Abstract
Mycobacterium tuberculosis and M. bovis-BCG induce potent expansions of human memory Vγ9+Vδ2+ T cells capable of IFN-γ production, cytolytic activity and mycobacterial growth inhibition. Certain phosphoantigens expressed by mycobacteria can stimulate γ9δ2 T cell expansions, suggesting that purified or synthetic forms of these phosphoantigens may be useful alone or as components of new vaccines or immunotherapeutics. However, we show that while mycobacteria-activated γ9δ2 T cells potently inhibit intracellular mycobacterial growth, phosphoantigen-activated γ9δ2 T cells fail to inhibit mycobacteria, although both develop similar effector cytokine and cytolytic functional capacities. γ9δ2 T cells receiving TLR-mediated co-stimulation during phosphoantigen activation also failed to inhibit mycobacterial growth. We hypothesized that mycobacteria express antigens, other than the previously identified phosphoantigens, that induce protective subsets of γ9δ2 T cells. Testing this hypothesis, we compared the TCR sequence diversity of γ9δ2 T cells expanded with BCG-infected versus phosphoantigen-treated DC. BCG-stimulated γ9δ2 T cells displayed a more restricted TCR diversity than phosphoantigen-activated γ9δ2 T cells. In addition, only a subset of phosphoantigen-activated γ9δ2 T cells functionally responded to mycobacteria-infected DC. Furthermore, differential inhibitory functions of BCG- and phosphoantigen-activated γ9δ2 T cells were confirmed at the clonal level and were not due to differences in TCR avidity. Our results demonstrate that BCG infection can activate and expand protective subsets of phosphoantigen responsive γ9δ2 T cells, and provide the first indication that γ9δ2 T cells can develop pathogen specificity similar to αβ T cells. Specific targeting of protective γ9δ2 T cell subsets will be important for future tuberculosis vaccines.
Keywords: gamma delta T cells, T cell receptors, Repertoire Development, Mycobacteria, Memory Immunity
Introduction
γδ T cells have been previously regarded as innate immune cells, responding to various generalized inflammatory markers. However, newer evidence suggests that the γ9δ2 T cell subset may exhibit pathogen specificity (1) as well as immune memory (2,3), two characteristics of adaptive immunity. We have demonstrated that γ9δ2 T cells from mycobacteria-immune individuals expand after in vitro Mycobacterium bovis bacillus Calmette-Guérin (BCG)3 stimulation with a memory-like recall response (2). Consistent with our observations enhanced secondary γ9δ2 T cell responses have been observed in non-human primates infected with virulent M. tuberculosis (3). In addition to activation by M. tuberculosis (4), γ9δ2 T cells proliferate and develop relevant effector functions during infections with virulent M. bovis (5), Listeria monocytogenes (6), Plasmodia (7) and herpesviruses (8). Furthermore, depletion of circulating γ9δ2 T cells has been associated with exacerbation of susceptibility to infectious diseases (9–11).
Human γ9δ2 T cells can be activated by nonpeptidic, phosphorylated biometabolites (phosphoantigens) and alkylamines. Natural phosphoantigens including biosynthetic precursors of lipid and steroid synthesis (e.g.-prenyl pyrophosphates) as well as phosphorylated nucleotides are secreted as by-products from infecting pathogens or are released from host cells damaged by infection (12). Although it is clear that optimal γ9δ2 T cell activation requires the presence of APC (13), the specific antigens expressed or induced by human pathogens that stimulate protective γ9δ2 T cell responses and the molecular details of how these antigens are presented to γ9δ2 TCR are unknown.
Because optimal doses of two natural phosphoantigens [isopentenyl pyrophosphate (IPP) (14) & (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate (HMB-PP) (15)] and a synthetic phosphoantigen [bromohydrin pyrophosphate (BrHPP) (16)] can induce the expansion of γ9δ2 T cells in vitro comparable to levels induced by live BCG stimulation, it has been proposed that these phosphoantigens may be valuable components of next generation TB vaccines or immunotherapeutics (17–19). However, the possibility that vaccination with purified phosphoantigens can induce protective TB immunity has not been reported. Furthermore, we have shown in functional in vitro assays that IPP-stimulated PBMC from PPD+ donors do not inhibit intracellular mycobacterial growth, while purified γ9δ2 T cells from these same donors expanded by stimulation with BCG-infected APC can potently inhibit the growth of intracellular mycobacteria (20). Here we expand these findings to show that BrHPP and HMB-PP also fail to stimulate γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth. In addition, co-stimulation through the TLR during phosphoantigen stimulation of γ9δ2 T cells failed to induce mycobacterial inhibitory activity.
We hypothesized that live mycobacteria express antigens, other than the previously identified phosphoantigens, that are required to induce a subset of protective γ9δ2 T cells. To analyze this protective subset, we have investigated differences in the diversity of the γ9δ2 T cell immunorepertoire by TCR spectratypic and sequence analyses of the expressed third Complementarity Determining Regions (CDR3). We found that live BCG induced a more restricted CDR3 spectratype and sequence diversity compared with IPP stimulation. These TCR studies combined with further differential functional results indicate that BCG-stimulated γ9δ2 T cells comprise a specific subset of IPP-reactive γ9δ2 T cells. These results have important implications for the development of prophylactic vaccines and immunotherapies designed to induce protective, pathogen-specific γ9δ2 T cell responses.
Materials and Methods
Reagents
Whole cell lysates of M. tuberculosis (MtbWL) were generated as previously described (2). Antigen concentrations for expansion were as follows: BCG MOI of 0.02–1 depending on BCG strain used, 5 μg/mL M. tuberculosis Erdman lysate, 2–1000 nM BrHPP (provided by Jean-Jacques Fournié, INSERM, Toulouse, France), 0.2 pM – 500 nM HMB-PP (HDMAPP, Echelon Bioscience, Salt Lake City, UT) and 5–100 μM of IPP (Echelon Bioscience) supplemented with 20 U/ml rhIL-2 (Hoffman-LaRoche, Basel, Switzerland). TLR 1/2, 3, 4, and 9 agonists were tested at the following ranges of concentrations, respectively: 1–100 μg/mL Pam3Cys (Echleon Bioscience), 10–1000 μg/mL PolyI:C (Sigma-Aldrich, St. Louis, MO), 1–100 ng/mL LPS (Sigma-Aldrich), 1–50 ng/mL CpG ODN (Coley Pharma, Wellesley, MA).
Intracellular Mycobacteria Growth Inhibition Assay
We obtained PBMC from healthy volunteers with evidence of mycobacteria-specific delayed type hypersensitivity (≥10mm of induration 48–72 hours after 5 TU tuberculin intradermal testing), by density gradient purification from whole blood samples or leukapheresis products following IRB approved protocols. Frozen samples were thawed in the presence of 20% normal human serum (Sigma-Aldrich). For most experiments, target and effector cell populations were prepared as previously described (20,21). Briefly, PBMC were plated to obtain macrophage targets which were infected with BCG (MOI=3) the day prior to addition of the T cells. Total PBMC and freshly purified γδ T cells were added to infected macrophages at an E:T ratio of 10:1. Long-term γδ T cell lines and γδ T cell clones were added at an E:T ratio of 1:1. To test the direct inhibitory effects of γδ T cell clones on intracellular mycobacterial growth without other T cells being present, target cells were prepared by plating 10 fold higher concentrations of PBMC and waiting until day 3 to wash away non-adherent cells. This latter method of preparing target macrophages was found to prevent the BCG growth-enhancing effects of the addition of 7 day rested PBMC described earlier (20,21), simplifying the interpretation of the results. Percent inhibition was calculated as 100-(100*(experimental cultures/control cultures)). Fold expansion of γδ T cells was determined by flow cytometric staining 7 days after stimulation. Absolute numbers (AN) of γδ T cells present were computed by multiplying the flow cytometric percentages times numbers of viable cells present after expansion, and fold expansions calculated as (AN of γδ T cells after antigen expansion)/(AN of γδ T cells after medium rest).
Generation of Long-Term Antigen-Specific γδ T cell Lines
PBMC were thawed and expanded with BCG or IPP as above. After 7 days of stimulation the cells were transferred to 24-well plates and expanded in fresh medium containing not more than 50 U/ml rhIL-2 replaced every 3–4 days as needed. After expansion with IL-2, the cells were co-cultured with BCG-infected or IPP-pulsed autologous DC as APC. DC were generated by incubation of adherent monocytes with GM-CSF (Immunex, Seattle, WA) and IL-4 (R&D Systems, Minneapolis, MN), and induced to mature with IL-6 (BD Pharmingen, San Jose, CA), TNF-α (Roche, Nutley, NJ), IL-1β (BD Pharmingen) and PGE2 (Sigma) as previously described (22). DC were infected overnight with BCG at an MOI of 5 or pulsed with 10 μM IPP and purified by centrifugation over Ficoll-Paque PLUS (Amersham-Pharmacia) to remove extracellular BCG and irradiated with 3000 rads prior to culture at a 1:5 APC:T cell ratio. 10 μM IPP was added to the culture medium of IPP-stimulated cultures to induce the optimal proliferation of IPP-reactive T cells. T cells and APC were co-cultured for 14 days replacing medium supplemented with rhIL-2 every 3–4 days. After a second antigen/DC expansion cycle, the resulting γδ T cells populations were purified by immunomagnetic bead selection (Miltenyi Biotech, Auburn, CA), routinely resulting in >95% purity. γ9δ2 T cells were purified as needed to maintain the purity of the populations as determined by flow cytometry. The antigen/IL-2 stimulation/expansion process was repeated every two weeks for at least 4 cycles before the cells were used in TCR spectratyping experiments. Some cells were frozen to preserve the populations for later use.
Limiting Dilution Cloning of Antigen-Specific γδ T cells
γδ T cell clones were generated from BCG and IPP primarily expanded γδ T cells. Antigen expanded γδ T cells were purified on day 7 as above with immunomagnetic beads and plated at 0.3, 3, 30, and 300 cells/well in 96-well round bottom tissue culture plates. Irradiated autologous feeder cells (PBMC or non-adherent cells) were added at 1E5 cells/well. The γδ T cells were stimulated with 2 μg/mL PHA-L (Sigma Aldrich) for 24 hours and then expanded by replenishing medium with 5 U/mL rhIL-2 every 3–4 days as needed beginning on day 1. Potential clones were identified visually by increase in the size of the cell pellet. As predicted by the Poisson distribution, cell growth was assumed to be due to clonal expansion if less than 1/3 of the wells plated for a given dilution showed cell growth. Positive clones were removed to a separate plate and restimulated with 2 μg/mL PHA every 2–3 weeks. Clonal γδ T cells were tested for the ability to inhibit intracellular mycobacterial growth by co-culture with BCG-infected macrophages as described above. Clones able to inhibit mycobacterial growth were defined as those clones suppressing intracellular mycobacterial growth to levels less than the mean minus standard error of growth detected in control wells without T cells added.
Cytotoxicity Assay
The cytolytic activities of BCG- and IPP-expanded γδ T cells were assessed by a standard 4 hour chromium release assay. 1×106 Daudi cells (ATCC, Manassas, VA) were labeled overnight with 100 μCi Cr51 (GE Healthcare) in 750μL RPMI supplemented with 2mM L-glutamine, 10 mM Hepes, 1mM sodium pyruvate, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, and 10% FBS (Sigma-Aldrich). The following day, these Daudi cells were washed and incubated for 15 minutes in medium to reduce spontaneous release. Purified γδ T cells and 5,000 Daudi cell targets were cultured at varying effector:target ratios for 4 hours at 37° C with 5% CO2. Spontaneous release was measured by incubation in medium alone. Maximum lysis was measured by lysis of the Daudi cells with 2% Triton X-100. Supernatants were harvested and the amount of Cr51 released was quantified in a Micro-beta scintillation counter (PerkinElmer, Waltham, MA). Percent lysis was calculated as (experimental cpm – spontaneous cpm)/(maximal cpm – spontaneous cpm)×100.
Flow Cytometric Analysis
The following antibodies from BD Pharmingen were used for flow cytometric analyses: anti-γδ TCR PE (clone 11F2), anti-αβ TCR FITC (clone B3), anti-CD3 PerCP (clone SK7), anti-γδ TCR FITC (clone WT31), anti-CD4 PE (clone SK3), anti-CD8 FITC (clone SK1), anti-δ2 TCR PE (clone B6), anti-γ9 TCR FITC (clone B1), anti-IFNγ APC or PE (clone B27), anti-Perforin PE (clone δG9), anti-granzyme A FITC (clone CB9), anti-CD134 PE (clone 1D11), anti-NKp46 PE (clone 9E2), anti-CD161 FITC (clone DX12), anti-KIR-NKAT2 PE (clone DX27), anti-CD94 FITC (clone HP-3D9), anti-NKB1 FITC (Clone DX9), anti-NKp44 PE (clone p44–8.1). The anti-granulysin PE (clone DH2) was purchased from EBioscience (San Diego, CA). Cells were stained for 20 minutes in 100 μL PBS supplemented with 4% FCS (Sigma) and 1% NaN3 at 4°C, and washed with 1 mL staining buffer. Cells were fixed in 1% methanol-free paraformaldehyde (Polyscience Inc., Warrington, PA) in PBS and analyzed on a BD Immunocytometry Systems FACSCalibur at the Flow Cytometry Core Facility at Saint Louis University. For intracellular cytokine studies, cells were stained as above for surface molecules and then fixed and permeablized for 15 minutes using BD CytoFix/CytoPerm (BD Bioscience) using the maufacturer’s recommended dosage. Staining for intracellular molecules was performed for 20 minutes in 100 μL PermWash (BD Bioscience) at 4°C washed and analyzed on a BD Immunocytometry Systems FACSAria or LSRII at the Core Flow Facility at Saint Louis University. Flow cytometric analysis of clonal γδ T cell responses to antigen titrations was preformed on Guava EasyCyte (Guava Technologies, Haywood, CA).
Spectratypic Analyses
Total RNA was harvested from γδ T cell populations following the Qiagen RNeasy protocol including the optional DNase treatment. Vγ9 and Vδ2 specific CDR3 sequences were amplified by RT-PCR (Sigma JumpStart AccuTaq). First strand synthesis was primed by anchored Oligo dT primers to generate a cDNA library representative of the entire cellular mRNA pool. Primer sequences complementary to upstream Variable regions and downstream Constant regions were used to amplify CDR3 regions [CCAGTACTAAAACGCTGTC & GTCGTTAGTCTTCATGGTGTTCCC (23) for TCRγ9 chains; GCACCATCAGAGAGAGATGAAGGG & AAACGGATGGTTTGTATGAGGC (24) for TCRδ2 chains]. The Constant region primers were 5′ end labeled with the D2 dye from Beckman Coulter (Fullerton, CA) by Proligo (Boulder, CO). Using the Beckman Coulter CEQ8000 genetic analysis system, the labeled fragments were separated by capillary gel electrophoresis and compared against size standards to estimate the nucleotide length for each fragment. The resulting fragment peaks were analyzed using the fragment analysis software package included with the CEQ8000, using the default settings to define peak threshold.
PCR Cloning and Sequencing
RNA harvested for Spectratyping was reverse transcribed as above. PCR was performed with the same primer sequences as above utilizing Taq polymerase (Sigma) to incorporate an adenosine at the 3′ end of the amplified DNA. The PCR fragments were ligated into the pCR2.1 TA cloning vector (Invitrogen) and the resulting plasmids were transfected by heat shock into TOP10 Competent E. coli (Invitrogen) for propagation. Glycerol stocks were frozen to maintain the clones. Colonies were picked and grown overnight in 1–2 mL LB broth containing carbenicillin (50 mg/ml). Plasmids were purified using the DNeasy Miniprep kit (Qiagen, Valencia, CA). The plasmid DNA was sequenced using the DTCS Quick Start kit (Beckman-Coulter) and the M13 reverse primer (CAGGAAACAGCTATGAC). Sequences were determined using the Beckman-Coulter CEQ8000 genetic analysis system. CDR3 lengths were calculated as four less than the number of amino acids between the last conserved Cys residue in the Variable region to the conserved GXG or AXG motif in the Joining segment as previously described (25).
Heterologous Stimulation of long-term γ9δ2 T cell lines and clones
A modification of a previously published method (26) to measure antigen-specific intracellular cytokine production was used to quantitate the degree of specificity of our long-term γ9δ2 T cell lines and clones. DC were infected with a titration of the Danish stain of BCG (MOI = 1–100) or pulsed with 100 μM IPP or a titration of HMB-PP (10–1000 pM) for 2 hours. Purified γ9δ2 T cells were cultured with BCG-infected or IPP-pulsed DC at an APC:T cell ratio of 1:1.2 in the presence of anti-CD28 and anti-CD49d (both at 1 μg/mL, BD Pharmingen). For the phosphoantigen stimulation, phosphoantigen was added to the culture medium in order to stimulate responsive γδ T cells. After 3 hours of stimulation at 37°C, 0.7 μL/mL GolgiStop (BD Pharmingen) was added and the cultures were incubated for 5 more hours at 37°C. Cells were surface-stained with anti-αβ TCR, anti-γδ TCR, anti-Vγ9 TCR or anti-CD3. Then cells were fixed and permeabilized with Cytofix/CytoPerm (BD Pharmingen) and stained for intracellular IFN-γ before 4-color analysis on a FACSCalibur flow cytometer or 3-color analysis on a Guava EasyCyte. A minimum of 10,000 events were acquired and analyzed using CellQuest or Guava EasyCyte software. For analyses of long-term γδ T cell lines, the percentages of γδ T cells staining positively for IFN-γ expression after homologous stimulation (i.e.-BCG stimulation of BCG-expanded γδ T cells and IPP stimulation of IPP-expanded γδ T cells) were defined as the 100% responses. Percent heterologous responses were calculated as the percentages of IFN-γ positive γδ T cells after heterologous stimulation (i.e.-IPP stimulation of BCG-expanded γδ T cells and BCG stimulation of IPP-expanded γδ T cells), divided by the percentages of IFN-γ positive γδ T cells after homologous stimulations, multiplied times 100. For analyses of γδ T cell clones, the percentage of γδ T cells staining positively for IFN-γ expression is reported.
Graphics, Statistics, and Correlations
Graphics and statistical results were generated using Microsoft Excel, Statistica, or SPSS. Percent inhibition is displayed as Mean ± Standard Error. Mann-Whitney U tests were used to compare TCR spectratyping peak number distributions. Wilcoxon matched pairs tests were used to compare intracellular BCG growth inhibition for: 1) matched primary cultures of PBMC stimulated with phosphoantigens and live BCG, 2) phosphoantigen- and BCG-expanded long-term γδ T cell lines generated from the same individuals, and 3) stimulations of γ9δ2 T cell lines with homologous and heterologous antigens (antigens used to expand long-term lines or not, respectively). The percentages of BCG- and IPP-expanded γ9δ2 T cell clones able to inhibit intracellular mycobacterial growth were compared using χ2 tests. To control for differential cloning efficiency, Spearman rank correlations were used to compare spectratyping and sequencing data. Both spectratyping and sequencing data were normalized by multiplying each nucleotide fragment length detected times the observed frequency in order to generate continuous variables for comparison by Spearman rank test.
Results
BCG-expanded γ9δ2 T cells inhibit intracellular mycobacterial growth whereas IPP-expanded γ9δ2 T cells do not
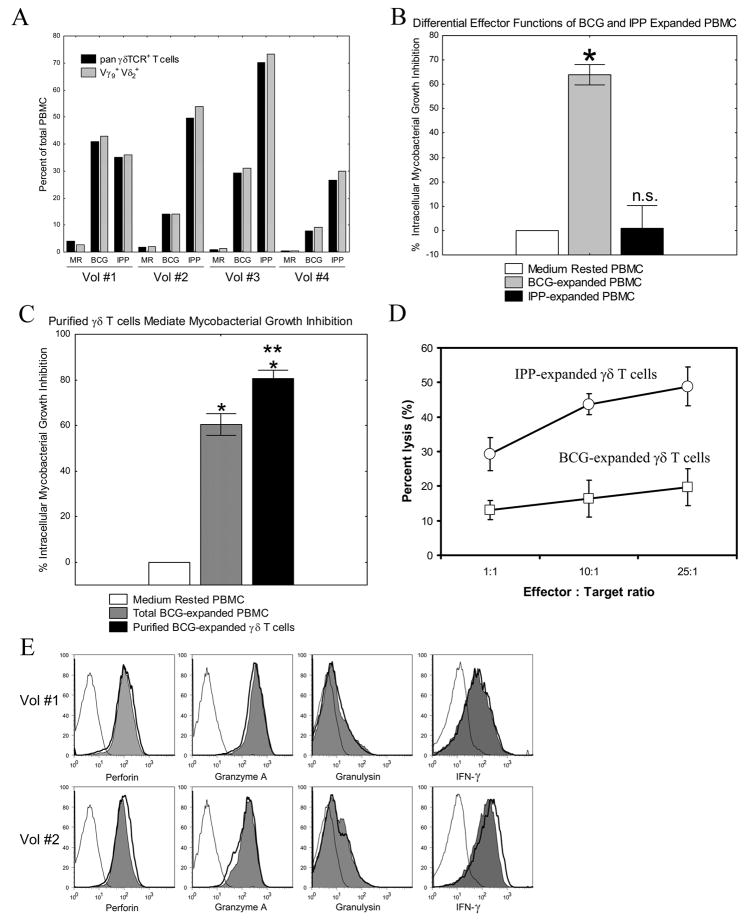
Despite the ability of mycobacteria and phosphoantigens to expand γ9δ2 T cells (Fig. 1A), we have found that BCG- and IPP-expanded PBMC have differential effects on intracellular mycobacterial growth. Results for the four volunteers studied in Fig. 1A demonstrate that IPP was at least as potent as BCG in stimulating the expansion of γ9δ2 T cells. However, PBMC expanded with BCG were capable of inhibiting the growth of intracellular mycobacteria by 65% compared with cultures containing only medium rested PBMC (Fig. 1B; *p< 0.005, n=12). In contrast, PBMC from the same volunteers expanded with IPP failed to inhibit intracellular mycobacterial growth (p = 0.81, n=12).
FIGURE 1.
Differential effector functions of BCG- and IPP-expanded γ9δ2 T cells. A, Both BCG and IPP expand Vγ9+Vδ2+ γδ T cells. After 1 week of rest in medium alone or expansion with either BCG or IPP, γδ T cells present were identified by staining with anti-CD3 and either a pan-γδ TCR-specific mAb, or Vγ9and Vδ2 specific mAb. Virtually all γδ T cells expanded with either BCG or IPP expressed both Vγ9 and Vδ2. None of the expanded γδ T cells expressed detectable Vγ1 or Vδ1 chains (data not shown). B, BCG- but not IPP-expanded PBMC inhibit intracellular mycobacteria. PBMC were cultured in vitro with BCG, IPP + IL-2, or in medium alone for 7 days, and then co-cultured with autologous BCG-infected macrophages. BCG viability was determined 3 days later by H3-Uridine incorporation. PBMC expanded with BCG could inhibit intracellular mycobacterial growth (n=12/group; *p<0.005). PBMC expanded with IPP did not inhibit intracellular BCG (NS, p = 0.81 by Wilcoxon matched-pairs tests). C, Purified BCG-expanded γ9δ2 T cells inhibit intracellular mycobacteria. BCG expanded and purified γδ T cells (purity > 98% γδ-TCR+, CD3+ T cells), or total BCG-expanded PBMC, were co-cultured with infected macrophages at an effector:target ratio of 10:1. Both total BCG-expanded PBMC and purified BCG-expanded γδ T cells significantly inhibited intracellular mycobacterial growth compared with cultures containing medium rested cells alone (n=9/group; *p<0.01 by Wilcoxon matched pairs test), and the inhibitory effects of purified γδ T cells were significantly greater than the effects of total BCG-expanded PBMC (n=9/group; **p=0.011). D, Both BCG- and IPP-expanded γδ T cells express cytolytic effector functions. γδ T cells were purified from PBMC after 7 days of expansion with BCG or IPP+IL-2. Cr51-labeled Daudi cells and purified γδ T cells were cultured at various Effector:Target ratios for 4 hours at 37°C. Supernatants were harvested and percent lysis calculated as described. E, Mycobacteria and phosphoantigen stimulation induce similar levels of key effector molecules. PBMC from 2 PPD+ volunteers (Vol#1 and Vol#2) were rested in medium (open histogram with thin line), stimulated with 5 μg/mL mycobacterial lysate (open histogram with thick line), or stimulated with 250 pM HMB-PP (filled histogram) for 7 days. The levels of intracellular perforin, granzyme A, granulysin and IFN-γ were assessed by intracellular cytokine staining. Results shown are gated on CD3+ Vδ2+ T cells.
It is well known that IPP exclusively stimulates γ9δ2 T cells, while BCG stimulates γ9δ2 T cells as well as CD4+ and CD8+ αβ T cells. Therefore, the differential effector functions shown in Fig. 1B could have been explained by the fact that only BCG can induce protective αβ T cell responses, while IPP fails to induce protective αβ T cells. However, the results shown in Fig. 1C clearly demonstrate that BCG-expanded γ9δ2 T cells have important mycobacterial inhibitory effects. Highly purified γ9δ2 T cells (>98% pure) from BCG-expanded PBMC inhibited intracellular mycobacterial growth significantly greater than both total BCG-expanded PBMC populations (**p=0.011, n=9) and control cultures (*p<0.01, n=9) (Fig. 1C). These results confirm that BCG-expanded γ9δ2 T cells develop protective immune functions directed against intracellular mycobacteria.
Another possible explanation for the failure of IPP to induce protective γ9δ2 T cells could be that this purified phosphoantigen did not fully induce activated effector functions. Effector γ9δ2 T cells have been shown previously to be capable of cytolytic activity directed against tumor cells such as the EBV-transformed Daudi cell line (27,28). Although IPP-expanded γ9δ2 T cells were unable to inhibit intracellular mycobacterial growth, they demonstrated higher levels of lytic activity directed against the Daudi cell line compared with BCG-expanded γ9δ2 T cells (Fig. 1D). In addition, γ9δ2 T cells stimulated with phosphoantigen had similar levels of intracellular staining for the effector molecules perforin, granzyme, granulysin and IFN-γ (Fig. 1E) effectively ruling out the possibility that purified phosphoantigen stimulation alone failed to induce γ9δ2 T cell effector functions. These results demonstrate that while 10 μM IPP does stimulate cytolytic activity and other effector molecules in γ9δ2 T cells, these γ9δ2 T cells fail to inhibit intracellular mycobacterial growth. In contrast, live BCG- and MtbWL-stimulated γ9δ2 T cells express the same effector molecules and functions but are potently able to inhibit intracellular mycobacterial growth.
Failure of IPP-expanded γ9δ2 T cells to inhibit mycobacterial growth is not related to the dose or phosphoantigen potency
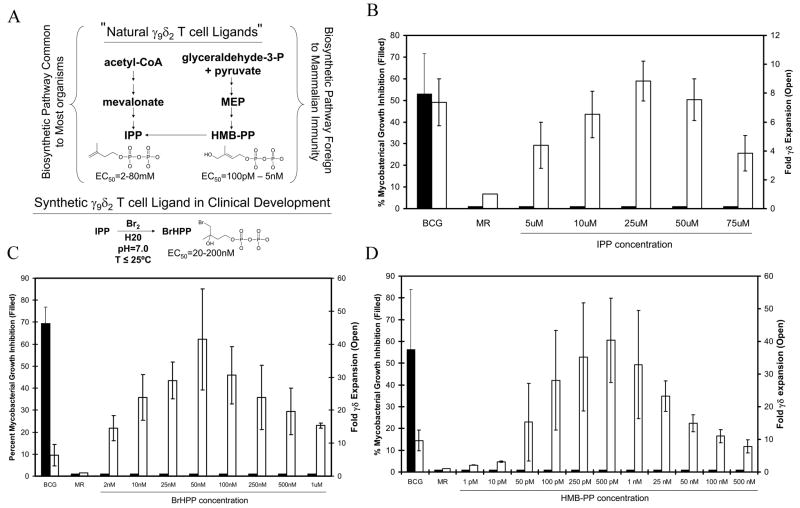
Although the 10 μM IPP concentration used in Fig. 1 induced greater expansions of γ9δ2 T cells than BCG stimulation in 3 out of 4 volunteer samples (Fig. 1A), we considered the possibility that optimal IPP concentrations for the induction of γ9δ2 T cell expansion and inhibitory function may be different, such that the concentrations used may have been optimal for expansion but not induction of inhibitory functions in γ9δ2 T cells. To address this possibility, we analyzed the inhibitory properties of PBMC expanded with a dose titration of IPP. As shown in Fig. 2B, γδ T cell expansion (Open bars) was maximal at an IPP concentration of 25 μM and then declined. While BCG-expanded PBMC inhibited intracellular mycobacterial growth by >50%, none of the IPP doses, even at concentrations much higher than required for optimal γδ T cell expansion, resulted in the generation of γ9δ2 T cells able to inhibit intracellular mycobacterial growth (Filled bars).
FIGURE 2.
Failure of IPP-expanded γ9δ2 T cells to inhibit mycobacterial growth is not related to IPP dose or phosphoantigen potency. A, Structure and synthesis of the 3 phosphoantigens used in these investigations. The mevalonate pathway of IPP biosynthesis (left side) is common to most organisms and converts acetyl Co-A to IPP through the intermediate mevalonate. In contrast, only bacteria and lower eukaryotes (right side) can convert gylceraldehyde-3-phosphate and pyruvate to IPP through the intermediates MEP, DOXP, and HMB-PP. A synthetic phosphoantigen has been developed for clinical applications by converting IPP into BrHPP. These phosphoantigens all stimulate the expansion of γ9δ2 T cells with the indicated potencies (EC50). B-D, The lack of inhibitory effects by IPP-expanded PBMC is independent of IPP concentration. PBMC were expanded with various concentrations of (B) IPP (5–75 μM), (C) BrHPP (2–1000 nM), or (D) HMB-PP (1 pM - 500 nM) and tested for their ability to inhibit mycobacterial growth (Filled Bars, left axis). The fold changes in expansion of γδ T cells detected by flow cytometry are depicted by Open Bars (right axis). Fold change was calculated as (%γδ T cells in stimulated cultures)/(%γδ T cells in medium rested cultures). Data shown are means ± SE (n=5 volunteers). BCG – BCG-expanded PBMC, MR – Medium Rested PBMC.
Currently, synthetic phosphoantigens with much more potent γ9δ2 T cell stimulatory activity than IPP are being developed clinically as potential new immunotherapies capable of inducing γ9δ2 T cells protective against tumors and infectious diseases. We next reasoned that IPP may not be potent enough to induce inhibitory γ9δ2 T cells, but more potent γ9δ2 T cell phosphoantigens (Fig. 2A) may be able to induce this inhibitory capacity. To address this possibility, we stimulated γ9δ2 T cells with the synthetic γ9δ2 TCR agonist in clinical development (BrHPP) which was previously shown to be 100–1,000 fold more potent for γ9δ2 T cell expansion than IPP (16), and assayed for the development of inhibitory γ9δ2 T cells (Fig. 2C). Maximal γδ T cell expansions (Open bars) were achieved with 50 nM concentrations of BrHPP. However, intracellular mycobacterial growth was unaffected by γδ T cells expanded with all doses of BrHPP (Filled bars).
Although IPP is known to be produced in mycobacteria-infected cells, concentrations of IPP needed to stimulate γ9δ2 T cell expansions are unlikely to be achieved in mycobacteria-infected cells. Furthermore, IPP is produced as a critical intermediate in the synthesis of isoprenoids in almost all living organisms via the mevalonate biosynthetic pathway (12). The ubiquitous nature of IPP and suboptimal IPP concentrations present in mammalian cells, strongly suggest that IPP is not the natural ligand responsible for inducing TB protective γ9δ2 T cells. The discovery of the MEP/DOXP pathway for IPP biosynthesis (Fig. 2A) present in bacteria (including mycobacteria) and lower eukaryotes (29), but not in mammalian cells, provided a potential alternative mechanism to explain mycobacteria-specific induction of TB protective γ9δ2 T cells. The intermediates produced in the MEP/DOXP pathway could be seen as “foreign” by the mammalian host. Furthermore, the penultimate metabolite in this pathway, HMB-PP, was shown to be at least 10,000 fold more potent for induction of γ9δ2 T cell expansion than IPP (15), such that physiologic concentrations of HMB-PP expressed in mycobacteria-infected cells could reasonably be expected to induce γ9δ2 T cells in vivo. After finding that IPP and BrHPP failed to induce γ9δ2 T cells inhibitory for intracellular mycobacteria, it was critically important to test whether γ9δ2 T cells stimulated with HMB-PP could generate γ9δ2 T cells with mycobacterial inhibitory effects (Fig. 2D). γδ T cells expanded (Open bars) in a HMB-PP concentration dependent manner with maximal proliferation seen at 500 pM. However, intracellular mycobacterial growth was unaffected by γδ T cells expanded with all doses of HMB-PP (Filled bars). The failure of these previously identified phosphoantigens to induce inhibitory γ9δ2 T cells was therefore not related to the doses used or the relative phosphoantigen potency.
Absence of secondary “danger signals” provided by viable mycobacteria or TLR signaling does not explain the failure of phosphoantigen-expanded γ9δ2 T cells to inhibit mycobacterial growth
We also considered the possibility that the induction of inhibitory γ9δ2 T cells requires additional signals provided by the metabolism of live BCG which would not be stimulated by purified phosphoantigens. We first tested whether viable mycobacteria were necessary for the induction of inhibitory γδ T cells. PBMC were stimulated for 7 days with live BCG or a whole killed lysate prepared from the M. tuberculosis Erdman strain, and then the expanded γδ T cells were purified and assayed for their ability to inhibit intracellular mycobacterial growth. γδ T cells stimulated with mycobacterial lysate and live BCG mediated similar inhibition of intracellular mycobacterial growth (Fig. 3A).
FIGURE 3.
The induction of inhibitory γ9δ2 T cells does not require viable mycobacteria or involve known TLR ligands expressed by mycobacteria. A, PBMC were either rested in medium or stimulated with live BCG or soluble M. tuberculosis (Mtb) lysates for 7 days. The expanded γδ T cells were immunomagnetically purified and then co-cultured at a 10:1 E:T ratio with BCG-infected macrophages to determine inhibitory effects against intracellular mycobacteria. *p< 0.05 comparing the effects of BCG- or MtbWL-expanded γδ T cells with control cultures by Wilcoxon matched pairs tests (n=5/group). B, TLR co-stimulation during IPP expansion does not generate inhibitory γ9δ2 T cells. PBMC were expanded with the indicated concentrations of TLR agonists in the presence or absence of 10 μM IPP for 7 days. Shown are fold expansions of γ9δ2 T cells (Open bars, right axis) and % inhibition of intracellular mycobacteria (Filled bars, left axis).
Live BCG and whole mycobacterial lysates contain many immunostimulatory molecules in addition to the phosphoantigens IPP and HMB-PP. For example, live BCG and mycobacterial lysates are capable of providing secondary signals through multiple different innate TLR. We next tested whether the non-inhibitory IPP-expanded γ9δ2 T cells could be converted to inhibitory γ9δ2 T cells by co-stimulation with TLR agonists. Specific TLR ligands were chosen for our studies because of previous reports that either 1) mycobacteria contain ligands for the same TLR signaling pathways [Pam3Cys, ligand for TLR1/2 (30–33); LPS, ligand for TLR4 (32,33); and CpG-containing oligonucleotides, ligands for TLR9 (34)], or 2) the expression of the corresponding TLR has been shown to be expressed by γ9δ2 T cells [polyI:C, ligand for TLR3 (35)]. PBMC were stimulated with dose titrations of the different TLR agonists in medium containing 20 U/mL of IL-2, and in the presence or absence of 10 μM IPP. Fig. 3B shows that co-stimulation of PBMC with high concentrations of the TLR ligands Pam3Cys, PolyI:C, and CpG oligos during IPP stimulation did not significantly alter the IPP-induced expansion of γ9δ2 T cells (Open bars) or result in γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth (Filled bars). PBMC co-stimulated with LPS were able to mediate low levels of intracellular mycobacterial growth inhibition. However, these inhibitory effects were independent of the presence of IPP and did not correlate with the expansion of γ9δ2 T cells, indicating that these effects were probably related to the induction of other innate immune functions induced by contamination of LPS in the co-culture and was independent of γ9δ2 T cell stimulation. Similar results were seen with all concentrations of these TLR ligands tested (as described in Materials and Methods). These results demonstrated that co-stimulation via the TLR signaling pathways known to be inducible by mycobacterial infections and/or expressed by γ9δ2 T cells were not sufficient to convert IPP-expanded γ9δ2 T cells into effector cells capable of inhibiting intracellular mycobacterial growth.
IPP and live BCG induce γ9δ2 T cells with different TCR spectratypes
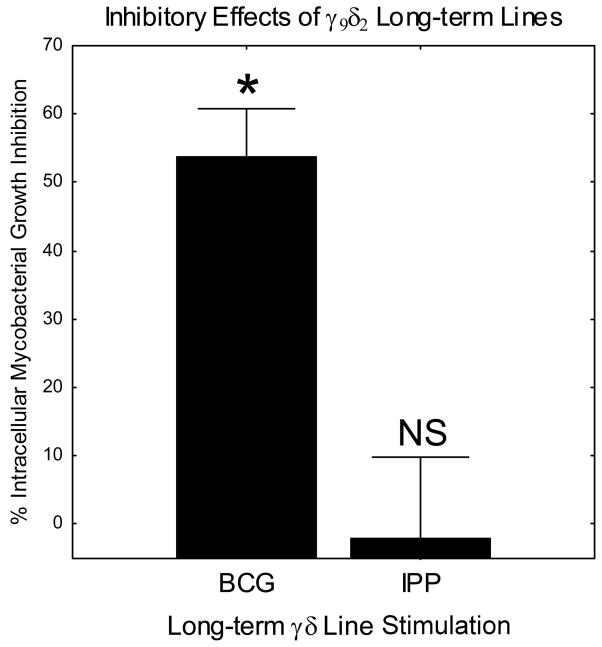
Since we were unable to generate inhibitory γ9δ2 T cells by stimulation with purified phosphoantigens in the presence or absence of TLR ligands, we hypothesized that BCG infection may in fact be stimulating a different subset of γ9δ2 T cells. In order to study this possibility, we generated long-term BCG- and IPP-expanded γ9δ2 T cell lines as described in Materials and Methods. By serial stimulation and expansion using DC infected with live BCG or cultured with IPP, we were successful in generating stable BCG- and IPP-stimulated γ9δ2 T cell lines, respectively. These BCG- and IPP-stimulated γ9δ2 T cell lines retained the differential inhibitory properties of the corresponding 7 day expansion cultures (Fig. 4). Therefore, we reasoned that we could use these paired γ9δ2 T cell lines to examine whether the TCR sequences expressed by inhibitory BCG-stimulated and non-inhibitory IPP-stimulated γ9δ2 T cells were different. Antigen specificity is largely determined by the sequences encoded by the hypervariable CDR3 regions of the TCR. To study differences in the specificities of the γ9δ2 T cell lines, we analyzed the diversity of the Vγ9 and Vδ2 TCR CDR3 loops expressed by these populations of T cells. TCR spectratyping generates a spectrum of PCR fragments of different lengths representative of the γ9δ2 TCR CDR3 length diversity in the cellular population.
FIGURE 4.
Long-term BCG- and IPP-stimulated γ9δ2 T cell lines also display differential inhibitory effector functions. Long-term γ9δ2 T cell lines were generated by serial stimulation with IPP-pulsed or BCG-infected autologous DC every two weeks adding fresh IL-2 every 2–3 days. Since DC cannot retain IPP following washing, 10 μM IPP was added to the culture medium during stimulation of IPP-expanded lines. γ9δ2 T cell lines at the end of a stimulation cycle were co-cultured with BCG-infected macrophages. *p< 0.05 comparing BCG-stimulated and IPP-stimulated inhibitory responses by Wilcoxon matched pairs test (n=5). Comparisons of IPP-stimulated inhibitory responses and medium rested control cultures were not significantly different (p = 0.89, n=5).
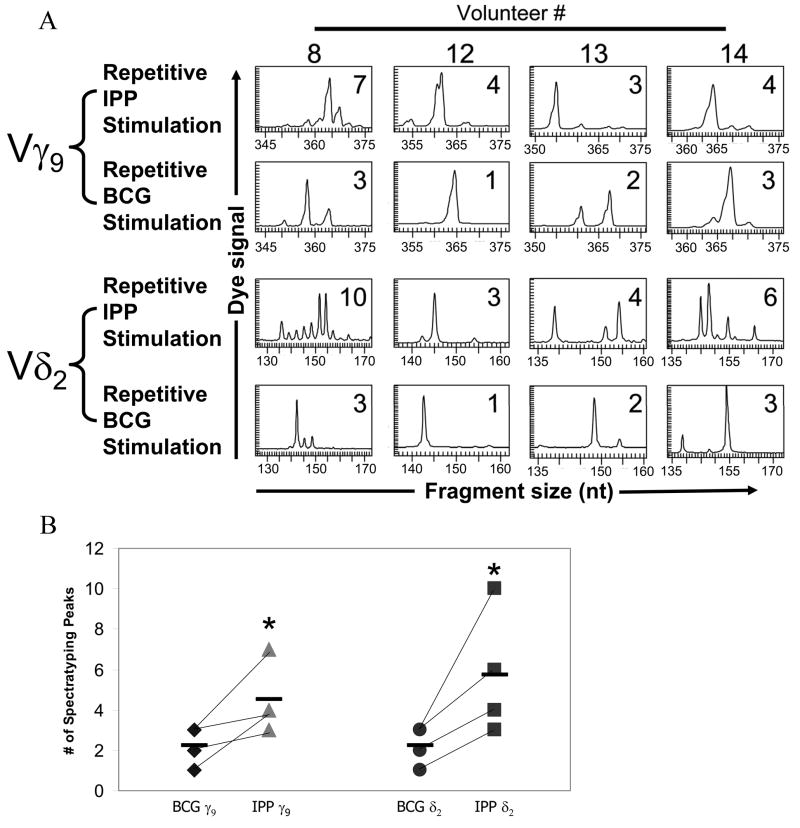
TCR spectratypic analyses of long-term γ9δ2 T cell lines identified dramatic differences in the CDR3 length distributions and frequencies utilized by BCG-stimulated and IPP-stimulated long-term γ9δ2 T cell lines. In four pairs of long-term γ9δ2 T cell lines, all BCG-expanded γ9δ2 T cell lines contained a more highly restricted spectratypic profile compared with the more polyclonal diversity present in the matching IPP-expanded γ9δ2 T cell lines (Fig. 5A). For both γ9 and δ2 TCR chains, BCG-expanded γ9δ2 long-term T cell lines had less diversity in their CDR3 regions indicating a more focused population. This difference was most pronounced comparing δ2 TCR chains presumably because of the increased potential for diverse sequences due to the additional D gene segment rearrangements and nucleotide additions/substitutions. TCR diversity was significantly lower in the BCG-stimulated γ9δ2 T cell lines (*p< 0.04) for both the γ9 and δ2 chains (Fig. 5B). The more limited CDR3 diversity expressed by BCG-expanded γ9δ2 T cell lines suggested that BCG stimulates only a limited subset of γ9δ2 T cells compared with the more broadly reactive IPP stimulation. Furthermore, paired BCG- and IPP-stimulated γ9δ2 T cell lines displayed spectratypes with distinctly different predominant CDR3 lengths. These results further suggest that while BCG induces the expansion of specific subsets of γ9δ2 T cells, IPP has a more polyclonal and perhaps nonspecific effect on γ9δ2 T cells reminiscent of a T cell mitogen or superantigen. In any case, it is evident that BCG and IPP expand γ9δ2 T cells expressing unique patterns of γ9δ2 TCR CDR3 rearrangements.
FIGURE 5.
TCR Spectratyping profile of BCG- and IPP-stimulated long-term γ9δ2 T cell lines. A, Total RNA was harvested from γδ T cell lines after a minimum of four stimulations with BCG-infected or IPP-pulsed dendritic cells. RT-PCR was used to specifically amplify the Vγ9 and Vδ2 chain CDR3 regions of the γδ T cell receptors. The sizes and frequencies of different PCR fragments were detected with the CEQ8000 genetic analysis system. The number of peaks detected by the CEQ8000 software is displayed in the upper right corner of each graph. B, A comparison of the number of CDR3 fragment peaks obtained with BCG- and IPP-stimulated Vγ9 and Vδ2 TCR spectratypic profiles from all 4 volunteers. BCG-stimulated Vγ9 and Vδ2 TCR spectratypes contained significantly fewer peaks compared with IPP-stimulated Vγ9 and Vδ2 TCR spectratypes (*p<0.04 by Mann-Whitney U tests), and the predominant peaks identified in BCG- and IPP-stimulated spectratypes were distinct.
Sequencing of the CDR3 regions confirm that BCG and IPP induce different populations of γ9δ2 T cells
We have shown that BCG and IPP stimulation expand γδ T cell populations that differ in their expressed CDR3 lengths. However, a single CDR3 length can encode multiple amino acid sequences and thus TCR spectratyping alone may not reveal the full levels of diversity present within T cell populations. To confirm that BCG stimulates a limited diversity of γ9δ2 T cells compared with IPP, we cloned the δ2 (Table I) and γ9 (Table II) CDR3 PCR fragments from selected pairs of BCG- and IPP-stimulated long-term γ9δ2 T cell lines generated from PPD+ volunteers, and sequenced at least ten randomly chosen clones for each to obtain a more complete understanding of the variations within the CDR3 regions of the expressed γδ TCR.
Table I.
Sequencing of the CDR3 regions of the TCR δ2 chain
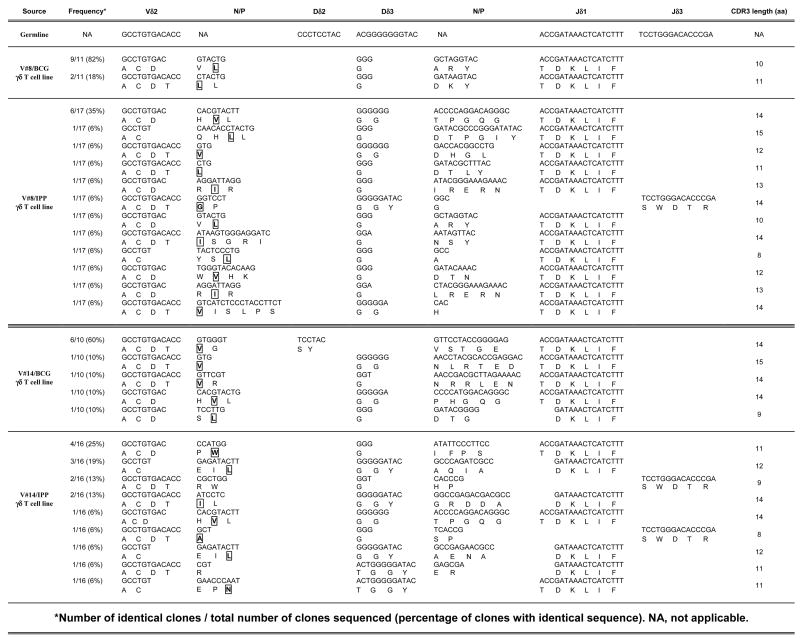
|
Number of identical clones/total number of clones sequenced (percentage of clones with identical sequence). NA, not applicable.
First strand cDNA from the reverse transcription step used to generate the data presented in Fig. 5 were amplified with specific Vδ2 and Cδ region primers spanning the CDR3 regions. The PCR fragments were cloned into a TA cloning vector and at least ten randomly selected colonies were picked for amplification. Plasmid DNA was harvested and sequenced across the insert. Sequences were detected by the CEQ8000 genetic analysis system. Conserved hydrophobic residues indicated with boxes and bold font. The presence of N/P nucleotides adds additional diversity to the TCR CDR3.
Table II.
Sequencing of the CDR3 regions of the TCR γ9 chain
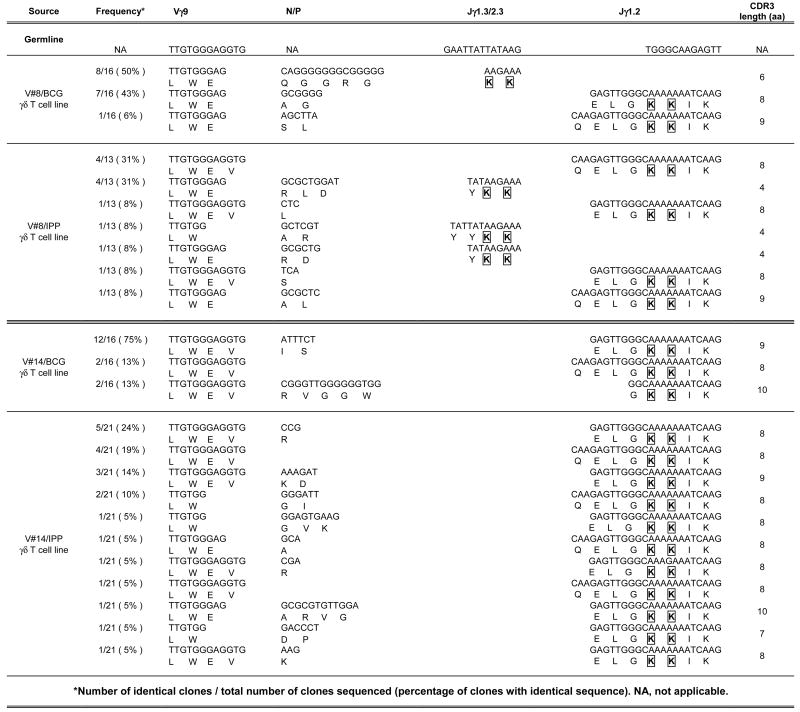
|
Number of identical clones/total number of clones sequenced (percentage of clones with identical sequence). NA, not applicable.
First strand cDNA from the reverse transcription step used to generate the data presented in Fig. 5 were amplified with specific Vγ9 and Cγ region primers spanning the CDR3 regions. The PCR fragments were cloned into a TA cloning vector and at least ten randomly selected colonies were picked for amplification. Plasmid DNA was harvested and sequenced across the insert. Sequences were detected by the CEQ8000 genetic analysis system. Conserved adjacent lysine residues are indicated with boxes and bold font. The presence of N/P nucleotides adds additional diversity to the TCR CDR3.
Our sequencing results confirm that BCG and IPP stimulation induced different subsets of γ9δ2 T cells. As seen by spectratyping, the CDR3 sequences of BCG-expanded populations were more oligoclonal while the CDR3 sequences of IPP-expanded populations were more polyclonal. For example, the randomly selected Vδ2 CDR3 clones shown in Table I from volunteer #8’s BCG-stimulated long-term γ9δ2 T cell line consisted of only 2 different CDR3 sequences with one predominant sequence present in 9 out of 11 clones sequenced (82%). Long-term γ9δ2 T cell lines generated from the same volunteer by IPP expansion produced 12 different Vδ2 CDR3 sequences, with the most common sequence present in only 6 out of 17 clones sequenced (35%). It should be noted that the predominant Vδ2 CDR3 sequence found in the BCG-expanded population from volunteer #8 was present among the more heterogeneous matched IPP-expanded population, but only at a frequency of 1 in 17 sequenced clones (6%). In addition, most of these sequences contained conserved hydrophobic residues (boxed and in bold font) upstream of the D region sequence previously shown to be associated with γ9δ2 T cell phosphoantigen reactivity (36), suggesting that the presence of these residues alone does not account for differential inhibitory activity
Similarly, Table II demonstrates that BCG-expanded γ9δ2 T cell lines expressed Vγ9 CDR3 regions with much less diversity than IPP-expanded γ9δ2 T cell lines. In agreement with data previously published showing that the Jγ1.2 segment was associated with phosphoantigen responsiveness (37), the majority of our expanded γ9δ2 T cells expressed the Jγ1.2 segment, while few γ9 TCR expressed the Jγ1.3/2.3 segments. In addition, all of the sequenced Jγ regions contained the conserved adjacent lysine motifs (boxed and in bold font) previously shown to be critical for phosphoantigen recognition (38). While these residues may be critical for binding of the phosphoantigen to the TCR, they do not appear to correlate with mycobacterial inhibitory activity.
These data demonstrate that IPP can stimulate a larger subset of γ9δ2 T cells inclusive of BCG-activated γ9δ2 T cells, also consistent with IPP having a mitogen-like effect. These results further support our hypothesis that BCG-infected APC stimulate a subset of γ9δ2 T cells, while IPP stimulates the nonspecific expansion of more polyclonal γ9δ2 T cells. A high degree of correlation between the frequencies of specific CDR3 fragment lengths detected was observed comparing our TCR spectratyping and sequencing results indicating that the clones sequenced were highly representative of the total heterogeneity present within the γ9δ2 T cell populations and not skewed by biased cloning efficiency (Fig. 6).
FIGURE 6.
High correlations between Sequencing and Spectratyping data rule out artifactual in vitro skewing during the cloning techniques used to obtain sequencing results. Continuous variables for comparative analysis were generated for both sequencing and spectratyping data by multiplying the nucleotide size of each fragment times its observed frequency within the population. Spearman-rank correlations were analyzed between these nt size * frequency products for each individual’s paired data as described in Experimental Procedures. R2 coefficients were 0.704, 0.983, and 0.951 for all three different volunteers (all p < 0.0001 by Spearman-rank tests).
Heterologous stimulations of BCG- and IPP-expanded γ9δ2 T cell lines confirm that BCG-activated inhibitory γδ T cells are a subset of IPP-responsive γ9δ2 T cells
Our results have led us to believe that BCG-infected DC can induce a pathogen-specific subset of IPP-responsive γ9δ2 T cells, while IPP acts more like a polyclonal mitogenic stimulus, broadly stimulating most, if not all peripherally circulating γ9δ2 T cells. If this hypothesis is correct, BCG-expanded γ9δ2 T cell lines should be fully responsive to IPP, while only a fraction of the more heterogeneous population of IPP-expanded γ9δ2 T cell lines would respond optimally to BCG unless of course BCG-specific γ9δ2 T cells have been expanded in vivo to represent the predominant γ9δ2 T cells circulating at the time of our PBMC harvests. However, all volunteers studied here were healthy PPD+ individuals with remote histories of BCG vaccination and/or chronic latent TB infection. Therefore, none of these volunteers would be expected to have had ongoing high level mycobacterial replication at the time of their lymphocyte harvests which might be expected to expand mycobacteria-specific γ9δ2 T cells in vivo as the predominant activated/memory population among circulating γ9δ2 T cells. Shown in Fig. 7 are comparisons of the intracellular IFN-γ response to homologous and heterologous stimulations of paired BCG- and IPP-expanded long-term γ9δ2 T cell lines generated from five different PPD+ individuals. The response to the homologous stimulus was defined as the “100%” response for each γδ T cell line. The % heterologous response was defined as the fraction of the response detected after stimulation with the heterologous antigen divided by the homologous response times 100. The mean heterologous response of BCG-expanded γδ T cells to IPP stimulation was 90% ± 5% (not significantly different from the homologous responses). In contrast, the mean heterologous response of IPP-expanded γδ T cells to BCG stimulation was only 26% ± 7% of the homologous IPP response (*p < 0.05 compared with homologous responses). These results indicate that virtually all γ9δ2 T cells expanded with BCG can respond to IPP stimulation, but only a minor fraction of the γ9δ2 T cells expanded with IPP can recognize BCG-infected DC.
FIGURE 7.
Only a subset of IPP-responsive γ9δ2 T cells recognize BCG. A, BCG-stimulated long-term γ9δ2 T cell lines respond to both BCG and IPP, while IPP-stimulated long-term γ9δ2 T cell lines preferentially respond to IPP. These data are consistent with the hypothesis that BCG induces pathogen-specific γ9δ2 T cells, while IPP has a polyclonal, non-specific mitogenic effect. Long-term γ9δ2 T cell lines were incubated with BCG-infected or IPP-pulsed dendritic cells for 16 hours. IFN-γ production was detected by intracellular cytokine staining. The homologous response (the response to the same antigen used for generation of the lines) was defined as the “100%” response for each γ9δ2 T cell line. Responses in BCG-stimulated long-term γ9δ2 T cell lines stimulated with BCG and IPP were not significantly different by Wilcoxon matched pairs test (n=5). *p<0.05 comparing IPP-stimulated long-term γ9δ2 T cell lines stimulated with BCG and IPP by Wilcoxon matched pairs test (n=5). B, Clonal analysis confirms differential functions in the majority of BCG- and IPP-expanded γ9δ2 T cells, providing further evidence that protective γ9δ2 T cells are a subset of IPP-responsive γ9δ2 T cells. γ9δ2 T cell clones were generated from matched BCG- and IPP-stimulated PBMC and the inhibitory effects of these clones on intracellular mycobacterial growth were measured. Clones able to inhibit mycobacterial growth were defined as those clones resulting in mycobacterial growth less than the mean minus standard error of growth detected in control wells without T cells added. *p<0.04 by χ2 test (n=11 and n=21 for BCG- and IPP-stimulated γ9δ2 T cell clones, respectively). **p<0.004 by χ2 test (n=21 for both BCG- and IPP-stimulated γ9δ2 T cell clones).
Limiting dilution cloning analyses further confirm that γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth are only a subset of IPP-responsive γ9δ2 T cells
Our results indicated that BCG-stimulated γ9δ2 T cells represent a subset of IPP-responsive γ9δ2 T cells which mediate inhibition of intracellular mycobacterial growth. These data imply that BCG-stimulated γ9δ2 T cells able to inhibit intracellular mycobacterial growth should be present as a minor fraction of phosphoantigen-reactive γ9δ2 T cells in vivo. In order to test this hypothesis, we generated γ9δ2 T cell clones by limiting dilution from matched BCG- and IPP-stimulated PBMC from two PPD+ volunteers and tested the ability of individual clonal populations of γ9δ2 T cells to inhibit intracellular mycobacterial growth (Fig. 7B). Clones able to inhibit mycobacterial growth were defined as those clones that suppressed mycobacterial growth to levels less than the mean minus standard error of growth detected in control wells without γδ T cell clones added. As predicted, only a minor fraction of the γ9δ2 T cell clones generated following IPP-expansion (33% and 23% for volunteers 1 and 2, respectively) were able to inhibit intracellular mycobacterial growth. In contrast, significantly more γ9δ2 T cell clones generated following BCG-expansion of PBMC (72% and 76% for volunteers 1 and 2, respectively) were able to inhibit intracellular mycobacterial growth (*p-value < 0.04 by χ2; **p-value < 0.004 by χ2). These data confirm at the clonal level that γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth are a minor fraction of IPP-responsive γ9δ2 T cells present in circulating lymphocytes.
γδ T cell clones capable of inhibiting intracellular mycobacterial growth do not have a higher TCR avidity for phosphoantigens than the non-inhibitory γδ T cell clones
Our results indicate that BCG-stimulated γ9δ2 T cells represent a subset of the more broadly reactive phosphoantigen-responsive γ9δ2 T cells. However, apparent “specificity” for BCG could be explained by selection for γ9δ2 TCR which have a higher affinity for the phosphoantigens produced in low concentrations by BCG infection and not a true difference in antigen specificity. In order to determine whether γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth respond to lower concentrations of phosphoantigen (i.e. have a higher avidity), we stimulated mycobacterial inhibitory and non-inhibitory γ9δ2 T cell clones with a dose titration of BCG and HMB-PP and compared the intracellular IFN-γ responses induced. Table III reports the percentages of γ9δ2 T cells that produced IFN-γ after overnight stimulation with DC infected with a dose titration of BCG (MOI = 1–100) or incubated with a dose titration of HMB-PP (10–1000 pM). Similar to the results obtained with the long-term heterogeneous γ9δ2 T cell lines (Fig. 7A), BCG-inhibitory γ9δ2 T cell clones produced IFN-γ after stimulation with BCG-infected DC while non-inhibitory γ9δ2 T cell clones did not produce IFN-γ responses detectable above background levels after stimulation with BCG-infected DC. Also similar to the results presented in Fig. 7A, both populations responded similarly to HMB-PP stimulation. Importantly, BCG-inhibitory γ9δ2 T cell clones did not respond to lower concentrations of HMB-PP than did non-inhibitory γ9δ2 T cell clones. If anything, the non-inhibitory γ9δ2 T cell clones seemed to have a higher avidity for HMB-PP than the inhibitory γ9δ2 T cell clones. These data confirm our previous results that BCG-stimulated γ9δ2 T cells are a subset of phosphoantigen-reactive γ9δ2 T cells and exclude the possibility that BCG-stimulated γ9δ2 T cells are selectively expanded due to a higher TCR avidity for phosphoantigens.
Table III.
Responses of BCG-inhibitory and non-inhibitory γ9δ2 T cell clones to dose titrations of BCG and phosphoantigen.
| BCG stimulation (MOI) |
HMB-PP stimulation (pM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 100 | 0 | 50 | 100 | 1000 | ||
| Inhibitory clones | 2C11 | 2.62 | 8.02 | 12.92 | 17.45 | 2.62 | 8.76 | 15.73 | 14.46 |
| 2E8 | 2.11 | 6.13 | 10.53 | 18.65 | 2.11 | 6.12 | 9.30 | 15.12 | |
| 2C8 | 2.46 | 6.31 | 11.06 | 12.81 | 2.46 | 4.97 | 7.19 | 8.62 | |
| Non-inhibitory clones | 2C4 | 2.53 | 4.56 | 5.31 | 4.97 | 2.53 | 16.63 | 12.14 | 16.12 |
| 2G7 | 2.23 | 4.18 | 4.95 | 3.75 | 2.23 | 6.45 | 12.35 | 14.33 | |
| 2C6 | 2.77 | 3.92 | 4.75 | 3.87 | 2.77 | 14.96 | 17.38 | 17.59 | |
Percentage of γδ T cells producing IFN-γ after overnight stimulation with DC infected with BCG at the indicated MOI or pulsed with HMB-PP at the indicated dosage.
Discussion
It is becoming increasingly recognized that γδ T cells, especially the phosphoantigen responsive Vγ9+Vδ2+ subset, are relevant for both innate and adaptive protective immunity against intracellular pathogens. Because both live mycobacteria and purified prenyl pyrophosphates, such as IPP, BrHPP and HMB-PP, induce similar expansions of γδ T cells capable of IFN-γ production and cytolytic activity, it has been suggested that phosphoantigens may be useful components of new TB vaccines or immunotherapies (17–19). However, our work indicates that synthetic phosphoantigens alone, or in combination with TLR ligands, will not induce TB-specific memory γ9δ2 T cells capable of inhibiting mycobacterial growth relevant for prophylactic vaccine development. On the other hand, γ9δ2 T cells expanded with mycobacteria-infected cells clearly can provide potent mycobacterial inhibitory effects important for protective TB immunity, and therefore the mechanisms mediating their induction should be investigated.
IPP was one of the first phosphoantigens shown to induce γ9δ2 T cell expansion, is known to be produced in mycobacteria-infected cells, and was the only purified γ9δ2 T cell stimulus readily available when we began the investigations reported here. However, concentrations of IPP needed to stimulate γ9δ2 T cell expansions are unlikely to be achieved in mycobacteria-infected cells. Furthermore, IPP is already produced as a critical intermediate in the synthesis of isoprenoids in almost all living organisms via the mevalonate biosynthetic pathway (12). The ubiquitous nature of IPP and suboptimal IPP concentrations present in mammalian cells strongly suggested that IPP alone is not the ligand responsible for inducing TB-protective γ9δ2 T cells. The discovery of a second pathway for IPP biosynthesis (MEP/DOXP pathway) present in bacteria (including mycobacteria) and lower eukaryotes (29), but not in mammalian cells, provided a potential alternative mechanism to explain mycobacteria-specific induction of TB-protective γ9δ2 T cells. The intermediates produced in the MEP/DOXP pathway could be seen as “foreign” by the mammalian host. Furthermore, the penultimate metabolite in this pathway, HMB-PP, was shown to be at least 10,000 fold more potent for induction of γ9δ2 T cell expansion than IPP (15), such that physiologic concentrations of HMB-PP expressed in mycobacteria-infected cells could reasonably be expected to induce γ9δ2 T cells in vivo. After finding that IPP failed to induce γ9δ2 T cells inhibitory for intracellular mycobacteria, it was critically important to test whether γ9δ2 T cells stimulated with HMB-PP, or another highly potent synthetic γ9δ2 T cell agonist currently in clinical development [BrHPP (16)], could generate γ9δ2 T cells with mycobacterial inhibitory effects. Similar to our results with IPP stimulation, neither HMB-PP nor BrHPP were able to stimulate the development of γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth, despite being much more potent in inducing expansion of γ9δ2 T cells even when compared with BCG stimulation. These combined results indicated that none of the previously identified phosphoantigens by themselves could induce γ9δ2 T cells relevant for protective mycobacterial immunity.
A possible explanation for the failure of IPP, BrHPP and HMB-PP to induce protective γ9δ2 T cells could be that these purified phosphoantigens were not fully inducing activated effector functions. Concentrations of IPP, BrHPP or HMB-PP optimal for γδ T cell expansion were unable to induce γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth. However, γ9δ2 T cells stimulated with IPP expressed similar levels of the effector molecules perforin, granzyme A, granulysin and IFN-γ and displayed more efficient cytolytic activity for Daudi target cells compared with mycobacteria-stimulated γ9δ2 T cells, effectively ruling out the possibility that purified phosphoantigen stimulation alone failed to induce γ9δ2 T cell effector functions. Recently, γ9δ2 T cells capable of lysing BCG-infected macrophages were identified by cell surface staining to belong to a subset of T cells identified as CD45RA− and CD27+ (39). If this population of T cells were uniquely responsible for mycobacterial inhibitory effects, it could be hypothesized that BCG induced differentiation of γ9δ2 T cells into this subset while IPP failed to do so. Therefore, we analyzed the frequency of CD45RA−CD27+ γ9δ2 T cells induced by stimulation with mycobacteria compared with phosphoantigen and observed that both stimuli induced similar frequencies of this γ9δ2 T cell subset (data not shown). Several reports have implicated receptors typically displayed on NK cells in the modulation of γ9δ2 T cells (40–43). We considered that these receptors may act as co-stimulatory molecules recognizing BCG-infected macrophages [esp. NKp44 which binds mycobacterium directly (43)]. Flow cytometric staining revealed no differences in the levels of NKp44, NKp46, CD94/NKG2D, KIR-NKAT2, NKB1 or CD161 expressed on BCG-expanded γ9δ2 T cell clones compared with IPP-expanded γ9δ2 T cell clones (data not shown). In addition, co-stimulation through TLR 1/2, 3, 4, and 9 during IPP expansion also failed to induce inhibitory γ9δ2 T cells. These combined results indicate that the failure of purified phosphoantigens to induce TB-protective γ9δ2 T cells could not be explained by inadequate phosphoantigen dose or potency, failure to induce effector functions or specific effector subsets, or lack of TLR mediated co-stimulation.
Another possible mechanism for the differential ability of mycobacteria- and phosphoantigen-stimulated γ9δ2 T cells to inhibit intracellular mycobacterial growth could be related to the recently published antigen presenting functions mediated by activated γ9δ2 T cells (44). Phosphoantigen-stimulated γ9δ2 T cells could become potent APC capable of activating protective effector functions in mycobacterial peptide-specific memory αβ T cells. Since the mycobacterial peptides are absent from purified phosphoantigen stimulated cultures, protective memory αβ T cell responses would not be induced. Indeed, we have shown that γ9δ2 T cells can enhance the effector functions provided by mycobacteria-specific αβ T cells (data submitted for publication). However, we also have demonstrated that both γ9δ2 T cells purified to greater than 98% after 7 day expansions with live mycobacteria, and BCG-stimulated long-term γ9δ2 T cell lines and clones not contaminated with αβ T cells, can directly inhibit intracellular mycobacteria. Therefore, APC and/or helper functions cannot explain the differential effector functions provided by highly purified γ9δ2 T cells stimulated with live BCG and purified phosphoantigens.
Selective expansion of a subset of γ9δ2 T cells expressing high affinity TCR also could explain the inhibitory effects mediated by BCG-stimulated, but not purified phosphoantigen-stimulated, γ9δ2 T cells. Lower levels of phosphoantigens expressed during mycobacterial infection might be capable of stimulating only a subset of γ9δ2 T cells with the highest TCR affinity and the possibility exists that only γ9δ2 T cells expressing TCR with the highest affinity can mediate protective functions against intracellular mycobacteria. On the other hand, exogenous phosphoantigen added at optimal concentrations might stimulate all γ9δ2 T cells regardless of TCR affinity. If this were true that only the γ9δ2 T cells with the highest affinity TCR were capable of inhibiting intracellular mycobacteria, we would predict that limiting doses of exogenously added purified phosphoantigen should preferentially stimulate high affinity γ9δ2 T cell subsets capable of mycobacteria-protective effects. However, in our dose titration experiments with the purified phosphoantigens IPP, BrHPP and HMB-PP, we did not observe inhibitory effects mediated by γ9δ2 T cells stimulated with suboptimal concentrations that still led to γ9δ2 T cell expansion. More directly, BCG-inhibitory γ9δ2 T cell clones stimulated with titrations of HMB-PP did not produce IFN-γ at lower HMB-PP concentrations compared with non-inhibitory γ9δ2 T cell clones. In fact, a greater percentage of BCG non-inhibitory γ9δ2 T cell clones produced IFN-γ at lower HMB-PP concentrations than did BCG-inhibitory γ9δ2 T cell clones. It also is conceivable that purified phosphoantigens could induce a selective depletion of inhibitory γ9δ2 T cells expressing high affinity TCR similar to the recently reported effects of repeated BrHPP injection in monkeys (18). However, we observed that a fraction of γ9δ2 T cell clones from IPP-expanded PBMC were capable of inhibiting intracellular mycobacterial growth demonstrating that inhibitory γ9δ T cells can be expanded with phosphoantigen. This suggests that phosphoantigen stimulation of γ9δ2 T cells does not result in the selective depletion of inhibitory γ9δ2 T cells. Therefore, while differences in affinity of γ9δ2 TCR for relevant mycobacterial antigens likely exist, selective expansion of γ9δ2 T cells based on TCR affinity does not appear to explain the differential inhibitory effects of mycobacteria- and purified phosphoantigen-stimulated γ9δ2 T cells.
Because none of the potential mechanisms discussed above can explain the differential protective effects of mycobacteria- and purified phosphoantigen-stimulated γ9δ2 T cells, we examined the antigenic specificity of BCG- and IPP-reactive γδ T cells. Crystallographic studies of the γ9δ2 TCR suggest that the same hypervariable regions critical for αβ TCR stimulation by antigen/MHC are important for γ9δ2 TCR stimulation (45). In addition, phosphoantigen reactivity has been shown to be dependent upon the sequence of the rearranged Vγ9/Jγ1.2 CDR3 gene segment (37) and co-expression of the Vγ9 and Vδ2 TCR chains (46). Therefore, we examined the antigen-specificity of BCG- and IPP-stimulated γ9δ2 T cells by comparing the diversity of Vγ9 and Vδ2 CDR3 regions of the TCR expressed by differentially expanded γ9δ2 T cell long-term lines. Our results demonstrate that BCG-expanded γ9δ2 T cells undergo a phenomenon consistent with “antigenic focusing” similar to αβ T cells, which in some cases resulted in the appearance of a single peak detected by TCR spectratyping. This apparent antigenic focusing indicates that only certain subsets of γ9δ2 T cells present in persons with mycobacterial immunity are relevant for protection. Consistent with this conclusion, we demonstrated that only a fraction of IPP-reactive γ9δ2 T cells were able to respond to BCG-infected DC by production of intracellular IFN-γ. In addition, only a minority of γ9δ2 T cell clones expanded with IPP were found to inhibit intracellular mycobacterial growth while most BCG-expanded γ9δ2 T cell clones could inhibit intracellular mycobacterial growth.
Previous sequencing of multiple human phosphoantigen-responsive γ9δ2 T cell clones, in combination with mutagenesis and transfection studies focused on a single phosphoantigen-responsive TCR, collectively demonstrated that 2 adjacent and highly conserved lysine residues within the Jγ1.2 regions of the Vγ9 CDR3 regions were critical for functional recognition of phosphoantigens (36,38). Molecular modeling studies suggested that these conserved lysines might form a positively charged pocket in which the negatively charged pyrophosphate moieties present on γ9δ2 T cell stimulatory phosphoantigens could dock (36). While a high frequency of our expanded γ9δ2 T cells expressed Jγ1.2, we also observed that both BCG- and IPP-expanded γ9δ2 T cell lines included clones expressing Jγ1.3. These Jγ1.3 expressing Vγ9 CDR3 regions also encode a pair of adjacent lysine residues that may provide for docking of pyrophosphate in the γ9δ2 TCR. The previous structural studies also suggested the importance of a conserved hydrophobic or neutral residue within the 5′ portion of the Vδ2 CDR3 region expressed by phosphoantigen-responsive γ9δ2 T cells (36). Consistent with this previous finding, almost all of the Vδ2 CDR3 clones we identified in BCG- and IPP-expanded γ9δ2 T cells expressed a 5′ hydrophobic or neutral amino acid residue.
The previous γ9δ2 TCR sequencing studies suggested that besides the conserved lysines in the Vγ9 CDR3 region and the conserved hydrophobic/neutral residue within the Vδ2 CDR3, phosphoantigen-responsive γ9δ2 TCR are highly diverse. This is also reflected in our findings with IPP-expanded γ9δ2 T cell lines. Thus purified phosphoantigens may interact with only a very small region of the γ9δ2 TCR. Our findings of more highly restricted diversity expressed by BCG-expanded γ9δ2 T cell lines suggest that mycobacterial antigens other than the previously identified phosphoantigens may be expressed that induce inhibitory γ9δ2 T cells. Alternatively, more complex antigenic structures containing phosphoantigen cores or intermolecular combinations of phosphoantigens with unknown presenting elements could alter the antigenic face of the presented phosphoantigens and induce the CDR3 focusing observed in inhibitory γ9δ2 T cell subsets. However, at this point we cannot rule out the possibility that phosphoantigen alone may contact the γ9δ2 TCR while an additional activating receptor present on only a subset of γ9δ2 T cells must be concurrently engaged to induce the more restricted populations with inhibitory activity for intracellular mycobacteria.
Our results also are reminiscent of the demonstration that 2 types of αβ T cells exist that appear to respond to different conformations of peptide epitopes generated by 1) stimulation with epitopes processed intracellularly and loaded onto class II MHC molecules with the aid of HLA-DM molecules in MIIC compartments, versus 2) synthetic peptide epitopes loaded onto empty MHC molecules at the cell surface or in endosomes without the need for intracellular processing (47). So-called type A T cells respond to both epitopes processed intracellularly from native antigen as well as purified peptide epitopes pulsed onto extracellular MHC. On the other hand, type B T cells only respond to peptide pulsed APC and not native antigens requiring intracellular processing. IPP-expanded γ9δ2 T cells may be similar to type B αβ T cells responding to purified phosphoantigens supplied exogenously and directly loaded onto unknown APC surface elements required for presentation, bypassing the normal processing of γ9δ2 T cell stimulatory antigens that are presented during natural infection of APC by mycobacteria. γ9δ2 T cells expanded with BCG-infected DC may be analogous to type A αβ T cells that are capable of responding to naturally processed antigens expressed in mycobacteria-infected cells. The natural processing pathways and restriction elements required for presenting mycobacterial antigens to γ9δ2 T cells in infected cells are unknown but likely are distinct from the lysosomal processing and MHC presentation involved in the stimulation of αβ T cells. Future research will be required to further explore the possibility that BCG- and IPP-expanded γ9δ2 T cells are analogous to type A and type B αβ T cells, respectively.
Our previous and current data suggest that γ9δ2 T cells can develop the characteristics of adaptive memory immunity (2) and now antigen specificity similar to αβ T cells. Although phosphoantigens have been used to study γ9δ2 T cell responses in nonhuman primates (18,19,48,49), their ability to protect against mycobacterial infection has not been reported. Utilizing an in vitro growth inhibition assay, we have demonstrated that although stimulation with the previously purified phosphoantigens alone can induce expansion of γ9δ2 T cells, these cells are unable to protect against intracellular mycobacterial replication. Our data indicate that mycobacterial protective immunity can be mediated by γ9δ2 T cells, but only by an antigen-specific subset of γ9δ2 T cells. These data also suggest that additional antigenic components, presenting elements, and/or activating receptors specifically involved in the induction of memory immune γ9δ2 T cells must be identified in order to harness the full potential of γ9δ2 T cells for pathogen-specific immunity.
Acknowledgments
We would like to thank Dr. Dana Oliver for assistance with spectratyping and sequencing correlations, Dr. John Tavis and Jeff Simmons for assistance in acquiring and analyzing spectratyping data, and the gift of BrHPP from Dr. Jean-Jacques Fournié.
Footnotes
This investigation was supported by National Institutes of Health Vaccine Treatment and Evaluation Unit Contract NO1-AI-25464 (D.F.H., co-investigator) and NIH R01-AI-48391 (D.F.H., Principal Investigator).
Abbreviations used in this paper: BCG – Mycobacterium bovis bacillus Calmette Guérin, BrHPP – Bromohydrin Pyrophosphate, DOXP – 1-deoxy-D-xylulose 5-phosphate, HMB-PP – (E)-4-hydroxy-3-methyl-but-enyl-pyrophosphate, IPP – Isopentenyl Pyrophosphate, MEP – 2-C-methyl-D-erythritol 4-phosphate, MtbWL – Mycobacterium tuberculosis whole lysate, N/P – Nontemplated/Palindromic nucleotides, PPD – Purified Protein Derivative, TB – Tuberculosis
Reference List
- 1.Worku S, Gorse GJ, Belshe RB, Hoft DF. Canarypox vaccines induce antigen specific human γδ T cells capable of IFN-γ production. J Infect Dis. 2001;184:525–532. doi: 10.1086/322792. [DOI] [PubMed] [Google Scholar]
- 2.Hoft DF, Brown R, Roodman S. Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 3.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janis EM, Kaufmann SHE, Schwartz RH, Pardoll DM. 89 A.D. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. Science. 244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 5.Alito A, McNair J, Girvin RM, Zumarraga M, Bigi F, Pollock JM, Cataldi A. Identification of Mycobacterium bovis antigens by analysis of bovine T-cell responses after infection with a virulent strain. Braz J Med Biol Res. 2003;36:1523–1531. doi: 10.1590/s0100-879x2003001100011. [DOI] [PubMed] [Google Scholar]
- 6.Jouen-Beades F, Paris E, Dieulois C, Lemeland JF, Barre-Dezelus V, Marret S, Humbert G, Leroy J, Tron F. In vivo and in vitro activation and expansion of gammadelta T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, Shafiq J, Suntharsamai P, Looareesuwan S, Webster HK, Elliott JF. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect Immun. 1994;62:855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verjans GM, Roest RW, van der KA, van Dijk G, van der Meijden WI, Osterhaus AM. Isopentenyl pyrophosphate-reactive Vγ9Vδ2 T helper 1-like cells are the major γδ T cell subset recovered from lesions of patients with genital herpes. J Infect Dis. 2004;190:489–493. doi: 10.1086/422393. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Lee CW, Biancaniello R, Carding SR. Disease-specific changes in γδ T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–4229. [PubMed] [Google Scholar]
- 10.Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AFO, Torres M, Sada E, Imir T, Carding SR. Involvement of the fas/fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ T cells: A mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis. J Immunology. 1998;161:1558–1567. [PubMed] [Google Scholar]
- 11.Zhou D, Lai X, Shen Y, Sehgal P, Shen L, Simon M, Qiu L, Huang D, Du GZ, Wang Q, Letvin NL, Chen ZW. Inhibition of adaptive Vγ2Vδ2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac-infected monkeys. J Virol. 2003;77:2998–3006. doi: 10.1128/JVI.77.5.2998-3006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 13.Wesch D, Marx S, Kabelitz D. Comparative analysis of αβ and γ/δ T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol. 1997;27:952–956. doi: 10.1002/eji.1830270422. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Morita CT, Tanaka YY, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 15.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 16.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagne F, Brailly H, Bonneville M, Fournie JJ. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–18344. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon ML, Malkovsky M, Casetti R, Agrati C, Poccia F. Innate T cell immunity to HIV-infection. Immunotherapy with phosphocarbohydrates, a novel strategy of immune intervention? Vaccine. 2002;20:1938–1941. doi: 10.1016/s0264-410x(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 18.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 19.Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, Sicard H, Fournie JJ, Poccia F. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct gammadelta and alphabeta T cell responses in primates. Eur J Immunol. 2007;37:549–565. doi: 10.1002/eji.200636343. [DOI] [PubMed] [Google Scholar]
- 20.Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun. 2003;71:1763–1773. doi: 10.1128/IAI.71.4.1763-1773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worku S, Hoft DF. In vitro measurement of protective mycobacterial immunity: Antigen specific expansion of T cells capable of inhibiting intracellular growth of BCG. Clin Infect Dis. 2000;30:S257–S261. doi: 10.1086/313887. [DOI] [PubMed] [Google Scholar]
- 22.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 23.Olive C, Gatenby PA, Serjeantson SW. Variable gene usage of T cell receptor gamma- and delta-chain transcripts expressed in synovia and peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1992;87:172–177. doi: 10.1111/j.1365-2249.1992.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmeier W, Hennemann A, May E, Duchmann R, Caspary WF. T cell receptor delta repertoire in inflamed and noninflamed colon of patients with IBD analyzed by CDR3 spectratyping. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1024–G1034. doi: 10.1152/ajpgi.00224.2001. [DOI] [PubMed] [Google Scholar]
- 25.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PA, Ress S, Hussey GD, Kaplan G. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Fisch P, Oettel K, Fudim N, Surfus JE, Malkovsky M, Sondel PM. MHC-unrestricted cytotoxic and proliferative responses of two distinct human gamma/delta T cell subsets to Daudi cells. J Immunol. 1992;148:2315–2323. [PubMed] [Google Scholar]
- 28.Halary F, Peyrat MA, Champagne E, Lopez-Botet M, Moretta A, Moretta L, Vie H, Fournie JJ, Bonneville M. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–2821. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 29.Schwender J, Seemann M, Lichtenthaler HK, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem J. 1996;316(Pt 1):73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 32.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 33.Chang JS, Huggett JF, Dheda K, Kim LU, Zumla A, Rook GA. Myobacterium tuberculosis induces selective up-regulation of TLRs in the mononuclear leukocytes of patients with active pulmonary tuberculosis. J Immunol. 2006;176:3010–3018. doi: 10.4049/jimmunol.176.5.3010. [DOI] [PubMed] [Google Scholar]
- 34.von Meyenn F, Schaefer M, Weighardt H, Bauer S, Kirschning CJ, Wagner H, Sparwasser T. Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette-Guerin by Flt3-ligand generated dendritic cells. Immunobiology. 2006;211:557–565. doi: 10.1016/j.imbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 36.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human γδ T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 37.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyagawa F, Tanaka Y, Yamashita S, Mikami B, Danno K, Uehara M, Minato N. Essential contribution of germline-encoded lysine residues in Jgamma1.2 segment to the recognition of nonpeptide antigens by human gammadelta T cells. J Immunol. 2001;167:6773–6779. doi: 10.4049/jimmunol.167.12.6773. [DOI] [PubMed] [Google Scholar]
- 39.Martino A, Casetti R, Sacchi A, Poccia F. Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guerin-infected dendritic cells kill mycobacterial-infected monocytes. J Immunol. 2007;179:3057–3064. doi: 10.4049/jimmunol.179.5.3057. [DOI] [PubMed] [Google Scholar]
- 40.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, Fournié JJ, Gougeon ML. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6017. [PubMed] [Google Scholar]
- 41.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 42.Lilienfeld-Toal M, Nattermann J, Feldmann G, Sievers E, Frank S, Strehl J, Schmidt-Wolf IG. Activated gammadelta T cells express the natural cytotoxicity receptor natural killer p 44 and show cytotoxic activity against myeloma cells. Clin Exp Immunol. 2006;144:528–533. doi: 10.1111/j.1365-2249.2006.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76:1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 45.Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 46.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells. J Immunol. 1998;161:286–293. [PubMed] [Google Scholar]
- 47.Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. doi: 10.1111/j.0105-2896.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 48.Casetti R, Perretta G, Taglioni A, Mattei M, Colizzi V, Dieli F, D’Offizi G, Malkovsky M, Poccia F. Drug-induced expansion and differentiation of V gamma 9V delta 2 T cells in vivo: the role of exogenous IL-2. J Immunol. 2005;175:1593–1598. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 49.Martino A, Casetti R, Poccia F. Enhancement of BCG-induced Th1 immune response through Vgamma9Vdelta2 T cell activation with non-peptidic drugs. Vaccine. 2007;25:1023–1029. doi: 10.1016/j.vaccine.2006.09.070. [DOI] [PubMed] [Google Scholar]