Abstract
Following injury to the nervous system, the activation of macrophages, microglia, and T-cells profoundly affects the ability of neurons to survive and to regenerate damaged axons. The primary visual pathway provides a well-defined model system for investigating the interactions between the immune system and the nervous system after neural injury. Following damage to the optic nerve in mice and rats, retinal ganglion cells, the projection neurons of the eye, normally fail to regenerate their axons and soon begin to die. Induction of an inflammatory response in the vitreous strongly enhances the survival of retinal ganglion cells and enables these cells to regenerate lengthy axons beyond the injury site. T cells modulate this response, whereas microglia are thought to contribute to the loss of retinal ganglion cells in this model and in certain ocular diseases. This review discusses the complex and sometimes paradoxical actions of blood-borne macrophages, resident microglia, and T-cells in determining the outcome of injury in the primary visual pathway.
Keywords: optic nerve, regeneration, oncomodulin, monocytes, central nervous system, inflammation
The optic nerve has long served as a model for understanding regenerative success or failure in the CNS (Benowitz and Yin, 2008). Under normal circumstances, mature retinal ganglion cells (RGCs) fail to regrow axons beyond the site of optic nerve injury, and if axotomy occurs within the orbit, most RGCs go on to die within a few weeks (Mey and Thanos, 1993; Berkelaar et al., 1994). Regenerative failure is not inevitable, however. In his classic work, “Degeneration and Regeneration of the Nervous System,” Ramon y Cajal (DeFelipe and Jones, 1991) described Tello’s discovery that mature RGCs can regenerate axons through a peripheral nerve graft sutured to the cut end of the optic nerve, and concluded that “the regenerative failure of the central paths is … an accidental condition due to the neuroglial environment.” Expanding on this observation, Aguayo and colleagues (Richardson et al., 1980; David and Aguayo, 1981; Aguayo et al., 1991) carried out many studies demonstrating that mature RGCs and other CNS neurons retain the potential to regenerate their axons in a more favorable environment, sparking renewed interest in the factors that support or inhibit nerve regeneration. Recent data show that the immune response dramatically affects the outcome of injury to the optic nerve. While neuro-immune crosstalk in CNS diseases is covered in another article in this issue by Kerschensteiner et al., 2009, this review discusses current knowledge on the role of blood-derived macrophages, resident microglia, and T-cells in RGC survival and/or axonal regeneration after injury to the primary visual pathway.
THE IMMUNE RESPONSE IN THE CNS AND PERIPHERAL NERVOUS SYSTEM (PNS)
The immune system operates via two separate but closely interacting subsystems: innate immunity as the antigen-independent arm and adaptive immunity as the antigen-specific arm. Inflammatory responses during traumatic and degenerative CNS diseases are dominated by cells of the innate immune system, most importantly resident microglia and blood-borne macrophages (Schroeter and Jander, 2005). After phagocytosing cellular debris, microglia/macrophages present antigens to lymphocytes, thereby activating the antigen-specific arm of the immune response (Suzumura et al., 1987; Abromson-Leeman et al., 1993; Ulvestad et al., 1994). Inflammation is the key component of host defense responses to injury, tissue ischemia, autoimmune reactions or infectious agents (Allan and Rothwell, 2003; Lucas et al., 2006). In general, the effects of inflammation are complex, and depending on the mode of stimulation, inflammation can have neuroprotective or neurotoxic effects or even a mixture of the two (David et al., 1990; Huitinga et al., 1990, 1995; Lu and Richardson, 1991; Dijkstra et al., 1992; Minghetti et al., 1999; Stoll et al., 2002; Wolf et al., 2002; Yin et al., 2003; Byram et al., 2004; Correale and Villa, 2004; Butovsky et al., 2005).
The inflammatory response in the CNS is attenuated relative to that in the PNS and other tissues. In fact, the CNS had long been thought to be an immune-privileged site, stemming from the properties of the blood–brain barrier (BBB) in restricting the passage of large molecules and cells into the CNS, the lack of draining lymphatics, and the apparent immunoincompetence of microglia in the CNS (Shrikant and Benveniste, 1996). However, recent evidence shows that both T and B lymphocytes can cross the BBB (Kajiwara et al., 1990; Hickey et al., 1991; Knopf et al., 1998; Becher et al., 2000), and that the CNS is far from passive in its interactions with the immune system (Ransohoff et al., 2003; Carson et al., 2006). Nonetheless, relative to the PNS, cellular infiltration is delayed and relatively weak in the CNS following traumatic injury or as a result of inflammatory responses (Andersson et al., 1992a,b; Avellino et al., 1995; Perry et al., 1995; Reichert and Rotshenker, 1996; Moalem et al., 1999; Allan and Rothwell, 2001; Jander et al., 2001). Following a crush injury of the optic nerve, infiltration of blood-borne macrophages into the degenerating distal segment of the nerve is considerably less than is seen in the sciatic nerve (Perry et al., 1987); this is also the case after the optic nerve is cut (Reichert and Rotshenker, 1996). One of the major roles that macrophages play after nervous system injury is to remove myelin debris (Stoll et al., 1989), thus facilitating axon regrowth and remyelination. The high capacity of PNS neurons to regrow injured axons is paralleled by the recruitment of large numbers of macrophages to the degenerating area, whereas the poor regenerative capacity of CNS neurons is correlated with a more limited presence of macrophages at the injury site. The phagocytic activity of macrophages or brain-derived microglia is enhanced upon exposure to sciatic nerve segments, but is inhibited by exposure to optic nerve segments (Zeev-Brann et al., 1998). The low microglia/macrophage activity in the CNS after injury may be related to the presence of specific inhibitors or to an absence of chemoattractants. A greater accumulation of T cells in the injured sciatic nerve than in the injured optic nerve has also been reported (Moalem et al., 1999).
INTRAVITREAL INFLAMMATION STIMULATES OPTIC NERVE REGENERATION
Prior to undergoing apoptotic cell death, axotomized RGCs show transitory axon sprouting at the injury site but almost no long-distance regeneration (DeFelipe and Jones, 1991; Ramon y Cajal, 1991). Macrophage-derived EphB3 mediates this sprouting (Liu X et al., 2006). The transfer of peripherally activated macrophages into the visual system was previously reported to enhance axonal regeneration (David et al., 1990; Lazarov-Spiegler et al., 1996; Rapalino et al., 1998). In more recent studies, the direct induction of an inflammatory response in the eye was shown to have dramatic effects on RGC survival and axon regeneration (Leon et al., 2000; Yin et al., 2003; Lorber et al., 2005; Pernet and Di Polo, 2006; Luo et al., 2007b; Fig. 1). As a result of either injuring the lens or injecting zymosan, a yeast cell-wall preparation, into the eye and subsequent activation of macrophages, the survival of axotomized RGCs increases eightfold to 10-fold and axon regeneration beyond the site of optic nerve injury increases 100-fold or more, with some axons extending almost as far as the optic chiasma (Leon et al., 2000; Fischer et al., 2001; Yin et al., 2003; Lorber et al., 2005; Pernet and Di Polo, 2006). It is important to note that this regeneration occurs through the optic nerve itself, a CNS environment that is normally hostile to axon regeneration. In addition, if one simultaneously counteracts the inhibitory effects of myelin and the glial scar that forms at the injury site, the amount of regeneration obtained increases several-fold, with numerous axons growing right through the scar (Fischer et al., 2004a,b). The effects of intravitreal inflammation on optic nerve regeneration have also been seen after treatment with another macrophage activator, oxidized galactin-1 (Okada et al., 2005). Earlier studies had demonstrated massive increases in RGC survival after vehicle injection into the eye (Mansour-Robaey et al., 1994) or implanting fragments of pre-injured peripheral nerve into the vitreous (Berry et al., 1996, 1999). These effects are probably due, at least in part, to intravitreal macrophage activation (Lorber et al., 2008). Lens injury and other methods of inducing inflammation in the eye also greatly increases the growth of RGC axons through the more permissive environment of a peripheral nerve grafted onto the cut end of the optic nerve (Fischer et al., 2000; Yin et al., 2003; Luo et al., 2007b).
Fig. 1.
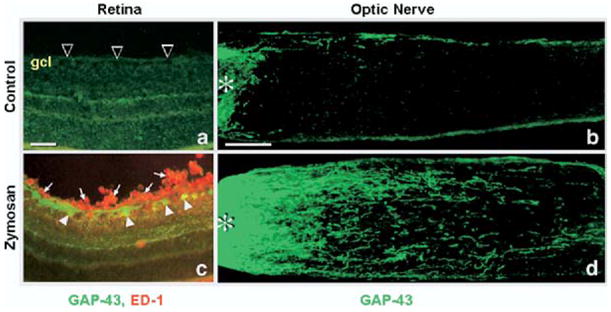
Intravitreal macrophage activation stimulates optic nerve regeneration. Macrophages were activated in vitreous by injecting zymosan into the eye after optic nerve crush in adult rats. Retina sections (a, c) or optic nerve sections (b, d) were immunostained with GAP-43 antibody to visualize regenerating RGCs and axons (green in a–d) and with ED-1 antibody to visualize macrophages (red in a, c) 2 weeks after the surgery. (a) Following optic nerve crush alone, there are no macrophages in retina and no GAP-43 immunostaining in RGCs (arrowheads), and (b) very few axons growing beyond the nerve crush site (asterisk). However, after zymosan treatment, (c) many macrophages are seen in the vitreous and near RGCs (arrows, red) and GAP-43 is dramatically upregulated in RGCs (arrowheads, green cells). As a result of this, (d) many GAP-43+ axons cross the nerve injury site and extend into the distal optic nerve. Scale bar=50 μm (a, c); 200 μm (b, d) (Yin et al., 2003).
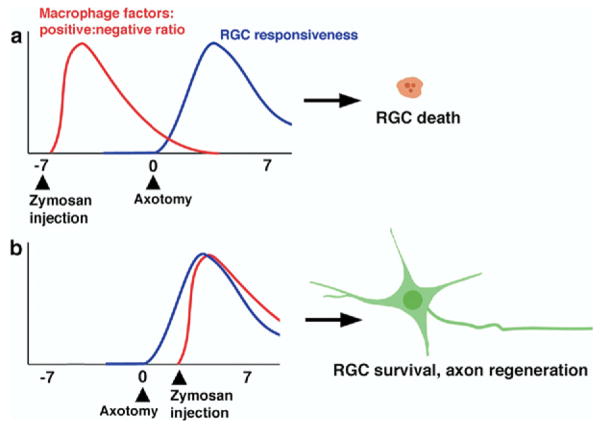
The magnitude of RGC protection and axon regeneration depends upon the timing and extent of macrophage influx (Yin et al., 2003; Luo et al., 2007b). Macrophages stimulated by zymosan at the time of optic nerve injury or 3 days afterward cause dramatic axon regeneration, whereas zymosan injections either 1 week before or 1 week after optic nerve injury have little value (Yin et al., 2003). After activation by zymosan, macrophages produce both beneficial and detrimental molecules (Yin et al., 2003), and it is possible that the outcome of an inflammatory response depends upon the balance between the two (Fig. 2). Both macrophages and microglial cells are the sources and targets of numerous factors, including pro-and anti-inflammatory cytokines (Giulian et al., 1986; Essner et al., 1989; de Waal Malefyt et al., 1991; Ballou and Lozanski, 1992; Brenneman et al., 1992; Rappolee and Werb, 1992; Kiefer et al., 1996; Schroeter et al., 1997; Asakura and Rodriguez, 1998; Batchelor et al., 1999; Dougherty et al., 2000; Leskovar et al., 2000; Hanisch, 2002; Tesseur et al., 2006; Campbell et al., 2007). Among the known factors with beneficial effects on neurons are brain-derived neurotrophic factor (BDNF), IL-6, PDGF, and glial-cell-line-derived neurotrophic factor (GDNF), whereas those with deleterious effects include nitric oxide, TNF-α and IL-1β (Ballou and Lozanski, 1992; Dougherty et al., 2000; Leskovar et al., 2000; Rappolee and Werb, 1992). Thus, in principle, the timing of macrophage activation, and/or activation under different pathological conditions, may alter the levels of various molecules that are produced, with profoundly different consequences on RGC survival and/or axonal regeneration. It is also possible that the proportions of different macrophage sub-populations may vary under different pathological conditions. There are at least three different populations of activated macrophages that possess different biological markers and distinct biological functions, including the production of different cytokines, chemokines, and cytotoxic free radicals such as NO and O2− (Mosser, 2003). Rats with neonatal thymectomies show a less sustained macrophage activation after injecting zymosan into the eye than normal rats, and this transitory response is associated with a reduction in axon regeneration, despite elevated RGC survival (Luo et al., 2007b). These data suggest that the early stage of macrophage activation may be sufficient to provide strong RGC protection, but that prolonged macrophage activation may be required for axonal regeneration. Macrophages normally become abundant at the site of an optic nerve crush (Berry et al., 1996). As in the spinal cord, macrophages accumulate at the core of the injury site in the optic nerve and are surrounded by fibroblasts and astrocytes (Blaugrund et al., 1992; Sellés-Navarro et al., 2001; Silver and Miller, 2004). These macrophages may provide some trophic support for RGCs, but it is clearly not enough to stimulate these cells to regrow axons, in view of the regenerative failure that normally occurs after nerve crush alone.
Fig. 2.
A possible schematic time course of factors released by macrophages and RGC responsiveness. Based on the results of our previous studies, we hypothesize that (i) the net positive effect of macrophage activation peaks shortly after macrophages are activated; and (ii) RGCs show an increased responsiveness to positive-acting factors a few days after axotomy. (a) A long delay between macrophage activation and axotomy results in no axon growth. (b) Macrophage activation 3 days after axotomy produces strong regeneration.
IDENTIFICATION OF ONCOMODULIN AS NOVEL MACROPHAGE-DERIVED GROWTH FACTOR
The small Ca2+-binding protein, oncomodulin, plays a key role in stimulating RGCs to regenerate their axons following intravitreal inflammation. Macrophages that enter the vitreous within 24 h of lens injury express high levels of oncomodulin mRNA and protein, whereas macrophages that enter the eye later show reduced levels. Concomitantly, Western blots reveal a strong elevation of the protein in the vitreous by day 1 and a decline to more modest levels by day 7 (Yin et al., 2006, and in press). Oncomodulin binds to a high-affinity receptor on RGCs in a cAMP-dependent manner. In the presence of elevated [cAMP]i and mannose, another essential co-factor, oncomodulin stimulates more outgrowth from RGCs in cell culture than ciliary neurotrophic factor (CNTF), BDNF, GDNF, or several other trophic factor known to act on these cells (Yin et al., 2003, 2006). Most strikingly, oncomodulin, when delivered from slow-release polymeric beads together with a cAMP analog, promotes extensive axon regeneration through the optic nerve itself (Yin et al., 2006). Depletion of oncomodulin from media conditioned by a macrophage cell line eliminates the axon-promoting effects of the conditioned media (Yin et al., 2006), and a peptide that competes with oncomodulin for receptor binding strongly reduces axon regeneration in vivo after lens injury (Yin et al., in press). These results indicate that oncomodulin plays an essential role in mediating the effects of intravitreal inflammation on optic nerve regeneration. However, additional inflammation-associated factors are presumably responsible for elevating intracellular [cAMP], thereby enabling oncomodulin to act, and perhaps for maintaining high levels of RGC survival as well.
Another group has recently claimed that the pro-regenerative effects of intravitreal inflammation are not mediated by either macrophages or oncomodulin (Müller et al., 2007; Hauk et al., 2008). The sources of disagreement between these authors and us are apparent in some cases but not others. Hauk et al. (2008) found that either a partial elimination of blood-borne macrophages or a local elimination of macrophages in the eye enhanced rather than reduced regeneration after lens injury. These studies used clodronate liposomes, which cause apoptotic death of blood monocytes and tissue macrophages when phagocytosed in sufficient numbers (van Rooijen et al., 1996). However, because these authors injected liposomes peritoneally, they would not be expected to enter the bloodstream in large numbers, and we have previously shown that limited macrophage activation produces better RGC survival than strong activation (Yin et al., 2003), presumably by producing a more favorable balance between cytotoxic and pro-regenerative products in the eye. Direct injection of clodronate liposomes into the eye may similarly shift the balance between positive factors that get released before macrophages die, including oncomodulin, and negative factors. Using the peripheral nerve graft model, we have found that eliminating blood monocytes by repeated i.v. injections of clodronate liposomes blocks the pro-regenerative effects of lens injury almost completely (Cui et al., in press). Müller et al. (2007) also reported an inability to diminish axon regeneration after injecting a polyclonal anti-oncomodulin antibody into the vitreous following lens injury, but this reagent has not been shown to act as a neutralizing antibody (Cui et al., 2008a). All in all, the evidence for oncomodulin being critical for the effects of lens injury seems compelling, i.e. the dramatic elevation of the mRNA and protein after lens injury, the ability of oncomodulin plus a cAMP analog to induce regeneration in vivo, and the effect of a competitive peptide in blocking the effects of lens injury on axon regeneration.
POSSIBLE PRO-REGENERATIVE EFFECTS OF MACROPHAGES IN OTHER TREATMENTS
The cytokine CNTF strongly enhances the ability of RGCs to regenerate axons through a peripheral nerve graft (Cui et al., 1999; Cui and Harvey, 2000), and high concentrations or viral delivery of CNTF have been reported to cause considerable (Leaver et al., 2006a,b; Müller et al., 2007), modest (Lingor et al., 2008) or no (Leon et al., 2000; Pernet and Di Polo., 2006) axon regeneration through the optic nerve itself. The effects of CNTF are enhanced by elevating intracellular cAMP levels (Cui et al., 2003; Park et al., 2004). Müller et al. (2007) have proposed that CNTF is the principal factor that mediates the effects of lens injury on optic nerve regeneration, based in part upon the observation that co-culturing retinal explants with lens or with zymosan increases CNTF expression in retinal astrocytes. However, these authors did not investigate whether this CNTF gets secreted, nor whether this astrocyte-derived CNTF or even exogenous CNTF can induce axon outgrowth in these cultures. It is unclear whether CNTF gets secreted from uninjured astrocytes (Stöckli et al., 1989; Winter et al., 1995), and exogenous CNTF exerts only modest effects on mature RGCs in dissociated culture (Lorber et al., 2002; Yin et al., 2003, 2006; Lingor et al., 2008) and little or no effect on retinal explants (Cohen et al., 1994; Cen et al., 2007). Recent results provide a likely resolution of the discrepancies concerning the role of CNTF in vivo and in culture. CNTF is a chemoattractant for blood-derived macrophages, and depletion of macrophages via i.v. or intravitreal application of clodronate liposomes nearly abolishes the effects of CNTF on RGC survival and axon regeneration in vivo (Cen et al., 2007).
Müller et al. (2007) report that once RGCs have been stimulated to regenerate their axons by injuring the lens in vivo, an anti-CNTF antibody or an inhibitor to the Jak-STAT signaling pathway partially diminishes axon regeneration when RGCs are placed in culture. However, other studies have reported that a function-blocking antibody to the CNTF receptor subunit gp130 did not diminish regeneration stimulated by exposing RGCs to factors derived from the injured lens despite its ability to block the effects of CNTF on rat RGC neurite outgrowth in culture (Lorber et al., 2002, 2008); and that an inhibitor of the Jak/STAT signaling pathway actually augments RGC survival and axon regeneration after optic nerve injury (Luo et al., 2007a). As with CNTF, this latter treatment stimulates macrophage entry into the eye, and removing macrophages with clodronate liposomes significantly reduced the effects of blocking this signaling pathway on RGC survival and axon regeneration (Luo et al., 2007a). Fischer et al. (2008) recently reported that crystallin can stimulate axon regeneration after lens injury, but crystallins also stimulate inflammation in the eye.
ROLE OF LOCAL MICROGLIA IN RGC SURVIVAL AND AXONAL REGENERATION
Blood- and bone marrow–derived monocytes can cross the BBB under physiological conditions and take up residence in the CNS parenchyma as microglial cells (Ling, 1979; Hailer et al., 1997; Wu et al., 2000; Hailer, 2008). Traumatic or ischemic injuries in the CNS result in the activation of local microglia that become morphologically and immunochemically similar to activated macrophages, losing their ramifications and acquiring the ability to migrate. The relationship between phagocytic cells in the retina and axon regeneration following optic nerve injury is unclear. Increased numbers of OX42 or NDPase-positive microglia/macrophages are commonly seen in the retina after optic nerve axotomy (Garcia-Valenzuela et al., 2005; Cen et al., 2007; Luo et al., 2007a; Sobrado-Calvo et al., 2007). While resting microglia are relatively inactive in secretion, activated ones produce a large number of substances including cytokines, proteases, free radicals and various trophic factors (Hanisch, 2002; Hailer, 2008). Owing to their secretion of proinflammatory cytokines and other neurotoxic substances, microglia are generally thought to aggravate neuronal damage after acute or chronic CNS disorders. In the developing retina, microglia-derived nerve growth factor (NGF) causes the death of RGCs (Frade and Barde, 1998). In the adult, treatment with Thr-Lys-Pro (also referred as tuftsin 1–3 or macrophage inhibitory factor, MIF), a tripeptide that inhibits microglia/macrophage activation, has been reported to retard axotomy-induced neuronal degradation and enhance axonal regeneration through a peripheral nerve graft; conversely, a tetrapeptide Thr-Lys-Pro-Arg (also referred as tusftin or macrophage stimulating factor, MSF) that stimulates monocytes has been reported to augment the devastating effects of optic nerve axotomy on RGCs (Thanos et al., 1993). Consequently, others have used tuftsin 1–3 to optimize RGC axon regeneration in peripheral nerve grafts and innervation of the superior colliculus (Whiteley et al., 1998; Avilés-Trigueros et al., 2000; Raibon et al., 2002). It is interesting to note that, when axotomized RGCs are protected from dying by intentionally enhancing inflammation or attaching a PN graft onto the axotomized ON, microglial activation is suppressed (Leon et al., 2000; Raibon et al., 2002). These data suggest that the activation of microglia may be secondary to RGCs becoming compromised, rather than being a primary factor that causes RGC loss. Recently, the Nogo receptor (NgR) has been found to be expressed on microglia/macrophages at PNS (Fry et al., 2007) or CNS (David et al., 2008) injury sites, and it is possible the presence of this receptor on phagocytic cells modulates the inflammatory responses and secondary damage after CNS injury (David et al., 2008).
ROLE OF MONOCYTES/MACROPHAGES IN GLAUCOMA
In other models of retinal disease, the role of monocytes can be quite different from that seen after optic nerve injury. Intraretinal microglia become activated after elevation of intraocular pressure (IOP), a model commonly used to study glaucoma, and this activation correlates with degeneration of RGCs (Naskar et al., 2002; Zhang and Tso, 2003). Nakazawa et al. (2006) showed that increasing IOP in mice by surgically impeding the flow of aqueous fluid leads to a rapid increase in TNF-α mRNA and protein in the eye. This increase is followed shortly afterward by increased microglial activation. Oligodendrocytes in the optic nerve begin to die after 2 weeks or so, and by 4 weeks, RGCs begin to die. The loss of oligodendrocytes and RGCs can be mimicked by injecting TNF-α into the eye. Conversely, injecting a function-blocking anti-TNF-α antibody into the eye or deleting the genes that encode TNF-α or its receptor, TNFR2, prevents the loss of oligodendrocytes and RGCs after elevating IOP. The deleterious effects of elevated IOP are also eliminated by deletion of genes required for monocyte activation (Nakazawa et al., 2006).
In contrast to what was seen after optic nerve injury, blood-derived macrophages in the eye play a deleterious role following acute elevation of IOP, a model that mimics ischemic/mechanical injury in acute glaucoma (Huang et al., 2007a). Inhibition of the PI3K/akt and JAK/STAT3 pathway resulted in macrophage recruitment into the eye following acute elevation of IOP, but the action of these blood-derived macrophages was detrimental to RGC survival under these conditions (Huang et al., 2007b, 2008).
Depending upon the nature of the injury, the modes of RGC death rendered by optic nerve axotomy versus acute IOP elevation are different (Berkelaar et al., 1994; Neufeld et al., 2002; Samardzija et al., 2006). The timing of macrophage activation and/or activation under different pathological conditions may lead to the production of different levels of beneficial and detrimental molecules, resulting in different actions on RGC survival and/or axonal regeneration. It is also possible that the proportions of different macrophage sub-populations vary under different pathological conditions.
ROLE OF T-CELLS IN RGC SURVIVAL AND AXONAL REGENERATION
Activated T-cells can enter the parenchyma of the CNS (Hickey et al., 1991) and accumulate at the site of injury (Popovich et al., 1996; Hirschberg et al., 1998; Raivich et al., 1998; Ling et al., 2006), including in the visual system (Moalem et al., 1999; Fisher et al., 2001; Johnson et al., 2007). T-cells have been widely implicated in inflammatory responses leading to axonal damage and neuronal death, probably via the production of factors such as proinflammatory cytokines, chemokines and nitric oxide (Brow and Bal-Price, 2003; Giraudon et al., 2005; Schroeter and Jander, 2005). However, CNS-infiltrating T-cells may be neuroprotective under certain circumstances (Hammarberg et al., 2000) depending on the subtype of T-cells involved and their activation state (Jones et al., 2002; Kipnis et al., 2002; Wolf et al., 2002). One way in which T-cells can be protective is through the production of neurotrophins (Ehrhard et al., 1993; Kerschensteiner et al., 1999; Moalem et al., 2000). The positive and negative effects of different lymphocyte subpopulations in stroke have been reviewed by Becker, 2009.
After optic nerve crush injury in rats and mice, T-cell-related autoimmunity has been reported to protect those RGCs whose axons were spared from degenerative events, though the effect of T-cells on the axon-injured RGC population was not determined (Moalem et al., 2000; Kipnis et al., 2001; Yoles et al., 2001). On the other hand, in evaluating the actions of CD4+ CD25+ regulatory T-cells, Kipnis et al. (2002) counted the survival of pre-labeled RGCs, whether their axons were injured or not. These latter T cells were found to be detrimental to RGC survival after optic nerve crush and to impair locomotor activity after spinal cord contusion injury (Kipnis et al., 2002).
In our own recent studies, we did not see an accumulation of T-cells in the eye after optic nerve injury in adult rats (Cui et al., 2007; Luo et al., 2007b), and neonatal thymectomy, which eliminates the production of T-cells, strongly enhanced RGC survival after axotomy (Luo et al., 2007b). These results suggest that, although some populations of T-cells may be protective, the overall effect of T-cell activation after axotomy appears to be detrimental to RGC survival. This result was further supported by observations in vitro showing that T-cells killed RGCs via a cell–cell contact mechanism in retinal explants (Luo et al., 2007b).
Activated monocytes present antigens to T-cells, and both types of cells produce molecules that influence each others’ functions. T-cell heterogeneity in macrophage activation is well established (Bach et al., 1997; Gordon, 2003; Ghasemlou et al., 2007). Conversely, macrophage-dependent modulation of T-cell activity has also been amply demonstrated, though mostly in other systems (Gallina et al., 2006; Siciliano et al., 2006). In the optic nerve model, where intravitreal zymosan induces a dramatic invasion of activated macrophages into the eyes, this invasion is not sustained if T-cells have been eliminated at birth by thymectomy (Luo et al., 2007b). Interactions of neurons with T-cells have also been reported (Liu Y et al., 2006), and hence the interactions among the cells of the immune and nervous systems become even more complex.
STRAIN DIFFERENCES IN REGENERATION
The differences in regeneration that are seen between strains of rats or mice provide another example of how the immune system influences outcome after CNS injury. Fischer 344 (F344) and Lewis rats are two inbred rat strains that are, respectively, resistant and susceptible to experimental allergic encephalomyelitis (EAE). EAE is an autoimmune disease model that reflects the functioning of the hypothalamic–pituitary–adrenal (HPA) axis (Wilder et al., 2000). In the disease-resistant F344 rats, intravitreal zymosan injections produce prolonged activation of macrophages in the eye, and, as noted above, a dramatic increase in RGC survival and axonal regeneration after optic nerve injury. In Lewis rats, the same treatment has no effect on RGC survival and a small detrimental effect on axon regeneration (Luo et al., 2007b). A similar correlation between EAE resistance and the ability of RGCs to survive after axotomy has been observed among different strains of mice (Kipnis et al., 2001). In another injury model, acute elevation of IOP results in a rapid and dramatic and prolonged increase in intravitreal macrophages in Lewis rats, but a more gradual and mild increase in macrophages in F344 rats (Huang et al., 2007a). In parallel to these differences in the inflammatory response, EAE-resistant rats showed better RGC survival than EAE-vulnerable rats (Bakalash et al., 2002; Huang et al., 2007a).
CONCLUSIONS
We have tried to convey some of the complexity of the interactions among cells of the nervous system and the immune system that influence outcome after neural injury in the primary visual pathway. As a whole, the field seems to be moving away from a simplistic debate of whether inflammation is good or bad for neural survival and axon regeneration, to a more nuanced discussion about which cells become activated under different circumstances, the profiles of gene expression induced in these cells, and the very complex interactions that take place among the various cell types. The optic nerve of mature rats or mice represents an instance in which inflammation can, under certain experimental conditions, dramatically enhance the survival and regenerative potential of injured neurons. Although we have identified oncomodulin as one molecule that plays a key role in this phenomenon, it is clear that other molecules participate in enabling RGCs to respond to oncomodulin by elevating intracellular cAMP and perhaps by enhancing cell survival. Studies in which the thymus is ablated at birth provide further insights into the complexity of the cellular interactions involved. On the other hand, it should be noted that the vitreous is a highly specialized environment that is normally highly suppressive to inflammation (Streilein, 2003; Kaplan, 2007), and it is possible that the combination of the anti- and pro-inflammatory molecules present when one injures the lens, injects zymosan, or carries out other manipulations creates conditions that are not readily reproduced in the parenchyma of the CNS. Like many other issues, this remains an empirical question. It is clear that further studies of how immune cells respond in vivo under different stimulatory conditions will be a rich area for future research, perhaps ultimately enabling us to fine-tune the immune response to optimize outcome after neural injury.
Abbreviations
- BBB
blood–brain barrier
- BDNF
brain-derived neurotrophic factor
- CNTF
ciliary neurotrophic factor
- EAE
experimental allergic encephalomyelitis
- F344
Fischer 344
- GDNF
glial-cell-line-derived neurotrophic factor
- IOP
intraocular pressure
- PNS
peripheral nervous system
- RGC
retinal ganglion cell
References
- Abromson-Leeman S, Hayashi M, Martin C, Sobel R, al-Sabbagh A, Weiner H, Dorf ME. T cell responses to myelin basic protein in experimental autoimmune encephalomyelitis-resistant BALB/c mice. J Neuroimmunol. 1993;45:89–101. doi: 10.1016/0165-5728(93)90168-x. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience. 1992a;48:169–186. doi: 10.1016/0306-4522(92)90347-5. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992b;176:255–259. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura K, Rodriguez M. A unique population of circulating autoantibodies promotes central nervous system remyelination. Mult Scler. 1998;4:217–221. doi: 10.1177/135245859800400324. [DOI] [PubMed] [Google Scholar]
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp Neurol. 1995;136:183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Avilés-Trigueros M, Sauvé Y, Lund RD, Vidal-Sanz M. Selective innervation of retinorecipient brainstem nuclei by retinal ganglion cell axons regenerating through peripheral nerve grafts in adult rats. J Neurosci. 2000;20:361–374. doi: 10.1523/JNEUROSCI.20-01-00361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Auguet M, Schreiber RD. The IFNγ receptor: a paradigm for cytokine receptor signalling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- Bakalash S, Kipnis J, Yoles E, Schwartz M. Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Invest Ophthalmol Vis Sci. 2002;43:2648–2653. [PubMed] [Google Scholar]
- Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- Becker K. Sensitization and tolerization to brain antigens in stroke. Neuroscience. 2009;158:1090–1097. doi: 10.1016/j.neuroscience.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A, Tsang W, Rosustrel P, Sievers J. Optic nerve regeneration after intravitreal peripheral nerve implants: trajectories of axons regrowing through the optic chiasm into the optic tracts. J Neurocytol. 1999;28:721–741. doi: 10.1023/a:1007086004022. [DOI] [PubMed] [Google Scholar]
- Benowitz L, Yin Y. Rewiring the injured CNS: Lessons from the optic nerve. Exp Neurol. 2008;209:389–398. doi: 10.1016/j.expneurol.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaugrund E, Duvdevani R, Lavie V, Solomon A, Schwartz M. Disappearance of astrocytes and invasion of macrophages following crush injury of adult rodent optic nerves: implications for regeneration. Exp Neurol. 1992;118:105–115. doi: 10.1016/0014-4886(92)90027-n. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Brow GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide glutamate and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Mol Cell Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Deacon RM, Jiang Y, Ferrari C, Pitossi FJ, Anthony DC. Overexpression of IL-1beta by adenoviral-mediated gene transfer in the rat brain causes a prolonged hepatic chemokine response, axonal injury and the suppression of spontaneous behaviour. Neurobiol Dis. 2007;27:151–163. doi: 10.1016/j.nbd.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen LP, Luo JM, Zhang CW, Fan YM, Song Y, So KF, van Rooijen N, Pang CP, Lam DSC, Cui Q. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Res. 2007;48:4257–4266. doi: 10.1167/iovs.06-0791. [DOI] [PubMed] [Google Scholar]
- Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25:953–959. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- Correale J, Villa A. The neuroprotective role of inflammation in nervous system injuries. J Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. doi: 10.1097/00001756-200012180-00019. [DOI] [PubMed] [Google Scholar]
- Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Cui Q, Hodgetts SI, Hu Y, Luo JM, Harvey AR. Strain-specific differences in the effects of cyclosporin A and FK506 on the survival and regeneration of axotomized retinal ganglion cells in adult rats. Neuroscience. 2007;146:986–999. doi: 10.1016/j.neuroscience.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Cui Q, Benowitz L, Yin Y. Does CNTF mediate the effect of intraocular inflammation on optic nerve regeneration? Brain. 2008a;131:e96, 1–2. doi: 10.1093/brain/awn027. [DOI] [PubMed] [Google Scholar]
- Cui Q, Yin Y, Li Z, Benowitz L. Macrophages and oncomodulin mediate the effects of lens injury in stimulating axon regeneration through a peripheral nerve graft. Abstract, annual meeting, Society for Neuroscience; Washington DC. Nov. 2008; 2008b. in press. [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- David S, Bouchard C, Tsatas O, Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990;5:463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- David S, Fry EJ, López-Vales R. Novel roles for Nogo receptor in inflammation and disease. Trends Neurosci. 2008;31:221–226. doi: 10.1016/j.tins.2008.02.002. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal’s degeneration and regeneration of the nervous system, history of neuroscience. Vol. 5. New York: Oxford; 1991. [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra CD, de Groot CJ, Huitinga I. The role of macrophages in demyelination. J Neuroimmunol. 1992;40:183–188. doi: 10.1016/0165-5728(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner R, Rhoades K, McBride WH, Morton DL, Economou JS. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989;142:3857–3861. [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004a;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004b;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Hauk TG, Müller A, Thanos S. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37:471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Fisher J, Levkovitch-Verbin H, Schori H, Yoles E, Butovsky O, Kaye JF, Ben-Nun A, Schwartz M. Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci. 2001;21:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Ho C, David S. A role for Nogo receptor in macrophage clearance from injured peripheral nerve. Neuron. 2007;53:649–662. doi: 10.1016/j.neuron.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Sharma SC, Piña AL. Multilayered retinal microglial response to optic nerve transection in rats. Mol Vis. 2005;11:225–231. [PubMed] [Google Scholar]
- Ghasemlou N, Jeong SY, Lacroix S, David S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia. 2007;55:294–302. doi: 10.1002/glia.20449. [DOI] [PubMed] [Google Scholar]
- Giraudon P, Vincent P, Vuaillat C. T-cells in neuronal injury and repair. Neuromol Med. 2005;7:207–216. doi: 10.1385/NMM:7:3:207. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Heppner FL, Haas D, Nitsch R. Fluorescent dye prelabelled microglial cells migrate into organotypic hippocampal slice cultures and ramify. Eur J Neurosci. 1997;9:863–866. doi: 10.1111/j.1460-9568.1997.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: It is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Lindå H, van Der Meide PH, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hauk TG, Müller A, Lee J, Schwendener R, Fischer D. Neuro-protective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol. 2008;209:469–482. doi: 10.1016/j.expneurol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hirschberg DL, Moalem G, He J, Mor F, Cohen IR, Schwartz M. Accumulation of passively transferred primed T cells independently of their antigen specificity following central nervous system trauma. J Neuroimmunol. 1998;89:88–96. doi: 10.1016/s0165-5728(98)00118-0. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li Z, van Rooijen N, Wang N, Pang CP, Cui Q. Different responses of macrophages in retinal ganglion cell survival after acute ocular hypertension in rats with different autoimmune backgrounds. Exp Eye Res. 2007a;85:659–666. doi: 10.1016/j.exer.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cen LP, Choy KW, van Rooijen N, Wang N, Pang CP, Cui Q. JAK/STAT pathway mediates retinal ganglion cell survival after acute ocular hypertension but not under normal conditions. Exp Eye Res. 2007b;85:684–695. doi: 10.1016/j.exer.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cen LP, Luo JM, Wang N, Zhang MZ, van Rooijen N, Pang CP, Cui Q. Differential roles of PI3K/akt pathway in retinal ganglion cell survival in rats with or without acute ocular hypertension. Neuroscience. 2008;153:214–225. doi: 10.1016/j.neuroscience.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Huitinga I, van Rooijen N, de Groot CJ, Uidehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I, Ruuls ISR, Jung S, van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995;100:344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Lausberg F, Stoll G. Differential recruitment of CD8+ macrophages during wallerian degeneration in the peripheral and central nervous system. Brain Pathol. 2001;11:27–38. doi: 10.1111/j.1750-3639.2001.tb00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Camras CB, Kipnis J. Bacterial DNA confers neuroprotection after optic nerve injury by suppressing CD4+ CD25+ regulatory T-cell activity. Invest Ophthalmol Vis Sci. 2007;48:3441–3449. doi: 10.1167/iovs.06-1351. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popocvich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Ito H, Fukumoto T. Lymphocyte infiltration into normal rat brain following hyperosmotic blood-brain barrier opening. J Neuroimmunol. 1990;27:133–140. doi: 10.1016/0165-5728(90)90062-r. [DOI] [PubMed] [Google Scholar]
- Kaplan HJ. Anatomy and function of the eye. Chem Immunol Allergy. 2007;92:4–10. doi: 10.1159/000099236. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122–1132. doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Funa K, Schweitzer T, Jung S, Bourde O, Toyka KV, Hartung HP. Transforming growth factor-beta 1 in experimental autoimmune neuritis. Cellular localization and time course. Am J Pathol. 1996;148:211–223. [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+ CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf PM, Harling-Berg CJ, Cserr HF, Basu D, Sirulnick EJ, Nolan SC, Park JT, Keir G, Thompson EJ, Hickey WF. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol. 1998;161:692–701. [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006a;24:3323–3332. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006b;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskovar A, Moriarty LJ, Turek JJ, Schoenlein IA, Borgens RB. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. J Exp Biol. 2000;203:1783–1795. doi: 10.1242/jeb.203.12.1783. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. 2006;26:731–741. doi: 10.1523/JNEUROSCI.3502-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling EA. Transformation of monocytes into amoeboid microglia in the corpus callosum of postnatal rats, as shown by labelling monocytes by carbon particles. J Anat. 1979;128:847–858. [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- Liu X, Hawkes E, Ishimaru T, Tran T, Sretavan DW. EphB3: an endogenous mediator of adult axonal plasticity and regrowth after CNS injury. J Neurosci. 2006;26:3087–3101. doi: 10.1523/JNEUROSCI.4797-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A, Tonge D. Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci. 2002;21:301–311. doi: 10.1006/mcne.2002.1175. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A. Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci. 2005;21:2029–2034. doi: 10.1111/j.1460-9568.2005.04034.x. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A. Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res. 2008;86:894–903. doi: 10.1002/jnr.21545. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JM, Cen LP, Zhang XM, Chiang SY, Huang Y, Lin D, Fan Y, van Rooijen N, Lam DSC, Pang CP, Cui Q. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci. 2007a;26:828–842. doi: 10.1111/j.1460-9568.2007.05718.x. [DOI] [PubMed] [Google Scholar]
- Luo JM, Zhi Y, Chen Q, Cen LP, Zhang CW, Lam DSC, Harvey AR, Cui Q. Influence of macrophages and lymphocytes on the survival and axon regeneration of injured retinal ganglion cells in rats from different autoimmune backgrounds. Eur J Neurosci. 2007b;26:3475–3485. doi: 10.1111/j.1460-9568.2007.05957.x. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Polazzi E, Nicolini A, Greco A, Levi G. Possible role of microglial prostanoids and free radicals in neuroprotection and neurodegeneration. Adv Exp Med Biol. 1999;468:109–119. doi: 10.1007/978-1-4615-4685-6_9. [DOI] [PubMed] [Google Scholar]
- Moalem G, Monsonego A, Shani Y, Cohen IR, Schwartz M. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. FASEB J. 1999;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Müller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962–2968. [PubMed] [Google Scholar]
- Neufeld AH, Kawai S, Das S, Vora S, Gachie E, Connor JR, Manning PT. Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase. Exp Eye Res. 2002;75:521–528. doi: 10.1006/exer.2002.2042. [DOI] [PubMed] [Google Scholar]
- Okada T, Ichikawa M, Tokita Y, Horie H, Saito K, Yoshida J, Watanabe M. Intravitreal macrophage activation enables cat retinal ganglion cells to regenerate injured axons into the mature optic nerve. Exp Neurol. 2005;196:153–163. doi: 10.1016/j.expneurol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Park K, Luo JM, Hisheh S, Harvey AR, Cui Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–10815. doi: 10.1523/JNEUROSCI.3532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Bell MD, Brown HC, Matyszak MK. Inflammation in the nervous system. Curr Opin Neurobiol. 1995;5:636–641. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Raibon E, Sauvé Y, Carter DA, Gaillard F. Microglial changes accompanying the promotion of retinal ganglion cell axonal regeneration into peripheral nerve grafts. J Neurocytol. 2002;31:57–71. doi: 10.1023/a:1022527800181. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and regeneration of the nervous system. New York: Oxford University Press; 1991. [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rappolee DA, Werb Z. Macrophage-derived growth factors. Curr Top Microbiol Immunol. 1992;181:87–140. doi: 10.1007/978-3-642-77377-8_4. [DOI] [PubMed] [Google Scholar]
- Reichert F, Rotshenker S. Deficient activation of microglia during optic nerve degeneration. J Neuroimmunol. 1996;70:153–161. doi: 10.1016/s0165-5728(96)00112-9. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Wenzel A, Augenberg S, Tgiersch M, Reme C, Grimm C. Differential role of Jak-STAT signalling in retinal degeneration. FASEB J. 2006;20:E1790–E1801. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S. T-cell cytokines in injury-induced neural damage and repair. Neuromol Med. 2005;7:1–13. doi: 10.1385/NMM:7:3:183. [DOI] [PubMed] [Google Scholar]
- Sellés-Navarro I, Ellezam B, Fajardo R, Latour M, McKerracher L. Retinal ganglion cell and nonneuronal cell responses to a microcrush lesion of adult rat optic nerve. Exp Neurol. 2001;167:282–289. doi: 10.1006/exnr.2000.7573. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Benveniste EN. The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol. 1996;157:1819–1822. [PubMed] [Google Scholar]
- Siciliano NA, Skinner JA, Yuk MH. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J Immunol. 2006;177:7131–7138. doi: 10.4049/jimmunol.177.10.7131. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sobrado-Calvo P, Vidal-Sanz M, Villegas-Pérez MP. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J Comp Neurol. 2007;501:866–878. doi: 10.1002/cne.21279. [DOI] [PubMed] [Google Scholar]
- Stöckli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Götz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Stoll G, Trapp BD, Griffin JW. Macrophage function during wallerian degeneration of rat optic nerve: clearance of degenerating myelin and Ia expression. J Neurosci. 1989;9:2327–2335. doi: 10.1523/JNEUROSCI.09-07-02327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Mezitis SG, Gonatas NK, Silberberg DH. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987;15:263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurode-generation and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56:732–740. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Whiteley SJ, Sauvé Y, Avilés-Trigueros M, Vidal-Sanz M, Lund RD. Extent and duration of recovered pupillary light reflex following retinal ganglion cell axon regeneration through peripheral nerve grafts directed to the pretectum in adult rats. Exp Neurol. 1998;154:560–572. doi: 10.1006/exnr.1998.6959. [DOI] [PubMed] [Google Scholar]
- Wilder RL, Griffiths MM, Cannon GW, Gaspi R, Remmers EF. Susceptibility to autoimmune disease and drug addiction in inbred rats. Are there mechanistic factors in common related to abnormalities in hypothalamic-pituitary-adrenal axis and stress response function? Ann N Y Acad Sci. 2000;917:784–796. doi: 10.1111/j.1749-6632.2000.tb05444.x. [DOI] [PubMed] [Google Scholar]
- Winter CG, Saotome Y, Levison SW, Hirsh D. A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci U S A. 1995;92:5865–5869. doi: 10.1073/pnas.92.13.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Fisher J, Bechmann I, Steiner B, Kwidzinski E, Nitsch R. Neuroprotection by T-cells depends on their subtype and activation state. J Neuroimmunol. 2002;133:72–80. doi: 10.1016/s0165-5728(02)00367-3. [DOI] [PubMed] [Google Scholar]
- Wu YP, McMahon E, Kraine MR, Tisch R, Meyers A, Frelinger J, Matsushima GK, Suzuki K. Distribution and characterization of GFP(+) donor hematogenous cells in twitcher mice after bone marrow transplantation. Am J Pathol. 2000;156:1849–1854. doi: 10.1016/S0002-9440(10)65058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Yin Y, Gilbert H-Y, Yang Y, Valle C, Cui Q, Petkova V, Benowitz LI. Oncomodulin mediates the effect of lens injury on optic nerve regeneration. Abstract, annual meeting, Society for Neuroscience; Washington DC. Nov. 2008; 2008. in press. [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeev-Brann AB, Lazarov-Spiegler O, Brenner T, Schwartz M. Differential effects of central and peripheral nerves on macrophages and microglia. Glia. 1998;23:181–190. doi: 10.1002/(sici)1098-1136(199807)23:3<181::aid-glia1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Tso MO. Characterization of activated retinal microglia following optic axotomy. J Neurosci Res. 2003;73:840–845. doi: 10.1002/jnr.10713. [DOI] [PubMed] [Google Scholar]