Abstract
Voltage sensors have been well studied in voltage-gated ion channels for neuronal excitation and muscle contraction. The recent discovery of a voltage-sensing phosphatase, VSP, has changed the idea that voltage sensors are unique to ion flux through membranes. Recent findings on mechanisms and potential applications of VSP are reviewed.
Voltage-gated ion channels have long been studied as key elements of membrane excitability in neurons and muscle cells (Bezanilla, 2000). Voltage-gated ion channels consist of two major domains: a voltage sensor domain (VSD) and a pore domain. The VSD consists of four transmembrane segments and confers voltage sensitivity to voltage-gated ion channels. In the VSD, multiple amino acid residues with positive and negative charges play key roles in sensing changes in the electric field across the cell membrane. Conformational change of VSD in response to depolarization leads to the opening (in most types of ion channels) or closing (in the case of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels) of channel pores. VSDs were originally thought to regulate exclusively the ion pores of voltage-gated ion channels, but this idea has recently been changed by discoveries of proteins with VSDs that do not contain pore domains (Murata et al. 2005; Sasaki et al. 2006; Ramsey et al. 2006).
Ci-VSP, a protein identified from the ascidian (Ciona intestinalis) genome based on sequencing (Dehal et al. 2002), consists of two modules; the VSD and the enzyme (Murata et al. 2005). The cytoplasmic region of VSP shows remarkable sequence homology to a tumour suppressor enzyme, called PTEN (phosphatase and tensin homologue deleted on chromosome 10). The cytoplasmic region of VSP has substrate specificity that is slightly distinct from that of PTEN (as discussed below); VSP dephosphorylates both phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) (Murata & Okamura, 2007; Iwasaki et al. 2008). This enzyme activity is induced by the action of depolarization on the VSD (Murata & Okamura, 2007). Another VSD protein that lacks a pore domain, VSOP/Hv1 (Sasaki et al. 2006; Ramsey et al. 2006), consists only of the VSD region with some cytoplasmic stretches at the N and C termini. Despite the lack of a pore domain, it exhibits proton-selective, voltage-dependent ion conductance. The discoveries of these VSD-containing proteins indicate that voltage sensing by VSDs is not only for generating action potentials, but also for more diverse physiological roles than previously appreciated (Okamura, 2007). In this article, recent findings on the molecular properties of VSP will be summarized and its emerging potential as a molecular tool will be discussed.
VSP senses membrane voltage
The VSD of VSP shows significant sequence homology to that of voltage-gated ion channels. As in voltage-gated ion channels, the fourth transmembrane segment (S4) of VSP contains a key motif: periodically aligned residues with positive charges and two intervening hydrophobic residues(Fig.1). Voltage-evoked asymmetrical capacitative currents (‘gating’ or sensing currents) indicated voltage-dependent transition of the voltage sensor in ascidian and zebrafish VSPs (Ci-VSP, Dr-VSP, respectively) (Murata et al. 2005; Murata & Okamura, 2007; Hossain et al. 2008). These measurements verified that the VSD of VSP operates as the voltage sensor. VSP lacking the whole cytoplasmic region still shows sensing currents, indicating that the VSD operates as a self-contained functional unit (Murata et al. 2005), consistent with the position of the VSD in voltage-gated K+ channels which was resolved by X-ray crystallography (Long et al. 2005,2007).
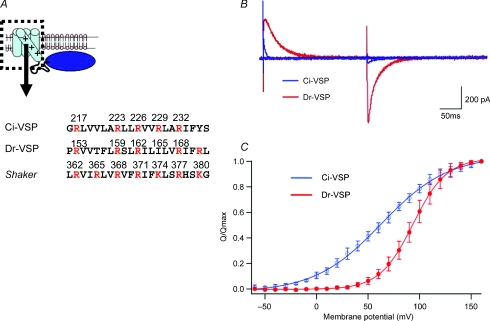
Figure 1. Topology of voltage-sensing phosphatase (VSP) and charge movements of the voltage sensor.
A, the S4 segment in the voltage sensor (dotted box) contains multiple positively charged residues (red). Amino acids from ascidian and teleost VSP (Ci-VSP and Dr-VSP) are compared with those of the Drosophila Shaker potassium channel. B, ‘gating’ or sensing currents evoked at 160 mV under whole-cell patch clamp from tsA201 cells transfected with Ci-VSP (blue) and Dr-VSP (red). C, charge–voltage (Q–V) relations of Ci-VSP (blue) and Dr-VSP (red) (modified from Hossain et al. 2008). Normalized values of Off-charges are shown as mean and standard deviations, collected from 6 and 5 cells for Ci-VSP and Dr-VSP, respectively. Maximum Off-charges were 2.89 ± 0.3 pC pF−1 and 0.11 ± 0.1 pC pF−1 for Ci-VSP and Dr-VSP, respectively.
Is the voltage-sensing mechanism in voltage-gated ion channels also shared by VSP? In voltage-gated ion channels, positive charges in S4 sense electric fields across cell membranes and their voltage-dependent translocation triggers protein conformational change in response to change of membrane potential (Bezanilla, 2000; Horn et al. 2000). Neutralization of positive charges of S4 eliminates (Murata et al. 2005) or causes a significant voltage shift of the charge–voltage (Q–V) curve and changes the kinetics of sensing currents (Murata & Okamura, 2007; Hossain et al. 2008; Kohout et al. 2008). Zebrafish Dr-VSP exhibits positively shifted voltage dependence of the Q–V curve and slower transition kinetics compared with Ci-VSP (Hossain et al. 2008). There exist some differences in the amino acids in the S4 domain, comparing Ci- and Dr-VSP (Fig. 1). In Ci-VSP, four positively charged residues are periodically aligned in S4, whereas in Dr-VSP one site has a hydrophobic residue, isoleucine (I165) instead of a basic amino acid. Changing I165 of Dr-VSP to arginine caused a shift of the voltage dependency in a negative direction and acceleration of sensing currents, mimicking the profiles of wild-type Ci-VSP (Hossain et al. 2008). Negative charges in the VSD are known to counterbalance positive charges of S4 in voltage-gated K+ channels. The VSD of VSP also contains several acidic residues in the S2 and S3 domains. Mutations of those negative charges (D151N or D164N/D186N) cause a voltage shift of the Q–V curve (Murata & Okamura, 2007). Further, it was demonstrated that a polypeptide of the extracellular half of S4 from Ci-VSP, corresponding to a region in the voltage-gated potassium channel called the paddle motif, can be transferred to the voltage-gated K+ channel to reconstitute channel gating (Alabi et al. 2007).
The voltage sensor of Ci-VSP has another property characteristic of voltage sensors in the channels: hysteresis of voltage dependence. The voltage-dependent behaviour of the voltage sensor has been known to be affected by the preceding level and duration of the holding potential in many voltage-gated ion channels including voltage-gated sodium channels (Bezanilla et al. 1982), Shaker K+ channels (Larsson & Elinder, 2000) and HCN channels (Mannikko et al. 2005; Bruening-Wright & Larsson, 2007). These properties have recently been studied in Ci-VSP by measuring sensing currents and site-directed fluorescence (Villalba-Galea et al. 2008). The voltage dependence of charge movements is shifted by about 30 mV in the negative direction by a preceding persistent (5 s) depolarization (to +80 mV) compared with that obtained with a holding potential of −60 mV, suggesting another stable state, called the ‘relaxed state’, in addition to the resting and activated state. Transitions reported by fluorescence directed to a site in S4 exhibited two phases (Kohout et al. 2008), and the slower phase corresponds to the transition into the ‘relaxed state’. The hysteresis of voltage dependence in Ci-VSP also confirmed that the previously characterized hysteresis of voltage dependence in diverse voltage-gated ion channels is derived from the intrinsic nature of the voltage sensor domain.
All of these studies support the idea that the molecular basis for the voltage-driven conformational change of the VSD is shared between VSPs and voltage-gated ion channels. In addition, VSP provides a simple model to study the mechanisms of the voltage sensor. The VSD operates as the voltage sensor without requiring other parts in VSP, whereas there is no report that voltage-gated ion channels lacking a pore domain show voltage sensor function.
The cytoplasmic region of VSP is a PI(4,5)P2/PI(3,4,5)P3 phosphoinositide phosphatase
The cytoplasmic region of VSP shows high sequence similarity to the tumour suppressor phosphatase PTEN. Like PTEN, it consists of a phosphatase domain and a C2 domain. Unlike PTEN, VSP lacks a PDZ-binding region at the C-terminus. The sequence similarity of the phosphatase domain of VSP to PTEN suggested that VSP has an enzyme activity similar to PTEN. In fact, a malachite green assay indicated that the cytoplasmic region of Ci-VSP dephosphorylates PI(3,4,5)P3, as does PTEN (Murata et al. 2005). This originally led us to speculate that the enzyme activity of VSP is activated upon hyperpolarization, based on the finding that the activities of PI(4,5)P2-sensitive potassium channels increased upon hyperpolarization in the presence of Ci-VSP (Murata et al. 2005). However, subsequent experiments using electrophysiology and fluorescence imaging with several phosphoinositide sensors (KCNQ2/3 potassium channels, GIRK/IRK potassium channels and pleckstrin homology domain (PHD)-fused with GFP) indicated that the phosphatase dephosphorylates not only PI(3,4,5)P3 but also PI(4,5)P2 (Murata & Okamura, 2007) and led us to conclude that VSP's enzyme activity is increased by depolarization.
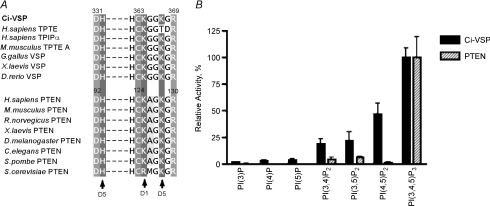
The substrate specificity of VSP was then systematically analysed by in vitro biochemical measurements (Iwasaki et al. 2008). This revealed that VSP has slightly broader substrate specificity than PTEN: in contrast with the rigid specificity of PTEN for PI(3,4,5)P3, VSP also dephosphorylates PI(4,5)P2 (Fig. 2). Such distinct substrate specificity from PTEN is probably due to a single amino acid change: alanine in the enzyme active centre of PTEN is changed to glycine in VSP (Fig. 2, left). Experiments using radiolabelled PI(4,5)P2 showed that VSP dephosphorylates the phosphate on the 5′ position of the inositol ring, but not that on the 4′ position. This indicates that PI(4,5)P2 is converted to PI(4)P by VSP (Iwasaki et al. 2008). Replacement of glycine by alanine in VSP converts its substrate specificity to be similar to that of PTEN. This glycine is also critical for VSP's actions in modulating ion channel activities: the G to A mutant of Ci-VSP does not induce any change in GIRK channel activities upon change of membrane potential. Dr-VSP with the same pattern of amino acid sequence in the active centre with Ci-VSP also shows enzyme activity toward PI(4,5)P2 (Hossain et al. 2008). However, paradoxically, it was reported that mammalian orthologues of VSP, originally called TPIP or PTEN2, do not dephosphorylate PI(4,5)P2, although they contain glycine instead of alanine (Walker et al. 2001; Wu et al. 2001). Further rigorous analysis of substrate specificity in mammalian orthologues is required to address substrate specificity of VSP orthologues and whether other sites are also involved in determining the different enzyme activities of VSP and PTEN. The appearance of shifted double bands by thin-layer chromatography using fluorescently labelled PI(3,4,5)P3 (H. Iwasaki & Y. Okamura, unpublished observations) shows that PI(3,4,5)P3 is dephosphorylated into PI(4,5)P2 (Iwasaki et al. 2008). It remains to be established whether only the 3′ phosphate on PI(3,4,5)P3 is removed, or whether phosphate on other positions is also removed.
Figure 2. Phosphoinositide consensus sequence and substrate specificity.
A, amino acid alignment of the active centres of PTENs and VSPs. Human has two orthologue genes of VSP, TPTE and TPIP. Both genes give rise to several alternatively spliced variants (Walker et al. 2001). Mouse genome has one VSP-like gene, mTPTE, that gives rise to at least three alternatively spliced variants including TPTE-A. There is one amino acid difference between VSP and PTEN (glycine for VSP and alanine, for PTEN). B, comparison of enzyme activities of Ci-VSP and human PTEN using a malachite green assay (from Iwasaki et al. 2008). Activities toward a panel of synthetic di-C16-phosphoinositides were determined. The relative activities of Ci-VSP and PTEN for the panel of phospholipids were calculated from their specific activities divided by the specific activities against PI(3,4,5)P3.
Coupling between VSD and enzyme: mechanisms of tranduction from electrical signal into chemical signal
How can the phosphatases be activated by the voltage sensor? Use of ion channels as biosensors for phosphoinositides has defined a quantitative relationship between VSD movement and enzyme activity (Murata & Okamura, 2007). Distinct types of potassium channels have distinct sensitivities to PI(4,5)P2 (Suh & Hille, 2008). GIRK2 channels coexpressed with G-proteins can report small changes of PI(4,5)P2 because they require a high concentration of PI(4,5)P2 for their activities (Huang et al. 1998). IRK1 potassium channels have a higher sensitivity to PI(4,5)P2, and can be used to monitor changes of PI(4,5)P2 level at lower concentrations than GIRK2 channels. KCNQ2/3 is a class of voltage-gated potassium channel for which the sensitivity to PI(4,5)P2 has been characterized in detail (Zhang et al. 2003; Suh & Hille, 2008). Studies using three types of PI(4,5)P2-sensitive ion channels have indicated that the enzyme activity of Ci-VSP is activated and changed over a wide range of membrane potential from −40 mV to 100 mV.
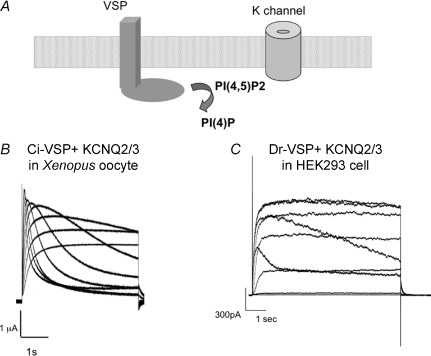
In the presence of VSP, a change of the holding membrane potential from −80 to −40 mV reduces GIRK2 activities (Murata et al. 2005). The decrease in GIRK2 channel activity saturates at around +20 mV (Murata et al. 2005). IRK1 currents measured with a similar pulse protocol to the experiments with GIRK2 channels showed a graded decrease from 0 mV to 60 mV (Murata & Okamura, 2007). An IRK1-R228Q mutant, which has a lower affinity for PI(4,5)P2 than the wild-type IRK1 channel, behaved in a similar manner to the GIRK2 channel (Murata et al. 2005). The decay of KCNQ2/3 currents results from activation of the VSP enzyme, thus reporting the temporal profile of PI(4,5)P2 level during a single depolarizing pulse (Murata & Okamura, 2007; Hossain et al. 2008) (Fig. 3). The decay of KCNQ2/3 currents induced by Ci-VSP becomes sharper as the voltage is set more positive from +50 mV to +100 mV (Murata & Okamura, 2007) (Fig. 3). These findings indicate that the magnitude of enzyme activity continues to increase over a wide range of membrane potentials, from −80 mV to +100 mV, where the maximum moving charges gradually increase. This suggests that enzyme activity correlates with the extent of voltage sensor movement.
Figure 3. KCNQ2/3 current decay induced by VSP expressed in oocyte and tsA201 cell.
A, schematic diagram of regulation of K+ channel activity by changes in the concentration of PI(4,5)P2 induced by the dephosphorylating activity of VSP. B and C, two examples of voltage-induced change of VSP enzyme activities reported by KCNQ2/3 channels are shown. B, traces of KCNQ2/3 currents from a Xenopus oocyte coexpressing KCNQ2/3 and Ci-VSP (Murata et al. 2005). Voltage was stepped in 10 mV increments ranging from −20 to +60 mV from a holding potential of −60 mV. Note that the decay becomes sharper as the voltage increases. C, traces of KCNQ2/3 currents from HEK293 cells coexpressing KCNQ2/3 and Dr-VSP (Hossain et al. 2008). Voltage was stepped in 20 mV increments ranging from −60 to +100 mV from a holding potential of −80 mV.
The coupling between the voltage sensor domain and enzyme has also been studied by fluorescence imaging of green fluorescent protein (GFP)-tagged sensors for phosphoinositides (Murata & Okamura, 2007). The pleckstrin homology domain (PHD) of the phospholipase C (PLC) δ subunit is known to selectively bind to PI(4,5)P2, and GFP fused with PHD-PLC(δ) translocates to the cell membrane dependent on the concentration of PI(4,5)P2 in the inner leaflet of cell membranes (Stauffer et al. 1998). The PHD of Bruton's tyrosine kinase (Btk) selectively binds to PI(3,4,5)P3, thus its GFP-fused form can be used to monitor the dynamics of PI(3,4,5)P3 (Salim et al. 1996; Varnai et al. 1999). Confocal imaging of these GFP-tagged PHDs in Xenopus oocytes showed that both PI(4,5)P2 and PI(3,4,5)P3 were reduced upon depolarization to 0 mV. These results confirmed that enzyme activity is activated by depolarization.
The coupling from the voltage sensor to the downstream effector has been well studied in voltage-gated ion channels. However, little is known about the reverse interaction: how the state of the pore domain affects the voltage sensor in voltage-sensitive proteins. Taking advantage of the robust sensing currents from zebrafish Dr-VSP, we examined whether the state of the enzyme could affect the operation of the voltage sensor. The kinetics of sensing currents were compared between wild-type and a catalytically inactive mutant (Hossain et al. 2008). In a protein with a mutation in the active centre, C302S in Dr-VSP or C363S in Ci-VSP, both On- and Off-sensing currents were faster than those of the wild-type. Pharmacological inhibition of enzyme activity using vanadates also resulted in a similar change in Dr-VSP properties (Hossain et al. 2008). Acceleration of the movement of VSD was not due to altered phosphoinositide level caused by the lack of basal enzyme activity. In addition, a similar effect by inhibition of enzyme activity can still be observed with S4 mutants of Dr-VSP with excessive positive charges that exhibit more rapid kinetics of sensing currents than the wild-type (Hossain et al. 2008). These findings indicate that the state of the enzyme can influence the movement of the voltage sensor. This also indicates that coupling between the two domains is extremely tight.
The stoichiometry of VSP is fundamental for understanding the operation mechanisms of the voltage sensor and its coupling to the enzyme. Recent single-molecule imaging of GFP-fused Ci-VSP expressed in Xenopus oocytes under total internal reflection fluorescence illumination has shown that photo-bleaching of individual fluorescence clusters occurred in a single step (Kohout et al. 2008). Coexpression of wild-type protein and a mutant protein with altered voltage dependence did not lead to the generation of a population of proteins with intermediate voltage dependence, strongly suggesting that single VSDs of VSP function independently (Kohout et al. 2008). These results suggest that Ci-VSP is expressed as a monomer. Since the kinetics of the fluorescence change with voltage sensor movement as measured by voltage-clamp fluorometry (Kohout et al. 2008; Villalba-Galea et al. 2008) are complex, the monomeric nature of VSP suggests that conformational change occurs in multiple steps in a unitary voltage sensor. In fact, a recent detailed study of the kinetics of fluorescence indicates an additional step where the protein enters into a stable, ‘relaxed state’, after the fast transition from the resting state to the activated state (Villalba-Galea et al. 2008). On the other hand, some PTEN-related phosphatases, Mtmr2, are known to be dimers (Berger et al. 2003). It will be intriguing to explore the possibility that VSP could form oligomers dependent on expression density or cell state.
Potential tools for studying phosphoinositide biophysics and neural circuits
PI(4,5)P2 is known to regulate many biological processes such as vesicle turnover, dynamics of the cytoskeleton, and activities of membrane proteins such as ion channels (Suh & Hille, 2005) and transporters. In heterologous expression systems, VSP can regulate ion channel activities through reducing the level of PI(4,5)P2 (Murata & Okamura, 2007; Iwasaki et al. 2008). When coexpressed with Ci-VSP, KCNQ2/3 channels exhibit a current decay that becomes sharper as membrane potential is stepped to more positive voltages (Murata & Okamura, 2007) (Fig. 3). The detailed kinetics of interactions between phosphoinositides and their target proteins have not been fully resolved. Since the levels of phosphoinositides can be acutely altered by VSP's enzyme activity through a simple jump of membrane potential, VSP will potentially serve as a tool to study the dynamics and biological roles of PI(4)P or PI(4,5)P2. Modification of the properties of VSP, such as voltage threshold, activation kinetics and substrate specificity, by mutagenesis, will further help to expand the range of such applications.
Measuring the electrical activity of a specific subpopulation of neurons is important for understanding the dynamics of neuronal networks. In the last decade, several voltage-sensitive probes have been made utilizing VSDs from voltage-gated ion channels (Miyawaki, 2003; Baker et al. 2008). VSP is a promising material for developing such voltage probes for several reasons. First, the VSD operates as a voltage sensor by itself: it shows robust sensing currents even when only the VSD without the enzyme region is expressed. In contrast, the VSD of voltage-gated ion channels does not show sensing or gating currents if the voltage sensor is separated from the pore region, thus making it difficult to monitor voltage-dependent properties of the engineered probe. Second, VSP expression in excitable cells such as neuron or muscle is low or absent. Although Ci-VSP is weakly expressed in the ascidian nervous system (Murata et al. 2005), VSP orthologues are not expressed in mammalian nervous systems (H. Iwasaki, unpublished observations). Thus, overexpression of a VSP-based probe is less likely to perturb native cellular functions. In contrast, voltage-sensitive reporters whose design is based on conventional voltage-gated ion channels may perturb cellular functions by interacting with natively expressed proteins.
Two voltage-sensitive probes have been designed by fusing the VSD of Ci-VSP with the fluorescence resonance energy transfer (FRET) pair cyan and yellow fluorescent proteins (CFP–YFP: VSFP) (Dimitrov et al. 2007; Lundby et al. 2008). A mutation was introduced into the S4 segment to modify the range of voltage sensitivity. These probes exhibited a voltage-dependent change of fluorescence intensity in the physiological range of membrane potential. Recently, a probe called Mermaid was designed by fusing two coral-derived fluorescent proteins as a bright and pH-insensitive FRET-pair to the whole VSD of Ci-VSP. Mermaid exhibits a surprisingly large change in YFP/CFP emission ratio, up to 40% per 100 mV in a heterologous expression system (Tsutsui et al. 2008). This large-ratio signal has enabled non-averaged imaging of membrane potential changes in isolated cardiac muscle cells and cortical neurons, providing the first demonstration of visualizing electrical activity in native cells with a protein-based voltage probe. Modification of properties (kinetics and voltage range) by site-directed mutagenesis or by making chimeric proteins will lead to the development of diverse voltage probes suitable for distinct purposes.
Further questions
To summarize, VSP is a depolarization-activated PI(3,4,5)P3/PI(4,5)P2 phosphatase. The voltage sensor of VSP is a self-contained module that drives enzyme activity inherent to the cytoplasmic region. Further, the voltage sensor of VSP can be transferred as an isolated unit to drive another protein, for example, a FRET pair of fluorescent proteins (Dimitrov et al. 2007; Lundby et al. 2008; Tsutsui et al. 2008).
Given that coupling mechanisms still remain unclear in voltage-gated ion channels, it is challenging to ask how a structural change of the VSD leads to activation of the enzyme in VSP, and how this coupling mechanism is shared by voltage-gated ion channels. A likely scenario for how VSP originated during evolution is that distinct genes encoding VSD and the PTEN-like enzyme region became located next to each other by exon shuffling, resulting in a new gene that encodes a hybrid protein with the novel function as a voltage-regulated enzyme. Then, is the VSP just a simple hybrid of the two protein modules? The linker region between VSD and enzyme is surprisingly short (Murata et al. 2005). The linker region does not show any organized structure based on the prediction of the secondary structure. The phosphoinositide activities of PTEN are regulated by its binding to PI(4,5)P2 via the N-terminal region (Campbell et al. 2003; Iijima et al. 2004; Walker et al. 2004) and phosphorylation of the C-terminal end (Odriozola et al. 2007). How are these regulations of the enzyme activity of PTEN related to the voltage-driven activation of the enzyme activity of VSP? The dual substrate activity of VSP (toward PI(4,5)P2 and PI(3,4,5)P3), unlike the rigid substrate specificity of PTEN toward PI(3,4,5)P3, also raises an intriguing possibility that dephosphorylation of distinct substrates could be coupled to distinct states of the voltage sensor. It also needs to be further investigated whether VSP's enzyme activity is completely silenced or weakly active at hyperpolarized membrane potentials where the voltage sensor is in the down-state, since a GST-fusion protein of the cytoplasmic region of Ci-VSP exhibits enzyme activity in vitro.
Acknowledgments
We thank Dr Laurinda Jaffe for critical reading of the manuscript and all members of the laboratory for helpful discussion.
References
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Mutoh H, Dimitrov D, Akemann W, Perron A, Iwamoto Y, Jin L, Cohen LB, Isacoff EY, Pieribone VA, Hughes T, Knopfel T. Genetically encoded fluorescent sensors of membrane potential. Brain Cell Biol. 2008;36:53–67. doi: 10.1007/s11068-008-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, Schaffitzel C, Berger I, Ban N, Suter U. Membrane association of myotubularin-related protein 2 is mediated by a pleckstrin homology-GRAM domain and a coiled-coil dimerization module. Proc Natl Acad Sci U S A. 2003;100:12177–12182. doi: 10.1073/pnas.2132732100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Taylor RE, Fernandez JM. Distribution and kinetics of membrane dielectric polarization. 1. Longterm inactivation of gating currents. J Gen Physiol. 1982;79:21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening-Wright A, Larsson HP. Slow conformational changes of the voltage sensor during the mode shift in hyperpolarization-activated cyclic-nucleotide-gated channels. J Neurosci. 2007;27:270–278. doi: 10.1523/JNEUROSCI.3801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knopfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS ONE. 2007;2:e440. doi: 10.1371/journal.pone.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Ding S, Gruber HJ. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283:18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279:16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2008;105:7970–7975. doi: 10.1073/pnas.0803936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- Larsson HP, Elinder F. A conserved glutamate is important for slow inactivation in K+ channels. Neuron. 2000;27:573–583. doi: 10.1016/s0896-6273(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Lundby A, Mutoh H, Dimitrov D, Akemann W, Knopfel T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS ONE. 2008;3:e2514. doi: 10.1371/journal.pone.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannikko R, Pandey S, Larsson HP, Elinder F. Hysteresis in the voltage dependence of HCN channels: conversion between two modes affects pacemaker properties. J Gen Physiol. 2005;125:305–326. doi: 10.1085/jgp.200409130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A. Fluorescence imaging of physiological activity in complex systems using GFP-based probes. Curr Opin Neurobiol. 2003;13:591–596. doi: 10.1016/j.conb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem. 2007;282:23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- Okamura Y. Biodiversity of voltage sensor domain proteins. Pflugers Arch. 2007;454:361–371. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, Waterfield MD, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:363–366. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- Villalba-Galea CA, Sandtner W, Starace DM, Bezanilla F. Inaugural Article: S4-based voltage sensors have three major conformations. Proc Natl Acad Sci U S A. 2008;105:17600–17607. doi: 10.1073/pnas.0807387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Downes CP, Leslie NR. TPIP: a novel phosphoinositide 3-phosphatase. Biochem J. 2001;360:277–283. doi: 10.1042/0264-6021:3600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Leslie NR, Perera NM, Batty IH, Downes CP. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J. 2004;379:301–307. doi: 10.1042/BJ20031839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Pisabarro MT, Dillard-Telm L, Koeppen H, Lasky LA. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J Biol Chem. 2001;276:21745–21753. doi: 10.1074/jbc.M101480200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]