Abstract
Recent work in biodemography has suggested that life-time exposure to infection and inflammation may be important determinants of later-life morbidity and mortality. Early exposure to infections during critical periods can predispose individuals to chronic disease, in part through the reallocation of energy away from development needed for immune and inflammatory responses. Furthermore, markers of inflammation are known to vary by socioeconomic status in adults and may contribute to overall socioeconomic health inequalities, but little is known about how the sources of this inflammation over the life course. This paper uses novel biomarker data from the Third National Health and Nutrition Examination Survey (NHANES III) to test the association of the burden of common chronic infections (Helicobacter pylori (H. pylori), cytomegalovirus (CMV), herpes simplex virus-1 (HSV-1), hepatitis A and hepatitis B) with height-for-age and asthma/chronic respiratory conditions in U.S. children ages 6 and older, and the association of these chronic infections to children’s socioeconomic status. A higher burden of infection is found to be associated with lower height-for-age as well as an increased likelihood of asthma net of race/ethnicity, family income, and parental education. Children with lower family income, lower parental education, and non-white race/ethnicity have a higher likelihood of infection with several individual pathogens as well as the overall burden of infection. Differential exposure and/or susceptibility to infections may be one mechanism through which early social factors get embodied and shape later life health outcomes.
Keywords: health inequalities, infections, children, NHANES III, socioeconomic status(SES), USA, lifecourse, biomarkers
Background
Recent work in biodemography has suggested that reductions in life-time exposure to infection and inflammation may have been an important determinant of cohort declines in later-life morbidity and mortality. Crimmins and Finch argue that cohorts with lower infectious disease mortality in childhood can be characterized by a “cohort morbidity phenotype” that links their early life experience to later life cohort mortality patterns(Crimmins & Finch, 2006; Finch & Crimmins, 2004). More broadly, life course epidemiology has drawn attention to the potential long-term impacts of early life exposures for the development of chronic disease(Ben-Shlomo & Kuh, 2002). Social scientists are also increasingly drawing links between early life conditons and later life outcomes(Case, Fertig, & Paxson, 2005; Hayward & Gorman, 2004; Heckman, 2006), however, the precise biological pathways linking early life conditions to later life outcomes are not well understood.
Early exposure to infections during critical periods is thought to predispose individuals to chronic disease, in part through the reallocation of energy away from development needed for immune and inflammatory responses (McDade, 2005). Early environments may model immune and inflammatory responses for the remainder of the life course. It is well known that socioeconomic status (SES) is consistently associated with adult health outcomes. Childhood socioeconomic status may shape early-life exposures such as chronic infections, with potentially important implications for later chronic disease. Infections may have a direct impact not only on adult health, but also on future socioeconomic outcomes. For example, in utero exposure to the 1918 flu pandemic has been found to increase the risk of health outcomes including cancer, hypertension, and heart disease, as well as lower educational attainment and income(Almond, 2006; Almond & Mazumder, 2005). These results illustrate the potential for early life infections to influence human capital accumulation as well as health, reinforcing health inequalities across the life course.
In contemporary cohorts, markers of inflammatory proteins such as C-reactive protein (CRP) have been found to vary by socioeconomic status in U.S. adults (Alley, Seeman, Kim, Karlamangla, Hu, & Crimmins, 2006; Loucks, Sullivan, Hayes, D’Agostino, Larson, Vasan et al., 2006; Ranjit, Diez-Roux, Shea, Cushman, Ni, & Seeman, 2007). The sources of these differences in adult inflammation are less clear, but differences in pathogen burden are one possibility(Zhu, Quyyumi, Norman, Csako, Waclawiw, Shearer et al., 2000b). Infections illict an inflammatory response from the innate immune system upon entry into the body, and chronic infections may elicit a persistent inflammatory response(Eskandari & Sternberg, 2002; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Segerstrom & Miller, 2004).
Seroprevalence rates of several persistent infections have been found to differ among adults by race/ethnicity and socioeconomic status in the U.S. (Dowd, Aiello, & Alley, 2008; McQuillan, Kruszon-Moran, Kottiri, Curtin, Lucas, & Kington, 2004; Zajacova, Dowd, & Aiello, 2008). If differences by socioeconomic status or race/ethnicity exist in the early acquisition of lifelong chronic infections, this might contribute to later life health inequalities in two ways; through direct links to later life health and/or through effects on cognitive functioning and human capital accumulation. Currently, little is known about whether differences in chronic infections exist during the critical early ages in U.S. children.
Infections and Chronic Disease
In addition to the idea that the lifelong burden of infection may help explain cohort changes in life expectancy over the least century(Finch & Crimmins, 2004), there is growing epidemiological evidence linking specific chronic infections to chronic disease outcomes in contemporaneous populations. For example, herpesviruses such as cytomegalovirus (CMV) and herpes simplex virus type 1 (HSV-1) have been linked to inflammatory processes, cardiovascular disease, frailty, cognitive outcomes, and Alzheimer’s disease (Aiello, Haan, Blythe, Moore, Gonzalez, & Jagust, 2006; Itzhaki, Wozniak, Appelt, & Balin, 2004; Liu, Moroi, Yamamoto, Kubota, Ono, Funatsu et al., 2006; Schmaltz, Fried, Xue, Walston, Leng, & Semba, 2005; Sorlie, Nieto, Adam, Folsom, Shahar, & Massing, 2000). Focusing earlier in the lifecourse, recent work has suggested a link between fetal exposure to herpesviruses and preterm birth(Gibson, Goldwater, MacLennan, Haan, Priest, & Dekker, 2008). Exposure to CMV and HSV-1 is very common in early life (Staras, Dollard, Radford, Flanders, Pass, & Cannon, 2006), with average seroprevalence in the U.S. close to 50% in the 20–29 age group, rising to 89% by ages 70–79(Staras et al., 2006). Although infection with CMV and HSV-1 often passes undiagnosed because of their asymptomatic properties, these viruses remain latent in the host for life, with risk of reactivation due to stress and aging (Koch, Solana, Rosa, & Pawelec, 2006). Most people will be infected with these viruses by the time they reach older ages, but it is possible that individuals infected earlier in life will face a greater pro-inflammatory toll over their life course.
In addition to herpesviruses, several other pathogens have been linked to the development of chronic disease. Helicobacter pylori (H. pylori) can lie dormant in the body for decades until the bacteria-host equilibrium is disturbed. Besides its well-known role in peptic ulcer disease, H pylori is the major risk factor for gastric cancer. H pylori has also been implicated in the development of stroke and ischemic heart disease through suggested pathways including chronic inflammation, lipid alterations, and endothelial dysfunction(Manolakis, Kapsoritakis, & Potamianos, 2007). H pylori has also been explicitly implicated in growth impairment in children(Mohammad, Hussein, Coward, & Jackson, 2008; Prentice & Darboe, 2008). Hepatitis B virus (HBV), known for its role in chronic liver disease, has been hypothesized to contribute to artherogenic diseases via systemic effects on immune response and colonization of vascular tissues, though the evidence for its association with stroke and myocardial infarction is mixed(Ishizaka, Ishizaka, Takahashi, Toda, Hashimoto, Ohno et al., 2002; Rong, Huang, Su, Li, Li, Zhang et al., 2007; Sung, Song, Choi, Ebrahim, & Davey Smith, 2007). Hepatitis A (HAV), though commonly thought to be eliminated from the body after acute infection, may also persist in the host or establish a chronic, subclinical inflammatory condition. Seropositivity to hepatitis A was found to be associated with both coronary artery disease (CAD) and elevated C-reactive protein (CRP) levels in U.S. adults, after controlling for age, race, sex, smoking, diabetes, cholesterol, hypertension, other infections, and occupational status(Zhu, Quyyumi, Norman, Costello, Csako, & Epstein, 2000a). Beyond the impact of individual infections, there is developing evidence that the presence of multiple chronic infections may contribute to disease through an overall downregulation of immune function and a systemic pro-inflammatory environment (Elkind & Cole, 2006; Espinola-Klein, Rupprecht, Blankenberg, Bickel, Kopp, Rippin et al., 2002; Fernandez-Real, Lopez-Bermejo, Vendrell, Ferri, Recasens, & Ricart, 2006; Zhu et al., 2000b).
Infections and health outcomes in children
Height
Although much prior research has focused on links between infections and adult chronic health outcomes, the health costs of early infections may manifest themselves earlier in the life course. One potential marker of the costs of infection is differences in growth, which has rarely been explored in children in developed countries. Crimmins and Finch suggest that cohort differences in infectious burden are reflected in differences in adult height as a result of the high metabolic demands of the inflammatory response (Crimmins & Finch, 2006). Pro-inflammatory cytokines such as TNF-alpha and Interleukin-6 released in response to infection may directly affect the process of bone-remodeling required for long bone growth, and direct viral infection of osteoclasts and osteoblasts has also been detected(Stephensen, 1999). Other mechanisms through which chronic infections are thought to affect growth include lower food intake, impaired nutrient absorption and direct nutrient loss (Stephensen, 1999). Height may also be a useful marker of broader health capital. Adult height has been of interest to economists due to its consistent relationship with wages, performance on cognitive tests, and longevity(Case & Paxson, 2006; Deaton, 2007).
Asthma causes considerable morbidity in U.S. children and is the third leading cause of hospitalization in persons 18 or under in the United States (Eder, Ege, & von Mutius, 2006). Debate around the secular trend in increased asthma and other allergic diseases has focused on the “hygiene hypothesis,” the idea that modern under-exposure to infectious agents may lead to immature and pro-allergic immune responses(Liu, 2007; Strachan, 1989). On the other hand, asthma is an inflammatory airway condition that may be exacerbated by infection-induced production of pro-inflammatory cytokines such as IL-6, which have been found to contribute to the structural remodeling of the airway wall in chronic asthma(Rodel, Woytas, Groh, Schmidt, Hartmann, Lehmann et al., 2000). The expected association of infectious burden with reported asthma in U.S. children is thus not clear a priori.
This paper seeks to bring together these different lines of research with novel biomarker data to test (1)whether the burden of common chronic infections including Helicobacter pylori (H pylori), Cytomegalovirus (CMV), Herpes simplex virus-1, Hepatitis A Virus (HAV) and Hepatitis B Virus (HBV) is related to socioeconomic status in U.S. children ages 6–16, and (2)whether this infectious burden is associated with height-for-age or reported asthma/chronic respiratory conditions in U.S. children.
DATA
The analyses are based on data from the National Health and Nutrition Examination Survey (NHANES III), collected between 1988 and 1994. NHANES III contains a cross-sectional representative sample of the U.S. civilian non-institutionalized population, with an oversample of Mexican Americans and non-Hispanic Blacks. Data were collected in household face-to-face interviews and medical examinations, which included the collection of blood and urine for laboratory tests. Data were collected in two phases, phase 1 from 1988–1991 and phase 2 from 1991–1994. Each phase was designed to be individually nationally representative. Details of the sampling design and protocol are available from National Center for Health Statistics(Gunter, Lewis, & Koncikowski, 1996). For the youth sample, there was over a 90% response rate to the initial interview portion of survey(Schafer, 1996) and 6,936 children age 6–16 were included in the interview sample. Of these, 606 (8.7%) were missing data on family income. Those missing income data were more likely to be Mexican-American and have less parental education than those not missing. Of the remaining 6330 respondents, 1,407 (22.2%) did not have their blood drawn during the medical examination. Higher child’s age and non-white race/ethnicity were associated with a greater likelihood of having blood drawn. Of the 4,923 respondents with blood drawn, 4,342 (88.2%) had data on at least one infection although 198 (4.6%) were missing data on one or more of the three infections that were tested in both survey waves and all age groups (CMV, HAV, HBV). Those missing data for one or more of these infections were slightly more likely to be black, but did not significantly differ in family income or education, nor did they significantly differ in average height-for-age or reported asthma/chronic respiratory conditions. H. pylori was only tested on samples from Phase 1 of the survey, so all analyses directly including H. pylori have a smaller samples size (N=1,962). Each phase of the survey was designed to be separately nationally representative, so missingness by phase can be considered missing completely at random. Similarly, HSV-1 was only tested on samples of children 12 and over, limiting analyses including HSV-1 to a sample size of 1,379. We excluded additional observations with missing data on parental education (N=23) and either one of the two health outcomes (N=7). There were no missing values on age, gender, race, and region. The final number of observations used in analyses was 4,319.
MEASURES
Sociodemographics
Childhood socioeconomic status was measured using the years of education of the household reference person and annual family income. Family income was coded as the midpoint of each of the 26 reported categories (using $65,000 for the incomes above $50,000) and adjusted for inflation between the two NHANES III phases using the Consumer Price Index. Income was log-transformed due to the skewness of the distribution. Education was measured as the highest completed year of schooling and was used in models as a continuous predictor or as a trichotomized measure, with less than 12 years, 12 years (reference), and more than 12 years. Race/ethnicity was categorized into four groups (non-Hispanic White (reference), non-Hispanic Black, Mexican-American, and Other). Other control variables included age (in years), sex (male as reference), and region of residence (Northeast as reference).
Individual Infections
We used seropositive status for H. pylori, CMV, HSV-1 HBV, and HAV, coded as 1=seropositive, 0=seronegative. H. pylori serologic testing was done using a commercial Immunoglobulin G (IgG) Enzyme Linked ImmunoSorbent Assay (ELISA) (Wampole Laboratories, Cranbury NJ)(“NHANES III Second Laboratory Data File Documentation, Series 11, No. 2A,” 1998). H. pylori testing was conducted on samples from Phase I only. CMV specific IgG seropositivity was measured with an ELISA (Quest International Inc., Miami FL)(NCHS, 2006). Solid-phase enzymatic immunodot assays were used to detect antibody seropositivity to HSV-1(NHANES III Second Laboratory Data File Documentation, Series 11, No. 2A,” 1998). HSV-1 serostatus was obtained only for respondents ages 12 and older. Hepatitis B serostatus was determined by core antigen enzyme-linked immunoassay (CORAB, Abbott Laboratories)(Gunter et al., 1996). Hepatitis A serostatus was determined using a commercially available enzyme immunoassay (HAVAB-EIA, Abbott Laboratories, Abbott Park, Illinois)(Bell, Kruszon-Moran, Shapiro, Lambert, McQuillan, & Margolis, 2005).
Burden of infection
Serostatus to H. pylori, CMV, HSV-1, HAV, and HBV were used to construct a latent infection burden variable using a confirmatory factor analysis.
Outcomes
Height was measured at the medical examination. The value was converted into a sex- and age-specific z-score, based on the 2000 CDC growth charts. Chronic respiratory conditions were measured by parental report of whether a doctor ever said the child had asthma or chronic bronchitis (0=no, 1= yes).
Descriptive statistics for the analysis sample are shown in Table 1. Among U.S. children aged 6–16, seroprevalence of H. pylori was 26.4%, CMV 38.5%, Hepatitis A 9.9%, and Hepatitis B 1.8%. Among children ages 12–16, seroprevalence of HSV-1 was 41.6%. Almost 15% of children have been told by their doctor that they have asthma or chronic bronchitis. The average educational attainment for a reference person in the household was 12.5 years and the mean family income was just over $37,000. The distribution of race reflects closely the total population distribution in this age group, with non-Hispanic white children comprising 67.8% of the sample, non-Hispanic black children 14.5%, Mexican-American children 8.3% and other race/ethnic groups 9.4%.
Table 1.
Descriptive statistics: children age 6–16, NHANES III.
| Mean or proportion | Standard error | |
|---|---|---|
| Age | 11.1 | (.10) |
| Household size | 4.7 | (.06) |
| Income (infl.-adjusted dollars) | $37,104.4 | (1273.9) |
| Education of head (years) | 12.5 | (.13) |
| Female | 49.0% | |
| Race | ||
| Non-Hispanic white | 67.8% | |
| Non-Hispanic black | 14.5% | |
| Mexican American | 8.3% | |
| Other race/ethnic groups | 9.4% | |
| Infections – proportion seropositive | ||
| H. pylori | 26.4% | |
| CMV | 38.5% | |
| HSV-1 | 41.6% | |
| HAV | 9.9% | |
| HBV | 1.8% | |
| Health outcomes | ||
| Asthma/Chronic bronchitis | 14.8% | |
| Height (age-specific z-scores) | .18 | (.03) |
Weighted, N=4,319.
Note: H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus.
METHODS
First, we calculated means (s.e.) and proportions for key analysis variables. Next we estimated tetrachoric (polychoric) correlations among the five infection seropositivity status indicators and used a likelihood ratio test to determine whether the correlations were statistically significant. Logit models were then used to estimate the association of race/ethnicity, education, and income with seropositivity to individual infections, as well as the association between the infections and the dichotomous asthma/chronic respiratory illness measure. Linear regression models were employed to estimate the relationship between socioeconomic status and infection burden, as well as the association of individual infections and the infection burden with the age-specific height z-scores.
Confirmatory factor analysis (CFA) was used to construct an infection burden index using information from the five individual infection serostatus dummies. The advantages of CFA to construct the infection burden, as opposed to alternatives such as a crude summation or mean index, relate to its handling of measurement error and missing data. Within the CFA framework, the burden of infection is conceptualized as a latent (unobserved) variable measured by a number of observed variables, referred to as factor indicators. The measurement error in the factor indicators is included in the regression model that describes their association with the latent variable. The second major advantage to CFA concerns the practical constraints of the NHANES III data, where some infections have only been measured in a subset of the sample (for instance, H. pylori was assessed only during phase I (1988–1991) of the survey). CFA allowed us to use all observations with one or more infection data points by using a full-information maximum likelihood estimation under the assumption of ignorable missingness. The model calculated a latent infection burden score for each individual using the posterior distribution of the burden variable, based on the model and the data specific to the person.
We also estimated a full structural equation model where the latent infection burden was a predictor of health outcomes. The findings were substantively equivalent to those shown here and are available from the authors.
Analyses were conducted using Stata 10.0 (2007, StataCorp, College Station, TX) and Mplus version 5.1 (2008, Muthén and Muthén, Los Angeles, CA), with proper adjustments for the NHANES III complex survey design.
RESULTS
Associations Among Individual Infections
Table 2a shows tetrachoric correlations among the five individual infections. The correlations show a moderate positive association between most infection pairs. Correlations among H. pylori, CMV, and HSV-1 are generally stronger (.25–.37) than those with Hepatitis A and B (ranging from .04 to .29). Overall, these results suggest some degree of clustering of individual infections that might indicate a shared environment of pathogen exposure or susceptibility.
TABLE 2.
| TABLE 2a. Correlations among individual infections: children age 6–16, NHANES III. | |||||
|---|---|---|---|---|---|
| H. pylori | CMV | HSV-1 | HAV | HBV | |
| H. pylori | 1 | ||||
| CMV | .25*** | 1 | |||
| HSV-1 | .37*** | .37*** | 1 | ||
| HAV | .29*** | .20*** | .04 | 1 | |
| HBV | .24*** | .16*** | .26*** | .09 | 1 |
| TABLE 2b. Confirmatory factor analysis of infection burden: children age 6–16, NHANES III. | |||
|---|---|---|---|
| Factor loadings | |||
| unstandardized | standardized | R2 | |
| H. pylori | 1.00 | .57 | .33 |
| CMV | .94*** | .54 | .29 |
| HSV-1 | 1.08*** | .62 | .38 |
| HAV | .64*** | .36 | .13 |
| HBV | .59** | .34 | .11 |
| Model fit indices | |||
| Chi square (d.f.) | 6.63(4) n.s. | ||
| CFI | .97 | ||
| RMSEA | .01 | ||
p<0.01,
p<0.05,
p<0.1
Note: weighted. The p-values are from a likelihood ratio test of no correlation. H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus.
p<0.01,
p<0.05,
p<0.1.
H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus.
Table 2b shows results from the confirmatory factor analysis (CFA) used to construct the index of latent infection burden. The factor loadings show that the burden construct explained a moderate but statistically significant proportion of variance in the observed infections. The R-squared values for the individual infections ranged from 11% for Hepatitis B to 38% for HSV-1. All factor loadings were statistically significant. The standardized factor loadings for H. pylori, CMV, and HSV-1 were higher (.54–.62), as might be expected from the correlation analysis above, while the loadings for Hepatitis A and B were smaller (.34–.36). The model had an adequate fit to the data based on multiple indices (Hu & Bentler, 1998). The chi square test was not significant (6.6 for 4 d.f.), CFI=.97, and RMSEA=.01.
Social Correlates of Infection
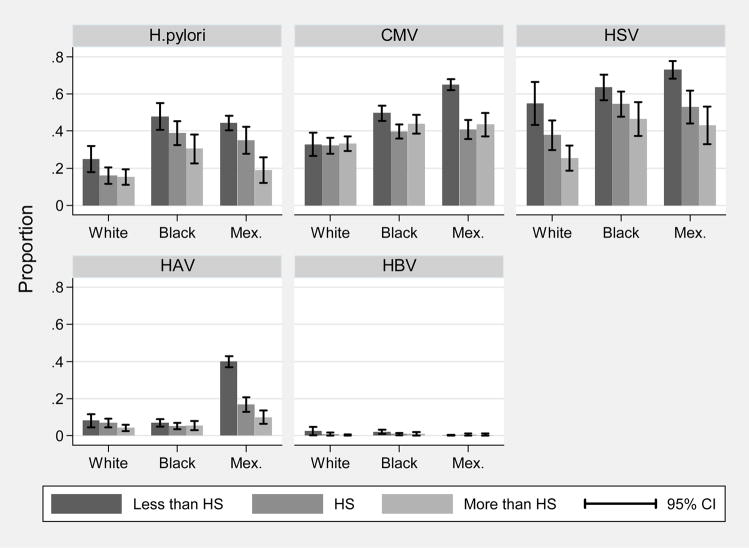
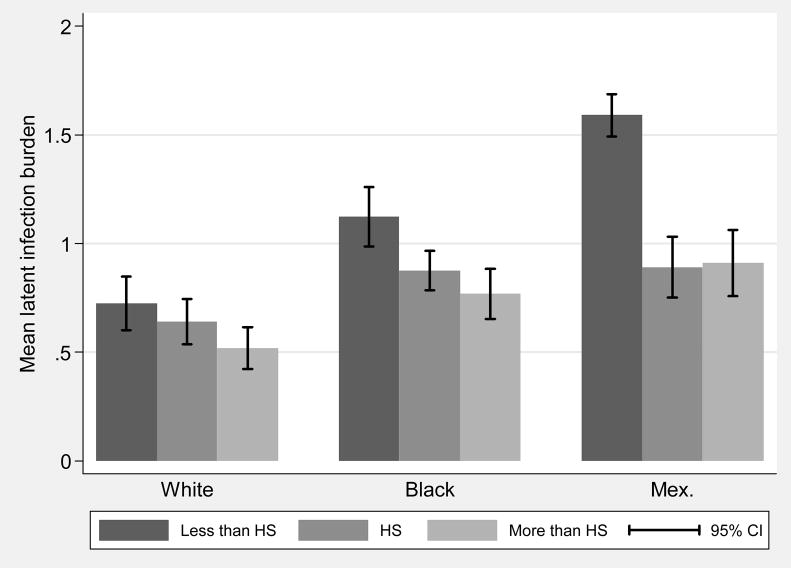
Figure 1 shows age- and sex-adjusted prevalence of the individual infections by race and education category. The figure shows educational gradients in prevalence for most individual infections, and higher overall levels of infection for non-Hispanic black and Mexican-American children. Figure 2 shows the mean burden of infection, operationalized as the mean latent factor score from the CFA, for each category of parental education by race. Educational gradients are evident within each race, as well as higher overall levels of infection burden for non-Hispanic blacks and Mexican-Americans.
Figure 1.
Age and sex-adjusted prevalence of persistent infections, by race and parental education: children age 6–16, NHANES III.
Note: H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus, white=non-Hispanic white, black=non-Hispanic black, Mex.=Mexican-American, parental education refers to the educational attainment of the household head.
Figure 2.
Mean infection burden by race and parental education: children age 6–16, NHANES III.
Note: white=non-Hispanic white, black=non-Hispanic black, Mex.=Mexican-American, parental education refers to the educational attainment of the household head.. The figure shows the mean infection burden factor score by race and education. The factor score indicating infection burden was calculated using the five infection indicators and standardized with a mean of one and a variance of one.
The first 5 columns in Table 3 report associations between sociodemographic characteristics and each infection based on individual logit models for seropositivity of each infection. Table 2 also shows similar results from linear regression models predicting the value of the latent infection burden. These models are all simultaneously adjusted for race/ethnicity, parental education, and family income, and additional control variables. The likelihood of being infected with H. pylori and HSV-1 is significantly higher for non-Hispanic black compared to non-Hispanic white children. The odds of seropositivity to CMV, HSV-1, and Hepatitis A, are also higher for Mexican-American children and ‘other’ race children compared to non-Hispanic white children. ‘Other race’ children also have a much higher likelihood of seropositivity for Hepatitis B. In addition to race/ethnicity, parental education is significantly associated with the likelihood of infection for three pathogens: H. pylori, HSV-1, and Hepatitis B. Controlling for parental education and race/ethnicity, increased family income is associated with lower odds of infection for CMV, HSV-1, and Hepatitis A. The results for the infection burden are in the same direction but stronger than those of the individual infections. All other race/ethnic groups have a significantly higher infection burden compared to non-Hispanic whites. Parental education and family income are both significantly inversely related to the burden of infection after adjustment for race/ethnicity.
TABLE 3.
Associations between infections and sociodemographic characteristics: children age 6–16, NHANES III.
| H. pylori | CMV | HSV-1 | HAV | HBV | Infection Burden | |
|---|---|---|---|---|---|---|
| Age | 0.09*** (0.02) | 0.04** (0.01) | 0.02 (0.07) | 0.03 (0.04) | 0.06 (0.06) | 0.02*** (0.01) |
| Female | −0.16 (0.14) | 0.24** (0.11) | 0.03 (0.14) | 0.13 (0.20) | −0.23 (0.33) | 0.06 (0.04) |
| Non-Hispanic Black | 0.83*** (0.19) | 0.12 (0.14) | 0.37** (0.14) | −0.38 (0.27) | 0.73 (0.54) | 0.15*** (0.05) |
| Mexican American | 0.12 (0.20) | 0.55*** (0.15) | 0.58*** (0.19) | 0.85*** (0.31) | −1.00 (0.67) | 0.33*** (0.07) |
| Other race | 0.43 (0.30) | 0.96*** (0.30) | 0.75** (0.36) | 1.00** (0.39) | 2.78*** (0.42) | 0.55*** (0.13) |
| Education of head | −0.08*** (0.03) | −0.03 (0.02) | −0.11** (0.04) | −0.05 (0.04) | −0.13* (0.07) | −0.03*** (0.01) |
| Income (log) | −0.15 (0.10) | −0.21*** (0.06) | −0.32*** (0.10) | −0.29** (0.11) | 0.19 (0.25) | −0.12*** (0.03) |
| Household size | 0.04 (0.05) | 0.14*** (0.04) | 0.11** (0.05) | 0.14*** (0.05) | 0.19* (0.11) | 0.07*** (0.01) |
| N | 1,962 | 4,185 | 1,379 | 4,258 | 4,257 | 4,319 |
p<0.01,
p<0.05,
p<0.1
Shown are coefficients and standard errors.
Note: Each column present results from a separate model. Results for single infections are from logistic models, results for infection burden from a linear regression model. Reference category for gender is male, and for race is non-Hispanic white. Infection burden was calculated using confirmatory factor analysis using the five individual infections and was standardized with a mean of zero and a variance of one. All models are adjusted for sampling design and control for region of residence. The sample sizes differ across infections because not all blood sera were tested for each infection.
H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus.
Relation of infections to asthma and height for age
Table 4 shows results from logit models of the individual infections’ associations with the likelihood of reporting asthma/chronic bronchitis. Linear regression is used to predict age-specific height z-scores. The first column for each outcome (model 1) reports age- and sex-adjusted relationships, the second column (model 2) reflects results adjusted for age, sex, race, education and income, household size, and region of residence. The coefficients on all infections are positive with regards to asthma, suggesting increased odds of reporting asthma in the presence of infections, though only one infection reaches statistical significance (Hepatitis A in model 2). In the models of height, the coefficients on all infections, both unadjusted and adjusted, are negative, suggesting a decrease in age-specific height with the presence of an infection. CMV and Hepatitis A significantly predict lower age-specific height in unadjusted models, with only Hepatitis A remaining significant after adjustment for race/ethnicity, income, and education.
TABLE 4.
Gross and net associations of individual infections with health outcomes: children age 6–16, NHANES III.
| Asthma/Chronic Bronchitis | Height (age-specific z-scores) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| H. pylori | 0.15 (0.34) | 0.21 (0.36) | −0.13 (0.09) | −0.09 (0.08) |
| CMV | 0.19 (0.18) | 0.25 (0.20) | −0.12** (0.05) | −0.04 (0.05) |
| HSV-1 | 0.19 (0.21) | 0.25 (0.23) | −0.13 (0.11) | −0.01 (0.11) |
| HAV | 0.38 (0.26) | 0.49* (0.28) | −0.45*** (0.11) | −0.33*** (0.10) |
| HBV | 0.62 (0.55) | 0.70 (0.45) | −0.42 (0.26) | −0.25 (0.25) |
p<0.01,
p<0.05,
p<0.1
Shown are coefficients and standard errors.
Note: Each coefficient reflects results from a separate regression model. Model 1 adjusts for age and gender. Model 2 adjusts for age, gender, race, household size, family income, education of the household head, and region. Results for asthma were estimated using logistic regression. Results for height were estimated using OLS. All models were adjusted for sampling design. H. pylori=Helicobacter pylori, CMV=cytomegalovirus, HSV-1= Herpes simplex virus type 1, HAV=Hepatitis A virus, HBV=Hepatitis B virus.
In Table 5, a higher infection burden is associated with an increased likelihood of reporting of asthma/chronic respiratory problems and lower age-specific height. Model 1 shows the association of infection burden with each health outcome adjusting for age and gender. The coefficients show that a one standard deviation increase on the infection burden scale is associated with a 16% increase in the odds of reporting asthma or chronic bronchitis. The second model shows that no demographic or socioeconomic factors included in the analyses are significantly associated with reported asthma. Adjusting for all predictors in model 3 does not change the coefficient of infection burden substantially. Adjusting for race and parental socioeconomic status, each standard deviation increase in infection burden increases the odds of asthma/chronic respiratory conditions by 22%. In the age and sex adjusted height model, each standard deviation increase in infection burden is associated with a .11standard deviation decrease in age-specific height. Race and household size, but not parental education and income, are associated with height, with non-Hispanic black children found to be taller and Mexican-American and ‘other race’ children shorter than their white counterparts. While these factors explain some of the effect of infection burden (model 3), the association remains significant. Since genetic potential is also an important determinant of height, we estimated an additional model (not shown) adding controls for mother’s and father’s height. Parental height, as expected, strongly predicts child height. Its inclusion reduces the coefficient of infection burden to − 0.035, with a p-value=.19. It is possible, however, that inclusion of parental height in this case is an over-adjustment, since in addition to genetic transmission of height, there is likely to be intergenerational correlation of pathogen burden via direct parent-child transmission or shared environments that affect both parental and child height.
TABLE 5.
The Association between infection burden and health outcomes: children age 6–16, NHANES III
| Asthma/Chronic Bronchitis | Height (age-specific z-scores) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Infection burden | 1.16* (0.10) | 1.22* (0.13) | −0.11*** (0.03) | −0.07* (0.03) | ||
| Age | 1.01 (0.03) | 1.01 (0.03) | 1.01 (0.03) | 0.01 (0.01) | 0.00 (0.01) | 0.01 (0.01) |
| Female | 0.75 (0.13) | 0.77 (0.13) | 0.76 (0.13) | −0.13* (0.06) | −0.13* (0.06) | −0.12* (0.06) |
| Non-Hispanic Black | 0.93 (0.17) | 0.90 (0.16) | 0.21*** (0.06) | 0.22*** (0.06) | ||
| Mexican American | 0.81 (0.18) | 0.76 (0.18) | −0.25*** (0.09) | −0.23** (0.09) | ||
| Other race | 1.08 (0.47) | 0.96 (0.46) | −0.24** (0.10) | −0.21** (0.10) | ||
| Education of head | 1.01 (0.03) | 1.02 (0.03) | 0.01 (0.01) | 0.01 (0.01) | ||
| Income (log) | 0.89 (0.07) | 0.92 (0.07) | 0.04 (0.04) | 0.03 (0.04) | ||
| Household size | 0.95 (0.05) | 0.93 (0.05) | −0.07*** (0.02) | −0.07*** (0.02) | ||
| N | 4,318 | 4,318 | 4,318 | 4,313 | 4,313 | 4,313 |
p<0.01,
p<0.05,
p<0.1
Shown are odds ratios for asthma, OLS regression coefficients for height and standard errors. Note: Results for asthma were estimated using logistic regression. Results for height were estimated using OLS. Model 1 estimates the effect of infection burden on a health outcome, adjusting for age and gender. Model 2 adjusts for age, gender, race, education of the household head, family income, household size, region, and rural/urban classification. Model 3 estimates the effect of infection burden on a health outcome, net of the same set of predictors. Reference category for gender is male, and for race is non-Hispanic white. Infection burden was calculated using confirmatory factor analysis using the five individual infections and was standardized with a mean of zero and a variance of one.
DISCUSSION
To our knowledge, this is the first study to examine the relationship between the burden of chronic infections and socioeconomic status in U.S. children. The results show that family income, parental education, and race/ethnicity are significantly associated with the likelihood of infection with several persistent infections in U.S. children aged 6–16, as well as the overall burden of multiple infections. These differences in the burden of infection at early ages might have important implications for chronic diseases later in life, through pathways such as an increase in lifetime immune and inflammatory burden. Since our data were cross-sectional, we could not directly examine the impact of these infections on later life outcomes. Instead, we examined whether the burden of infection is associated with outcomes at younger ages. While a relationship between these infections and health in childhood is not necessary for the emergence of a relationship between early infections and chronic disease later in the life course, these analyses allow us to look for evidence any early biological costs of infection. We found evidence that the burden of infection was associated with shorter height-for-age and an increased likelihood of reporting asthma or chronic respiratory conditions. These results suggests that even in the context of relatively contemporary cohorts (aged 6–16 in 1988–1994) of U.S. children, infectious environments encountered early in life may be affecting growth and future health outcomes. Since height has also been found to be associated with educational attainment and wages, early infectious burden may contribute to future economic disadvantage as well. Prospective studies that measure early life infectious exposure and inflammation should in the future be linked to educational and economic outcomes to test these pathways, similarly to work that has investigated the association of low birth weight to these outcomes(Black, Devereux, & Salvanes, 2007; Case et al., 2005; Conley & Bennett, 2000).
This paper seeks to bring together several disparate literatures to shed light on a novel life-course risk factor for health inequalities in the United States. Crimmins and Finch have suggested that life-long chronic inflammation resulting from early infectious environments might contribute to cohort differences in mortality. Their work focused on differential infectious environments over time or across countries with different levels of development. We extend this approach by looking for potential sources of differences in inflammatory burden within cohorts, specifically differences by race/ethnicity and family income and education. One strength of this study is the use of individual data measuring seropositivity to infections, as opposed to previous work looking at infant mortality rates as a proxy for early life infectious exposures. A limitation of the current study comes from the fact that the particular infections available to us are imperfect proxies for the overall pathogen and inflammatory burden. Depending on how representative these infections are of the overall pathogen environment of an individual, our infection burden index could have considerable measurement error, potentially biasing our estimates downward. We have used confirmatory factor analysis in order to model these infections as imperfect proxies of the overall pathogen burden of an individual.
Epidemiological research suggests a potential role for persistent infections in the development of inflammation-related diseases of aging. Given our finding of significant socioeconomic differences in U.S. children in several life-long persistent pathogens, future work should examine the sources of differential rates of seropositivity among U.S. children. With current NHANES III data, it is impossible to distinguish whether different rates are a result of increased exposure, increased susceptibility, or both. While H Pylori, Hepatitis A and Hepatitis B all have some hygiene and sanitation related etiologies, CMV and HSV-1 are extremely prevalent pathogens spread through very casual contact similar to other common viruses such as colds. It is therefore less obvious why rates of these viruses would differ by social factors in the U.S. While household size was associated with an increased likelihood of several infections, it did not alter the relationship between SES or race/ethnicity and the infections. It is possible that in groups with historically higher rates of infection who predominantly live and work together, higher levels would persist over time. Environmental factors associated with socioeconomic status such as household crowding or use of public transportation, could contribute directly to exposure risk. Suppressed immune function as a result of stress, poor nutrition, smoking, or other environmental exposures could increase susceptibility to infections given equal levels of exposure. Low social status as well as indicators of psychosocial stress have been linked to increased risk of respiratory infections in humans and other primates in experimental studies (Cohen, 1999; Cohen, 2005; Cohen, Doyle, Turner, Alper, & Skoner, 2004; Cohen, Line, Manuck, Rabin, Heise, & Kaplan, 1997). Much less is known about the links between social status, stress, and susceptibility to infections in the broader U.S. population. Low social class was associated with lower secretory immunoglobulin (sIgA), cited as a first line of defense against infection, in a large community sample in Scotland (Evans, Der, Ford, Hucklebridge, Hunt, & Lambert, 2000). Taken together, these studies suggest that psychological stress associated with low SES could down-regulate various aspects of the cellular immune response, increasing susceptibility to infection. These ties are speculative at this time with respect to the current findings, and future work should aim to build evidence regarding the sources of such early differences in infection rates.
NHANES III biological specimen testing also pre-dated high sensitivity C-reactive Protein testing, and therefore we were unable to directly test the links between SES, infections, and inflammation in U.S. children. As new data become available, we are eager to directly test whether differences in chronic infections contribute to differences in inflammatory burden amongst U.S. children, and whether these differences might help explain differences in chronic disease later in life.
With regards to height-for-age, our results suggest that the relationship between infectious environments and height may not be a historical relic or exist only in developing countries. The relationship between early environments and height has often been expressed in terms of net nutrition, to which infections detract. In theory infections might take less of a toll on height in countries or cohorts where undernutrition is less of a concern. It is perhaps more surprising then to find any evidence that infections and height are linked in U.S. children in 1988–1994, though we did not examine food intake directly. It is possible that particular micronutrients affected by infections are more important in this regard than total calories, or it could be that other mechanisms regarding the effects of inflammation on bone remodeling might be at play.
One important limitation of this work is that there are other explanations for the relationship between infections and height that are difficult to exclude with these data. Rather than a direct link between infections and height in the children measured, the association could reflect an association between the overall health of the mother and the height of the child. The mother herself may have been affected by early infectious environments, and these infections may also be more likely to be passed on to the child without directly affecting his height. In sensitivity analysis, the relationship between infectious burden and height-for-age weakened with the inclusion of both mother’s and father’s height. We also tested whether the child’s infectious burden predicted the mother’s height, which would have suggested a more intergenerational story, but these results were not significant (not shown). To test whether general frailty that could be related to both susceptibility to infections and height might be a factor, we tested an indicator for the child being born low birth weight (<2500 grams). While being born low birth weight was associated with lower height for age, it did not alter the association between burden of infection and height-for-age and was not predictive of pathogen burden. While these tests suggest that our results are robust to some alternative explanations for our results, we cannot causally link infections and height from these data.
Our finding that an increased burden of infection is associated with a higher likelihood of reporting asthma or chronic respiratory problems is consistent with recent evidence from the U.K. that a decline in cold viruses was associated with declining rates of asthma between 1993 and 2003, thought to be due to the role these viruses played in exacerbating respiratory problems (Urquhart, Anderson, & McKenzie, 2008). While our infection burden measure did not include any cold or respiratory viruses, to the extent that they share common exposure, transmission, or susceptibility pathways with our measured pathogens, they might have been picked up in our latent infection burden index. Evidence regarding the hygiene hypothesis is decidedly mixed, and other research has shown an inverse relationship between infections such as H Pylori and asthma and allergy (Chen & Blaser, 2007; Ponsonby & Kemp, 2008). Our measure included reported asthma combined with other chronic respiratory problems that by definition might have involved respiratory infections sharing common pathways with our measure of infection burden. Moreover, it is likely that the timing and intensity of exposure to pathogens is important for the relationship between infections and atopic diseases such as asthma, which prospective data would be better-suited to investigate.
In sum, a high lifetime burden of chronic infections may lead to overall heightened inflammation and earlier development of chronic disease and mortality. The social distribution of these infections and their combined burden is thus an important topic for research on health disparities. This paper suggests that disparities in infectious burden may begin early in life in the U.S, and these infections may also manifest themselves in children’s growth and development early in life, contributing to the intergenerational transmission of health inequalities.
Acknowledgments
Jennifer Dowd acknowledges funding from the Robert Wood Johnson Health and Society Scholars Program, the University of Michigan during the writing of the paper. Anna Zajacova acknowledges funding from the National Institute of Aging and Population Studies Center, University of Michigan. Revision of this paper was also supported by NIH grant R21 NR011181-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Haan MN, Blythe L, Moore K, Gonzalez JM, Jagust W. The Influence of Latent Viral Infection on Rate of Cognitive Decline over 4 Years. Journal of the American Geriatrics Society. 2006;54(7):1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu PF, Crimmins EM. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behavior and Immunity. 2006;20(5):498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Almond D. Is the 1918 Influenza Pandemic Over? Long Term Effects of In Utero Influenza Exposure in the Post 1940 U.S. Population. Journal of Political Economy. 2006;114(4):672–712. [Google Scholar]
- Almond D, Mazumder D. The 1918 influenza pandemic and subsequent health outcomes: An analysis of SIPP data. American Economic Review. 2005;95(2):258–262. doi: 10.1257/000282805774669943. [DOI] [PubMed] [Google Scholar]
- Antibody to Cytomegalovirus IgG and IgM. NCHS NHANES III Documentation, Codebook and Frequencies page. (2006).
- Bell BP, Kruszon-Moran D, Shapiro CN, Lambert SB, McQuillan GM, Margolis HS. Hepatitis A virus infection in the United States: Serologic results from the Third National Health and Nutrition Examination Survey. Vaccine. 2005;23(50):5798–5806. doi: 10.1016/j.vaccine.2005.03.060. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- Black SE, Devereux PJ, Salvanes KG. From the Cradle to the Labor Market? The Effect of Birth Weight on Adult Outcomes. Quarterly Journal of Economics. 2007;122(1):409–439. [Google Scholar]
- Case A, Paxson C. National Bureau of Economic Research Working Paper #12466. 2006. Stature and Status: Height, Ability and Labor Market Outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. Journal of Health Economics. 2005;24(2):365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Blaser MJ. Inverse Associations of Helicobacter pylori With Asthma and Allergy. Arch Intern Med. 2007;167(8):821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social Status and Susceptibility to Respiratory Infections. Ann NY Acad Sci. 1999;896(1):246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. The Pittsburgh common cold studies: Psychosocial predictors of susceptibility to respiratory infectious illness. International Journal of Behavioral Medicine. 2005;12(3):123–131. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood Socioeconomic Status and Host Resistance to Infectious Illness in Adulthood. Psychosom Med. 2004;66(4):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med. 1997;59(3):213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Conley D, Bennett NG. Is Biology Destiny? birthweight and life chances. American Sociological Review. 2000;65(3):458–467. [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. PNAS. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton A. From the Cover: Economics of Health and Mortality Special Feature: Height, health, and development. Proceedings of the National Academy of Sciences. 2007;104(33):13232–13237. doi: 10.1073/pnas.0611500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE, Alley DE. Socioeconomic Gradients in Cytomegalovirus Seropositivity in the U.S.: NHANES III. Epidemiology and Infection 2008 [Google Scholar]
- Eder W, Ege MJ, von Mutius E. The Asthma Epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- Elkind MSV, Cole JW. Do common infections cause stroke? Seminars in Neurology. 2006;26(1):88–99. doi: 10.1055/s-2006-933312. [DOI] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-Immune Interactions in Health and Disease. Ann NY Acad Sci. 2002;966(1):20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, et al. Impact of Infectious Burden on Extent and Long-Term Prognosis of Atherosclerosis. Circulation. 2002;105(1):15–21. doi: 10.1161/hc0102.101362. [DOI] [PubMed] [Google Scholar]
- Evans P, Der G, Ford G, Hucklebridge F, Hunt K, Lambert S. Social Class, Sex, and Age Differences in Mucosal Immunity in a Large Community Sample. Brain, Behavior, and Immunity. 2000;14(1):41–48. doi: 10.1006/brbi.1999.0571. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of Infection and Insulin Resistance in Healthy Middle-Aged Men. Diabetes Care. 2006;29(5):1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory Exposure and Historical Changes in Human Life-Spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Gibson CS, Goldwater PN, MacLennan AH, Haan EA, Priest K, Dekker GA. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population*. BJOG: An International Journal of Obstetrics and Gynaecology. 2008;115(4):492–500. doi: 10.1111/j.1471-0528.2007.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter E, Lewis B, Koncikowski S. Laboratory Procedures Used for the Third National Health and Nutrition Examination Surveys (NHANES III), 1988–1994. National Center for Health Statistics; Hyattsville, MD: 1996. [Google Scholar]
- Hayward MD, Gorman BK. The Long Arm of Childhood: The Influence of Early-Life Social Conditions on Men’s Mortality. Demography. 2004;41(1):87. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- Heckman JJ. Skill Formation and the Economics of Investing in Disadvantaged Children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hu L-t, Bentler PM. Fit Indices in Covariance Structure Modeling: Sensitivity to Underparameterized Model Misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- Ishizaka N, Ishizaka Y, Takahashi E, Toda E-i, Hashimoto H, Ohno M, et al. Increased Prevalence of Carotid Atherosclerosis in Hepatitis B Virus Carriers. Circulation. 2002;105(9):1028–1030. doi: 10.1161/hc0902.105718. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiology of Aging. 2004;25(5):619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 2002;64(1):15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Koch S, Solana R, Rosa OD, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: A mini review. Mechanisms of Ageing and Development. 2006;127(6):538–543. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Liu AH. Hygiene theory and allergy and asthma prevention. Paediatric and Perinatal Epidemiology. 2007;21(s3):2–7. doi: 10.1111/j.1365-3016.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Moroi M, Yamamoto M, Kubota T, Ono T, Funatsu A, et al. Presence and severity of chlamydia pneumoniae and cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. International Heart Journal. 2006;47(4):511–519. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, Hayes LJ, D’Agostino RB, Sr, Larson MG, Vasan RS, et al. Association of Educational Level with Inflammatory Markers in the Framingham Offspring Study. Am J Epidemiol. 2006;163(7):622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]
- Manolakis A, Kapsoritakis AN, Potamianos SP. A review of the postulated mechanisms concerning the association of Helicobacter pylori with ischemic heart disease. Helicobacter. 2007;12(4):287–297. doi: 10.1111/j.1523-5378.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. American Journal of Human Biology. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, Kington RS. Racial and Ethnic Differences in the Seroprevalence of 6 Infectious Diseases in the United States: Data From NHANES III, 1988–1994. Am J Public Health. 2004;94(11):1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MA, Hussein L, Coward A, Jackson SJ. Prevalence of Helicobacter pylori infection among Egyptian children: impact of social background and effect on growth. Public Health Nutr. 2008;11(3):230–236. doi: 10.1017/S1368980007000481. [DOI] [PubMed] [Google Scholar]
- NHANES III Second Laboratory Data File Documentation, Series 11, No. 2A. (1998). National Center for Health Statistics.
- Ponsonby AL, Kemp A. Investigation of the hygiene hypothesis: current issues and future directions. Allergy. 2008;63(5):506–508. doi: 10.1111/j.1398-9995.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Darboe MK. Growth and host-pathogen interactions. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:197–210. doi: 10.1159/000113495. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic Position, Race/Ethnicity, and Inflammation in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;116(21):2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- Rodel J, Woytas M, Groh A, Schmidt KH, Hartmann M, Lehmann M, et al. Production of Basic Fibroblast Growth Factor and Interleukin 6 by Human Smooth Muscle Cells following Infection with Chlamydia pneumoniae. Infect Immun. 2000;68(6):3635–3641. doi: 10.1128/iai.68.6.3635-3641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Q, Huang J, Su E, Li J, Li J, Zhang L, et al. Infection of hepatitis B virus in extrahepatic endothelial tissues mediated by endothelial progenitor cells. Virol J. 2007;4:36. doi: 10.1186/1743-422X-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J, Ezzati-Rice TM, Johnson W, Khare M, Little RJA, Rubin DB. The NHANES III Multiple Imputation Project. National Center For Health Statistics; 1996. [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A Prospective Study of Cytomegalovirus, Herpes Simplex Virus 1, and Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2000;160(13):2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- Staras SAS, Dollard SC, Radford KW, Dana Flanders W, Pass RF, Cannon MJ. Seroprevalence of Cytomegalovirus Infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Burden of Infection on Growth Failure. J Nutr. 1999;129(2):534. doi: 10.1093/jn/129.2.534S. [DOI] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J, Song YM, Choi YH, Ebrahim S, Davey Smith G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke. 2007;38(5):1436–1441. doi: 10.1161/STROKEAHA.106.466268. [DOI] [PubMed] [Google Scholar]
- Urquhart DS, Anderson AK, McKenzie SA. Fewer colds, less asthma? A hypothesis to explain the fall in childhood asthma in the UK. J Epidemiol Community Health. 2008;62(10):921–925. doi: 10.1136/jech.2007.068965. [DOI] [PubMed] [Google Scholar]
- Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. Journal of Gerontology: Medical Sciences. 2008 doi: 10.1093/gerona/gln012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Quyyumi A, Norman J, Costello R, Csako G, Epstein S. The Possible Role of Hepatitis A Virus in the Pathogenesis of Atherosclerosis. The Journal of Infectious Diseases. 2000a;182(6):1583–1587. doi: 10.1086/317613. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000b;85(2):140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]