo et al. 1987; Piepoli et al. 1995).
With acclimatization, the ventilatory response to exercise was enhanced (Calbet et al. 2003b), but proportionally the same magnitude during both exercise models. Since the relatively higher exercise hyperventilation during exercise in hypoxia with a small muscle mass had a minor influence on  and
and  , the differences in pulmonary ventilation could barely contribute to the higher
, the differences in pulmonary ventilation could barely contribute to the higher  observed during exercise with a small compared to a large muscle mass either in acute or chronic hypoxia.
observed during exercise with a small compared to a large muscle mass either in acute or chronic hypoxia.
Pulmonary gas exchange
This study confirms our hypothesis and shows that exercise-induced impairment of pulmonary gas exchange (Hopkins et al. 1994, 2000) is attenuated when the exercise is performed with a small muscle mass. Although acute hypoxia exacerbates the exercise-induced impairment of pulmonary gas exchange (Hammond et al. 1986; Calbet et al. 2003a), we show that this effect is blunted when the exercise is performed with a small muscle mass. With acclimatization, the impairment of pulmonary gas exchange is reduced (Bebout et al. 1989; Calbet et al. 2003b) leading to an almost perfect (i.e an  close to 0 mmHg) pulmonary gas exchange during peak leg extension exercise. Several factors can influence the pulmonary gas exchange, such as ventilation perfusion (
close to 0 mmHg) pulmonary gas exchange during peak leg extension exercise. Several factors can influence the pulmonary gas exchange, such as ventilation perfusion ( , where
, where  is alveolar ventilation and
is alveolar ventilation and  cardiac output) inequality, diffusion limitation, and to a lesser extend right-to-left shunts (Vogiatzis et al. 2008). Ventilation perfusion inequality increases with exercise intensity (Hammond et al. 1986; Hopkins et al. 1994) and duration (Hopkins et al. 1998), and has been shown to account for a large part of the
cardiac output) inequality, diffusion limitation, and to a lesser extend right-to-left shunts (Vogiatzis et al. 2008). Ventilation perfusion inequality increases with exercise intensity (Hammond et al. 1986; Hopkins et al. 1994) and duration (Hopkins et al. 1998), and has been shown to account for a large part of the  during maximal exercise in normoxia (Hopkins et al. 1994) and hypoxia (Gale et al. 1985; Torre-Bueno et al. 1985). However, during exercise in hypoxia, diffusion limitation appears to be the most important mechanism accounting for the
during maximal exercise in normoxia (Hopkins et al. 1994) and hypoxia (Gale et al. 1985; Torre-Bueno et al. 1985). However, during exercise in hypoxia, diffusion limitation appears to be the most important mechanism accounting for the  (Torre-Bueno et al. 1985; Hammond et al. 1986). The diffusion limitation is supposedly caused by too short a transit time of the blood through the pulmonary capillaries, which does not allow for a complete gas equilibration between the alveolar gas and the capillary blood (Calbet et al. 2008). This reduction in mean transit time has been attributed to high values of cardiac output based on the negative correlation that exists between pulmonary transit time and cardiac output (Hopkins et al. 1996). By combining the different experimental conditions included in our studies it becomes clear that cardiac output does not explain the observed difference in pulmonary gas exchange between the large and small muscle mass models (Calbet et al. 2008). For example, during submaximal exercise on the cycle ergometer in severe acute hypoxia (intensity ∼120 W) cardiac out was 16 l min−1 and the
(Torre-Bueno et al. 1985; Hammond et al. 1986). The diffusion limitation is supposedly caused by too short a transit time of the blood through the pulmonary capillaries, which does not allow for a complete gas equilibration between the alveolar gas and the capillary blood (Calbet et al. 2008). This reduction in mean transit time has been attributed to high values of cardiac output based on the negative correlation that exists between pulmonary transit time and cardiac output (Hopkins et al. 1996). By combining the different experimental conditions included in our studies it becomes clear that cardiac output does not explain the observed difference in pulmonary gas exchange between the large and small muscle mass models (Calbet et al. 2008). For example, during submaximal exercise on the cycle ergometer in severe acute hypoxia (intensity ∼120 W) cardiac out was 16 l min−1 and the  was 23 mmHg (Calbet et al. 2003a). However, during peak knee extension exercise in acute hypoxia the corresponding values were 14 l min−1 and 15 mmHg. Thus, despite an almost similar cardiac output and lower mean arterial pressure during exercise with a large muscle mass, the
was 23 mmHg (Calbet et al. 2003a). However, during peak knee extension exercise in acute hypoxia the corresponding values were 14 l min−1 and 15 mmHg. Thus, despite an almost similar cardiac output and lower mean arterial pressure during exercise with a large muscle mass, the  was 53% higher during exercise with a large muscle mass. Moreover, an increase in cardiac output from 14 to 16 l min−1 during submaximal exercise (∼120 W) with hypoxia in altitude-acclimatized humans induced by isovolaemic haemodilution (Calbet et al. 2002) was not accompanied by an impairment of pulmonary gas exchange, suggesting that for cardiac outputs up to 14–16 l min−1 pulmonary gas exchange is not limited by cardiac output (Calbet et al. 2008). In chronic hypoxia, peak leg extension exercise elicited a cardiac output of 12 l min−1 while the accompanying
was 53% higher during exercise with a large muscle mass. Moreover, an increase in cardiac output from 14 to 16 l min−1 during submaximal exercise (∼120 W) with hypoxia in altitude-acclimatized humans induced by isovolaemic haemodilution (Calbet et al. 2002) was not accompanied by an impairment of pulmonary gas exchange, suggesting that for cardiac outputs up to 14–16 l min−1 pulmonary gas exchange is not limited by cardiac output (Calbet et al. 2008). In chronic hypoxia, peak leg extension exercise elicited a cardiac output of 12 l min−1 while the accompanying  was only 2 mmHg. This condition can be compared with the values obtained during submaximal exercise on the cycle ergometer after acclimatization (∼120 W) which elicited a cardiac output of 14 l min−1 and a
was only 2 mmHg. This condition can be compared with the values obtained during submaximal exercise on the cycle ergometer after acclimatization (∼120 W) which elicited a cardiac output of 14 l min−1 and a  of 15 mmHg. The 13 mmHg difference in
of 15 mmHg. The 13 mmHg difference in  between knee extension and cycle ergometer exercise is too large to be explained by the small difference in cardiac output alone between these two conditions (Calbet et al. 2008).
between knee extension and cycle ergometer exercise is too large to be explained by the small difference in cardiac output alone between these two conditions (Calbet et al. 2008).
Since the main two factors that explain the  during exercise in acute hypoxia are diffusion limitation and
during exercise in acute hypoxia are diffusion limitation and  mismatch, the most likely is that the degree of
mismatch, the most likely is that the degree of 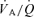 mismatch induced by exercise is attenuated during exercise with a small compared with a large muscle mass. However, this needs to be tested specifically.
mismatch induced by exercise is attenuated during exercise with a small compared with a large muscle mass. However, this needs to be tested specifically.
Dissociation curve of the haemoglobin
This study shows that one of the main mechanisms by which exercise with a small muscle mass is associated with a markedly larger  than exercise with a large muscle mass is the less accentuated right-shift of the O2 haemoglobin dissociation (ODC) curve during exercise with a small muscle mass. This difference in the position of ODC at the lungs accounts for 50% and ∼25% of the difference in
than exercise with a large muscle mass is the less accentuated right-shift of the O2 haemoglobin dissociation (ODC) curve during exercise with a small muscle mass. This difference in the position of ODC at the lungs accounts for 50% and ∼25% of the difference in  between small and large muscle mass exercise in acute and chronic hypoxia, respectively. Thus, mechanisms allowing an attenuation of exercise-induced hyperthermia and blood pH drop during exercise in hypoxia may have a rather favourable effect on exercise capacity, particularly when the exercise is performed in severe acute hypoxia, confirming our previous findings (Calbet et al. 2003a; Holmberg & Calbet, 2007).
between small and large muscle mass exercise in acute and chronic hypoxia, respectively. Thus, mechanisms allowing an attenuation of exercise-induced hyperthermia and blood pH drop during exercise in hypoxia may have a rather favourable effect on exercise capacity, particularly when the exercise is performed in severe acute hypoxia, confirming our previous findings (Calbet et al. 2003a; Holmberg & Calbet, 2007).
Summary
The amount of active muscle mass engaged in the exercise has a major impact on the cardiovascular and respiratory response to exercise. The reductions in whole body and leg peak 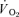 are blunted by more than 50% during exercise in hypoxia with a small muscle mass, like leg extension exercise which recruits about 3 kg of muscle compared to exercise on the cycle ergometer which recruits more than 10 kg of muscle mass. Moreover, acclimatization abolishes the hypoxic effect in small muscle mass whereas whole body
are blunted by more than 50% during exercise in hypoxia with a small muscle mass, like leg extension exercise which recruits about 3 kg of muscle compared to exercise on the cycle ergometer which recruits more than 10 kg of muscle mass. Moreover, acclimatization abolishes the hypoxic effect in small muscle mass whereas whole body  and leg
and leg  are still remarkably reduced. The two main mechanisms that explain these findings are that during exercise with a small muscle mass pulmonary gas exchange is better preserved and the oxygen dissociation curve of the haemoglobin less shifted to the right as compared to exercise with a large muscle mass. These two mechanisms act together to enhance O2 uploading in the lungs and, hence,
are still remarkably reduced. The two main mechanisms that explain these findings are that during exercise with a small muscle mass pulmonary gas exchange is better preserved and the oxygen dissociation curve of the haemoglobin less shifted to the right as compared to exercise with a large muscle mass. These two mechanisms act together to enhance O2 uploading in the lungs and, hence,  , permitting a relative larger O2 delivery when exercise is performed with a small muscle mass. In addition our study shows that, in contrast with cycle ergometer exercise, knee extension exercise in severe acute hypoxia does not reduce peak leg blood flow and peak cardiac output. Consequently, the amount of flow available to perfuse the active muscles is similar in acute hypoxia and normoxia when the recruited muscle mass is small. The fact that
, permitting a relative larger O2 delivery when exercise is performed with a small muscle mass. In addition our study shows that, in contrast with cycle ergometer exercise, knee extension exercise in severe acute hypoxia does not reduce peak leg blood flow and peak cardiac output. Consequently, the amount of flow available to perfuse the active muscles is similar in acute hypoxia and normoxia when the recruited muscle mass is small. The fact that  and perfusion pressure are much larger during peak knee extension exercise implies that the amount of O2 delivery available to perfuse other vascular beds apart from the active muscles is markedly greater, reducing the likelihood of an imbalance between O2 demand and delivery in critical regions such as the central nervous system, the myocardium or respiratory muscles. Thus, the altitude-acclimatized human has potentially a similar exercising capacity as at sea level when the exercise model allows for an adequate oxygen delivery (blood flow ×
and perfusion pressure are much larger during peak knee extension exercise implies that the amount of O2 delivery available to perfuse other vascular beds apart from the active muscles is markedly greater, reducing the likelihood of an imbalance between O2 demand and delivery in critical regions such as the central nervous system, the myocardium or respiratory muscles. Thus, the altitude-acclimatized human has potentially a similar exercising capacity as at sea level when the exercise model allows for an adequate oxygen delivery (blood flow × ), with only a minor role of
), with only a minor role of 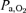 per se, when
per se, when  is more than 55 mmHg.
is more than 55 mmHg.
Acknowledgments
Special thanks go to Harriet Wagner, Carsten Nielsen, Karin Hansen and Birgitte Jessen for their excellent technical assistance. We also acknowledge the Academia de Ciencias de Bolivia and especially Dr Carlos Aguirre for all the help and support to set our expeditionary lab at the heights of Mt Chacaltaya. All the help and support provided by Dr Mauricio Araoz and Dr Hilde Spielvogel is also greatly acknowledged. This study was supported by a grant from The Danish National Research Foundation (504-14) and by the Carlsberg Foundation.
References
- Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Dempsey JA. The concept of peripheral locomotor muscle fatigue as a regulated variable. J Physiol. 2008 in press. [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008a;1059:1714–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2008b doi: 10.1113/jphysiol.2008.163303. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen E, Nielsen M. Studies on the regulation of respiration in heavy work. Acta Physiol Scand. 1946;12:171–188. [Google Scholar]
- Bebout DE, Story D, Roca J, Hogan MC, Poole DC, Gonzalez-Camarena R, Ueno O, Haab P, Wagner PD. Effects of altitude acclimatization on pulmonary gas exchange during exercise. J Appl Physiol. 1989;67:2286–2295. doi: 10.1152/jappl.1989.67.6.2286. [DOI] [PubMed] [Google Scholar]
- Boushel R, Calbet JA, Radegran G, Sondergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol. 2003;551:379–386. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003a;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
-
Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Why is
 after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003b;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003b;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar] - Calbet JA, De Paz JA, Garatachea N, Cabeza De Vaca S, Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol. 2003c;94:668–676. doi: 10.1152/japplphysiol.00128.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, Van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004a;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Calbet JA, Radegran G, Boushel R, Sondergaard H, Saltin B, Wagner PD. Effect of blood haemoglobin concentration on
 and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar] -
Calbet JA, Radegran G, Boushel R, Sondergaard H, Saltin B, Wagner PD. Plasma volume expansion does not increase maximal cardiac output or
 in lowlanders acclimatized to altitude. Am J Physiol Heart Circ Physiol. 2004b;287:H1214–H1224. doi: 10.1152/ajpheart.00840.2003. [DOI] [PubMed] [Google Scholar]
in lowlanders acclimatized to altitude. Am J Physiol Heart Circ Physiol. 2004b;287:H1214–H1224. doi: 10.1152/ajpheart.00840.2003. [DOI] [PubMed] [Google Scholar] - Calbet JA, Robach P, Lundby C, Boushel R. Is pulmonary gas exchange during exercise in hypoxia impaired with the increase of cardiac output? Appl Physiol Nutr Metab. 2008;33:593–600. doi: 10.1139/H08-010. [DOI] [PubMed] [Google Scholar]
- Cerretelli P. Limiting factors to oxygen transport on Mount Everest. J Appl Physiol. 1976;40:658–667. doi: 10.1152/jappl.1976.40.5.658. [DOI] [PubMed] [Google Scholar]
- Dow P. Estimations of cardiac output and central blood volume by dye dilution. Physiol Rev. 1956;36:77–102. doi: 10.1152/physrev.1956.36.1.77. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Wilson G, Astrand PO. Central circulation during exercise after venesection and reinfusion of red blood cells. J Appl Physiol. 1976;40:379–383. doi: 10.1152/jappl.1976.40.3.379. [DOI] [PubMed] [Google Scholar]
- Fulco CS, Lewis SF, Frykman PN, Boushel R, Smith S, Harman EA, Cymerman A, Pandolf KB. Muscle fatigue and exhaustion during dynamic leg exercise in normoxia and hypobaric hypoxia. J Appl Physiol. 1996;81:1891–1900. doi: 10.1152/jappl.1996.81.5.1891. [DOI] [PubMed] [Google Scholar]
- Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GE, Torre-Bueno JR, Moon RE, Saltzman HA, Wagner PD. Ventilation-perfusion inequality in normal humans during exercise at sea level and simulated altitude. J Appl Physiol. 1985;58:978–988. doi: 10.1152/jappl.1985.58.3.978. [DOI] [PubMed] [Google Scholar]
- Garner SH, Sutton JR, Burse RL, McComas AJ, Cymerman A, Houston CS. Operation Everest II: neuromuscular performance under conditions of extreme simulated altitude. J Appl Physiol. 1990;68:1167–1172. doi: 10.1152/jappl.1990.68.3.1167. [DOI] [PubMed] [Google Scholar]
- Hallman H, Farnebo LO, Hamberger B, Johnsson G. A sensitive method for the determination of plasma catecholamines using liquid chromatography with electrochemical detection. Life Sci. 1978;23:1049–1052. doi: 10.1016/0024-3205(78)90665-3. [DOI] [PubMed] [Google Scholar]
- Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J Appl Physiol. 1986;61:1749–1757. doi: 10.1152/jappl.1986.61.5.1749. [DOI] [PubMed] [Google Scholar]
- Holmberg HC, Calbet JAL. Insufficient ventilation as a cause of impaired pulmonary gas exchange during submaximal exercise. Respir Physiol Neurobiol. 2007;157:348–359. doi: 10.1016/j.resp.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol. 2000;89:721–730. doi: 10.1152/jappl.2000.89.2.721. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Belzberg AS, Wiggs BR, McKenzie DC. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol. 1996;103:67–73. doi: 10.1016/0034-5687(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Gavin TP, Siafakas NM, Haseler LJ, Olfert IM, Wagner H, Wagner PD. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol. 1998;85:1523–1532. doi: 10.1152/jappl.1998.85.4.1523. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, McKenzie DC, Schoene RB, Glenny RW, Robertson HT. Pulmonary gas exchange during exercise in athletes. I. Ventilation-perfusion mismatch and diffusion limitation. J Appl Physiol. 1994;77:912–917. doi: 10.1152/jappl.1994.77.2.912. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol. 1997a;272:H2655–H2663. doi: 10.1152/ajpheart.1997.272.6.H2655. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Roach RC, Calbet JA, Radegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol Heart Circ Physiol. 1997b;273:H1787–H1793. doi: 10.1152/ajpheart.1997.273.4.H1787. [DOI] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008a;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Calbet JA, van Hall G, Saltin B, Sander M. Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4,100 m and in high-altitude Aymara natives. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1202–R1208. doi: 10.1152/ajpregu.00725.2003. [DOI] [PubMed] [Google Scholar]
- Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JA. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008b;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- Lundby C, Sander M, van Hall G, Saltin B, Calbet JA. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and in high altitude natives. J Physiol. 2006;573:535–547. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Savard GK, Areskog N-H, Lundby C, Saltin B. Skeletal muscle adaptations to prolonged exposure to extreme altitude: a role of physical activity? High Alt Med Biol. 2008 doi: 10.1089/ham.2008.1009. in press. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol Heart Circ Physiol. 1995;269:H1428–H1436. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- Pugh LGCE. Cardiac output in muscular exercise at 5800 m. (19000 ft) J Appl Physiol. 1964;19:441–447. [Google Scholar]
- Rådegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol. 1987;63:531–539. doi: 10.1152/jappl.1987.63.2.531. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol Heart Circ Physiol. 1995;268:H1453–H1461. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Savard GK, Areskog NH, Saltin B. Maximal muscle activation is not limited by pulmonary ventilation in chronic hypoxia. Acta Physiol Scand. 1996;157:187–190. doi: 10.1046/j.1365-201X.1996.493234000.x. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol Heart Circ Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Stenberg J, Ekblom B, Messin R. Hemodynamic response to work at simulated altitude, 4,000 m. J Appl Physiol. 1966;21:1589–1594. doi: 10.1152/jappl.1966.21.5.1589. [DOI] [PubMed] [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol. 1994;44:197–219. doi: 10.1016/0301-0082(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE, Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol. 1985;58:989–995. doi: 10.1152/jappl.1985.58.3.989. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, Wagner H, Roussos C, Wagner PD. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol. 2008;586:2381–2391. doi: 10.1113/jphysiol.2007.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. Algebraic analysis of the determinants of VO2,max. Respir Physiol. 1993;93:221–237. doi: 10.1016/0034-5687(93)90007-w. [DOI] [PubMed] [Google Scholar]
- Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- Wagner PD, Araoz M, Boushel R, Calbet JA, Jessen B, Radegran G, Spielvogel H, Sondegaard H, Wagner H, Saltin B. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol. 2002;92:1393–1400. doi: 10.1152/japplphysiol.00093.2001. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Nitric oxide in the ventrolateral medulla regulates sympathetic responses to systemic hypoxia in pigs. Am J Physiol Regul Integr Comp Physiol. 1998;275:R33–R39. doi: 10.1152/ajpregu.1998.275.1.R33. [DOI] [PubMed] [Google Scholar]