Abstract
The cutaneous silent period (CSP) is a spinal inhibitory reflex mediated by Aδ fibres. The postinhibitory rebound of electromyographic (EMG) activity following the CSP has been mainly attributed to resynchronization of motoneurons, but the possibility of startle reflex activity contributing to the EMG burst has also been suggested. Several types of reflexes may be suppressed by a preceding weak stimulus – a phenomenon called prepulse inhibition (PPI). Our aim was to study whether PPI would diminish the EMG rebound, thereby providing further evidence for excitatory reflex activity contained within the postinhibitory EMG rebound following the CSP. Ten healthy subjects underwent CSP testing following noxious digit II stimulation in two conditions, with and without a prepulse applied to digit III. Rectified surface EMG recordings were obtained from right orbicularis oculi, sternocleidomastoid and thenar muscles of the dominant hand during thumb abduction with 25% of maximum force. The area of the EMG rebound and the EMG reflex responses in orbicularis oculi and sternocleidomastoid were significantly smaller in recordings where a prepulse stimulus was applied 100 ms before the stimulus as compared to control responses without prepulse. CSP onset and end latency, CSP duration, and the degree of EMG suppression were not influenced. Prepulses significantly reduced subjective discomfort as based on visual analog scale scores. Inhibition of the EMG rebound by prepulse stimulation supports the hypothesis that the excitatory EMG activity following the CSP contains not only resynchronization of motoneuronal firing, but also an excitatory reflex component. The most probable type of reflex seems to be a somatosensory startle reflex, a defence reaction which is generated in structures located in the caudal brainstem following an unexpected intense stimulus. Reduction of the discomfort associated with high-intensity electrical fingertip stimulation by a prepulse without affecting CSP parameters underlines the utility of PPI in the context of CSP testing.
The cutaneous silent period (CSP) is a spinal inhibitory reflex mediated by Aδ fibres (Uncini et al. 1991; Leis et al. 1992; Kofler, 2003; Floeter, 2003; Romaniello et al. 2004). Upper limb CSPs constitute the inhibitory part of a complex preattentional protective reflex (Inghilleri et al. 1997; Leis et al. 2000; Kofler, 2003; Kofler et al. 2004), which operates in a timely manner together with excitatory withdrawal flexor reflexes serving to retract the hand away from a noxious stimulus (Floeter et al. 1998; Rossi et al. 2003). Both inhibitory and excitatory components seem to share common spinal neural circuitry, which is activated by high-threshold, low-diameter Aδ fibres.
Post-inhibition excitatory electromyographic (EMG) activity following the CSP occurs regularly at latencies exceeding those typical of transcortical long loop reflexes (Deuschl & Eisen, 1999). This EMG rebound period has been mainly attributed to resynchronization of motoneuronal firing (Kranz et al. 1973), but in the lower limbs has also been suggested to represent a spino-bulbo-spinal reflex mediated by group III afferents (Gassel & Ott, 1970). Recently, Kofler & Poustka (2005) suggested that there may be startle reflex activity coinciding in time with the EMG rebound. It is likely that the size of the rebound depends on both resynchronized motoneuron firing following suppression (Fetz & Gustafsson, 1983) and reflex activity (Gassel & Ott, 1970; Kofler & Poustka, 2005). While a resynchronized burst of activity would be related to motoneuronal firing properties underlying ongoing muscle activation, reflex activity would be related to the stimulus properties eliciting such activity.
The magnitude of reflex activity can be experimentally decreased, together with the perceived intensity of the stimulus, when the reflex-triggering stimulus is preceded by a weak prepulse (Graham, 1975; Blumenthal, 1999). Prepulse inhibition (PPI) is well known in the domain of the startle (Graham, 1975), but it has also been described in other exteroceptive reflexes such as the blink reflex (Ison et al. 1990; Rossi & Scarpini, 1992; Valls-Soléet al. 1994) and the masseteric inhibitory reflex (Gómez-Wong & Valls-Solé, 1996). PPI is maximal at interstimulus intervals around 100 ms and operates both within and across sensory modalities. For example, acoustic prepulses can reduce both the magnitude of the startle reflex elicited by an intense noise or air puff and the perceived intensity of the startling stimuli (Blumenthal et al. 1996; Swerdlow et al. 1999). Electrical prepulses have been reported to inhibit the auditory blink reflex (Valls-Soléet al. 1999) and the reflex elicited by intense electrical stimuli (Ison et al. 1986; Blumenthal & Swerdlow, 2002).
The aim of the present study was to investigate prepulse modulation of the EMG rebound following the CSP. We hypothesized that a weak electrical prepulse presented to the third digit would suppress part of the EMG rebound activity following the CSP elicited by a noxious stimulus to the index finger. The amount of PPI of the EMG activity would determine the amount of reflex activity and discern it from the activity related to posthyperpolarization resynchronization of motoneuronal firing.
Methods
Subjects
Twelve healthy subjects with a mean age of 33 ± 5.9 years (8 men, 4 women) with no history of neurological disorders underwent repeated CSP testing after granting informed consent. Data of two subjects had to be excluded from analysis. In one of them, thenar recordings showed a previously unrecognized tremor at 10 Hz, compatible with enhanced physiological tremor. In the other subject, orbicularis oculi and sternocleidomastoid recordings contained artefacts due to metallic material implanted in the zygomatic bone following a fracture. The study was approved by the local ethics committee of the Instituto Guttmann, Hospital de Neurorehabilitació in compliance with the Declaration of Helsinki.
Experimental set-up
Subjects were seated upright in a comfortable chair. The dominant arm (8 right, 2 left) was positioned on a table in front of the subject, with the shoulder in a neutral position, the elbow joint maintained at a 90 deg angle, the hand held in a slightly pronated position, and the fingers extended. The subjects were then asked to perform thumb abduction as if ‘hitch-hiking’, but the movement was blocked by a custom-made force transducer when the thumb reached a 45 deg angle. Maximum voluntary thumb abduction was determined three times over 5 s and was monitored by visual feedback of the applied force (in kiloponds) on a display as measured by the force transducer. Rectified surface electromyographic (EMG) recordings were obtained from thenar muscles on the dominant side for CSPs, and from right orbicularis oculi and sternocleidomastoid for eventual startle reflexes. Electrodes were attached in a belly tendon fashion with a 3 cm interelectrode distance (thenar and sternocleidomastoid), or below and lateral to the eye (orbicularis oculi), respectively. Routine electrodiagnostic equipment (Medelec Synergy, Cardinal Health, Surrey, UK) was used in all experiments.
Stimulus characteristics
Sensory thresholds (ST) were determined as previously described (Kofler, 2003) for digit II, and subsequently for digit III of the dominant hand, using constant current square wave electrical stimuli of 0.5 ms duration delivered through ring electrodes attached around the distal two phalanges. Subjects were then asked to evaluate subjective pain perception on a visual analog scale (VAS; 0 = no pain; 10 = most severe pain) when stimuli intended to induce the silent period were applied at rest to digit II, either alone or preceded 100 ms earlier by a prepulse applied to digit III. Based on previous studies, we used an intensity of 25 times sensory threshold (25ST) for induction of CSPs (Kofler, 2003, 2004; Kofler et al. 2004) and 2 times ST (2ST) for induction of prepulse effects (Valls-Soléet al. 2005).
Experimental procedure
Subjects were asked to activate the thenar muscles for periods up to 10 s with 25% of individual maximum force, which was continuously monitored by visual feedback of the force transducer, and by audio feedback of the EMG signal recorded from thenar muscles, followed by rest periods of 10 s. Stimuli were applied after 3–7 s of continuous voluntary contraction when the force level had stabilized, but subjects were asked to maintain contraction for a further 2 s following stimulation. Subjects received at random either single stimuli of 25ST to digit II (control trials, CSP-only, number of trials 15) or paired stimuli of 25ST to digit II preceded 100 ms earlier by a prepulse stimulus of 2ST applied to digit III (test trials, CSP-PP, n= 15), in order to avoid habituation. Additionally, we applied single unexpected stimuli at rest with 25ST to digit II (startle reflex, SR-only; n= 7), and with 2ST to digit III (PP-only; n= 3), randomly interspersed among control and test trials.
Data acquisition and measurement
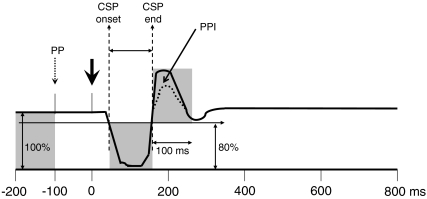
Single sweeps of 1000 ms were recorded including a 200 ms prestimulus delay before digit II stimulation (Fig. 1). Off-line averaging of thenar EMG responses was done separately for control and test trials using Matlab (The MathWorks, Natick, MA, USA). Mean rectified EMG amplitudes were established in each subject for the following periods: during the first 100 ms (baseline EMG); during the CSP, which was defined by a drop of rectified EMG below 80% of baseline EMG and the final return above 80% of baseline EMG at appropriate latencies (Kofler, 2003); and during a 100 ms period following the CSP end latency (EMG rebound). As the exact duration of the postinhibitory EMG rebound is often difficult to define with certainty, we decided to measure a fixed window of 100 ms to avoid as much contamination with eventual voluntary reactions as possible, as in previous studies on the startle reflex (Kofler et al. 2001b, 2006). In prepulse containing traces, a 100 ms period following the prepulse was also measured (postprepulse EMG) (Fig. 1). The amount of EMG attenuation during the CSP was expressed as the percentage of the average rectified EMG amplitude during the CSP divided by the average rectified baseline EMG amplitude (index of suppression). The amount of EMG rebound following the CSP was expressed as the percentage of the average rectified EMG rebound amplitude divided by the average rectified baseline EMG amplitude.
Figure 1. Rectified averaged EMG trace with a 200 ms prestimulus delay preceding the noxious stimulus.
Schematic representation of the rectified averaged EMG trace with a 200 ms prestimulus delay preceding the noxious stimulus (thick arrow); including the period of baseline EMG preceding the prepulse (PP, small broken arrow), and the postprepulse EMG period (between prepulse and noxious stimulus). The cutaneous silent period (CSP, between CSP onset and CSP end) is followed by an EMG rebound. This EMG rebound is reduced by prepulse inhibition (PPI).
Latency and area under the curve of reflex responses in orbicularis oculi and sternocleidomastoid were measured in non-averaged single rectified traces, and the resulting values were arithmetically averaged according to the four experimental conditions (CSP-only, CSP-PP, SR-only, PP-only). Missing responses were assigned a zero area value, but no latency value.
One-way ANOVA was used to assess the effect of condition on the latency of orbicularis oculi and sternocleidomastoid reflex responses. Wilcoxon's test was used to compare CSP onset latency, end latency and duration, as well as index of suppression, area of orbicularis oculi and sternocleidomastoid reflex responses, baseline EMG, postprepulse EMG, and EMG rebound. The level of statistical significance for all comparisons was set at P < 0.05.
Results
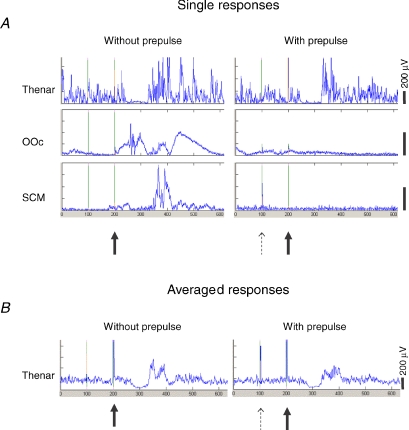
All subjects were able to complete the experiments without difficulty. VAS score at rest was 6.1 ± 1.5 (mean ±s.d.) in trials without prepulse, and 4.5 ± 1.6 in trials with prepulse (P < 0.001). MVC for thumb abduction was 1.6 ± 0.6 kp (corresponding to 15.7 ± 5.9 N), and 25% MVC was 0.4 ± 0.1 kp (corresponding to 3.9 ± 1.0 N). Sensory thresholds were 1.0 ± 0.4 mA following digit II stimulation, and 0.7 ± 0.3 mA following digit III stimulation. Figure 2 shows trials from a representative subject, showing CSPs with and without prepulse.
Figure 2. Responses recorded from thenar, orbicularis oculi and sternocleidomastoid muscles.
A and B, representative examples of single (A) and averaged (B) responses recorded from thenar, orbicularis oculi (OOc) and sternocleidomastoid (SCM) muscles following digit II with 25 times sensory threshold intensity, without (left) and with (right) a prepulse delivered to digit III with 2 times sensory threshold intensity.
Mean baseline EMG was not significantly different in CSP-only and CSP-PP (Table 1). In order to exclude an effect of the prepulse on the baseline EMG activity immediately preceding the noxious stimulus (e.g. in form of a cutaneomuscular reflex, which could in turn cause reduced thenar contraction and lead to less EMG rebound activity), we also compared mean baseline EMG with mean postprepulse EMG in those traces containing a prepulse. There was, however, no significant difference between baseline EMG and postprepulse EMG (21.7 ± 11.6 mV versus 21.2 ± 11.8 mV; P= 0.39).
Table 1.
EMG amplitude, CSP onset latency, CSP end latency, CSP duration, index of suppression, and EMG rebound amplitude obtained in thenar muscles following noxious digit II stimulation
| CSP-only | CSP-PP | P | |
|---|---|---|---|
| Baseline EMG amplitude (mV s) | 22.1 (4.1) | 21.7 (3.7) | 0.33 |
| CSP onset latency (ms) | 44.9 (3.0) | 45.0 (1.4) | 0.91 |
| CSP end latency (ms) | 124.1 (3.9) | 124.1 (3.5) | 0.73 |
| CSP duration (ms) | 79.2 (5.7) | 79.1 (4.0) | 0.91 |
| Index of suppression (%) | 40.4 (3.0) | 38.9 (3.0) | 0.44 |
| EMG rebound amplitude (mV s) | 31.5 (4.3) | 27.6 (3.3) | 0.02 |
Mean and standard error of baseline EMG amplitude (from −200 ms until −100 ms preceding the noxious stimulus), CSP onset latency, CSP end latency, CSP duration, index of suppression, and EMG rebound amplitude (during 100 ms following the end of the CSP) obtained in thenar muscles following noxious digit II stimulation (25 times sensory threshold intensity). Note the significant decrease in EMG rebound amplitude in traces containing a prepulse (CSP-PP)
Effect of a prepulse on CSPs and EMG rebound in thenar muscles
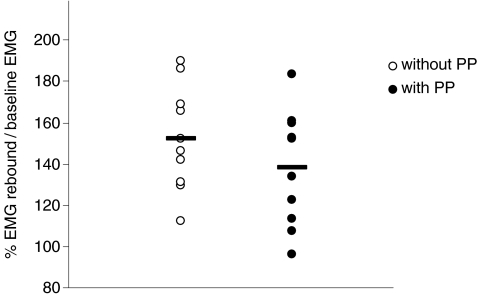
Group average CSP onset and end latency, CSP duration, and the index of suppression were not influenced by the prepulse (P > 0.3) (Table 1). However, EMG rebound was significantly smaller in CSP-PP than in CSP-only (P= 0.02) (Table 1, Fig. 3). EMG rebound values did not systematically change over time in either condition (with or without prepulse), indicating no habituation with repeated stimulation.
Figure 3. Distribution of individual values of EMG rebound amplitudes relative to baseline EMG amplitudes in 10 subjects.
Effect of a prepulse on reflexes in orbicularis oculi and sternocleidomastoid muscles
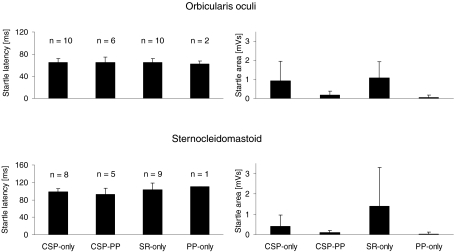
Reflex responses were recorded in orbicularis oculi (F3,23= 0.069; P= 0.98) and in sternocleidomastoid muscles (F3,19= 1.06; P= 0.39) to stimuli delivered in all conditions. However, responses recorded in trials containing prepulses were smaller and inconsistent. The area under the curve was significantly smaller in CSP-PP and PP-only than in CSP-only and SR-only in both orbicularis oculi (P < 0.03) and sternocleidomastoid (P < 0.04), but it did not differ significantly between SR-only and CSP-only (Fig. 4).
Figure 4. Mean latency and area of orbicularis oculi and sternocleidomastoid muscles.
Mean latency and area of orbicularis oculi and sternocleidomastoid muscles in the experimental conditions ‘CSP-only’ (stimuli of 25ST intensity to digit II during target muscle contraction), ‘CSP-PP’ (paired stimuli of 25ST intensity to digit II preceded 100 ms earlier by a prepulse stimulus of 2ST intensity applied to digit III during target muscle contraction), ‘SR-only’ (unexpected stimuli at rest with 25ST intensity to digit II) and ‘PP- only’ (unexpected stimuli at rest with 2ST intensity to digit III). Numbers in the panels on the left side indicate the number of subjects who had at least one response in the various experimental conditions.
Discussion
This study reports for the first time an inhibitory effect of a prepulse on the EMG rebound following the CSP induced in a hand muscle, thus supporting the hypothesis that the post-CSP EMG rebound contains an excitatory reflex component, which, based on the latency and the nature of the underlying stimulus, is compatible with a somatosensory startle reflex. Furthermore, our results indicate that prepulse stimuli significantly reduced the subjective sensation, so that weak lead stimuli may be applied in CSP testing in order to reduce the discomfort associated with noxious electrical stimuli delivered to fingertips without affecting CSP parameters.
PPI is a basic neurophysiological mechanism elicited by a subthreshold stimulus preceding a suprathreshold stimulus by a brief interval, thereby suppressing excitatory reflex activity evoked by the suprathreshold stimulus. The underlying loop is located in the upper brainstem and involves the pedunculopontine nucleus. PPI has been demonstrated for the R2 component of the blink reflex elicited by electrical supraorbital nerve stimulation (Ison et al. 1990; Rossi & Scarpini, 1992; Valls-Soléet al. 1994), the auditory blink reflex (Valls-Soléet al. 1999), the auditory startle reflex in the orbicularis oculi muscle (Valls-Soléet al. 1999), and the second masseteric suppression period (SP2) (Gómez-Wong & Valls-Solé, 1996). PPI has only rarely been demonstrated in extracranial muscles following auditory stimuli (Müller et al. 2003). Suppression of the EMG rebound following the CSP is the first demonstration of PPI upon a putative spinal reflex. The lack of effect on the CSP and the concomitant presence of an effect on the EMG rebound resembles what is seen with the blink reflex, i.e. no or little effect on the blink reflex component R1, but clear suppression of the R2 component, and with the masseteric inhibitory reflex, i.e. lack of effect on the masseteric suppression period SP1 but a clear effect on the masseteric suppression period SP2.
The CSP is considered to be a protective spinal inhibitory reflex mediated by Aδ fibres and is present in both upper and lower limb muscles (Uncini et al. 1991; Leis et al. 1992; Kofler, 2003; Floeter, 2003; Romaniello et al. 2004; Tataroglu et al. 2005; Svilpauskaite et al. 2006). The distinct timing and magnitude of EMG suppression in different upper limb muscles indicate a functional organization of the underlying spinal circuitry (Kofler, 2003; Kofler et al. 2004). Studies using H-reflexes, F-waves, and motor evoked potentials suggest that motoneuron inhibition is mediated by spinal inhibitory interneurons (Uncini et al. 1991; Walk & Fisher, 1993; Inghilleri et al. 1995; Leis et al. 1995; Leis et al. 1996; Inghilleri et al. 1997; Manconi et al. 1998; Logigian et al. 1999; Kofler et al. 2001a; Floeter, 2003). Only very few synapses seem to be interspersed as habituation is virtually absent (Kranz et al. 1973; Uncini et al. 1991; Floeter, 2003). Supraspinal influence upon the CSP mediating pathways has been postulated based on studies in patients with spinal cord injury, minor stroke, amyotrophic lateral sclerosis, and compressive cervical myelopathy, which revealed either less EMG suppression or a delayed CSP onset (Logigian et al. 1999; Gilio et al. 2008; Štetkárová & Kofler, 2009).
Nociceptive EMG modulation as tested by means of motor evoked potentials conditioned by high intensity electrical finger stimulation (Inghilleri et al. 1995; Kofler et al. 2001a) revealed motor evoked potential amplitude facilitation in muscles involved in withdrawing the hand from a noxious stimulus, suggesting that withdrawal reflexes represent the underlying neurophysiological principle of the observed facilitatory effects. In contrast, CSPs seem to reflect part of the inhibitory effects seen as motor evoked potential amplitude suppression in muscles involved in reaching and grasping (Kofler et al. 2001a). CSPs and motor evoked potential amplitude suppression, however, are not completely interchangeable (Kofler et al. 2008). Based on a different time course of maximum changes in motor evoked potential amplitude and latency induced by conditioning noxious finger stimulation, a possible role of modulatory brainstem influence on spinal nociceptive circuitry has previously been suggested (Kofler et al. 2001a).
The EMG rebound following the CSP has scarcely been investigated, and its nature remains a matter of debate. The first to note that an excitatory reflex activity was present following the CSP were Gassel & Ott (1970), who suggested a spino-bulbo-spinal reflex mediated by group III afferents in the lower limbs. In contrast, applying single motor unit recordings, Kranz et al. (1973) found increased firing probability following the CSP, but only 1 of 62 motoneurons showed an increase in discharge frequency following inhibition; recruitment of additional motoneurons following inhibition was considered to be unlikely due to the Henneman principle (Henneman et al. 1965). Kranz et al. (1973) attributed the EMG rebound mainly to resynchronization of motoneuronal activity following a period of reduced firing probability during the CSP. This concept was taken up by Fetz & Gustafsson, 1983), but was further elaborated and critically discussed by Türker & Powers (1999, 2001, 2005). Resynchronization of motoneuronal activity is also consistent with the lack of significant difference in the magnitude of the EMG rebound between normal controls and patients with brachial dystonia or Parkinson's disease, despite a longer CSP duration in both patient groups (Pullman et al. 1996).
A potential influence of low-threshold afferents on the ensuing EMG trace in form of a cutaneomuscular reflex induced by the prepulse has to be considered as well. The typical cutaneomuscular reflex contains alternating phases of inhibition and excitation, usually termed E1, I1, E2, I2 and E3 (Türker & Powers, 2005). While some of these EMG undulations may be true reflexes, others may be due to ‘electro-technical’ artefacts (Türker & Powers, 2005). The first phase of altered excitability to appear with low stimulus intensity is the I2 phase with latencies around 70 ms (Caccia et al. 1973). A later phase of excitation termed cLLR III (with a mean latency of 76 ms following radial nerve stimulation) should – if present – also appear before the noxious stimulus (Deuschl & Eisen, 1999). However, as there was no significant difference between baseline EMG and postprepulse EMG, neither immediate inhibition nor delayed inhibition due to resynchronization following a preceding EMG peak (e.g. cLLR III) can be accounted for the observed reduction in postinhibitory rebound EMG.
Nonetheless, a few reported findings cast doubts on attributing the EMG rebound solely to resynchronization of motoneuronal activity and favour the possibility that it involves true reflex activity: Uncini et al. (1991) reported the lack of EMG rebound despite the presence of a distinct CSP in patients with Friedreich's ataxia and chronic idiopathic ataxic neuropathy. Furthermore, the fact that nociceptive motor evoked potential modulation and tonic EMG differ in various hand muscles during and following the CSP (Kofler et al. 2001a; Kofler et al. 2008) casts doubt on pure passive resynchronization. Although no ‘action’ was asked from the subjects other than to maintain constant voluntary muscle contraction, we cannot entirely rule out voluntary ‘re-action’ to the stimulus. However, nociceptive MEP suppression during the period of EMG rebound (Kofler et al. 2008) renders active voluntary reaction rather unlikely, but is consistent with subcortical reflex activity.
Recently, Kofler & Poustka (2005) suggested that the EMG rebound period may contain startle reflex activity. Speculating about its origin, the authors described features resembling startle reflexes in that: (1) responses appeared ipsi- and contralateral to the side of stimulation; (2) response latencies lay in the range of auditory startle responses (Kofler et al. 2006), particularly when adjusted for a longer afferent conduction time to the brain-stem from the digit as compared to the ear; (3) responses were inconsistent in their presence and latency even within a given subject; and (4) they habituated rapidly with repeated stimulation (Kofler & Poustka, 2005).
The startle reflex is an involuntary polysynaptic brainstem response to an unexpected intense stimulus. Various afferent modalities converge in the brainstem structure in which the startle reflex is generated, i.e. the nucleus reticularis pontis caudalis (Koch, 1999; Yeomans et al. 2002). Muscle responses following high-intensity auditory stimulation involve eye closure, facial grimacing, neck flexion, and abduction or flexion of the arms. To our knowledge, however, there are no data available on somatosensory startle responses in human upper limb muscles, and they have scarcely been studied in orbicularis oculi and sternocleidomastoid (Alvarez et al. 2007). There may be, however, a large overlap between startle reflexes and withdrawal reflexes in the upper limbs following high-intensity fingertip stimulation. One may speculate that if stimuli are strong enough to elicit an overt withdrawal reflex in the upper limb, they may also induce a concomitant startle reflex as evidenced by responses in orbicularis oculi and sternocleidomastoid muscles, or in contralateral muscles. In fact, noxious fingertip stimulation revealed bilateral motor evoked potential amplitude facilitation 100 ms later in proximal upper limb muscles (Kofler et al. 2001a), this being in fact compatible with a somatosensory startle reflex. On the contrary, not every somatosensory startle reflex induced by fingertip stimulation is unvaryingly accompanied by an overt withdrawal reflex. Perhaps there is a continuum in the spectrum of protective reflexes, from ‘subthreshold’ changes seen in motor evoked potential amplitudes to somatosensory startle reflexes – being most prominent in orbicularis oculi – to ‘suprathreshold’ overt withdrawal reflexes. Floeter et al. (1998) suggested latencies around 80–100 ms for withdrawal reflexes in hand muscles, while startle reflexes following high-intensity auditory stimulation occur in a range of 70–140 ms, median 89 ms, in the same age group as studied here (Kofler et al. 2001b). The difference in afferent conduction time to the brainstem between somatosensory and auditory stimuli is indeed some 10 ms. Given the fact of profound inhibition in hand muscles induced by noxious fingertip stimulation and ensuing resynchronization of EMG activity, perhaps withdrawal reflexes could also be shifted towards longer latencies, giving rise to the possibility of close overlap between withdrawal and startle reflexes in upper limb muscles. Certainly, further studies are needed on this interesting aspect of motor control.
One important physiological characteristic of startle reflexes is their susceptibility to PPI. Any weak stimulus that is unable to cause a recordable response by itself may modulate the response to a subsequent suprathreshold stimulus. In two subjects, the prepulse stimulus was able to induce a small response in orbicularis oculi and sternocleidomastoid, respectively, on 3 out of 60 occasions. Therefore, the stimulus was not always subthreshold. The possibility of a weak stimulus giving rise to a startle reflex has previously been published (Blumenthal & Goode, 1991), as well as its possibility of triggering a prepared voluntary motor programme (Valls-Soléet al. 2005). The functional anatomy of PPI is not completely known, although there is evidence from various sources that the pedunculopontine nucleus may be involved (Koch et al. 1993; Reese et al. 1995; Inglis & Winn, 1995; Costa et al. 2006). The pedunculopontine nucleus is the main structure within the first level of higher order hierarchical circuits governing the primary startle circuit and mediating both intermodal and cross-modal PPI (Kofler et al. 2006). The suppressing effect of a prepulse on the auditory startle reflex induced in orbicularis oculi and sternocleidomastoid has previously been shown (Valls-Soléet al. 2005).
The reduction in VAS scores seen in our subjects (6.1 without prepulse versus 4.5 with prepulse, i.e. mean reduction by 26.2%) was more than the mean reduction of 17.5% previously reported by Blumenthal et al. (2001) on pain induced by electrical shocks. Differences may be based on the location of stimuli (fingertip versus upper arm), interstimulus intervals (100 ms versus 40 and 60 ms), and lower baseline VAS scores (6.1 versus approximately 7), respectively. Blumenthal et al. (2001), however, reported significantly larger prepulse effects (reduction by 25.9%) in subjects with lower pain thresholds, hence higher VAS scores. This is consistent with a previous study seeking the influence of therapeutic cutaneous electrical nerve stimulation on CSPs (Kofler, 2004), which revealed slight shortening of CSPs, while not reducing the subjective discomfort associated with noxious fingertip stimulation. Baseline VAS scores were also lower than in the present study, 4.5 versus 6.1 (Kofler, 2004). Blumenthal & Swerdlow (2002) showed that weak prepulses of 1ST were unable to reduce pain perception, while significantly suppressing startle blink responses in orbicularis oculi to both acoustic and somatosensory stimulation.
We assume that PPI was effective in our paradigm as sternocleidomastoid and orbicularis oculi reflex responses were markedly suppressed; hence we also deduce that the observed reduction in EMG rebound following the CSP may be due to a similar mechanism. As startle reflexes are affected by PPI, this reduction may represent PPI of the startle component contained within the EMG rebound. The remaining excitatory activity is compatible with ‘passive’ EMG resynchronization. Furthermore, applying prepulses in CSP testing may reduce the discomfort associated with activation of pain afferents by high-intensity electrical fingertip stimulation without affecting CSP parameters such as CSP onset and end latency, duration or index of suppression.
Acknowledgments
The authors express their gratitude to Mrs Ellen Quirbach, Hochzirl, Austria, for her help with editing the manuscript.
This work was in part accomplished thanks to grant 071931 of Fundació la Marató de TV3 2006 (HK).
References
- Alvarez S, Marchetti P, Valls-Solé J. Startle reactions to somatosensory inputs. Different response pattern to stimuli of upper and lower limbs. Clin Neurophysiol. 2007;118:2817. doi: 10.1007/s00221-009-1784-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Short lead interval startle modification. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle Modification. Implications for Neuroscience, Cognitive Science, and Clinical Science. Cambridge: Cambridge University Press; 1999. pp. 51–71. [Google Scholar]
- Blumenthal TD, Burnett TT, Swerdlow CD. Prepulses reduce the pain of cutaneous electrical shocks. Psychosom Med. 2001;63:275–281. doi: 10.1097/00006842-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Goode CT. The startle eyeblink response to low intensity acoustic stimuli. Psychophysiology. 1991;28:296–306. doi: 10.1111/j.1469-8986.1991.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Schicatano EJ, Chapman JG, Norris CM, Ergenzinger ER., Jr Prepulse effects on magnitude estimation of startle-eliciting stimuli and startle responses. Perception Psychophysics. 1996;58:73–80. doi: 10.3758/bf03205477. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Swerdlow CD. Electrical shocks to the arm elicit and inhibit startle eyeblinks. Psychophysiology. 2002;39:218–221. [PubMed] [Google Scholar]
- Caccia MR, McComas AJ, Upton ARM, Blogg T. Cutaneous reflexes in the small muscles of the hand. J Neurol Neurosurg Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Valls-Solé J, Valldeoriola F, Pech C, Rumià J. Single subthalamic nucleus deep brain stimuli inhibit the blink reflex in Parkinson's disease patients. Brain. 2006;129:1758–1767. doi: 10.1093/brain/awl143. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Eisen A. Long-latency reflexes following electrical nerve stimulation. In: Deuschl G, Eisen A, editors. Recommendations for the Practice of Clinical Neurophysiology: Guidelines of the International Federation of Clinical Neurophysiology. Amsterdam, BV: Elsevier Science; 1999. pp. 263–268. [PubMed] [Google Scholar]
- Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003;28:391–401. doi: 10.1002/mus.10447. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Gerloff C, Kouri J, Hallett M. Cutaneous withdrawal reflexes of the upper extremity. Muscle Nerve. 1998;21:591–598. doi: 10.1002/(sici)1097-4598(199805)21:5<591::aid-mus5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gassel M, Ott KH. Local sign and late effect on motor neuron excitability of cutaneous stimulation in man. Brain. 1970;93:95–106. doi: 10.1093/brain/93.1.95. [DOI] [PubMed] [Google Scholar]
- Gilio F, Bettolo CM, Conte A, Iacovelli E, Frasca V, Serrao M, Giacomelli E, Gabriele M, Prencipe M, Inghilleri M. Influence of the corticospinal tract on the cutaneous silent period: a study in patients with pyramidal syndrome. Neurosci Lett. 2008;433:109–113. doi: 10.1016/j.neulet.2007.12.055. [DOI] [PubMed] [Google Scholar]
- Gómez-Wong E, Valls-Solé J. Effects of a prepulse stimulus on the masseteric inhibitory reflex in humans. Neurosci Lett. 1996;208:183–186. doi: 10.1016/0304-3940(96)12574-x. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurones. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M, Priori A, Rothwell JC. Inhibition of hand muscle motoneurones by peripheral nerve stimulation in the relaxed human subject. Antidromic versus orthodromic input. Electroencephalogr Clin Neurophysiol. 1995;97:63–68. doi: 10.1016/0924-980x(94)00225-v. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Cruccu G, Argenta M, Polidori L, Manfredi M. Silent period in upper limb muscles after noxious cutaneous stimulation in man. Electroencephalogr Clin Neurophysiol. 1997;105:109–115. doi: 10.1016/s0924-980x(97)96579-6. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculo-pontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Ison JR, Foss JA, Falcone P, Sakovits L, Adelson AA, Burton RI. Reflex modification: a method for assessing cutaneous dysfunction. Percept Psychophys. 1986;40:164–170. doi: 10.3758/bf03203012. [DOI] [PubMed] [Google Scholar]
- Ison JR, Sanes JN, Foss JA, Pinckney LA. Facilitation and inhibition of the human startle blink reflexes by stimulus anticipation. Behav Neurosci. 1990;104:418–429. doi: 10.1037//0735-7044.104.3.418. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- Kofler M. Functional organization of exteroceptive inhibition following nociceptive electrical fingertip stimulation in humans. Clin Neurophysiol. 2003;114:973–980. doi: 10.1016/s1388-2457(03)00060-9. [DOI] [PubMed] [Google Scholar]
- Kofler M. Influence of transcutaneous electrical nerve stimulation on cutaneous silent periods in humans. Neurosci Lett. 2004;360:69–72. doi: 10.1016/j.neulet.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Kofler M, Fuhr P, Leis AA, Glocker FX, Kronenberg MF, Wissel J, Štetkárová I. Modulation of upper extremity motor evoked potentials by cutaneous afferents in humans. Clin Neurophysiol. 2001a;112:1053–1063. doi: 10.1016/s1388-2457(01)00540-5. [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Reggiani L, Valls-Solé J. Influence of age on auditory startle responses in humans. Neurosci Lett. 2001b;307:65–68. doi: 10.1016/s0304-3940(01)01908-5. [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Valls-Solé J. Auditory startle responses as a probe of brainstem function in healthy subjects and patients with movement disorders. Clin Neurophysiol. 2006;58(Suppl.):232–248. doi: 10.1016/s1567-424x(09)70072-8. [DOI] [PubMed] [Google Scholar]
- Kofler M, Poustka K. Ipsi- and contralateral exteroceptive EMG modulation in uni- and bilaterally activated thenar muscles. Clin Neurophysiol. 2005;116:300–307. doi: 10.1016/j.clinph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kofler M, Štetkárová I, Wissel J. Nociceptive EMG suppression in triceps brachii muscle in humans. Clin Neurophysiol. 2004;115:1052–1056. doi: 10.1016/j.clinph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kofler M, Valls-Solé J, Fuhr P, Schindler C, Zaccaria BR, Saltuari L. Sensory modulation of voluntary and TMS-induced activation in hand muscles. Exp Brain Res. 2008;188:399–409. doi: 10.1007/s00221-008-1372-2. [DOI] [PubMed] [Google Scholar]
- Kranz H, Adorjani C, Baumgartner G. The effect of nociceptive cutaneous stimulation on human motoneurons. Brain. 1973;96:571–590. doi: 10.1093/brain/96.3.571. [DOI] [PubMed] [Google Scholar]
- Leis AA, Kofler M, Ross MA. The silent period in pure sensory neuronopathy. Muscle Nerve. 1992;15:1345–1348. doi: 10.1002/mus.880151209. [DOI] [PubMed] [Google Scholar]
- Leis AA, Štetkárová I, Beric A, Stokic DS. Spinal motor neuron excitability during the cutaneous silent period. Muscle Nerve. 1995;18:1464–1470. doi: 10.1002/mus.880181218. [DOI] [PubMed] [Google Scholar]
- Leis AA, Štetkárová I, Beric A, Stokic DS. The relative sensitivity of F wave and H reflex to changes in motoneuronal excitability. Muscle Nerve. 1996;19:1342–1344. doi: 10.1002/(SICI)1097-4598(199610)19:10<1342::AID-MUS13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Leis AA, Stokic DS, Fuhr P, Kofler M, Kronenberg MF, Wissel J, Glocker FX, Seifert C, Štetkárová I. Nociceptive fingertip stimulation inhibits synergistic motoneuron pools in the human upper limb. Neurology. 2000;55:1305–1309. doi: 10.1212/wnl.55.9.1305. [DOI] [PubMed] [Google Scholar]
- Logigian EL, Plotkin GM, Shefner JM. The cutaneous silent period is mediated by spinal inhibitory reflex. Muscle Nerve. 1999;22:467–472. doi: 10.1002/(sici)1097-4598(199904)22:4<467::aid-mus7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Manconi FM, Syed NA, Floeter MK. Mechanisms underlying spinal motor neuron excitability during the cutaneous silent period in humans. Muscle Nerve. 1998;21:1256–1264. doi: 10.1002/(sici)1097-4598(199810)21:10<1256::aid-mus3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Müller J, Kofler M, Wenning GK, Seppi K, Valls-Solé J, Poewe W. Auditory startle response in cervical dystonia. Mov Disord. 2003;18:1522–1526. doi: 10.1002/mds.10609. [DOI] [PubMed] [Google Scholar]
- Pullman SL, Ford B, Elibol B, Uncini A, Su PC, Fahn S. Cutaneous electromyographic silent period findings in brachial dystonia. Neurology. 1996;46:503–508. doi: 10.1212/wnl.46.2.503. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus – auditory input, arousal and pathophysiology. Prog Neurobiol. 1995;42:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- Romaniello A, Truini A, Galeotti F, De Lena C, Willer JC, Cruccu G. Cutaneous silent period in hand muscle is evoked by laser stimulation of the palm, but not the hand dorsum. Muscle Nerve. 2004;29:870–872. doi: 10.1002/mus.20040. [DOI] [PubMed] [Google Scholar]
- Rossi P, Pierelli F, Parisi L, Perrotta A, Bartolo M, Amabile G, Serrao M. Effect of painful heterotopic stimulation on the cutaneous silent period in the upper limbs. Clin Neurophysiol. 2003;114:1–6. doi: 10.1016/s1388-2457(02)00321-8. [DOI] [PubMed] [Google Scholar]
- Rossi A, Scarpini C. Gating of trigemino-facial reflex from low-threshold trigeminal and extratrigeminal cutaneous fibres in humans. J Neurol Neurosurg Psychiatry. 1992;55:774–780. doi: 10.1136/jnnp.55.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štetkárová I, Kofler M. Cutaneous silent periods in assessment of mild compressive cervical spondylotic myelopathy. Spine. 2009 doi: 10.1097/BRS.0b013e31818f8be3. in press. [DOI] [PubMed] [Google Scholar]
- Svilpauskaite J, Truffert A, Vaiciene N, Magistris MR. Electrophysiology of small peripheral nerve fibers in man. A study using the cutaneous silent period. Medicina (Kaunas) 2006;42:300–313. [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Blumenthal TD, Hartman PL. Effects of discrete acoustic prestimuli on perceived intensity and behavioral responses to startling and tactile stimuli. Psychobiology. 1999;27:547–556. [Google Scholar]
- Tataroglu C, Uludag B, Karapinar N, Bademkiran F, Ertekin C. Cutaneous silent periods of the vastus medialis evoked by the stimulation of lateral femoral cutaneous nerve. Clin Neurophysiol. 2005;116:1335–1341. doi: 10.1016/j.clinph.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol. 1999;82:829–840. doi: 10.1152/jn.1999.82.2.829. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Effects of common excitatory and inhibitory inputs on motoneuron synchronization. J Neurophysiol. 2001;86:2807–2822. doi: 10.1152/jn.2001.86.6.2807. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Black box revisited: a technique for estimating postsynaptic potentials in neurons. Trends Neurosci. 2005;28:379–386. doi: 10.1016/j.tins.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Uncini A, Kujirai T, Gluck B, Pullman S. Silent period induced by cutaneous stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:344–352. doi: 10.1016/0168-5597(91)90023-q. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Cammarota A, Alvarez R, Hallett M. Orbicularis oculi responses to stimulation of nerve afferents from upper and lower limbs in normal humans. Brain Res. 1994;650:313–316. doi: 10.1016/0006-8993(94)91797-3. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegre M. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Valldeoriola F, Molinuevo JL, Cossu G, Nobbe F. Prepulse modulation of the startle reaction and the blink reflex in normal human subjects. Exp Brain Res. 1999;129:49–56. doi: 10.1007/s002210050935. [DOI] [PubMed] [Google Scholar]
- Walk D, Fisher MA. Effects of cutaneous stimulation on ipsilateral and contralateral motoneuron excitability. An analysis using H reflexes and F waves. Electromyogr Clin Neurophysiol. 1993;33:259–264. [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]